Fetal Programming of Brain and Behavior through Ionizing Radiation
Abstract
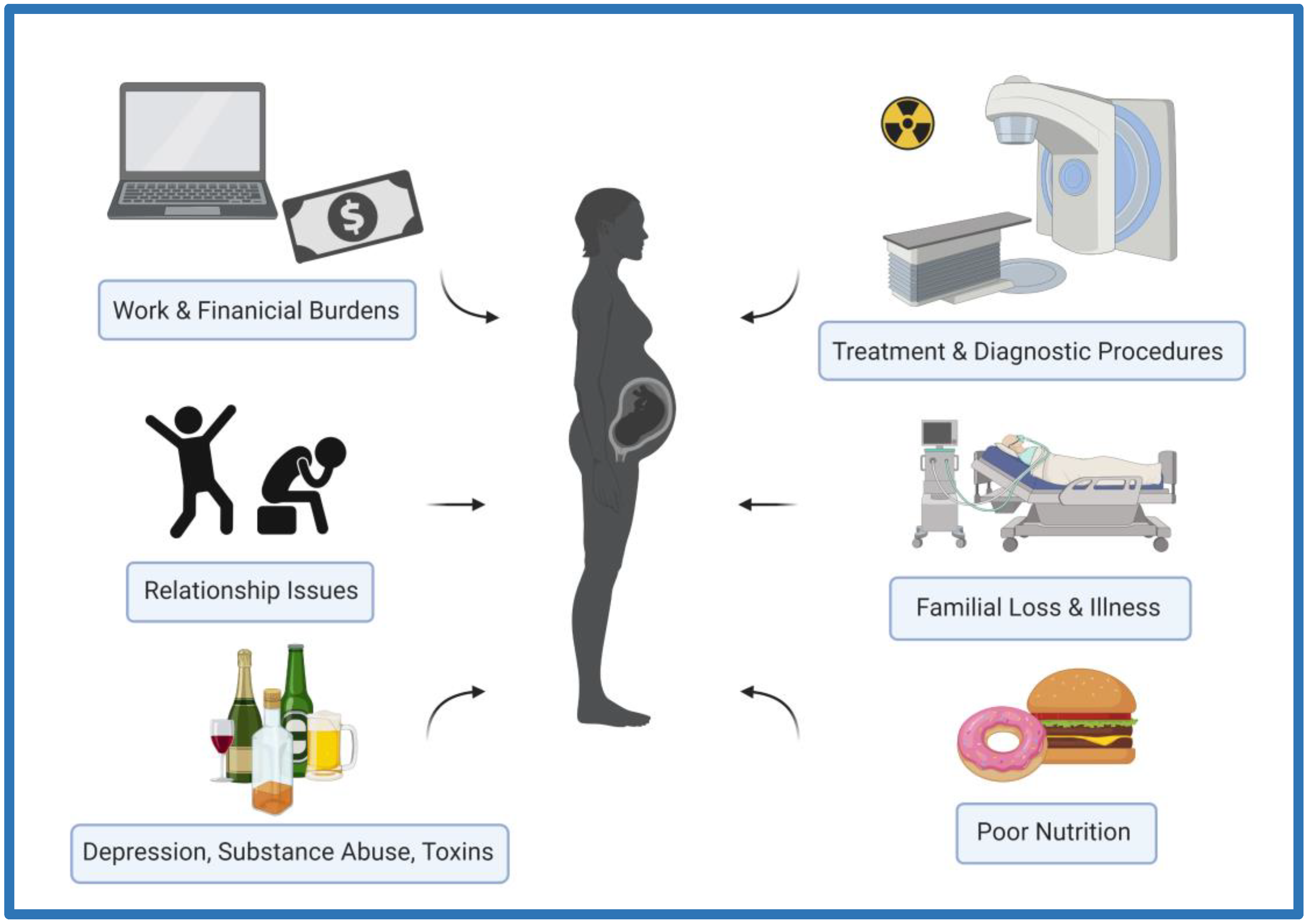
1. Introduction: Brain and Behavior
2. Fetal Programming of Brain and Behavior
3. Ionizing Radiation as a Stressor
3.1. The Prefrontal and Cerebral Cortices
3.2. The Hippocampus
3.3. The Cerebellum
3.4. The Hypothalamic–Pituitary–Adrenal Axis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell, R.A.; Honey, P.L.; Symbaluk, D.G. Introduction to Learning and Behavior, 5th ed.; Wadsworth Publishing: Belmont, CA, USA, 2016. [Google Scholar]
- Hughes, R.N.; Kaiser, M.J.; Mackney, P.A.; Warburton, K. Optimizing foraging behaviour through learning. J. Fish Biol. 1992, 41, 77–91. [Google Scholar] [CrossRef]
- Thornton, A.; Clutton-Brock, T. Social learning and the development of individual and group behaviour in mammal societies. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Morand-Ferron, J. Why learn? The adaptive value of associative learning in wild populations. Curr. Opin. Behav. Sci. 2017, 16, 73–79. [Google Scholar] [CrossRef]
- Plath, M.; Liu, K.; Umutoni, D.; Gomes-Silva, G.; Wei, J.-F.; Cyubahiro, E.; Chen, B.-J.; Sommer-Trembo, C. Predator-induced changes of male and female mating preferences: Innate and learned components. Curr. Zool. 2019, 65, 305–316. [Google Scholar] [CrossRef]
- Black, A.H.; Nadel, L.; O’Keefe, J. Hippocampal function in avoidance learning and punishment. Psychol. Bull. 1977, 84, 1107–1129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Narayanan, S.; Kumar, N.; Nayak, S. Exposure to enriched environment restores altered passive avoidance learning and ameliorates hippocampal injury in male albino Wistar rats subjected to chronic restraint stress. Int. J. Appl. Basic Med. Res. 2018, 8, 231–236. [Google Scholar] [CrossRef]
- Judah, G.; Gardner, B.; Kenward, M.G.; DeStavola, B.; Aunger, R. Exploratory study of the impact of perceived reward on habit formation. BMC Psychol. 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Brust, V.; Schindler, P.M.; Lewejohann, L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 2015, 12, S17. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 2005, 8, 1481–1489. [Google Scholar] [CrossRef]
