Proboscis Extension Response of Three Apis mellifera Subspecies toward Water and Sugars in Subtropical Ecosystem
Abstract
1. Introduction
2. Results
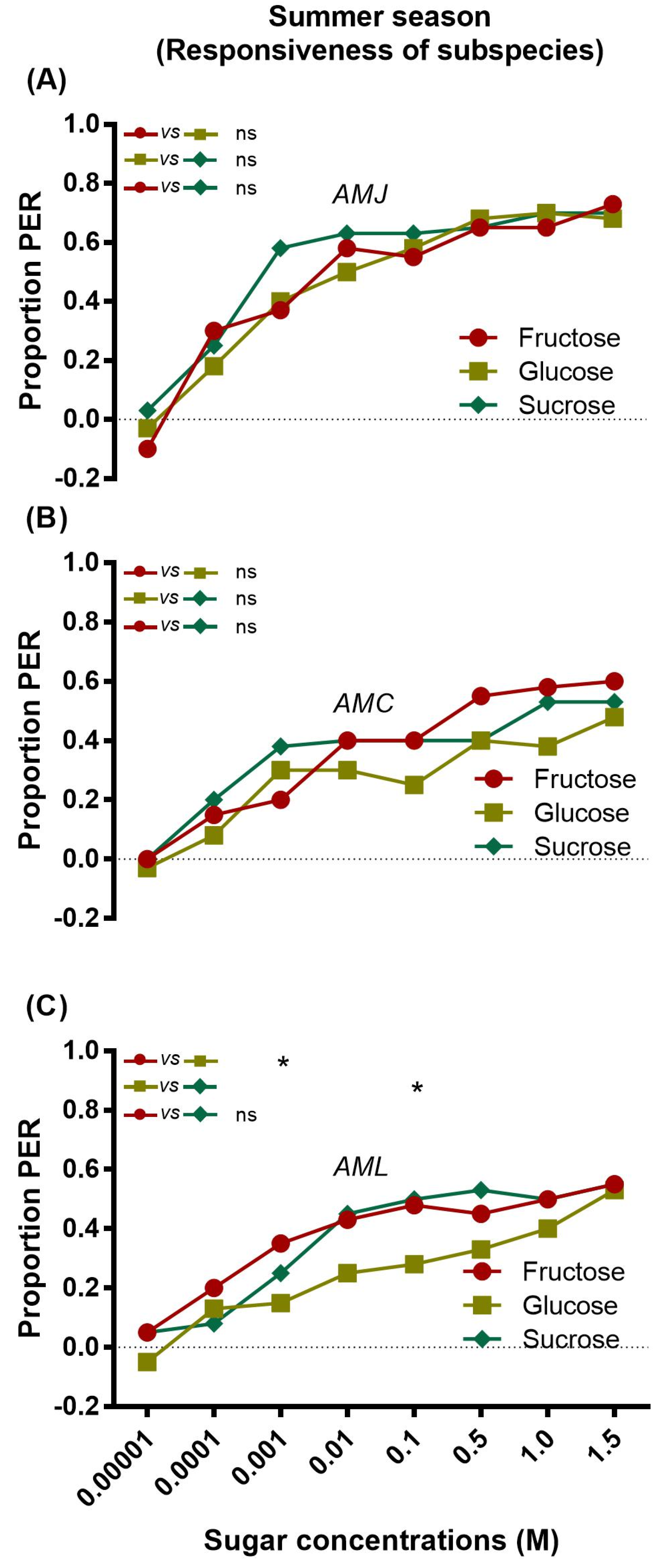
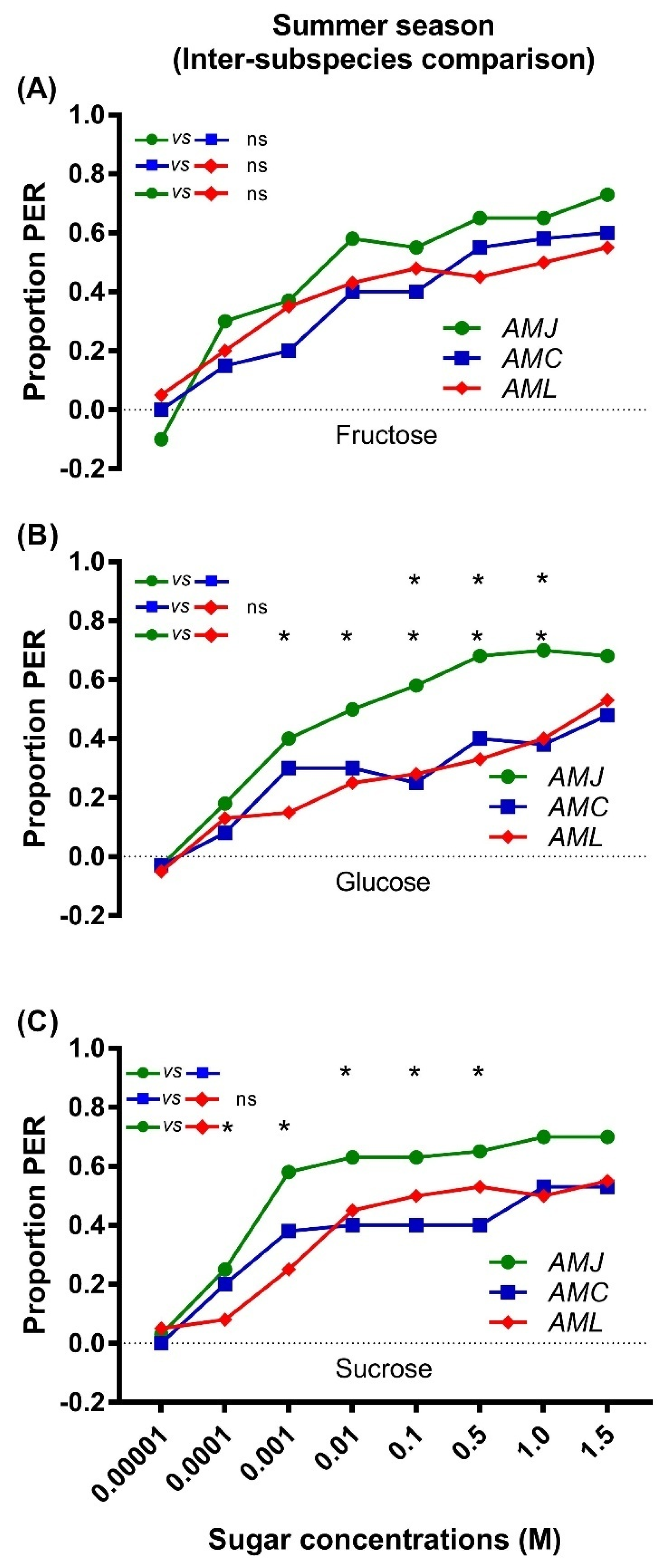
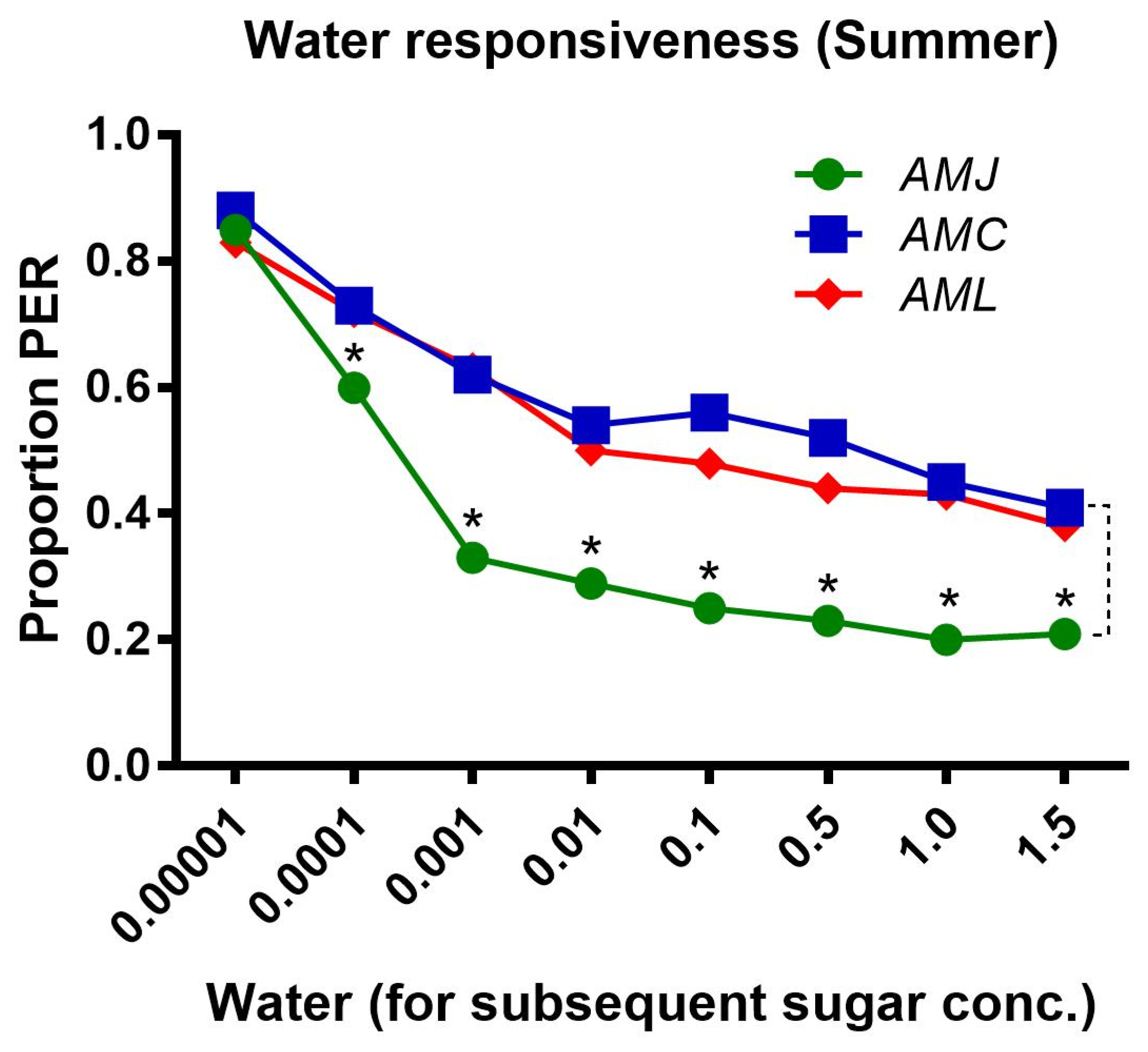
2.1. Responsiveness of Bees (Summer Season)
2.1.1. Responsiveness of a Single Subspecies to Sugars
2.1.2. Inter Subspecies Comparison of Responsiveness to Sugars
2.1.3. Water Responsiveness during the Summer Season
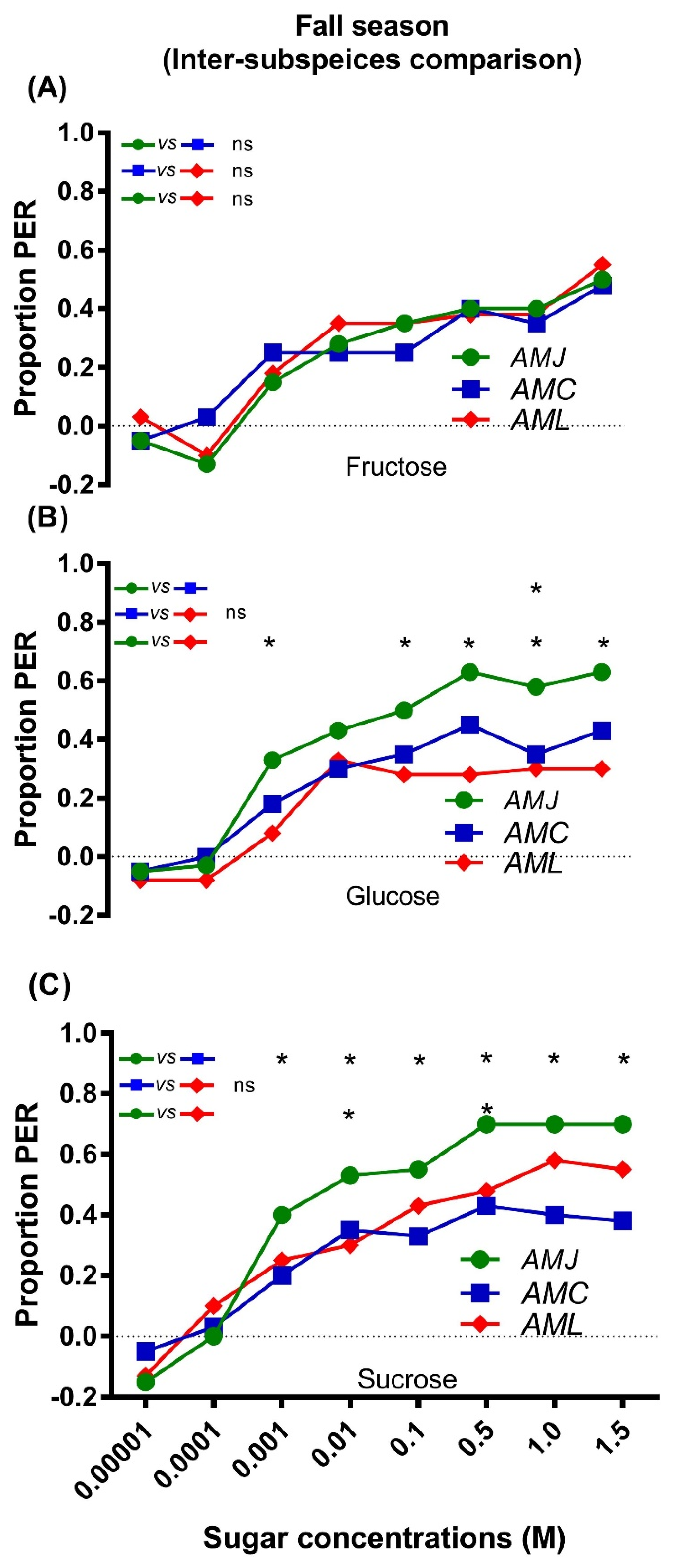
2.2. Responsiveness of Bees (Fall Season)
2.2.1. Responsiveness of a Single Subspecies to Sugars
2.2.2. Inter Subspecies Comparison of Responsiveness to Sugars
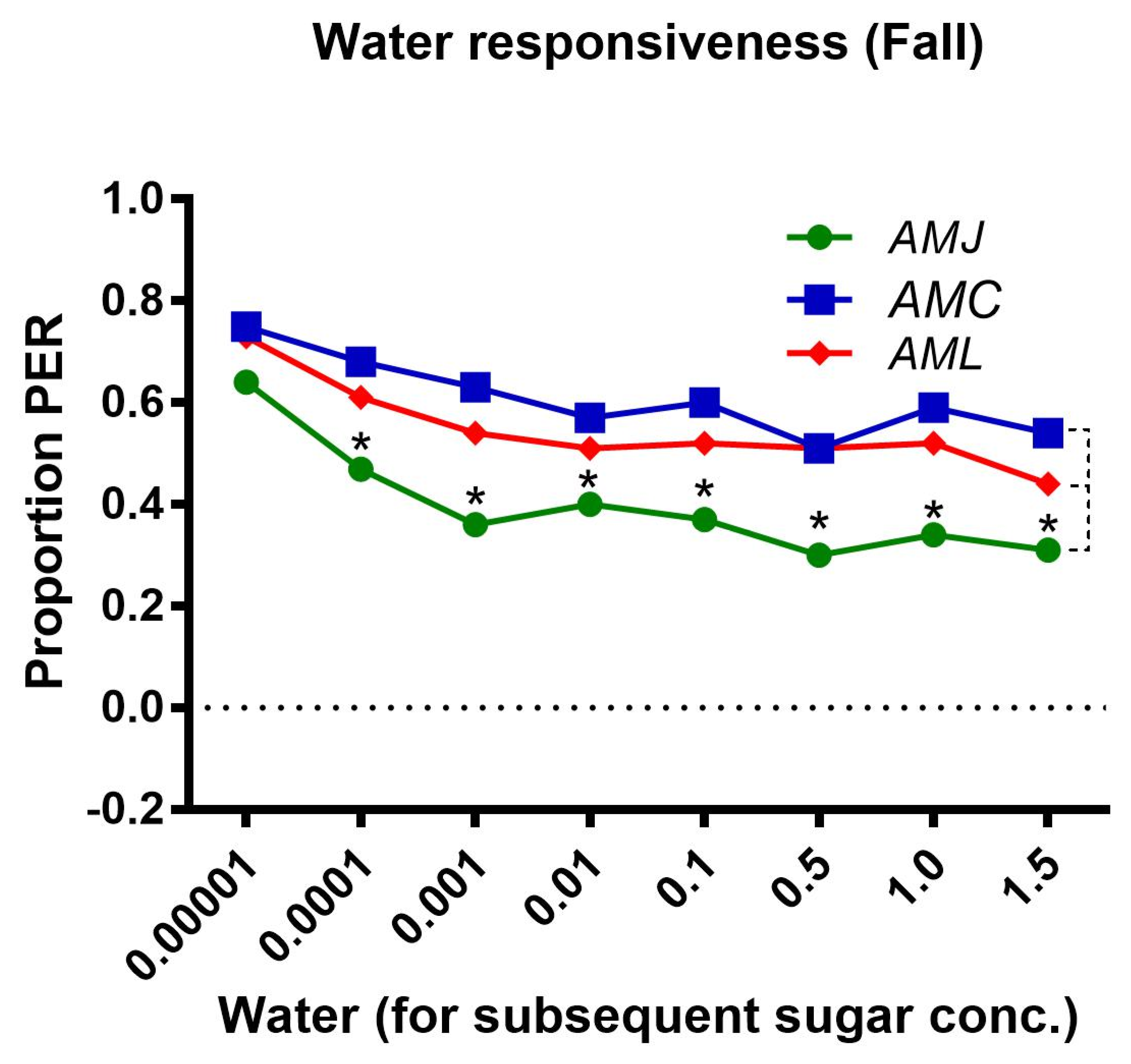
2.2.3. Water Responsiveness during the Fall Season
2.3. PER of Nectar vs. Pollen Foragers during the Fall Season
2.3.1. Sugar Responsiveness
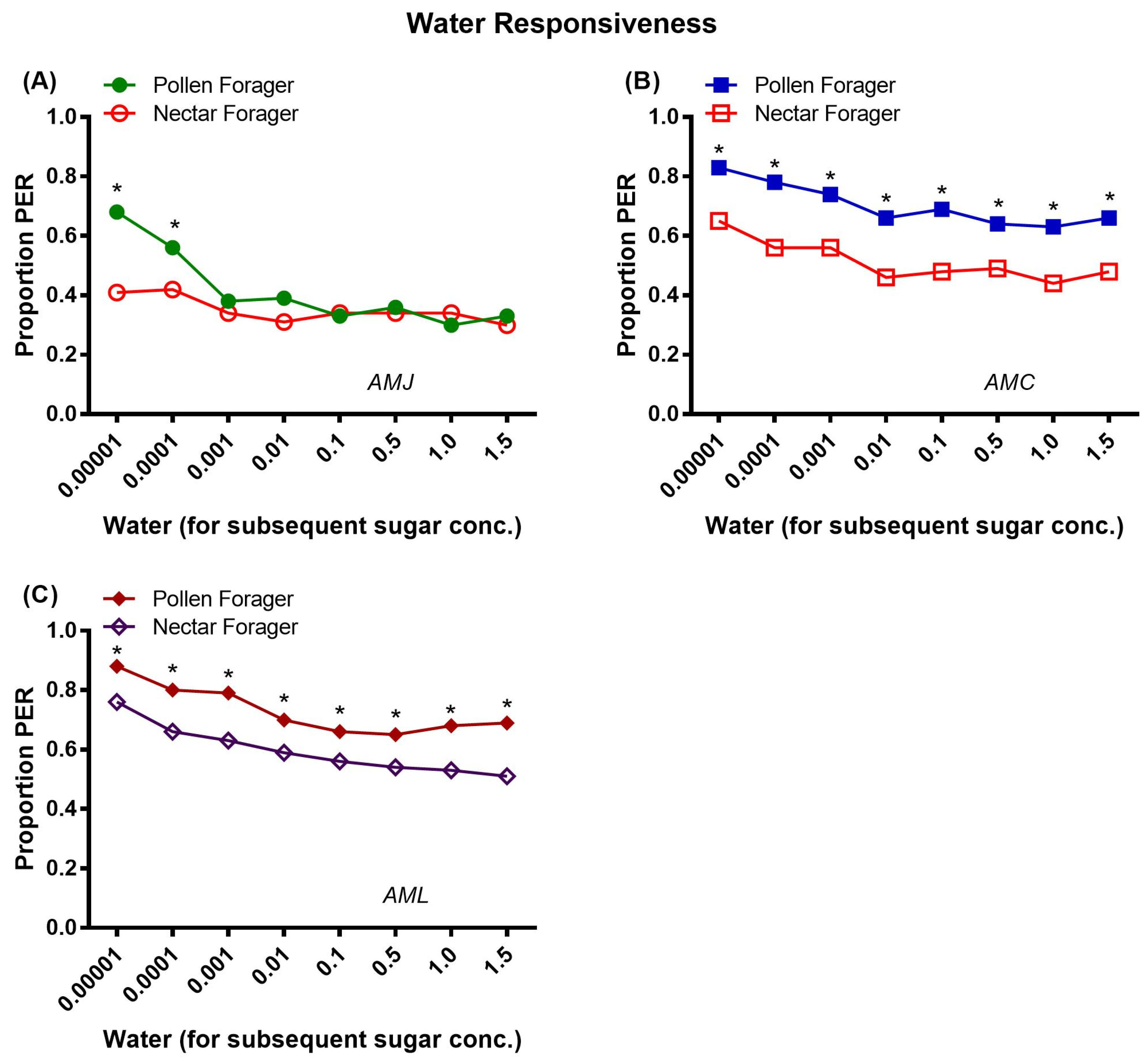
2.3.2. Water Responsiveness
3. Discussion
Nectar and Pollen Foragers
4. Materials and Methods
4.1. Honey bee Subspecies
4.2. Sample Collection and Behavioral Assay
4.3. Behavioral Tests for Bees’ Responsiveness
4.4. Meteorological Data
