Evolution of Gait Biomechanics During a Nine-Month Exercise Program for Parkinson’s Disease: An Interventional Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
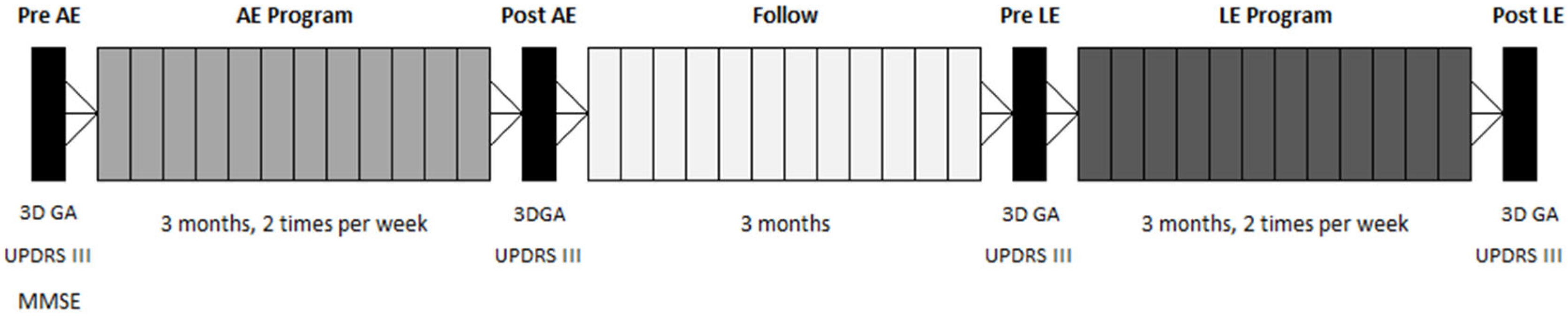