- Miller, R.R.; Polack, C.W. Sources of maladaptive behavior in ‘normal’ organisms. Behav. Process. 2018, 154, 4–12. [Google Scholar] [CrossRef]
- Ersche, K.D.; Gillan, C.M.; Jones, P.S.; Williams, G.B.; Ward, L.H.E.; Luijten, M.; de Wit, S.; Sahakian, B.J.; Bullmore, E.T.; Robbins, T.W. Carrots and sticks fail to change behavior in cocaine addiction. Science 2016, 352, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.E.; Sheynin, J.; Balsdon, T.; Luzardo, A.; Beck, K.D.; Hogarth, L.; Haber, P.; Moustafa, A.A. Probabilistic reward- and punishment-based learning in opioid addiction: Experimental and computational data. Behav. Brain Res. 2016, 296, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hair, P.; Hampson, S.E. The role of impulsivity in predicting maladaptive behaviour among female students. Pers. Individ. Differ. 2006, 40, 943–952. [Google Scholar] [CrossRef]
- Loxton, N.J.; Nguyen, D.; Casey, L.; Dawe, S. Reward drive, rash impulsivity and punishment sensitivity in problem gamblers. Pers. Individ. Differ. 2008, 45, 167–173. [Google Scholar] [CrossRef]
- Barch, D.M.; Ceaser, A. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends Cogn. Sci. 2012, 16, 27–34. [Google Scholar] [CrossRef]
- Diehl, M.M.; Lempert, K.M.; Parr, A.C.; Ballard, I.; Steele, V.; Smith, D.V. Toward an integrative perspective on the neural mechanisms underlying persistent maladaptive behaviors. Eur. J. Neurosci. 2018, 48, 1870–1883. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Circuit dynamics of adaptive and maladaptive behaviour. Nature 2014, 505, 309–317. [Google Scholar] [CrossRef]
- DeFelipe, J. Sesquicentenary of the birthday of Santiago Ramón y Cajal, the father of modern neuroscience. Trends Neurosci. 2002, 25, 481–484. [Google Scholar] [CrossRef]
- DeFelipe, J. Brain plasticity and mental processes: Cajal again. Nat. Rev. Neurosci. 2006, 7, 811–817. [Google Scholar] [CrossRef]
- Phenotypic Plasticity: Functional and Conceptual Approaches-Google Books. Available online: https://books.google.ca/books?hl=en&lr=&id=7A3AdwmEi-UC&oi=fnd&pg=PA1&dq=plasticity+biology&ots=5kH-wBb3JZ&sig=nUqObPEHWWZqbBPqwHAnQ7wFV0A#v=onepage&q=plasticity%20biology&f=false (accessed on 14 April 2020).
