Hylotelephium maximum from Coastal Drift Lines Is a Promising Zn and Mn Accumulator with a High Tolerance against Biogenous Heavy Metals
Abstract
1. Introduction
2. Results
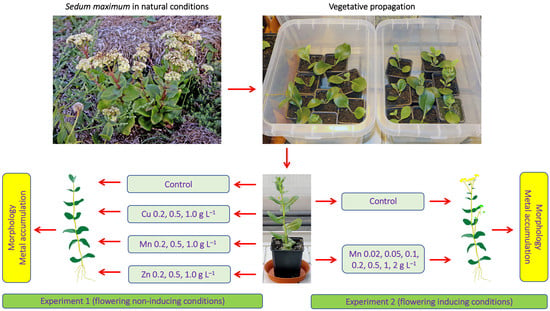
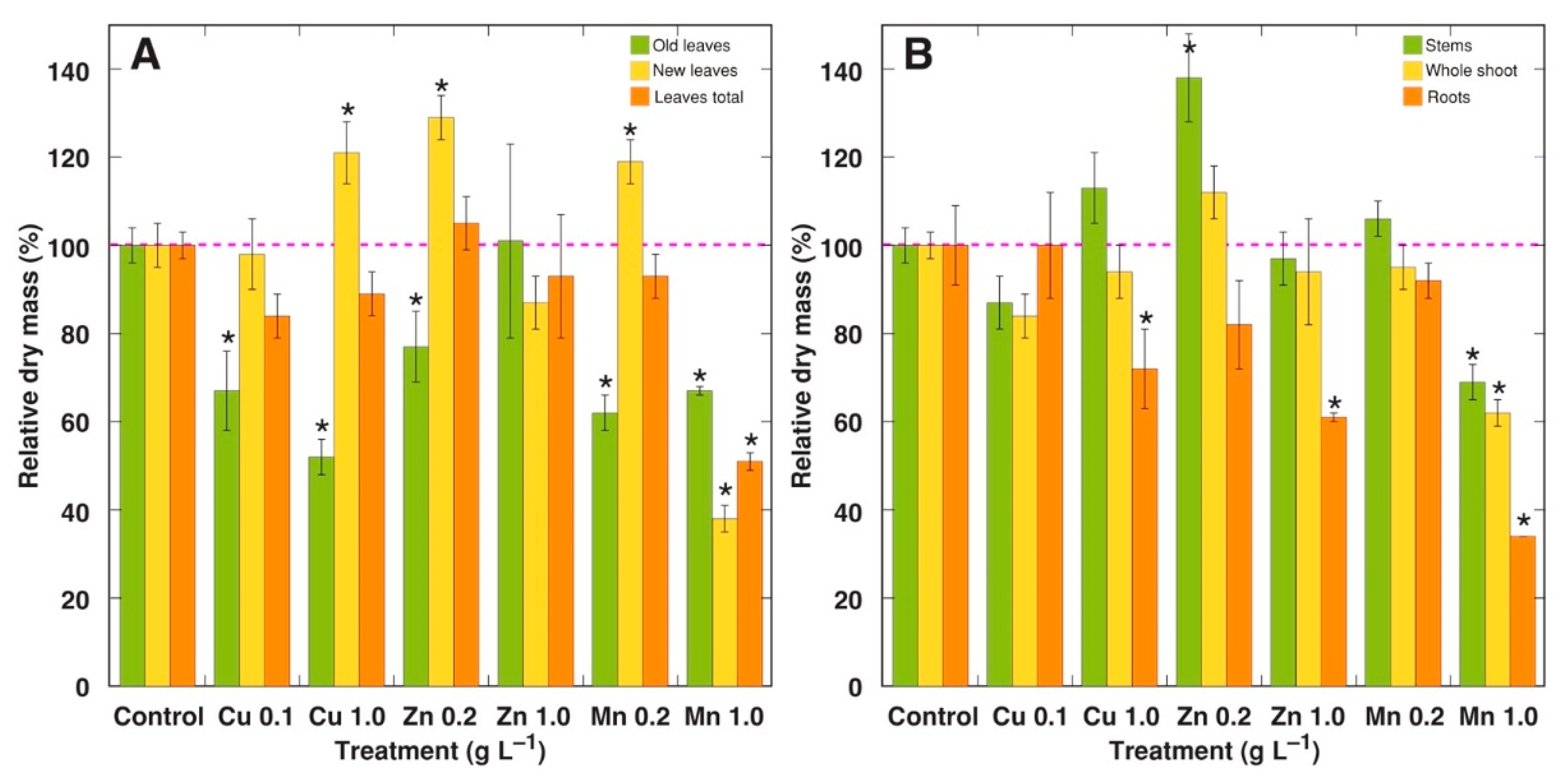
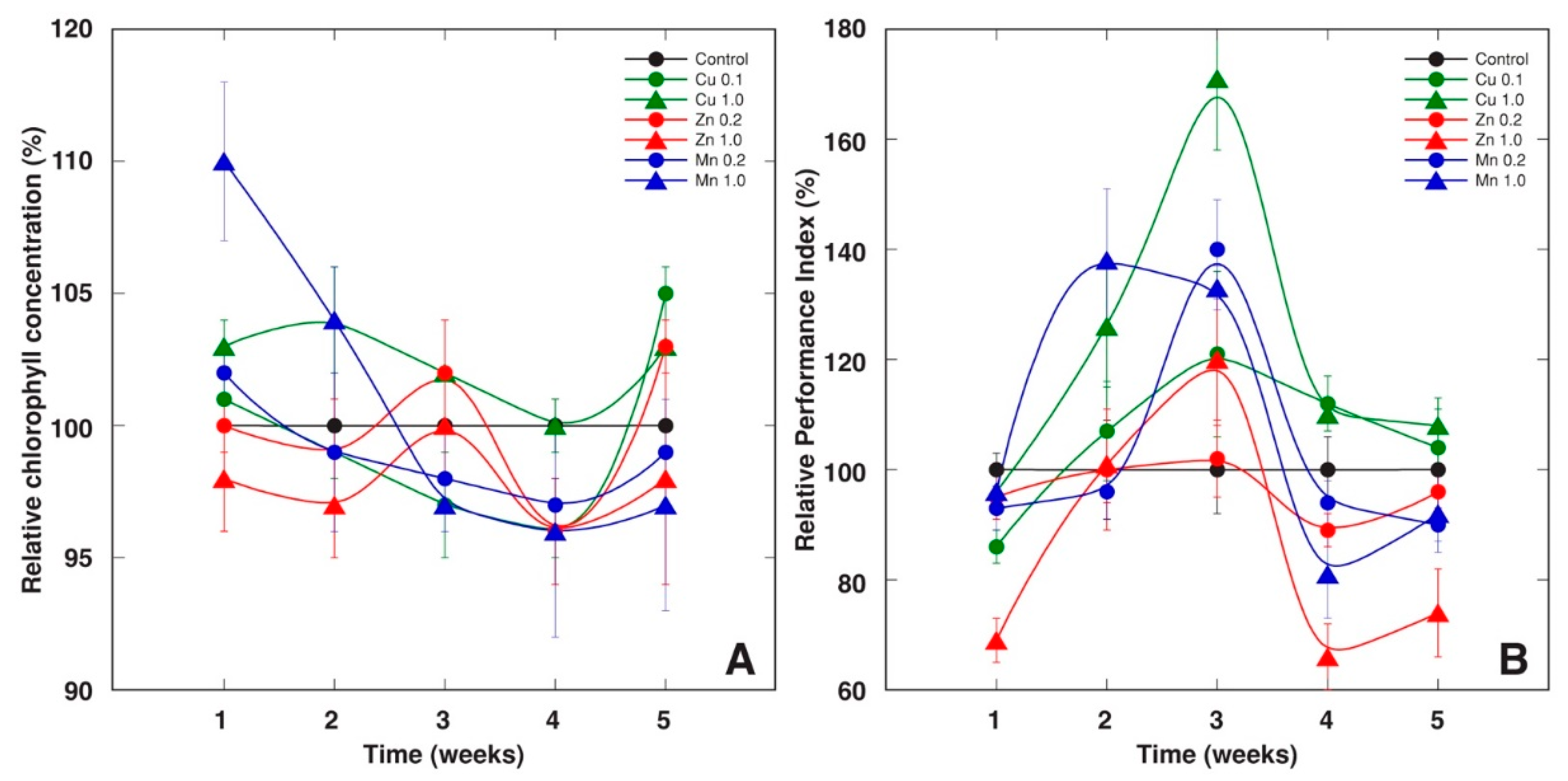
2.1. Experiment 1
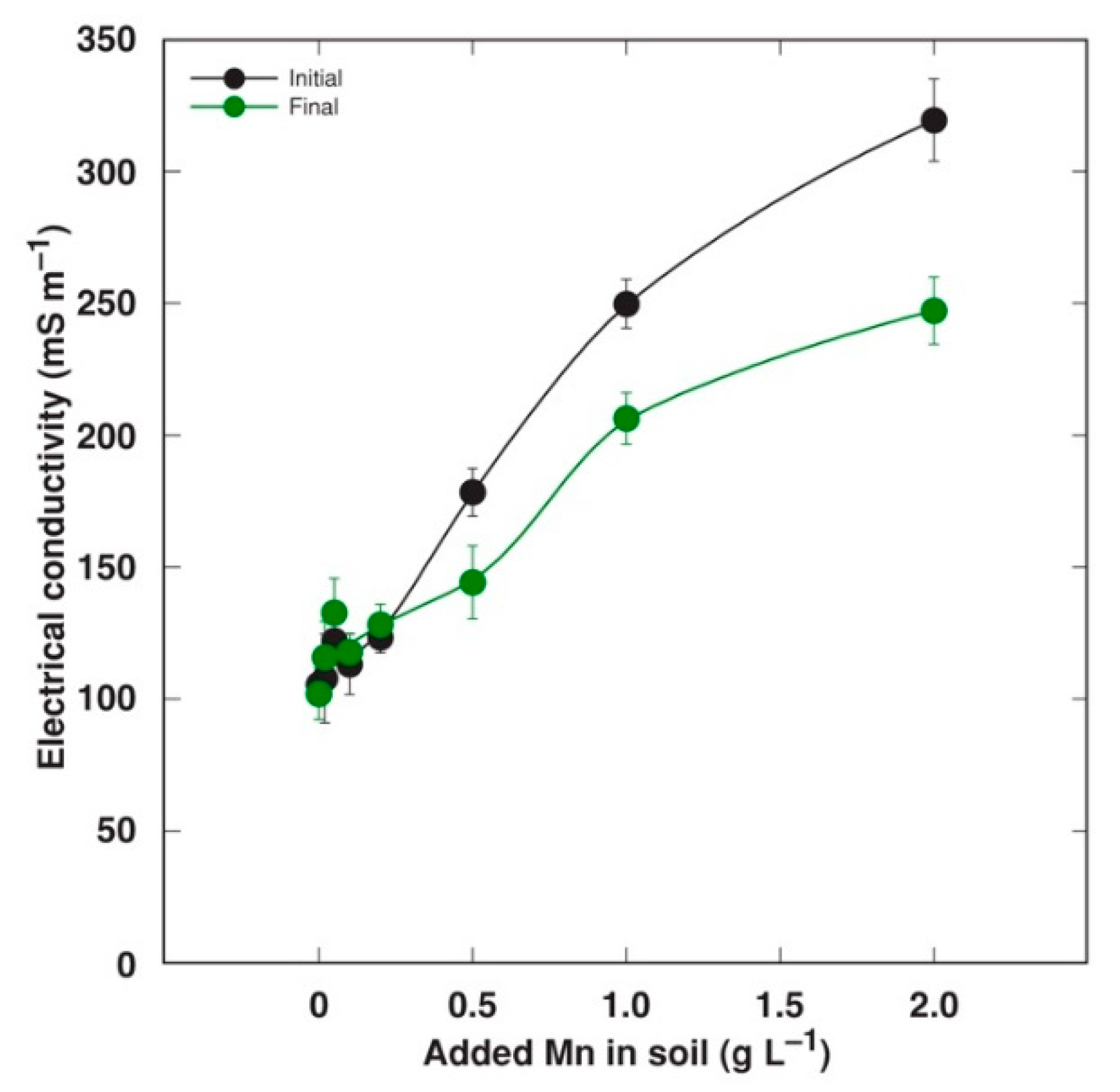
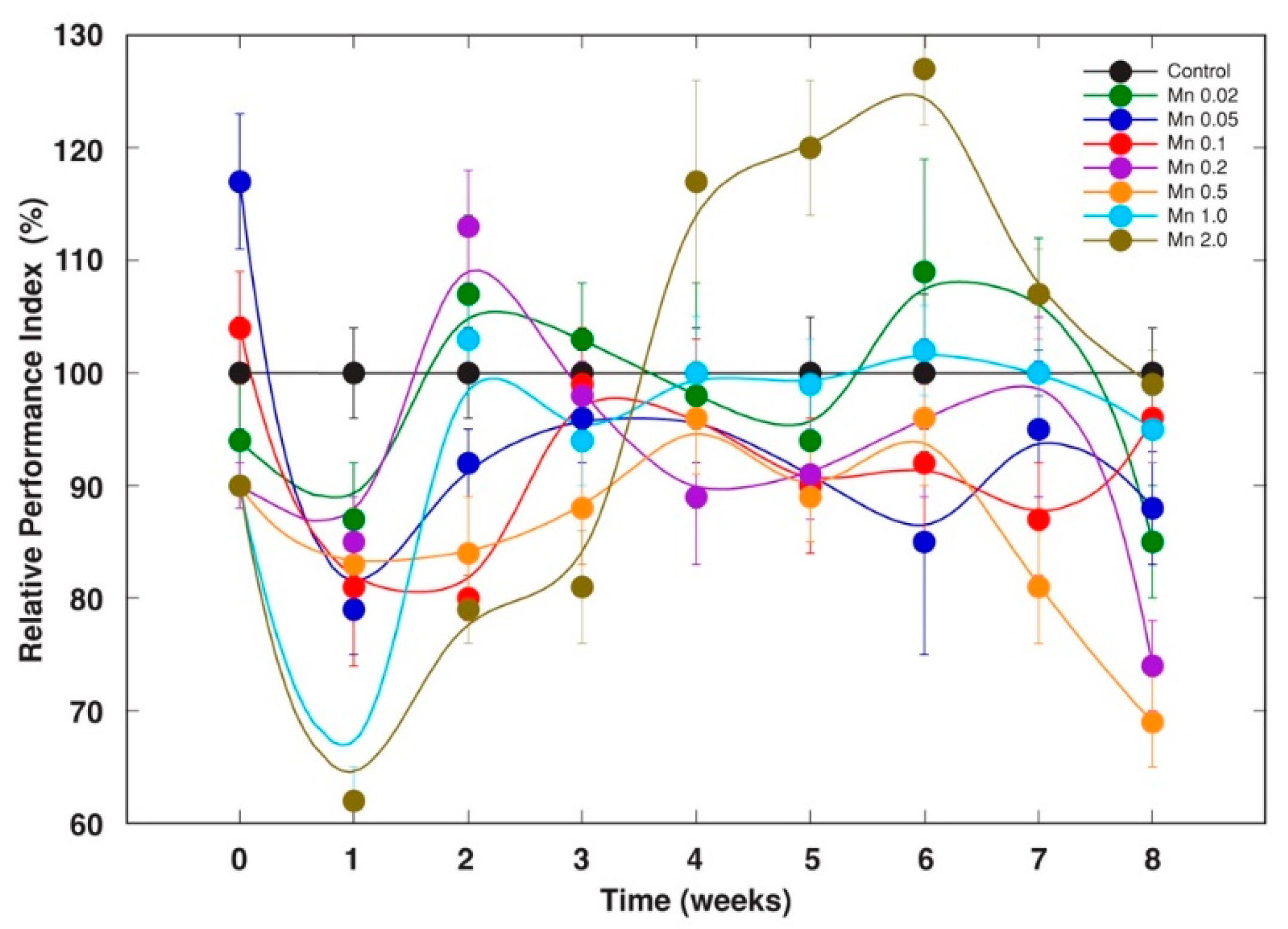
2.2. Experiment 2
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Amėricano-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef] [PubMed]
- Skuza, L.; Szućko-Kociuba, I.; Filip, E.; Bożek, I. Natural molecular mechanisms of plant hyperaccumulation and hypertolerance towards heavy metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef]
- Raskin, I.; Smith, R.D.; Salt, D.E. Phytoremediation of metals using plants to remove pollutants from environment. Curr. Opin. Biotechnol. 1997, 8, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Goolsby, E.W.; Mason, C.M. Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Goolsby, E.W.; Mason, C.M. Response: Commentary: Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2016, 6, 1252. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 136–146. [Google Scholar] [CrossRef]
- Anjum, N.A.; Duarte, B.; Caçador, I.; Sleimi, N.; Duarte, A.C.; Pereira, E. Biophysical and biochemical markers of metal/metalloid-impacts in salt marsh halophytes and their implications. Front. Environ. Sci. 2016, 4, 24. [Google Scholar] [CrossRef]
- Debez, A.; Huchzermeyer, B.; Abdelly, C.; Koyro, H.-W. Current Challenges and Future Opportunities for a Sustainable Utilization of Halophytes. In Sabkha Ecosystems. Tasks for Vegetation Science; Ozturk, M., Böer, B., Barth, H.-J., Clüsener-Godt, M., Khan, M.A., Breckle, S.-W., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 46, pp. 59–77. [Google Scholar]
