Abstract
Trees in urban areas provide important ecosystem services and are an essential element of urban green space. The constant increase in artificial light from anthropogenic activities around the world creates photopollution that affects the phenology and physiology of plants. Here we conducted a field study to investigate the anthropogenic impacts on six urban trees (Saraca asoca, Terminalia catappa, Bauhinia variegata, Holoptelea integrifolia, Ficus benjamina and Thevetia peruviana) using chlorophyll fluorescence analysis. OJIP curve, maximum quantum yield of primary photochemistry (ΦPo), quantum yield of electron transport (ΦEo), probability that an absorbed photon will be dissipated (ΦDo), photosynthetic performance index (PIcsm) and reaction center photochemistry were assessed. According to the results, various parameters of chlorophyll fluorescence showed significant and important effects on different tree species. T. peruviana and F. benjamina were found to be tolerant to street lighting, while on the other hand, S. asoca, T. catappa, B. variegata and H. integrifolia were found to be sensitive to artificial light induced by street lamps. This study clearly indicates that chlorophyll fluorescence analysis is a potent method for screening the tolerance of tree species to photopollution induced by artificial lights.
1. Introduction
The growing human population and its activities are imposing strong pressures on living organisms and their ecosystems [1]. These pressures might be direct (e.g., habitat fragmentation and modification) or indirect (e.g., altered temperature and air, noise and light pollution) [2]. Among these stresses, light pollution caused by the overuse of artificial light exerts a strong selective force on biodiversity [3]. According to studies on light pollution, the use of artificial lighting is increasing at a rate of 6% every year [4]. The increasing use of artificial lights at night has led light pollution to emerge as a threat to ecosystems. Plants need light for photosynthesis, information (e.g., when to bud, flower, germinate, etc.) and to maintain their growth form. The process of photosynthesis occurs within photosynthetic active radiation (PAR); however, its excess may reduce the photosynthetic efficiency or even cause damage to the photosynthetic apparatus [5]. Recent studies have shown that artificial light sources disrupt the natural cycle of light and darkness, potentially damaging both plants and the ecosystems that support them [6]. These artificial light sources are concentrated along road borders and among trees in parks and urban areas, potentially presenting a significant and currently undetected hazard [7]. Plants need periods of darkness to recover and repair from environmental stress; therefore, the disruption of their natural cycles by artificial light can inhibit their recovery from stress and increase the risk of leaf injury [8]. Previous studies demonstrating how artificial light and plants interact were mostly performed in laboratories and were based on the impact of the quantity, quality and duration of light on plant development processes [9,10,11,12,13].
The response to light depends on different factors, such as leaf structure, tree crown depth and canopy height, as the leaves on the lower side of the tree canopy receive low photosynthetic photon flux density (PPFD) light, whereas the upper leaves get more PPFD [14]. Absorption of light results in the singlet-state excitation of a Chlorophyll (Chl) a molecule (1Chl*), which can return to its ground state by one of several pathways, including (i) re-emitting excitation energy as Chl fluorescence, (ii) energy used to drive photochemistry or (iii) thermal dissipation processes, e.g., the non-photochemical process (NPQ) [15]. When nearby road lights are bright enough to generate a physiological response in plants, their photosynthesis, growth rate and photosynthetic assimilation are affected [16,17,18]. If the plants cannot manage the continuous illumination absorption from artificial light sources, this can result in the production of chemical intermediates that cause biological damage and photo-oxidative damage to the two photosynthetic reaction centers, photosystem (PS) II and PSI, or different cellular biomolecules [19]. The leaves of trees grown under continuous lighting can be considerably larger and more prone to water stress and air pollution than other trees since the stomatal pores in the leaves are continually open for longer periods. Night illumination causes necrosis and chlorosis in plants and enhances the effect of ozone injury and recovery [20]. Plants growing near artificial light sources exhibit a delay in leaf senescence [16]. Plants of Traganum moquinii have shown a reduction in their reproduction potential using seeds when exposed to high-intensity artificial lights [21]. Some plant species growing near artificial light sources demonstrate earlier flower induction [22] and reduced photosynthetic efficiency [8].
The ability to survive in urban environments determines the effectiveness of plants in minimizing certain aspects of the urban environment, including air temperature, flooding and pollution, that are stressful to human residents [23]. Therefore, the selection of the species that possess the efficiency to utilize excess light energy in photosynthetic processes will be necessary to the accumulation of higher biomass. Study of the physiological mechanisms by which plants adapt to artificial light at night will help to select the species suitable for urban plantations and, consequently, to determine the success of urban plantations. Indeed, few previous studies were conducted in the natural environment, and most of the earlier findings on artificial light at night (ALAN) were mainly focused on plant ecosystem sustainability, while the studies showing the effects of ALAN on plant physiological processes were reported in very small numbers [24,25,26,27]. Trees growing under street-light conditions experience an extremely heterogeneous environment; therefore, the use of traditional analytical approaches in the early detection of stress would be expensive and time-consuming [28].
Chlorophyll fluorescence is closely linked to the photosynthesis process, and its analysis has become an effective, practical and common method for accessing the changing physiology of the plants surviving under stress conditions [29,30]. Chlorophyll fluorescence is a very beneficial technique for studying the physiological state of a plant, as its measurement is non-destructive, non-invasive and obtained from lightweight portable devices and its readings are obtained in <1 s, allowing many plants to be examined in a single day [31,32]. The JIP-test method is an add-on to the quality assessment process that allows for the detection of most biological deregulations and is commonly used in experiments where only one or a limited number of stress factors are dominant [33,34]. According to the chlorophyll fluorescence OJIP curve, the O–J normalized phase is related to the electron donation from the oxygen-evolving complex (OEC) to the oxidized PSII reaction center chlorophyll (P680+) [35]. The J–I and I–P normalized phases provide information about an imbalance between the oxidation and reduction of QA and the plastoquinone (PQ) pool, respectively [36]. We hypothesized that ALAN can induce functional and structural changes in the components of the photosynthetic apparatus of plants that cause stress. The adjustments of photosynthetic reactions are necessary to avoid the deleterious effects of excess light. The objective of this study was to determine the effect of artificial light exposure on primary photosynthetic reactions in selected tree species using chlorophyll fluorescence analysis.