- Verreet, T.; Verslegers, M.; Quintens, R.; Baatout, S.; Benotmane, R. Current Evidence for Developmental, Structural, and Functional Brain Defects following Prenatal Radiation Exposure. Neural Plast. 2016, 2016, 1243527. [Google Scholar] [CrossRef]
- Zucchi, F.C.R.; Yao, Y.; Ward, I.D.; Ilnytskyy, Y.; Olson, D.M.; Benzies, K.; Kovalchuk, I.; Kovalchuk, O.; Metz, G.A.S. Maternal Stress Induces Epigenetic Signatures of Psychiatric and Neurological Diseases in the Offspring. PLoS ONE 2013, 8, e56967. [Google Scholar] [CrossRef] [PubMed]
- Korosi, A.; Naninck, E.; Oomen, C.; Schouten, M.; Krugers, H.; Fitzsimons, C.; Lucassen, P. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 2012, 227, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J.; Aranceta, J.; Serra-Majem, L.; Ribas, L.; Pérez-Rodrigo, C. Fetal programming and adult health. Public Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef]
- Sallout, B.; Walker, M. The fetal origin of adult diseases. J. Obstet. Gynaecol. 2003, 23, 555–560. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.P. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Vaag, A.A.; Grunnet, L.G.; Arora, G.P.; Brøns, C. The thrifty phenotype hypothesis revisited. Diabetologia 2012, 55, 2085–2088. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Chivers, E.K.; Wyrwoll, C.S. Maternal Malnutrition, Glucocorticoids, and Fetal Programming: A Role for Placental 11β-Hydroxysteroid Dehydrogenase Type 2. In Diet, Nutrition, and Fetal Programming; Springer International Publishing: Cham, Switzerland, 2017; pp. 543–555. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Buss, C.; Wadhwa, P.D.; Entringer, S. The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biol. Psychiatry 2018, 85, 135–149. [Google Scholar] [CrossRef]
- Lesage, J.; Blondeau, B.; Grino, M.; Bréant, B.; Dupouy, J.P. Maternal Undernutrition during Late Gestation Induces Fetal Overexposure to Glucocorticoids and Intrauterine Growth Retardation, and Disturbs the Hypothalamo-Pituitary Adrenal Axis in the Newborn Rat1. Endocrinology 2001, 142, 1692–1702. [Google Scholar] [CrossRef]
- Correia-Branco, A.; Keating, E.; Martel, F. Maternal Undernutrition and Fetal Developmental Programming of Obesity. Reprod. Sci. 2014, 22, 138–145. [Google Scholar] [CrossRef]
- Seckl, J.R. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol. Cell Endocrinol. 2001, 185, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J.R.; Meaney, M.J. Glucocorticoid Programming. Ann. N.Y. Acad. Sci. 2004, 1032, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.G.; Owen, D.; Kalabis, G.; Banjanin, S.; Setiawan, E.B.; Dunn, E.A.; Andrews, M.H. Fetal Glucocorticoid Exposure and Hypothalamo-Pituitary-Adrenal (HPA) Function After Birth. Endocr. Res. 2004, 30, 827–836. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Matthews, S.G. Prenatal Stress, Glucocorticoids, and Developmental Programming of the Stress Response. Endocrinology 2018, 159, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Petropoulos, S.; Matthews, S.G. Fetal programming of hypothalamic–pituitary–adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res. Rev. 2008, 57, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, G.-Q.; Peng, J.-P.; Shen, C. Glucocorticoids induce apoptosis and matrix metalloproteinase-13 expression in chondrocytes through the NOX4/ROS/p38 MAPK pathway. J. Steroid Biochem. Mol. Biol. 2018, 181, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.L.; Owen, M.; Fagan-Murphy, A.; Intabli, H.; Healy, D.; Patel, A.; Allen, M.C.; Patel, B.A.; Flint, M.S. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017, 19, 35. [Google Scholar] [CrossRef]