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alqarni, A. Beekeeping in Saudi Arabia: Current and future (in Arabic), 1st ed.; Saudi Society for Agricultural Sciences, King Saud University: Riyadh, Saudi Arabia, 2011; Volume 21, p. 40. [Google Scholar]
- Attfield, H.H.D. A Beekeeping Guide for the Tropics and Subtropics; AVITA: Tokyo, Japan, 2001. [Google Scholar]
- Abrol, D.P. Beekeeping: A Compressive Guide to Bees and Beekeeping; Scientific Publishers: Rajasthan, India, 2013; p. 896. [Google Scholar]
- Ilyasov, R.A.; Lee, M.-l.; Takahashi, J.-i.; Kwon, H.W.; Nikolenko, A.G. A revision of subspecies structure of western honey bee Apis mellifera. Saudi J. Biol. Sci. 2020, 27, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Taxonomy and distribution of different honeybee species. In Beekeeping for Poverty Alleviation and Livelihood Security; Gupta, R.K., Reybroeck, W., Veen, J.W.V., Gupta, A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 63–103. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Härtel, S.; Neumann, P. Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Ecoscience 2005, 12, 289–301. [Google Scholar] [CrossRef]
- Kim, K.T.; Oguro, J. Update on the status of Africanized honey bees in the western states. West. J. Med. 1999, 170, 220–222. [Google Scholar]