2. Results
2.1. Chlorophyll a Fluorescence
Photopollution significantly altered the chlorophyll a fluorescence OJIP kinetics in tree species. In normal-light-period-grown plants, two intermediate peaks, FJ (chlorophyll fluorescence at 2 ms) and Fi (chlorophyll fluorescence at 300 ms), were formed between FO and FM, forming a typical chlorophyll a fluorescence OJIP curve (Figure 1). However, in the artificial-light-growing plant, the OJIP curve was suppressed. In B. variegata and F. benjamina, the JI to IP phases were reduced and not able to complete the fluorescence curve (Figure 1C,E). In S. asoca, the OJ to JI phase was complete but the IP phase was reduced under artificial night light (Figure 1A). In the artificial-light-grown H. integrifolia, the IP phase of the OJIP curve was reduced in comparison to control plants, whereas in the T. catappa, it was raised (Figure 1B,D).
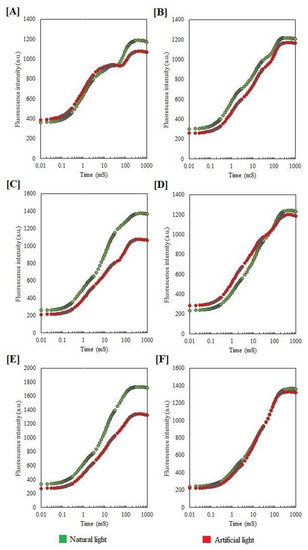
Figure 1.
Raw OJIP chlorophyll a transient and kinetic differences of S. asoca (A), T. catappa (B), B. variegata (C), H. integrifolia (D), F. benjamina (E) and T. peruviana (F) growing under artificial-light and natural-light conditions. Each curve represents the average of three independent measurements.
2.2. Density of Active Reaction Centers
The number of active PS II reaction centers (RC/CS) was not altered by artificial night light in B. variegata, F. benjamina and T. peruviana in comparison to control plants (Figure 2C,E,F). Photopollution led to a reduction in the active reaction centers in S. asoca, T. catappa and H. integrifolia (Figure 2A,B,D). Among all of the plants, the lowest number of active reaction centers was found in S. asoca (Figure 2A).
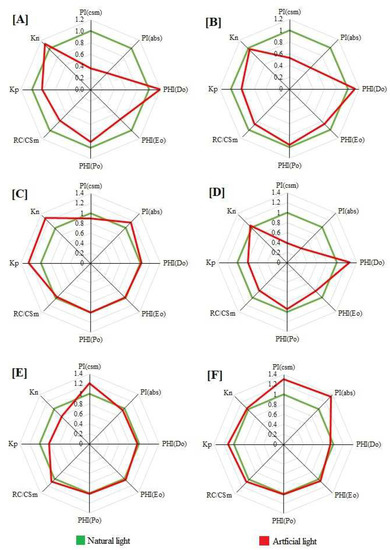
Figure 2.
A radar plot of selected JIP parameters derived from chlorophyll a fluorescence S. asoca (A), T. catappa (B), B. variegata (C), H. integrifolia (D), F. benjamina (E) and T. peruviana (F) growing under artificial-light and natural-light conditions. Values presented are the average of three replicates.
2.3. Yield and Flux Ratio
PHI Po (ΦPo), which reflects the overall photosynthetic potential of the active PSII reaction centers, was not affected by artificial light in B. variegata, F. benjamina and T. peruviana (Figure 2C,E,F). However, a significant decline in FV/FM was recorded in S. asoca, H. integrifolia and T. catappa, while the lowest value was recorded in S. asoca (Figure 2A,B,D).
PHI Eo (ΦEo), which reflects the overall electron transport potential of the active PSII reaction centers, was not affected by photopollution in B. variegata and F. benjamina (Figure 2C,E). In T. peruviana, a significant enhancement was observed under artificial-light conditions (Figure 2F). However, a significant decline in φEo was recorded in S. asoca, H. integrifolia and T. catappa, and the lowest value was in S. asoca growing in artificial light (Figure 2A,B,D).
PHI Do (ΦDo), which reflects the overall dissipation potential of active PSII reaction centers, was not altered in B. variegata, F. benjamina. and T. peruviana grown under street-light conditions (Figure 2C,E,F). In T. peruviana, a significant reduction in ΦDo was observed (Figure 2F). However, a significant enhancement in φDo was recorded in S. asoca, H. integrifolia and T. catappa, and the lowest value was observed in S. asoca plants grown in nighttime artificial light (Figure 2A,B,D).
Photopollution significantly altered the photochemistry of the plants grown under artificial-light conditions (Figure 2). The rate constant of primary photochemistry (kP) was reduced in artificial-light-grown S. asoca, H. integrifolia, T. catappa and F. benjamina plants (Figure 2A,B,E). The value of kP was enhanced by artificial light in T. peruviana and B. variegata compared to control plants, and the highest value was found in B. variegata (Figure 2C,F).