- Stress Science: Neuroendocrinology-Google Books. Available online: https://books.google.ca/books?hl=en&lr=&id=HJwqWQhQELMC&oi=fnd&pg=PA3&dq=stress+definition&ots=ooqPf-2b_-&sig=R_3UlYwxjQM-33ayUW_CmLRogJU#v=onepage&q=stress%20definition&f=false (accessed on 14 April 2020).
- Mora, F.; Segovia, G.; del Arco, A.; de Blas, M.; Garrido, P. Stress, neurotransmitters, corticosterone and body–brain integration. Brain Res. 2012, 1476, 71–85. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Tharmalingam, S.; Sreetharan, S.; Kulesza, A.V.; Boreham, D.R.; Tai, T.C. Low-Dose Ionizing Radiation Exposure, Oxidative Stress and Epigenetic Programing of Health and Disease. Radiat. Res. 2017, 188, 525–538. [Google Scholar] [CrossRef]
- Sreetharan, S.; Thome, C.; Tharmalingam, S.; Jones, D.E.; Kulesza, A.V.; Khaper, N.; Lees, S.J.; Wilson, J.Y.; Boreham, D.R.; Tai, T.C. Ionizing Radiation Exposure During Pregnancy: Effects on Postnatal Development and Life. Radiat. Res. 2017, 187, 647–658. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Production of low-energy electrons by ionizing radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Limoli, C.; Ponnaiya, B.; Corcoran, J.; Giedzinski, E.; Kaplan, M.; Hartmann, A.; Morgan, W. Genomic instability induced by high and low let ionizing radiation. Adv. Space Res. 2000, 25, 2107–2117. [Google Scholar] [CrossRef]
- He, C.-Q.; Mao, L.; Yao, J.; Zhao, W.-C.; Huang, B.; Hu, N.; Long, D.-X. The Threshold Effects of Low-Dose-Rate Radiation on miRNA-Mediated Neurodevelopment of Zebrafish. Radiat. Res. 2021, 196, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose-Response 2018, 16, 1559325818796331. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.-J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef]
- Lemon, J.A.; Phan, N.; Boreham, D.R. Multiple CT Scans Extend Lifespan by Delaying Cancer Progression in Cancer-Prone Mice. Radiat. Res. 2017, 188, 495–504. [Google Scholar] [CrossRef]
- Lemon, J.A.; Phan, N.; Boreham, D.R. Single CT Scan Prolongs Survival by Extending Cancer Latency in Trp53 Heterozygous Mice. Radiat. Res. 2017, 188, 505–511. [Google Scholar] [CrossRef]
- Carlén, M. What constitutes the prefrontal cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Gee, D.; Humphreys, K.L.; Flannery, J.; Goff, B.; Telzer, E.H.; Shapiro, M.; Hare, T.; Bookheimer, S.; Tottenham, N. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. J. Neurosci. 2013, 33, 4584–4593. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Klavir, O.; Prigge, M.; Sarel, A.; Paz, R.; Yizhar, O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat. Neurosci. 2017, 20, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhong, P.; Qin, L.; Tan, T.; Yan, Z. Chemicogenetic Restoration of the Prefrontal Cortex to Amygdala Pathway Ameliorates Stress-Induced Deficits. Cereb. Cortex 2018, 28, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Vujaskovic, Z.; Down, J.D. Revisiting Strain-Related Differences in Radiation Sensitivity of the Mouse Lung: Recognizing and Avoiding the Confounding Effects of Pleural Effusions. Radiat. Res. 2010, 173, 10–20. [Google Scholar] [CrossRef]