- Ji, Y. The geographical origin, refugia, and diversification of honey bees (Apis spp.) based on biogeography and niche modeling. Apidologie 2021, 52, 367–377. [Google Scholar] [CrossRef]
- Dogantzis, K.A.; Tiwari, T.; Conflitti, I.M.; Dey, A.; Patch, H.M.; Muli, E.M.; Garnery, L.; Whitfield, C.W.; Stolle, E.; Alqarni, A.S.; et al. Thrice out of Asia and the adaptive radiation of the western honey bee. Sci. Adv. 2021, 7, eabj2151. [Google Scholar] [CrossRef] [PubMed]
- Pankiw, T.; Waddington, K.D.; Page, R.E., Jr. Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): Influence of genotype, feeding, and foraging experience. J. Comp. Physiol. A 2001, 187, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Page, R.E., Jr.; Erber, J.; Fondrk, M.K. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J. Comp. Physiol. A 1998, 182, 489–500. [Google Scholar] [CrossRef]
- Pankiw, T. Brood pheromone regulates foraging activity of honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2004, 97, 748–751. [Google Scholar] [CrossRef]
- Scheiner, R.; Barnert, M.; Erber, J. Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie 2003, 34, 67–72. [Google Scholar] [CrossRef]
- Kovac, H.; Stabentheiner, A.; Schmaranzer, S. Thermoregulation of water foraging honeybees—Balancing of endothermic activity with radiative heat gain and functional requirements. J. Insect Physiol. 2010, 56, 1834–1845. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee colony thermoregulation–regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R.; Müller, U. Learning and memory in honeybees: From behavior to neural substrates. Annu. Rev. Neurosci. 1996, 19, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Ali, H.; Owayss, A.A.; Raweh, H.S.A.; Engel, M.S.; Alqarni, A.S.; Smith, B.H. Olfactory associative behavioral differences in three honey bee Apis mellifera L. races under the arid zone ecosystem of central Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R.; Greggers, U.; Smith, A.; Berger, S.; Brandt, R.; Brunke, S.; Bundrock, G.; Watzl, S. Honey bees navigate according to a map-like spatial memory. Proc. Natl. Acad. Sci. USA 2005, 102, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Corbet, S.A. Nectar sugar content: Estimating standing crop and secretion rate in the field. Apidologie 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Chalcoff, V.R.; Aizen, M.A.; Galetto, L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006, 97, 413–421. [Google Scholar] [CrossRef]