The rate constant of the non-photochemical deexcitation events (kN) was depicted in H. integrifolia, T. catappa and F. benjamina (Figure 2B,D,E) grown under night- and lamp-light conditions, whereas in S. asoca and B. variegata it was enhanced in comparison to control plants (Figure 2A,C). The value of kN was reduced in F. benjamina under artificial-light conditions (Figure 2E).
2.4. Performance Index
Photopollution had a significant effect on the performance index on the absorption basis (PIABS) and cross-section basis (PICS) (Figure 2). PIABS and PICS declined sharply in S. asoca, H. integrifolia and T. catappa grown under street-light conditions, whereas the largest decrease was found in S. asoca (Figure 2A,B,D). In B. variegata, PIcsM was reduced under artificial-light conditions compared to control plants, whereas the PIabs were enhanced (Figure 2C). Conversely, in F. benjamina, PIcsM was enhanced and the PIabs were reduced in artificial light compared to natural-light-growing plants. Enhancement was found in both PIcsM and PIabs in artificial-light-growing T. peruviana in comparison to control plants (Figure 2F).
2.5. Phenomenological Energy Fluxes
Phenomenological energy fluxes, including absorption flux per cross section (ABS/CS), electron transport flux per cross section (ET/CS) and dissipated energy flux per cross section (DI/CS), were strongly affected by photopollution in various tree species (Figure 3A–C). The ABS/CS had not changed in the F. benjamina growing under artificial-light conditions compared to control plants (Figure 3A), whereas it was reduced in the other tree species growing in artificial light. The lowest value of ABS/CS was noticed in artificial-light-grown B. variegata (Figure 3A).
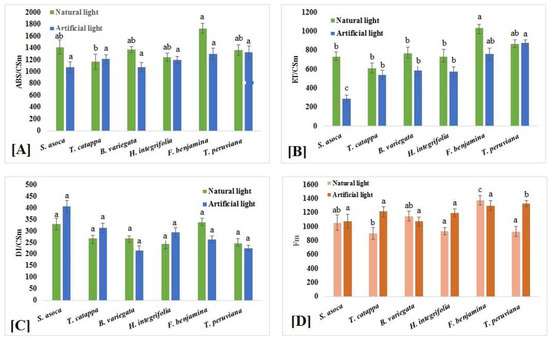
Figure 3.
(A) Absorption per cross section (ABS/CSM), (B) electron transport per cross section (ABS/CSM), (C) dissipation per cross section (ABS/CSM) and (D) maximum fluorescence of S. asoca, T. catappa, B. variegata, H. integrifolia, F. benjamina and T. peruviana leaves exposed to artificial-light and natural-light conditions. Values presented are the average of three replicates, and standard errors are represented by error bars. Different characters indicate significant differences among the results (p ≤ 0.05).
The electron-transport efficiency of plants was found to be sensitive to artificial light. Artificial light drastically reduced the electron-transfer system in thylakoid membranes (Figure 3). Nighttime-artificial-light-grown T. peruviana showed an enhancement in the ET/CS (Figure 3B), whereas it was decreased in S. asoca and F. benjamina (Figure 3B), and the lowest value was found in S. asoca compared to control plants (Figure 3B). In T. catappa, B. variegata and H. integrifolia, the ET/CS was not much affected by excess light. The DI/CS was not significantly altered in the plants grown under street-light conditions (Figure 3C). Hence, an enhancement was observed in T. peruviana and a reduction was observed in S. asoca grown under artificial-light conditions compared to control plants (Figure 3C).
A significant change in FM was reported in the plants exposed to artificial light (Figure 3D). The results clearly indicate that in F. benjamina and B. variegate, the FM was significantly reduced under artificial-light conditions compared to control plants (Figure 3D). In T. peruviana, S. asoca and T. catappa, a significate rise the FM value was found (Figure 3D). In H. integrifolia, the FM value was not significantly affected in artificial-light-grown plants (Figure 3D).
3. Discussion
Photopollution is a major global issue for sustainable urban areas as it affects plant growth and development at all growth stages by disturbing cellular and physiological processes [37]. In this study, artificial light reduced the photosynthesis (fluorescence analysis) of different tree species. However, these adverse effects were varied in different tree species. Based on fluorescence parameters under light stress, light-tolerant (F. benjamina and T. peruviana) and light-sensitive trees (S. asoca, T. catappa, B. variegata and H. integrifolia) were identified. Similar variation in light tolerance has been observed in different tree species [38,39,40]. Such variation in light tolerance could be due to variation in different physiological processes, such as photosynthetic capacity and antioxidant potential [41].
The Chl a fluorescence induction transient is known to be influenced by environmental changes and the physiological state of the plant [42], since PSII is often the primary target under stress [43,44]. The rapid rise from O to J is photochemically controlled and the J–I rise is restricted by thermal reactions [45]. In this study, we observed a decline in the I–P phase and the complementary area of the OJIP curve. Light stress lowered the redox potential of PSI, as seen by the decreased I-P stage in S. asoca, T. catappa and F. benjamina under artificial-light conditions [46,47]. Degradation and denaturation of chlorophyll proteins at the PSI acceptor side, as well as a decline in the number of closed PSII RCs, both contributed to the lower P value [48,49]. The disturbance in I-P phase also indicated a poor redox state of the pool of the QB, cytb6f and the acceptor end of PSI [50]. Similar results have additionally been found in other plant studies [51,52]. Studies have also shown that an increase in cyclic electron flow (CEF) around the PSI is accompanied by a reduction in the I–P phase, which is caused by a barrier of electron transfer at the PSI’s electron acceptor side [53,54]. These findings suggest that artificial light restricted both the probability of electron transport from the donor end of PSII to the acceptor side of PSII and the transfer of absorbed energy from the light-harvesting complex to the reaction center [33,55]. Similar results were found by Yao et al., who studied the effects of night light on Hevea brasiliensis [56].