- Liang, L.; Liu, W.; Sun, W.; Huo, X.; Li, S.; Zhou, G. Phytoremediation of heavy metal contaminated saline soils using halophytes: Current progress and perspectives. Environ. Rev. 2017, 25, 269–281. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Xu, H.; Wang, D.; Xu, J. Accumulation of Cd, Pb and Zn by 19 wetland plant species in constructed wetland. J. Haz. Mater. 2007, 147, 947–953. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Z. Metal accumulation and tolerance in wetland plants. Front. Biol. China 2009, 4, 282–288. [Google Scholar] [CrossRef]
- Santos, M.S.S.; Pedro, C.A.; Gonçalves, S.C.; Ferreira, S.M.F. Phytoremediation of cadmium by the facultative halophyte plant Bolboschoenus maritimus (L.) Palla, at different salinities. Environ. Sci. Pollut. Res. 2015, 22, 15598–15609. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, G.; Yang, J.; Wan, X.; Song, B.; Cai, W.; Guo, J. Phytoaccumulation of heavy metals (Pb, Zn, and Cd) by 10 wetland plant species under different hydrological regimes. Ecol. Eng. 2017, 107, 56–64. [Google Scholar] [CrossRef]
- Laime, B.; Tjarve, D. Coastal plant communities of drift lines in the Lake Engure Nature Park, Latvia. Latvijas Veģetācija 2012, 23, 137–151. (In Latvian) [Google Scholar]
- Ievinsh, G.; Andersone-Ozola, U.; Landorfa-Svalbe, Z.; Karlsons, A.; Osvalde, A. Wild Plants from Coastal Habitats as a Potential Resource for Soil Remediation. In Soil Health. Soil Biology; Giri, B., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 59, pp. 121–144. [Google Scholar]
- Anjum, N.A.; Singh, H.P.; Khan, M.I.R.; Masood, A.; Per, T.S.; Negi, A.; Batish, D.R.; Khan, N.A.; Duarte, A.C.; Pereira, E.; et al. Too much is bad – an appraisal of phytotoxicity of elevated plant beneficial heavy metal ions. Environ. Sci. Pollut. Res. 2015, 22, 3361–3382. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Visioli, G.; Marmiroli, N. The proteomics of heavy metal hyperaccumulation in plants. J. Proteom. 2013, 79, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Osvalde, A. Optimization of plant mineral nutrition revisited: The roles of plant requirements, nutrient interactions, and soil properties in fertilization management. Environ. Exp. Biol. 2011, 9, 1–8. [Google Scholar]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.B.; Eisenhut, M.; Schneider, A. Chloroplast transition metal regulation for efficient photosynthesis. Trends Plant Sci. 2020, 25, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Guerinot, M.L. A question of balance: Facing the challenges of Cu, Fe and Zn homeostasis. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef]
- Puig, S.; Peñrrubia, L. Placing metal micronutrients in context: Transport and distribution in plants. Curr. Opin. Plant Biol. 2009, 12, 299–306. [Google Scholar] [CrossRef]
- Rodrigo-Moreno, A.; Poschenrieder, C.; Shabala, S. Transition metals: A double edge sward in ROS generation and signaling. Plant Signal. Behav. 2013, 8, e23425. [Google Scholar] [CrossRef]
- Wairich, A.; De Conti, L.; Lamb, T.I.; Keil, R.; Neves, L.O.; Brunetto, G.; Sperotto, R.A.; Ricachenevsky, F.K. Throwing copper around: How plants control uptake, distribution, and accumulation of copper. Agronomy 2020, 12, 994. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zn hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Li, J.; Lia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Deng, D.M.; Shu, W.S.; Zhang, J.; Zou, H.L.; Lin, Z.; Ye, Z.H.; Wong, M.B. Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environ. Pollut. 2007, 147, 381–386. [Google Scholar] [CrossRef]
- Huang, H.; Li, T.; Tian, S.; Gupta, D.K.; Zhang, X.; Yang, X. Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii Hance. Bioresour. Technol. 2008, 99, 6088–6096. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhang, H.; Wang, Y.; Zheng, L. Accumulation and distribution characteristics of zinc and cadmium un the hyperaccumulator plant Sedum plumbizincicola. Bull. Environ. Contam. Toxicol. 2014, 93, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Liu, X.; Zhang, J.; Chen, G.; Liu, S.; Zhang, P.; Wang, Y. Analysis of the tolerance of four ecotype plants against copper stress in soil. Procedia Environ. Sci. 2011, 10, 1802–1810. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E.; Barceló, J. Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 2001, 230, 247–256. [Google Scholar] [CrossRef]