- Ali, H.; Iqbal, J.; Raweh, H.S.; Alqarni, A.S. Proboscis Behavioral Response of four honey bee Apis species towards different concentrations of sucrose, glucose, and fructose. Saudi J. Biol. Sci. 2021, 28, 3275–3283. [Google Scholar] [CrossRef]
- Iqbal, J.; Mueller, U. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B. 2007, 274, 1517–1521. [Google Scholar] [CrossRef]
- Smith, B.H.; Burden, C.M. A proboscis extension response protocol for investigating behavioral plasticity in insects: Application to basic, biomedical, and agricultural research. J. Vis. Exp. 2014, e51057. [Google Scholar] [CrossRef]
- Scheiner, R.; Page, R.E.; Erber, J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 2004, 35, 133–142. [Google Scholar] [CrossRef]
- Pankiw, T.; Page, J.R.E. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav. Ecol. Sociobiol. 2000, 47, 265–267. [Google Scholar] [CrossRef]
- Pankiw, T.; Page, R.E. Brood pheromone modulates honeybee (Apis mellifera L.) sucrose response thresholds. Behav. Ecol. Sociobiol. 2001, 49, 206–213. [Google Scholar] [CrossRef]
- Scheiner, R.; Page, R.E., Jr.; Erber, J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol. Learn. Mem. 2001, 76, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Pankiw, T.; Page, R.E.J. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J. Comp. Physiol. A 1999, 185, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.S. Differential foraging of indigenous and exotic honeybee (Apis mellifera L.) races on nectar-rich flow in a subtropical ecosystem. Insects 2020, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.S.; Iqbal, J.; Raweh, H.S.; Hassan, A.M.A.; Owayss, A.A. Beekeeping in the Desert: Foraging Activities of Honey Bee during Major Honeyflow in a Hot-Arid Ecosystem. Appl. Sci. 2021, 11, 9756. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Hannan, M.A.; Owayss, A.A.; Engel, M.S. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): Their natural history and role in beekeeping. Zookeys 2011, 134, 83–98. [Google Scholar] [CrossRef]
- Pankiw, T.; Nelson, M.; Page, R.E.; Fondrk, M. The communal crop: Modulation of sucrose response thresholds of pre-foraging honey bees with incoming nectar quality. Behav. Ecol. Sociobiol. 2004, 55, 286–292. [Google Scholar] [CrossRef]
- Alqarni, A.S. Tolerance of summer temperature in imported and indigenous honeybee Apis mellifera L. races in central Saudi Arabia. Saudi J. Biol. Sci. 2006, 13, 123–127. [Google Scholar]