S. asoca, T. catappa, B. variegata and H. integrifolia exhibited very low FM under light stress. This low FM value suggests that inactive RCs have accumulated at PSII [57], implying that the PSII donor end degrades under light stress and that its efficiency in donating electrons also declines as a result of an increase in closed PSII RCs [58]. Light stress significantly reduced Fv/Fm in S. asoca, T. catappa, B. variegata and H. integrifolia when compared to F. benjamina and T. peruviana. The decrease in ΦPo (Fv/FM) indicated the fact that PSII RCs were damaged or photochemically inactive under light stress. The decline in Fv/FM ratio was related to a decrease in FM values under light stress, indicating disruption of the antenna complex of PSII and increased dissipated energy, destruction of the reaction center at PSII and impaired ribulose-1,5-bisphosphate (RuBP) regeneration ability. Thus, the electron-transport capacity of PSII is reduced [59]. These results are similar to earlier studies on grass [60], cannabis [24] and fresh-water plants [27].
The parameters, such as ΦEo, mainly reflect the changes on the acceptor side of PSII. Reduction in ΦEo in artificial-light-grown S. asoca, T. catappa, B. variegata and H. integrifolia indicate that light stress reduced the capability of the photosynthetic electron transport from the PSII donor side to the PSI end acceptors, decreasing the photosynthetic capacity of the leaves. These findings suggest that both donor and acceptor sides of photosystems were the target sites under light stress in S. asoca, T. catappa, B. variegata and H. integrifolia. These results are consistent with the conclusions suggested by Kwak et al. in yellow poplar (Liriodendron tulipifera) grown under nighttime artificial light [61]. Contrary to ΦEo, ΦDo is associated with non-photochemical processes [62]. It was inferred that artificial light inhibited the absorption of light and electron (or exciton) transport (ΦEo), while evoking the dissipation of light energy (ΦDo) in S. asoca, T. catappa, B. variegata and H. integrifolia. The decrease in photosynthetic-light-use efficiency in light pollution is due, in part, to photoprotective processes that convert absorbed light energy to heat, rather than allowing it to be used for electron transport in the light reactions of photosynthesis [63]. Similar inactivation of RCs was reported in Arabidopsis leaves exposed to low light [64], as well as pulsed-light-induced photoinhibition in wheat leaves [65].
The phenomenological energy flux (ABS/CS, ET/CS and DI/CS) suggests several sensitive sites of PSII that respond to light stress [66]. The decreases in ABS/CS and ET/CS of S. asoca, T. catappa, B. variegata and H. integrifolia due to light stress are indications that the light energy absorbed per leaf area, energy captured in PSII and the energy transferred by electron transfer have decreased [67]. On the contrary, increases in DI/CS under artificial-light conditions indicate an increase in the energy that is not being used for electron transfer. Similarly, Umar et al. found a depreciation in these parameters and reported photosystem damage in sunflowers under stress conditions [28]. Artificial light resulted in a decrease in the total number of active reaction centers per absorption (RC/CS) in S. asoca, T. catappa and H. integrifolia leaves. It is known that inactive PSII RCs can prevent further damage to themselves and protect neighboring active PSII RCs by acting as heat sinks [68].
The performance index is one of the most important parameters in the JIP expressions constellation. This parameter shows the changes in fluorescence caused by antenna-conformation alteration and energy fluctuations. Consequently, the PI is useful in the high-resolution estimation of plant vitality [69]. The PIcsm combines the density of working photosystems (reaction centres per chlorophyll, RC/ABS) with the performance of the light reactions (ΦPO) and the performance of the dark reactions (ψE0), whereas the expression Fv/FM = (ΦPO) contains only information about the quantum yield of primary photochemistry [70]. Increased PI values in T. peruviana and F. benjamina indicate an adaptation strategy for plants to cope with the stress caused by artificial light and to maintain photosynthetic activity in order to survive in these unfavorable conditions [71]. By comparison, the low PI values in B. variegata, S. asoca and H. integrifolia suggest sensitivity towards artificial light. Performance index was also found to be important in previous studies describing the physiological status of plants exposed to abiotic stress [72,73,74]. In conclusion, the performance index, which was measured in a short time and provides a general overview of the photochemical events, correlates well with other, highly time-consuming quality-assessment methods [75]. Moreover, through the PI calculation, the heterogeneity of the vitality of trees in urban areas could be described.
The higher non-photochemical constants (kN) suggest that energy dissipation in S. asoca, T. cattpa and B. variegata was a more physiologically controlled process than that of F. benjamina [67]. The higher maximal fluorescence emissions (FM) and the lower photochemical constants (kP) in S. asoca, T. cattpa and B. variegata indicate processes able to disturb Chl efficiency [76]. The increase of the photochemical constant (kP) in T. peruviana indicates that its leaves tend to compensate for the loss of entire cells (and thus the overall quantity of chlorophyll) by increasing the efficiency of the remaining chlorophyll in unaffected cells. Similar results were found by Kruger, who reported changes in kP and kN under light stress in Camellia leaves [67].
Plants have developed different mechanisms to manage the various levels of irradiance. These include modifications of leaf structure, chloroplast structure, arrangement of the photosynthetic electron transport chain and regulation of photosynthetic light utilization [77]. Smooth surfaces, inorganic deposits on the leaf surface (such as salt crystals) and the development of air-filled hairs are all possible forms of adaptation [78]. Previous studies have also reported the light acclimation ability of F. benjamina, which includes well-developed palisade tissue and higher stomatal density [79,80]. T. peruviana also has a thick cell and palisade layer [81], which may enable it to prevent absorbing excess light [82].