- Ponnaiya, B.; Cornforth, M.N.; Ullrich, R.L. Radiation-Induced Chromosomal Instability in BALB/c and C57BL/6 Mice: The Difference Is as Clear as Black and White. Radiat. Res. 1997, 147, 121. [Google Scholar] [CrossRef]
- Roderick, T.H. The Response of Twenty-Seven Inbred Strains of Mice to Daily Doses of Whole-Body X-Irradiation. Radiat. Res. 1963, 20, 631. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H. Prefrontal–hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017, 18, 547–558. [Google Scholar] [CrossRef]
- Faa, G.; Manchia, M.; Pintus, R.; Gerosa, C.; Marcialis, M.A.; Fanos, V. Fetal programming of neuropsychiatric disorders. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 207–223. [Google Scholar] [CrossRef]
- Goyal, N.; Siddiqui, S.V.; Chatterjee, U.; Kumar, D.; Siddiqui, A. Neuropsychology of prefrontal cortex. Indian J. Psychiatry 2008, 50, 202–208. [Google Scholar] [CrossRef]
- Laubach, M.; Amarante, L.; Swanson, T.K.; White, S. What, If Anything, Is Rodent Prefrontal Cortex? Eneuro 2018, 5, 315–333. [Google Scholar] [CrossRef]
- Muir, J.L.; Everitt, B.J.; Robbins, T.W. The Cerebral Cortex of the Rat and Visual Attentional Function: Dissociable Effects of Mediofrontal, Cingulate, Anterior Dorsolateral, and Parietal Cortex Lesions on a Five-Choice Serial Reaction Time Task. Cereb. Cortex 1992, 6, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Narducci, R.; Baroncelli, L.; Sansevero, G.; Begenisic, T.; Prontera, C.; Sale, A.; Cenni, M.C.; Berardi, N.; Maffei, L. Early impoverished environment delays the maturation of cerebral cortex. Sci. Rep. 2018, 8, 1187. [Google Scholar] [CrossRef]
- Burgos, H.; Hernández, A.; Constandil, L.; Ríos, M.; Flores, O.; Puentes, G.; Hernández, K.; Morgan, C.; Valladares, L.; Castillo, A.; et al. Early postnatal environmental enrichment restores neurochemical and functional plasticities of the cerebral cortex and improves learning performance in hidden-prenatally-malnourished young-adult rats. Behav. Brain Res. 2019, 363, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.H.; Kostaki, A.; Setiawan, E.; McCabe, L.; Owen, D.; Banjanin, S.; Matthews, S.G. Developmental regulation of the 5-HT7 serotonin receptor and transcription factor NGFI-A in the fetal guinea-pig limbic system: Influence of GCs. J. Physiol. 2004, 555, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 2: Mechanisms. Nat. Rev. Endocrinol. 2014, 10, 403–411. [Google Scholar] [CrossRef]
- Norton, S.; Kimler, B.F. Comparison of functional and morphological deficits in the rat after gestational exposure to ionizing radiation. Neurotoxicology Teratol. 1988, 10, 363–371. [Google Scholar] [CrossRef]
- Norton, S.; Donoso, J. Forebrain damage following prenatal exposure to low-dose X-irradiation. Exp. Neurol. 1985, 87, 185–197. [Google Scholar] [CrossRef]
- Selemon, L.D.; Wang, L.; Nebel, M.B.; Csernansky, J.G.; Goldman-Rakic, P.S.; Rakic, P. Direct and indirect effects of fetal irradiation on cortical gray and white matter volume in the macaque. Biol. Psychiatry 2005, 57, 83–90. [Google Scholar] [CrossRef]
- Selemon, L.D.; Zecevic, N. Schizophrenia: A tale of two critical periods for prefrontal cortical development. Transl. Psychiatry 2015, 5, e623. [Google Scholar] [CrossRef]