- Guo, J.-M.; Lei, M.; Yang, J.-X.; Yang, J.; Wan, X.-M.; Chen, T.-B.; Zhou, X.-Y.; Gu, S.-P.; Guo, G.-H. Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L.) as potential Cd accumulators. Ecol. Eng. 2017, 106, 409–414. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yang, J. Cadmium accumulation and subcellular distribution in populations of Hylotelephium spectabile (Boreau) H. Ohba. Environ. Sci. Pollut. Res. 2018, 25, 30917–30927. [Google Scholar] [CrossRef]
- Zhou, C.; Xiao, X.; Guo, Z.; Peng, C.; Zemg, P.; Bridget, A.F. Physiological responses, tolerance efficiency, and phytoextraction potential of Hylotelephium spectabile (Boreau) H. Obha under Cd stress in hydroponic condition. Int. J. Phytoremed. 2021, 23, 80–88. [Google Scholar] [CrossRef]
- Bomble, F.W. Ein Beitrag zur Taxonomie der Hylotelephium-Gruppe in der Eifel und angrenzenden Flusstälern. Jahrb. Bochumer Bot. Ver. 2011, 2, 87–97. [Google Scholar]
- Rūsiņa, S. 6120* Xeric Sand Calcareous Grasslands. In European Union Protected Habitats in Latvia. Interpretation Manual; Auniņš, A., Ed.; Latvian Fund for Nature; Ministry of Environmental Protection and Regional Development: Riga, Latvia, 2013; pp. 165–168. [Google Scholar]
- Jusselme, M.D.; Pruvost, C.; Motard, E.; Giusti-Miller, S.; Frechault, S.; Alphonse, V.; Balland-Bolou-Bi, C.; Dajoz, I.; Mora, P. Increasing the ability of a green roof to provide ecosystem services by adding organic matter and earthworms. Appl. Soil Ecol. 2019, 143, 61–69. [Google Scholar] [CrossRef]
- Liu, R.; Stanford, R.L.; Deng, Y.; Liu, D.; Liu, Y.; Yu, S.L. The influence of extensive green roofs on rainwater runoff quality: A field-scale study in southwest China. Environ. Sci. Pollut. Res. 2020, 27, 12932–12941. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practicioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Liang, H.M.; Lin, T.-H.; Chiou, J.-M.; Yeh, K.-C. Model evaluation of the phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environ. Pollut. 2009, 157, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- van der Ent, A.; Echevarria, G.; Pollard, A.J.; Erskine, P.D. X-Ray fluorescence ionomics of herbarium collections. Sci. Rep. 2019, 9, 4746. [Google Scholar] [CrossRef] [PubMed]