The results of this study indicate that photo-oxidative damage was inhibited and the function of PSII was maintained in photopollution-tolerant tree species under light stress. Finally, the measurement of chlorophyll fluorescence is a useful technique for demonstrating the early phases of photosynthesis. It offers a clear illustration of the primary energy differences across samples and allows for the visual comparison of various trees or groups of trees (e.g., tolerant versus sensitive).
4. Materials and Methods
4.1. Site Description
This field study was conducted at the University College of Science (24°34′51″ N, 73°42′42″ E), located in Udaipur in the Rajasthan province of India. The area in which the study was conducted is characterized by deciduous trees. Winter (November to February), summer (April to mid-June) and a rainy season (mid-June to mid-September) are the three principal seasons that occur each year. The transitional seasons of autumn and spring are marked by the months of October and March, respectively. Udaipur experiences a tropical climate, with summer highs of 41.77 °C and lows of 15.10 °C. The minimum temperature drops to 3.82 °C and the highest temperature may reach 26.8 °C during the winter. The average annual rainfall in the area is less than 700 mm. The soil is alluvial, sandy-loam type at the study site.
4.2. Plant Selection
Healthy and mature leaf samples were collected from the six dicotyledonous deciduous trees (Saraca asoca (Roxb.) W. J. de Wilde, Terminalia catappa Linn, Bauhinia variegata L., Holoptelea integrifolia (Roxb.) Planch, Ficus benjamina L., Thevetia peruviana (Pers.) K. Schum) that were growing under street-light conditions (mentioned as artificial light) (Figure 4). Leaves that were collected from the trees growing without exposure to streetlights were used as controls (mentioned as natural light). The samples were taken from a total of five individuals from each species growing with and without exposure to street lighting, so a total of 60 individuals from six species were sampled. The samples were collected at morning times (~8:00 a.m.) in August 2021.
Figure 4.
Photographs of six species grown at the study site, university area, Udaipur. Study site (Image source: Google Maps; https://earth.google.com/web/, accessed on 12 August 2021 ); S. asoca (A), T. catappa (B), B. variegata (C), H. integrifolia (D), F. benjamina (E) and T. peruviana (F).
Leaves from each of the six species were collected from the field and placed in a large plastic polybag and brought to the laboratory within 15 min. The leaves were then used for chlorophyll fluorescence analysis.
4.3. Measurement of Chlorophyll a Fluorescence
Chlorophyll a fluorescence signals were measured using a plant efficiency analyzer (Handy-PEA fluorimeter, Hansatech Instruments Ltd., Norfolk, England). Prior to measurement, the leaves were subjected to dark conditions for 1 h. Fluorescence transients were induced by a red light (650 nm) and 3000 µmol m−2s−1, provided by a high intensity LED array of three light emitting diodes. The fluorescence intensities were measured at 50, 100 and 300 µs (F50 µs F100 µs and F300 µs) and 2 and 30 ms (FJ and FI), respectively. The minimal fluorescence F0 was observed at 50 µs. Following this, other Chl a fluorescence parameters, such as specific flux, phenomenological flux and Fv/Fm, were analysed using the Biolyzer software (developed by the Laboratory of Bioenergetics, University of Geneva, Geneva Switzerland).
4.4. Statistical Analysis
Statistical analyses were conducted using one-way analysis of variance (ANOVA) and the Tukey HSD test (p = 0.05) using SPSS (22.0) statistical software (IBM, Armonk, NY, USA). In the figures, only the statistically significant measurement results (p ≤ 0.05) are shown.
5. Conclusions
The effect of artificial light on plants is species-specific and depends on light perception, distance from the light source, canopy size and environmental-habitat conditions. According to the results, T. peruviana and F. benjamina perform well in artificial-light conditions, whereas B. variegata, S. asoca and H. integrifolia are affected by artificial light as observed by chlorophyll fluorescence analysis. The mature leaves of the tree species T. peruviana and F. benjamina stably maintained the balance between light energy absorption and light-energy utilization through the photosynthetic capacity of their leaves. In the context of green infrastructure development, it would be valuable to assess the influence of light on the physiology of trees growing near roadsides in order to appropriately include light pollution as a criterion for defining ecological corridors. At the local and mid-level, outdoor-lighting planning could be modified to include dark havens and low-intensity ecological corridors for urban-plantation success. Lighting design should first be considered on a relatively large scale, coherent with a lighting-management scale such as Municipalities. The methodology presented in this publication could be used to define potential dark corridors for trees and adapted to assess such corridors for other tree species. Regarding the chlorophyll fluorescence technique, this technique can be used as an efficient tool to detect PSII activity under artificial-light conditions and to select species that are resistant or susceptible to light pollution.