- Selemon, L.D.; Ceritoglu, C.; Ratnanather, J.T.; Wang, L.; Harms, M.P.; Aldridge, K.; Begović, A.; Csernansky, J.G.; Miller, M.I.; Rakic, P. Distinct abnormalities of the primate prefrontal cortex caused by ionizing radiation in early or midgestation. J. Comp. Neurol. 2013, 521, 1040–1053. [Google Scholar] [CrossRef]
- Fushiki, S.; Hyodo-Taguchi, Y.; Kinoshita, C.; Ishikawa, Y.; Hirobe, T. Short- and long-term effects of low-dose prenatal X-irradiation in mouse cerebral cortex, with special reference to neuronal migration. Acta Neuropathol. 1997, 93, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.L.; Lent, R. Effects of prenatal irradiation on the development of cerebral cortex and corpus callosum of the mouse. J. Comp. Neurol. 1987, 264, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Furini, C.; Zinn, C.; Cavalcante, L.; Ferreira, F.; Behling, J.; Myskiw, J.; Izquierdo, I. Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiol. Learn. Mem. 2017, 142, 48–54. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Yavas, E.; Gonzalez, S.; Fanselow, M.S. Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory. F1000Research 2019, 8, 1292. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Yao, X.; Huang, J.; Bai, S. Prenatal Stress Up-Regulated Hippocampal Glucocorticoid Receptor Expression in Female Adult Rat Offspring Estrés Prenatal Expresión del Receptor de Glucocorticoides del Hipocampo Regulado por Aumento en Crías de Ratas Hembras Adultas. Int. J. Morphol. 2020, 38, 400–405. [Google Scholar] [CrossRef]
- Constantinof, A.; Moisiadis, V.G.; Kostaki, A.; Szyf, M.; Matthews, S.G. Antenatal Glucocorticoid Exposure Results in Sex-Specific and Transgenerational Changes in Prefrontal Cortex Gene Transcription that Relate to Behavioural Outcomes. Sci. Rep. 2019, 9, 764. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Gibb, R.; Kolb, B. Prenatal Stress Produces Sexually Dimorphic and Regionally Specific Changes in Gene Expression in Hippocampus and Frontal Cortex of Developing Rat Offspring. Dev. Neurosci. 2011, 33, 531–538. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Hellstrom, I.; Bagot, R.C.; Wen, X.; Diorio, J.; Meaney, M.J. Maternal Care and DNA Methylation of a Glutamic Acid Decarboxylase 1 Promoter in Rat Hippocampus. J. Neurosci. 2010, 30, 13130–13137. [Google Scholar] [CrossRef]
- Bagot, R.C.; Tse, Y.C.; Nguyen, H.-B.; Wong, A.S.; Meaney, M.J.; Wong, T.P. Maternal Care Influences Hippocampal N-Methyl-D-Aspartate Receptor Function and Dynamic Regulation by Corticosterone in Adulthood. Biol. Psychiatry 2012, 72, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bagot, R.C.; Zhang, T.-Y.; Wen, X.; Nguyen, T.T.T.; Nguyen, H.-B.; Diorio, J.; Wong, T.P.; Meaney, M.J. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc. Natl. Acad. Sci. USA 2012, 109, 17200–17207. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.L.; Bagot, R.C.; Van Hasselt, F.; Ramakers, G.; Meaney, M.J.; de Kloet, R.; Joels, M.; Krugers, H. Maternal Care and Hippocampal Plasticity: Evidence for Experience-Dependent Structural Plasticity, Altered Synaptic Functioning, and Differential Responsiveness to Glucocorticoids and Stress. J. Neurosci. 2008, 28, 6037–6045. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-B.; Bagot, R.C.; Diorio, J.; Wong, T.P.; Meaney, M.J. Maternal Care Differentially Affects Neuronal Excitability and Synaptic Plasticity in the Dorsal and Ventral Hippocampus. Neuropsychopharmacology 2015, 40, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- van Hasselt, F.N.; Cornelisse, S.; Zhang, T.Y.; Meaney, M.J.; Velzing, E.H.; Krugers, H.J.; Joëls, M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus 2012, 22, 255–266. [Google Scholar] [CrossRef]