- Purwadi, I.; Gei, V.; Echeverria, G.; Erskine, P.D.; Mesjasz-Przybyłowicz, J.; Przybyłowicz, W.J.; van der Ent, A. Tools for Discovery of Hyperaccumulator Plant Species in the Field and in the Herbarium. In Agromining: Farming for Metals. Mineral Resource Reviews; van der Ent, A., Baker, A.J., Echevarria, G., Simonnot, M.O., Morel, J.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 183–195. [Google Scholar]
- Aziz, I.; Mujeeb, A. Halophytes for phytoremediation of hazardous metal(oid)s: A review on metal tolerance, bio-indication and hyperaccumulation. J. Hazard. Mater. 2022, 424, 127309. [Google Scholar] [CrossRef] [PubMed]
- Long, X.X.; Yang, X.E.; Ni, W.Z.; Ye, Z.Q.; He, Z.L.; Calvert, D.V.; Stoffella, J.P. Assessing zinc thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Commun. Soil Sci. Plant Anal. 2003, 34, 1421–1434. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Robson, A.D.; Reuter, D.J. Diagnosis of Copper Deficiency and Toxicity. In Copper in Soils and Plants; Loneragan, J.F., Robson, A.D., Graham, R.D., Eds.; Academic Press: New York, NY, USA, 1981; pp. 287–312. [Google Scholar]
- Reichman, S. The Responses of Plant to Metal Toxicity. A review Focusing on Copper, Manganese and Zinc; Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002; Volume 14. [Google Scholar]
- Jin, X.F.; Yang, X.E.; Islam, E.; Liu, D.; Mahmood, Q.; Li, H.; Li, J. Ultrastructural changes, zinc hyperaccumulation and its relation with antioxidants in two ecotypes of Sedum alfredii Hance. Plant Physiol. Biochem. 2008, 46, 997–1006. [Google Scholar] [CrossRef]
- Sghaier, D.B.; Duarte, B.; Bankaji, I.; Caçador, I.; Sleimi, N. Growth, chlorophyll fluorescence and mineral nutrition in the halophyte Tamarix gallica cultivated in combined stress conditions: Arsenic and NaCl. J. Photochem. Photobiol. B Biol. 2015, 149, 204–214. [Google Scholar] [CrossRef]