Author Contributions
D.K. conceived the idea and designed the experiment. D.K., H.S. and U.B. performed the experimental work and data analysis. V.S. supervised the experiment work. D.K. wrote the initial manuscript and prepared figures. D.K., H.S., U.B. and V.S. contributed to discussing, reviewing and approving the final version of the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors are thankful to Mohanlal Sukhadia University, Udaipur for providing the necessary facilities during the course of the study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Jiao, L.; Zhong, J.; Jia, Q.; Liu, J.; Liu, Z. Artificial Light Pollution Inhibits Plant Phenology Advance Induced by Climate Warming. Environ. Pollut. 2021, 291, 118110. [Google Scholar] [CrossRef] [PubMed]
- Longcore, T.; Rich, C. Ecological Light Pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light Pollution as a Biodiversity Threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, A.; Trtílek, M.; Niewiadomska, E. Thermographic Study of Plant Response to Excessive Light. Acta Phys. Pol. Ser. A 2021, 139, 257–260. [Google Scholar] [CrossRef]
- Crump, M.C.; Brown, C.; Griffin-Nolan, R.J.; Angeloni, L.; Lemoine, N.P.; Seymoure, B.M. Effects of Low-Level Artificial Light at Night on Kentucky Bluegrass and an Introduced Herbivore. Front. Ecol. Evol. 2021, 9, 732959. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Nelson, R.J. Artificial Light at Night as an Environmental Pollutant: An Integrative Approach across Taxa, Biological Functions, and Scientific Disciplines. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 387. [Google Scholar] [CrossRef]
- Meravi, N.; Prajapati, S.K. Effect Street Light Pollution on the Photosynthetic Efficiency of Different Plants. Biol. Rhythm. Res. 2020, 51, 67–75. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of Light Quality on CO2 Assimilation, Chlorophyll-Fluorescence Quenching, Expression of Calvin Cycle Genes and Carbohydrate Accumulation in Cucumis Sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and Physiological Stress Responses of Lettuce to Different Intensities of Continuous Light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many Hands Make Light Work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, P.; Wu, Y. Effects of Different Light Intensities on Chlorophyll Fluorescence Characteristics and Yield in Lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Bhatt, U.; Soni, V.; Allakhverdiev, S. Effect of Continuous Light on Antioxidant Activity, Lipid Peroxidation, Proline and Chlorophyll Content in Vigna radiata L. Funct. Plant Biol. 2021, 49, 145–154. [Google Scholar] [CrossRef]
- Liu, P.; Cao, B.; Wang, Y.; Wei, Z.; Ye, J.; Wei, H. Spectral Effect of Streetlamps on Urban Trees: A Simulated Study on Tissue Water, Nitrogen, and Carbohydrate Contents in Maple and Oak. PLoS ONE 2021, 16, e0248463. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Bennie, J.; Davies, T.W.; Cruse, D.; Gaston, K.J. Ecological Effects of Artificial Light at Night on Wild Plants. J. Ecol. 2016, 104, 611–620. [Google Scholar] [CrossRef]
- Briggs, W.R. Physiology of Plant Responses to Artificial Lighting. In Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006; pp. 389–411. [Google Scholar]
- Massetti, L. Assessing the Impact of Street Lighting on Platanus x Acerifolia Phenology. Urban For. Urban Green. 2018, 34, 71–77. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection Revisited: Genetic and Molecular Approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Vollsnes, A.V.; Eriksen, A.B.; Otterholt, E.; Kvaal, K.; Oxaal, U.; Futsaether, C.M. Visible Foliar Injury and Infrared Imaging Show that Daylength Affects Short-Term Recovery after Ozone Stress in Trifolium subterraneum. J. Exp. Bot. 2009, 60, 3677–3686. [Google Scholar] [CrossRef]
- Viera-Pérez, M.; Hernández-Calvento, L.; Hesp, P.A.; Santana-del Pino, A. Effects of Artificial Light on Flowering of Foredune Vegetation. Ecology 2019, 100, e02678. [Google Scholar] [CrossRef]
- Han, B.-H.; Kim, J.-Y.; Kwak, J.-I.; Choi, T.-Y. Correlation between the Illuminance and the Flowering and Leaf Growth of Trees at Night1a. Korean J. Environ. Ecol. 2015, 29, 441–453. [Google Scholar] [CrossRef]
- Pataki, D.E.; Alberti, M.; Cadenasso, M.L.; Felson, A.J.; McDonnell, M.J.; Pincetl, S.; Pouyat, R.V.; Setälä, H.; Whitlow, T.H. The Benefits and Limits of Urban Tree Planting for Environmental and Human Health. Front. Ecol. Evol. 2021, 9, 603757. [Google Scholar] [CrossRef]
- Gajdošik, M.S.; Vicić, A.; Gvozdić, V.; Galić, V.; Begović, L.; Mlinarić, S. Effect of Prolonged Photoperiod on Light-Dependent Photosynthetic Reactions in Cannabis. Int. J. Mol. Sci. 2022, 23, 9702. [Google Scholar] [CrossRef] [PubMed]
- Hey, M.H.; DiBiase, E.; Roach, D.A.; Carr, D.E.; Haynes, K.J. Interactions between Artificial Light at Night, Soil Moisture, and Plant Density Affect the Growth of a Perennial Wildflower. Oecologia 2020, 193, 503–510. [Google Scholar] [CrossRef]
- Anic, V.; Gaston, K.J.; Davies, T.W.; Bennie, J. Long-Term Effects of Artificial Nighttime Lighting and Trophic Complexity on Plant Biomass and Foliar Carbon and Nitrogen in a Grassland Community. Ecol. Evol. 2022, 12, e9157. [Google Scholar] [CrossRef]
- Segrestin, J.; Mondy, N.; Boisselet, C.; Guigard, L.; Lengagne, T.; Poussineau, S.; Secondi, J.; Puijalon, S. Effects of Artificial Light at Night on the Leaf Functional Traits of Freshwater Plants. Freshw. Biol. 2021, 66, 2264–2271. [Google Scholar] [CrossRef]