- Verreet, T.; Quintens, R.; Van Dam, D.; Verslegers, M.; Tanori, M.; Casciati, A.; Neefs, M.; Leysen, L.; Michaux, A.; Janssen, A.; et al. A multidisciplinary approach unravels early and persistent effects of X-ray exposure at the onset of prenatal neurogenesis. J. Neurodev. Disord. 2015, 7, 3. [Google Scholar] [CrossRef]
- Kempf, S.J.; von Toerne, C.; Hauck, S.M.; Atkinson, M.J.; Benotmane, M.A.; Tapio, S. Long-term consequences of in utero irradiated mice indicate proteomic changes in synaptic plasticity related signalling. Proteome Sci. 2015, 13, 26. [Google Scholar] [CrossRef]
- Koturbash, I.; Zemp, F.; Kolb, B.; Kovalchuk, O. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 722, 114–118. [Google Scholar] [CrossRef]
- Saito, S.; Sawada, K.; Aoki, I. Prenatal Irradiation-Induced Hippocampal Abnormalities in Rats Evaluated Using Manganese-Enhanced MRI. Front. Neural Circuits 2018, 12, 112. [Google Scholar] [CrossRef]
- Baskar, R.; Devi, P. Influence of gestational age to low-level gamma irradiation on postnatal behavior in mice. Neurotoxicology Teratol. 2000, 22, 593–602. [Google Scholar] [CrossRef]
- Ganapathi, R.; Manda, K. Later Life Changes in Hippocampal Neurogenesis and Behavioral Functions After Low-Dose Prenatal Irradiation at Early Organogenesis Stage. Int. J. Radiat. Oncol. 2017, 98, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. The Cerebellum and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Chadman, K.K.; Brown, W.T.; Junaid, M.A. Microarray Analysis Reveals Higher Gestational Folic Acid Alters Expression of Genes in the Cerebellum of Mice Offspring—A Pilot Study. Brain Sci. 2015, 5, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Pourié, G.; Martin, N.; Bossenmeyer-Pourié, C.; Akchiche, N.; Guéant-Rodriguez, R.M.; Geoffroy, A.; Jeannesson, E.; Chehadeh, S.E.H.; Mimoun, K.; Brachet, P.; et al. Folate- and vitamin B12–deficient diet during gestation and lactation alters cerebellar synapsin expression via impaired influence of estrogen nuclear receptor α. FASEB J. 2015, 29, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Davis, E.P.; Muftuler, L.T.; Head, K.; Sandman, C.A. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 2010, 35, 141–153. [Google Scholar] [CrossRef]
- A Sandman, C.; Glynn, L.M.; Davis, E.P. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013, 75, 327–335. [Google Scholar] [CrossRef]
- Pascual, R.; Valencia, M.; Larrea, S.; Bustamante, C. Single course of antenatal betamethasone produces delayed changes in morphology and calbindin-D28k expression in a rat’s cerebellar Purkinje cells. Acta Neurobiol. Exp. 2014, 74, 415–423. [Google Scholar]
- Darmanto, W.; Inouye, M.; Murata, Y. Risk of Gamma X-Rays Irradiation and Cyclotron Exposure in the Brain Development of Rats. Pros. SNasPPM 2017, 1, 1–7. [Google Scholar]
- McCormick, C.M.; Mathews, I.Z. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 2007, 86, 220–233. [Google Scholar] [CrossRef]
- Watson, S.; Mackin, P. HPA axis function in mood disorders. Psychiatry 2006, 5, 166–170. [Google Scholar] [CrossRef]