- Awad, A.M.; Owayss, A.A.; Alqarni, A.S. Performance of two honey bee subspecies during harsh weather and Acacia gerrardii nectar-rich flow. Sci. Agric. 2017, 74, 474–480. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Ali, H.; Iqbal, J.; Owayss, A.A.; Smith, B.H. Expression of heat shock proteins in adult honey bee (Apis mellifera L.) workers under hot-arid subtropical ecosystems. Saudi J. Biol. Sci. 2019, 26, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Ferneyhough, B. Seasonal variation of proboscis extension reflex conditioning in the honey bee (Apis Mellifera). J. Apic. Res. 1997, 36, 108–110. [Google Scholar] [CrossRef]
- Iqbal, J.; Alqarni, A.S.; Raweh, H.S.A. Effect of sub-lethal doses of imidacloprid on learning and memory formation of indigenous Arabian bee (Apis mellifera jemenitica Ruttner) adult foragers. Neotrop. Entomol. 2019, 48, 373–380. [Google Scholar] [CrossRef]
- Raweh, H.S.A.; Ahmed, A.Y.B.H.; Iqbal, J.; Alqarni, A.S. Monitoring and evaluation of free acidity levels in Talh honey originated from Talh tree Acacia gerrardii Benth. J. King Saud Univ. Sci. 2022, 34, 101678. [Google Scholar] [CrossRef]