- Umar, M.; Uddin, Z.; Siddiqui, Z.S. Responses of Photosynthetic Apparatus in Sunflower Cultivars to Combined Drought and Salt Stress. Photosynthetica 2019, 57, 627–639. [Google Scholar] [CrossRef]
- Logan, B.A. Chlorophyll a Fluorescence: A Signature of Photosynthesis. J. Torrey Bot. Soc. 2005, 132, 650. [Google Scholar] [CrossRef]
- Lagorio, M.G. Chlorophyll Fluorescence Emission Spectra in Photosynthetic Organisms. In Chlorophyll: Structure, Production and Medicinal Uses; Nova Science Publishers: New York, NY, USA, 2011; pp. 115–150. [Google Scholar]
- Bucher, S.F.; Bernhardt-Römermann, M.; Römermann, C. Chlorophyll Fluorescence and Gas Exchange Measurements in Field Research: An Ecological Case Study. Photosynthetica 2018, 56, 1161–1170. [Google Scholar] [CrossRef]
- Moustakas, M.; Calatayud, Á.; Guidi, L. Chlorophyll Fluorescence Imaging Analysis in Biotic and Abiotic Stress. Front. Plant Sci. 2021, 12, 658500. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. In Vivo Assessment of Stress Impact on Plant’s Vitality: Applications in Detecting and Evaluating the Beneficial Role of Mycorrhization on Host Plants. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 679–703. [Google Scholar]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) Response to Ozone Exposure Assessed with a Chlorophyll a Fluorescence Performance Index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.J. Donor Side Capacity of Photosystem II Probed by Chlorophyll a Fluorescence Transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. The Energy Flux Theory 35 Years Later: Formulations and Applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Torriglia, A.; Attia, D.; Viénot, F.; Gronfier, C.; Behar-Cohen, F.; Martinsons, C.; Hicks, D. Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems. Front. Neurosci. 2020, 14, 602796. [Google Scholar] [CrossRef]
- Chaney, W.R. Does Night Lighting Harm Trees. Purdue Univ. For. Nat. Resour. FAQ 2002, 17, 1–4. [Google Scholar]
- Škvareninová, J.; Tuhárska, M.; Škvarenina, J.; Babálová, D.; Hana, S.; Mária, T.; Jaroslav, Š.; Jana, Š. Effects of Light Pollution on Tree Phenology in the Urban Environment. Morav. Geogr. Rep. 2017, 25, 282–290. [Google Scholar] [CrossRef]
- Singhal, R.K.; Kumar, M.; Bose, B. Eco-Physiological Responses of Artificial Night Light Pollution in Plants. Russ. J. Plant Physiol. 2019, 66, 190–202. [Google Scholar] [CrossRef]
- Sysoeva, M.I.; Markovskaya, E.F.; Shibaeva, T.G. Plants under Continuous Light: A Review. Plant Stress 2010, 4, 5–17. [Google Scholar]
- Luo, H.; Merope, T.; Zhang, Y.; Zhang, W. Combining Gas Exchange and Chlorophyll a Fluorescence Measurements to Analyze the Photosynthetic Activity of Drip-Irrigated Cotton under Different Soil Water Deficits. J. Integr. Agric. 2016, 15, 1256–1266. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.D. Heterogeneity of Photosystem II Probed by the Numerically Simulated Chlorophyll a Fluorescence Rise (O–J–I–P). Math. Comput. Simul. 1998, 48, 3–9. [Google Scholar] [CrossRef]
- Ranjbar, F.A.; Dehghani, B.R. Impact of Salinity Stress on Photochemical Efficiency of Photosystem Ii, Chlorophyll Content and Nutrient Elements of Nitere Bush (Nitraria schoberi L.) Plants. J. Rangel. Sci. 2016, 6, 1–9. [Google Scholar]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Schansker, G.; Srivastava, A.; Strasser, R.J. Characterization of the 820-Nm Transmission Signal Paralleling the Chlorophyll a Fluorescence Rise (OJIP) in Pea Leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R.J. Delayed Fluorescence in Photosynthesis. Photosynth. Res. 2009, 101, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, W.; Wang, X.; Yang, L.; Han, S.; Chen, S.; Strasser, R.J.; Valverde, B.E.; Qiang, S. Comparative Phytotoxicity of Usnic Acid, Salicylic Acid, Cinnamic Acid and Benzoic Acid on Photosynthetic Apparatus of Chlamydomonas Reinhardtii. Plant Physiol. Biochem. 2018, 128, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, V.; Zaharieva, I.; Lambrev, P.; Yordanov, I.; Strasser, R. Simultaneous Analysis of Prompt and Delayed Chlorophyll a Fluorescence in Leaves during the Induction Period of Dark to Light Adaptation. J. Theor. Biol. 2003, 225, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Stirbet, A.D. Estimation of the Energetic Connectivity of PS II Centres in Plants Using the Fluorescence Rise O–J–I–P: Fitting of Experimental Data to Three Different PS II Models. Math. Comput. Simul. 2001, 56, 451–462. [Google Scholar] [CrossRef]
- Solovchenko, A.; Solovchenko, O.; Khozin-Goldberg, I.; Didi-Cohen, S.; Pal, D.; Cohen, Z.; Boussiba, S. Probing the Effects of High-Light Stress on Pigment and Lipid Metabolism in Nitrogen-Starving Microalgae by Measuring Chlorophyll Fluorescence Transients: Studies with a Δ5 Desaturase Mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae). Algal Res. 2013, 2, 175–182. [Google Scholar] [CrossRef]