- Rentesi, G.; Antoniou, K.; Marselos, M.; Fotopoulos, A.; Alboycharali, J.; Konstandi, M. Long-term consequences of early maternal deprivation in serotonergic activity and HPA function in adult rat. Neurosci. Lett. 2010, 480, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.G.; Cervoni, N.; A Champagne, F.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Alt, S.R.; Cao, L.; Vernocchi, S.; Trifonova, S.; Battello, N.; Muller, C.P. Transcriptional control of the glucocorticoid receptor: CpG islands, epigenetics and more. Biochem. Pharmacol. 2010, 12, 1860. [Google Scholar] [CrossRef]
- de Vries, A.; Holmes, M.C.; Heijnis, A.; Seier, J.V.; Heerden, J.; Louw, J.; Wolfe-Coote, S.; Meaney, M.J.; Levitt, N.S.; Seckl, J.R. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. Am. Soc. Clin. Investig. 2007, 117, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Heyer, D.B.; Meredith, R.M. Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 2017, 58, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Borovitskaya, A.E.; Evtushenko, V.I.; Sabol, S.L. Gamma-radiation-induced cell death in the fetal rat brain possesses molecular characteristics of apoptosis and is associated with specific messenger RNA elevations. Mol. Brain Res. 1996, 35, 19–30. [Google Scholar] [CrossRef]
- Giarola, R.S.; De Almeida, G.H.O.; Hungaro, T.H.; De Paula, I.R.; Godinho, A.F.; Acencio, M.L.; Mesa, J.; Lemke, N.; Vieira, L.D.; Delicio, H.C. Low-dose ionizing radiation exposure during pregnancy induces behavioral impairment and lower weight gain in adult rats. arXiv 2019. [Google Scholar] [CrossRef]
- Bratt, A. Long term modulation of the HPA axis by the hippocampus Behavioral, biochemical and immunological endpoints in rats exposed to chronic mild stress. Psychoneuroendocrinology 2001, 26, 121–145. [Google Scholar] [CrossRef]
- Kern, S.; Oakes, T.R.; Stone, C.K.; McAuliff, E.M.; Kirschbaum, C.; Davidson, R.J. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology 2008, 33, 517–529. [Google Scholar] [CrossRef]
- Swaab, D.; Fliers, E.; Hoogendijk, W.; Veltman, D.; Zhou, J. Interaction of prefrontal cortical and hypothalamic systems in the pathogenesis of depression. Prog. Brain Res. 2000, 126, 369–396. [Google Scholar] [CrossRef]
- Schutter, D.J. The cerebello-hypothalamic–pituitary–adrenal axis dysregulation hypothesis in depressive disorder. Med. Hypotheses 2012, 79, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Effects of Radiation on the Embryo and Fetus | Radiology Key. Available online: https://radiologykey.com/effects-of-radiation-on-the-embryo-and-fetus/ (accessed on 28 July 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalonde, C.; Boreham, D.; Tai, T.C. Fetal Programming of Brain and Behavior through Ionizing Radiation. Stresses 2023, 3, 198-209. https://doi.org/10.3390/stresses3010015
Lalonde C, Boreham D, Tai TC. Fetal Programming of Brain and Behavior through Ionizing Radiation. Stresses. 2023; 3(1):198-209. https://doi.org/10.3390/stresses3010015
Chicago/Turabian StyleLalonde, Christine, Douglas Boreham, and T. C. Tai. 2023. "Fetal Programming of Brain and Behavior through Ionizing Radiation" Stresses 3, no. 1: 198-209. https://doi.org/10.3390/stresses3010015
APA StyleLalonde, C., Boreham, D., & Tai, T. C. (2023). Fetal Programming of Brain and Behavior through Ionizing Radiation. Stresses, 3(1), 198-209. https://doi.org/10.3390/stresses3010015