- Loneragan, J.F. Distribution and Movement of Manganese in Plants. In Manganese in Soils and Plants. Developments in Plant and Soil Sciences; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 33, pp. 113–124. [Google Scholar]
- Santos, E.F.; Santini, J.M.K.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef]
- Losfeld, G.; L’Huillier, L.; Fogliani, B.; Mc Coy, S.; Grison, C.; Jaffré, T. Leaf-age and soil-plant relationships: Key factors for reporting trace-elements hyperaccumulation by plants and design applications. Environ. Sci. Pollut. Res. 2015, 2, 5620–5632. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shang, W.; Zhang, X.; Zhu, Y.; Yu, K. Mn accumulation and tolerance in Celosia argentea Linn.: A new Mn-hyperaccumulating plant species. J. Haz. Mater. 2014, 267, 136–141. [Google Scholar] [CrossRef]
- Yang, X.-J.; Deng, D.-M.; Liu, K.-H.; Yu, F.-M. Response of enzymatic and non-enzymatic antioxidant defense systems of Polygonum hydropiper to Mn stress. J. Cent. South Univ. 2016, 23, 793–797. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, R. Mechanism of zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Longnecker, N.E.; Robson, A.D. Distribution and Transport of Zinc in Plants. In Zinc in Soils and Plants. Developments in Plant and Soil Sciences; Robson, A.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 55, pp. 79–91. [Google Scholar]
- Lange, B.; van der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verruggen, N.; Faucon, M.-P. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; van der Ent, A.; Baker, A.J.M. Global Distribution and Ecology of Hyperaccumulator Plants. In Agromining: Farming for Metals. Mineral Resource Reviews; van der Ent, A., Baker, A.J., Echevarria, G., Simonnot, M.O., Morel, J.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 75–92. [Google Scholar]