- Balarinová, K.; Barták, M.; Hazdrová, J.; Hájek, J.; Jílková, J. Changes in Photosynthesis, Pigment Composition and Glutathione Contents in Two Antarctic Lichens during a Light Stress and Recovery. Photosynthetica 2014, 52, 538–547. [Google Scholar] [CrossRef]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the Cyclic Electron Flow around PSI (CEF-PSI) and O2-Dependent Alternative Pathways in Regulation of the Photosynthetic Electron Flow in Short-Term Fluctuating Light in Arabidopsis Thaliana. Plant Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Zhu, X.-G. Changes in the Photosynthesis Properties and Photoprotection Capacity in Rice (Oryza sativa) Grown under Red, Blue, or White Light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Stirbet, A. On the Relation between the Kautsky Effect (Chlorophyll a Fluorescence Induction) and Photosystem II: Basics and Applications of the OJIP Fluorescence Transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Yao, X.C.; Tu, H.Q.; Wang, X.L.; Wang, J. The Effect of Supplemental LED Night Lighting on the Growth and Physiology of the Para Rubber Tree. J. Rubber Res. 2021, 24, 321–326. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Hossain, Z. Chlorophyll a Fluorescence—A Useful Tool for the Early Detection of Temperature Stress in Spring Barley (Hordeum vulgare L.). OMICS J. Integr. Biol. 2011, 15, 925–934. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in Vivo Recording of Prompt and Delayed Fluorescence and 820-Nm Reflection Changes during Drying and after Rehydration of the Resurrection Plant Haberlea Rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A Non-Invasive Assay of the Plastoquinone Pool Redox State Based on the OJIP-Transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Vyas, D.K.; Sher, A.A.; Grenis, K. Light Pollution Affects Invasive and Native Plant Traits Important to Plant Competition and Herbivorous Insects. Biol. Invasions 2022, 24, 599–602. [Google Scholar] [CrossRef]
- Kwak, M.J.; Je, S.M.; Cheng, H.C.; Seo, S.M.; Park, J.H.; Baek, S.G.; Khaine, I.; Lee, T.; Jang, J.; Li, Y.; et al. Night Light-Adaptation Strategies for Photosynthetic Apparatus in Yellow-Poplar (Liriodendron tulipifera L.) Exposed to Artificial Night Lighting. Forests 2018, 9, 74. [Google Scholar] [CrossRef]
- Mendes, M.M.; Pinheiro, A.C.R.; Pires, F.R.; Fernandes, A.A.; de Menezes, L.F.; Pereira, I.A.P.; Dos Santos, V.F.; de Almeida Leite, L.; Cassol, D.; Falqueto, A.R. Photosynthesis and Leaf Traits of Tree Species Influenced by Green Manure Associated with Soil Treatments. Commun. Soil Sci. Plant Anal. 2022, 53, 2064–2081. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. Regulation of Photosynthetic Electron Transport and Photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef]
- Tian, Y.; Sacharz, J.; Ware, M.A.; Zhang, H.; Ruban, A.V. Effects of Periodic Photoinhibitory Light Exposure on Physiology and Productivity of Arabidopsis Plants Grown under Low Light. J. Exp. Bot. 2017, 68, 4249–4262. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive Light Pulse-Induced Photoinhibition of Photosystem I Severely Affects CO2 Assimilation and Photoprotection in Wheat Leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence Parameters as Early Indicators of Light Stress in Barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krüger, G.H.J.; Tsimilli-Michael, M.; Strasser, R.J. Light Stress Provokes Plastic and Elastic Modifications in Structure and Function of Photosystem II in Camellia Leaves. Physiol. Plant. 1997, 101, 265–277. [Google Scholar] [CrossRef]
- Heber, U.; Soni, V.; Strasser, R.J. Photoprotection of Reaction Centers: Thermal Dissipation of Absorbed Light Energy vs. Charge Separation in Lichens. Physiol. Plant. 2011, 142, 65–78. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a Fluorescence Kinetics of Mung Bean (Vigna radiata L.) Grown under Artificial Continuous Light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef]
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; den Ende, W.; Ceusters, J. Performance Index and PSII Connectivity under Drought and Contrasting Light Regimes in the CAM Orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt Chlorophyll Fluorescence as a Tool for Crop Phenotyping: An Example of Barley Landraces Exposed to Various Abiotic Stress Factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a Fluorescence Parameters as Indicators of a Particular Abiotic Stress in Rice. Photosynthetica 2019, 57, 108–115. [Google Scholar]
- Hermans, C.; Smeyers, M.; Rodriguez, R.M.; Eyletters, M.; Strasser, R.J.; Delhaye, J.-P. Quality Assessment of Urban Trees: A Comparative Study of Physiological Characterisation, Airborne Imaging and on Site Fluorescence Monitoring by the OJIP-Test. J. Plant Physiol. 2003, 160, 81–90. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Schöttler, M.A.; Tóth, S.Z. Photosynthetic Complex Stoichiometry Dynamics in Higher Plants: Environmental Acclimation and Photosynthetic Flux Control. Front. Plant Sci. 2014, 5, 188. [Google Scholar] [PubMed]
- Ruban, A.V. Plants in Light. Commun. Integr. Biol. 2009, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Fails, B.S.; Lewis, A.J.; Barden, J.A. Light Acclimatization Potential of Ficus Benjamina1. J. Am. Soc. Hortic. Sci. 1982, 107, 762–766. [Google Scholar] [CrossRef]
- Fails, B.S.; Lewis, A.J.; Barden, J.A. Anatomy and Morphology of Sun-and Shade-Grown Ficus Benjamina1. J. Am. Soc. Hortic. Sci. 1982, 107, 754–757. [Google Scholar] [CrossRef]
- Fjell, I. Anatomy of the Xeromorphic Leaves of Allamanda Neriifolia, Thevetia Peruviana and Vinca Minor (Apocynaceae). Nord. J. Bot. 1983, 3, 383–392. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Day, T.A.; Vogelman, T.C. Ultraviolet-B and Visible Light Penetration into Needles of Two Species of Subalpine Conifers during Foliar Development. Plant Cell Environ. 1992, 15, 921–929. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).