- Scheiner, R. Responsiveness to sucrose and habituation of the proboscis extension response in honey bees. J. Comp. Physiol. A Sens. Neural. Behav. Physiol. 2004, 190, 727–733. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, A.S.; Ali, H.; Iqbal, J.; Raweh, H.S.A. Proboscis Extension Response of Three Apis mellifera Subspecies toward Water and Sugars in Subtropical Ecosystem. Stresses 2023, 3, 182-197. https://doi.org/10.3390/stresses3010014
Alqarni AS, Ali H, Iqbal J, Raweh HSA. Proboscis Extension Response of Three Apis mellifera Subspecies toward Water and Sugars in Subtropical Ecosystem. Stresses. 2023; 3(1):182-197. https://doi.org/10.3390/stresses3010014
Chicago/Turabian StyleAlqarni, Abdulaziz S., Hussain Ali, Javaid Iqbal, and Hael S. A. Raweh. 2023. "Proboscis Extension Response of Three Apis mellifera Subspecies toward Water and Sugars in Subtropical Ecosystem" Stresses 3, no. 1: 182-197. https://doi.org/10.3390/stresses3010014
APA StyleAlqarni, A. S., Ali, H., Iqbal, J., & Raweh, H. S. A. (2023). Proboscis Extension Response of Three Apis mellifera Subspecies toward Water and Sugars in Subtropical Ecosystem. Stresses, 3(1), 182-197. https://doi.org/10.3390/stresses3010014