- Küpper, H.; Gotz, B.; Mijovilovich, A.; Küpper, F.C.; Meyer-Klaucke, W. Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol. 2009, 151, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Arain, B.A.; Abbasi, M.S.; Jahangir, T.M.; Amin, F. Potential for phytoextraction of Cu by Sesamum indicum L. and Cyamopsis tetragonoloba L.: A green solution to decontaminate soil. Earth Syst. Environ. 2018, 2, 133–143. [Google Scholar] [CrossRef]
- Zhang, C.; Sale, P.W.G.; Clark, G.J.; Liu, W.; Doronila, A.I.; Kolev, S.D.; Tang, C. Succulent species differ substantially in their tolerance and phytoextraction potential when grown in the presence of Cd, Cr, Cu, Mn, Ni, Pb, and Zn. Environ. Sci. Pollut. Res. 2015, 22, 18824–18838. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, L.; Hu, P.; Luo, Y.; Christie, P. Copper changes the yield and cadmium/zinc accumulation and cellular distribution in the cadmium/zinc hyperaccumulator Sedum plumbizincicola. J. Hazard. Mater. 2013, 261, 332–341. [Google Scholar] [CrossRef]
- Frey, B.; Keller, C.; Zierold, K.; Schulin, R. Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2000, 23, 675–687. [Google Scholar] [CrossRef]
- Lu, L.; Tian, S.; Zhang, J.; Yang, X.; Labavitch, J.M.; Webb, S.M.; Latimer, M.; Brown, P.H. Efficient xylem transport and phloem remobilization of Zn in the hyperaccumulator plant species Sedum alfredii. New Phytol. 2013, 198, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-Q.; Yang, X.-E.; Yang, J.-Y.; He, Z.-L. Zn accumulation and subcellular distribution in the Zn hyperaccumulator Sedum alfredii Hance. Pedosphere 2006, 16, 616–623. [Google Scholar] [CrossRef]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes (Review). Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Ievinsh, G.; Dišlere, E.; Karlsons, A.; Osvalde, A.; Vikmane, M. Physiological responses of wetland species Rumex hydrolapathum to increased concentration of biogenous heavy metals Zn and Mn in substrate. Proc. Latv. Acad. Sci. B 2020, 74, 35–47. [Google Scholar] [CrossRef]
- Zhuang, P.; Wang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Commentary: Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 554. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, S.; Wu, F.; Leung, H.M.; Lin, X.; Wong, M.H. Arbuscular mycorrhizal fungi enhance both absorption and stabilization of Cd by Alfred stonecrop (Sedum alfredii Hance) and perennial ryegrass (Lolium perenne L.) in a Cd-contaminated acidic soil. Chemosphere 2013, 93, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Escarré, J.; Lefebvre, C.; Gruber, W.; Leblanc, M.; Lepart, J.; Riviere, Y.; Delay, B. Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: Implications for phytoremediation. New Phytol. 2000, 145, 429–437. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.J.; Folsom, T.C.; Lee, T.; Park, F.; Price, C.; Roeder, D.; Scmitz, J.; Stockwell, C. Heavy metal tolerance in Typha latifolia without the evolution of tolerant races. Ecology 1974, 55, 1163–1165. [Google Scholar] [CrossRef]
- Bert, V.; Bonnin, I.; Saumitou-Laprade, P.; de Laguérie, P.; Petit, D. Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol. 2002, 155, 47–57. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ievinsh, G.; Osvalde, A.; Karlsons, A.; Andersone-Ozola, U. Hylotelephium maximum from Coastal Drift Lines Is a Promising Zn and Mn Accumulator with a High Tolerance against Biogenous Heavy Metals. Stresses 2022, 2, 450-466. https://doi.org/10.3390/stresses2040031
Ievinsh G, Osvalde A, Karlsons A, Andersone-Ozola U. Hylotelephium maximum from Coastal Drift Lines Is a Promising Zn and Mn Accumulator with a High Tolerance against Biogenous Heavy Metals. Stresses. 2022; 2(4):450-466. https://doi.org/10.3390/stresses2040031
Chicago/Turabian StyleIevinsh, Gederts, Anita Osvalde, Andis Karlsons, and Una Andersone-Ozola. 2022. "Hylotelephium maximum from Coastal Drift Lines Is a Promising Zn and Mn Accumulator with a High Tolerance against Biogenous Heavy Metals" Stresses 2, no. 4: 450-466. https://doi.org/10.3390/stresses2040031
APA StyleIevinsh, G., Osvalde, A., Karlsons, A., & Andersone-Ozola, U. (2022). Hylotelephium maximum from Coastal Drift Lines Is a Promising Zn and Mn Accumulator with a High Tolerance against Biogenous Heavy Metals. Stresses, 2(4), 450-466. https://doi.org/10.3390/stresses2040031