2.2. Assessment Sessions—Data Collection
2.3. Exercise Programs
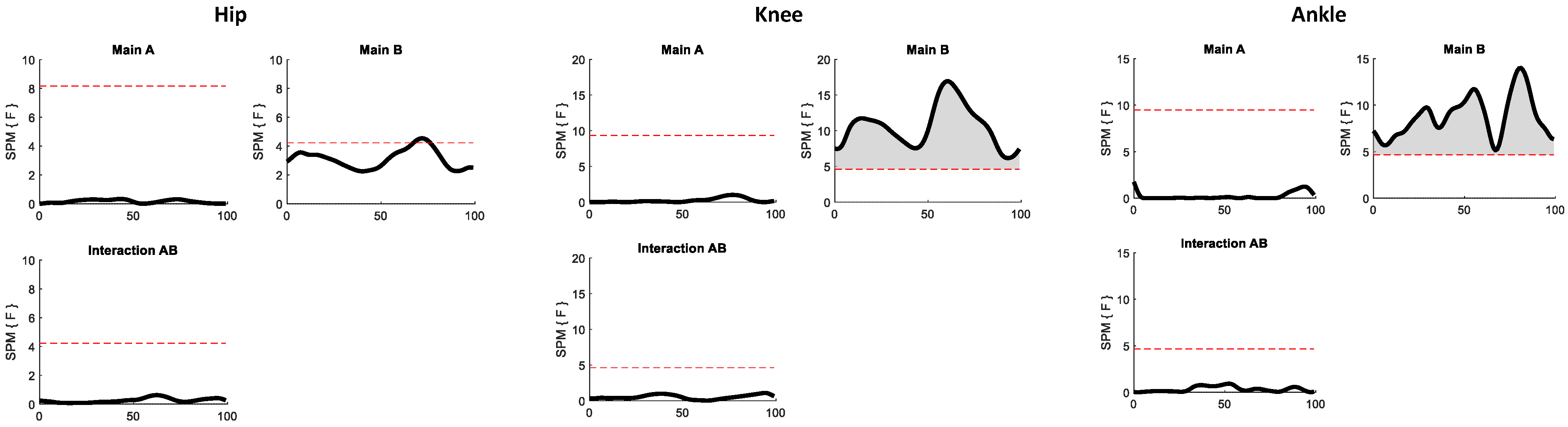
2.4. Statistical Analyses
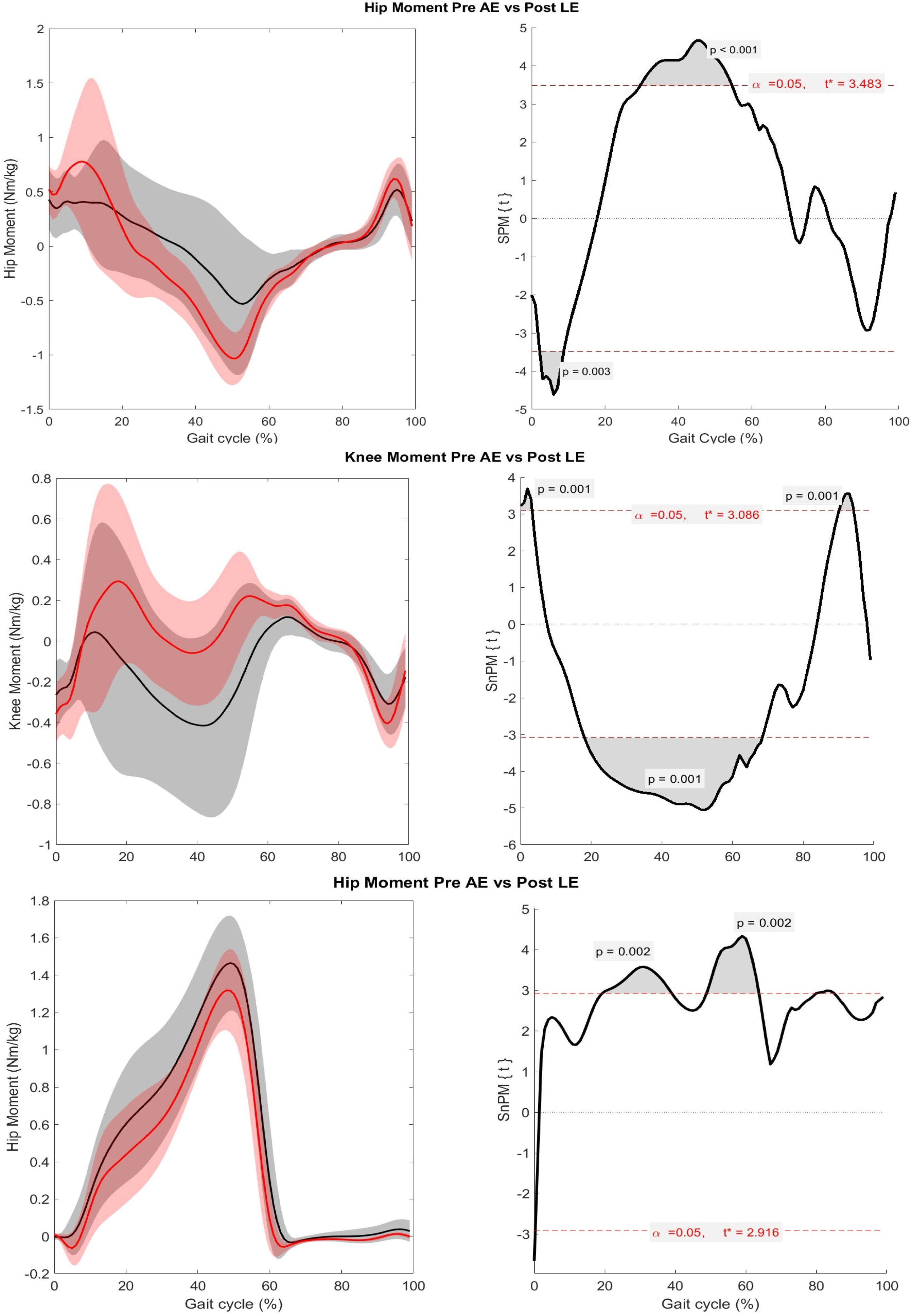
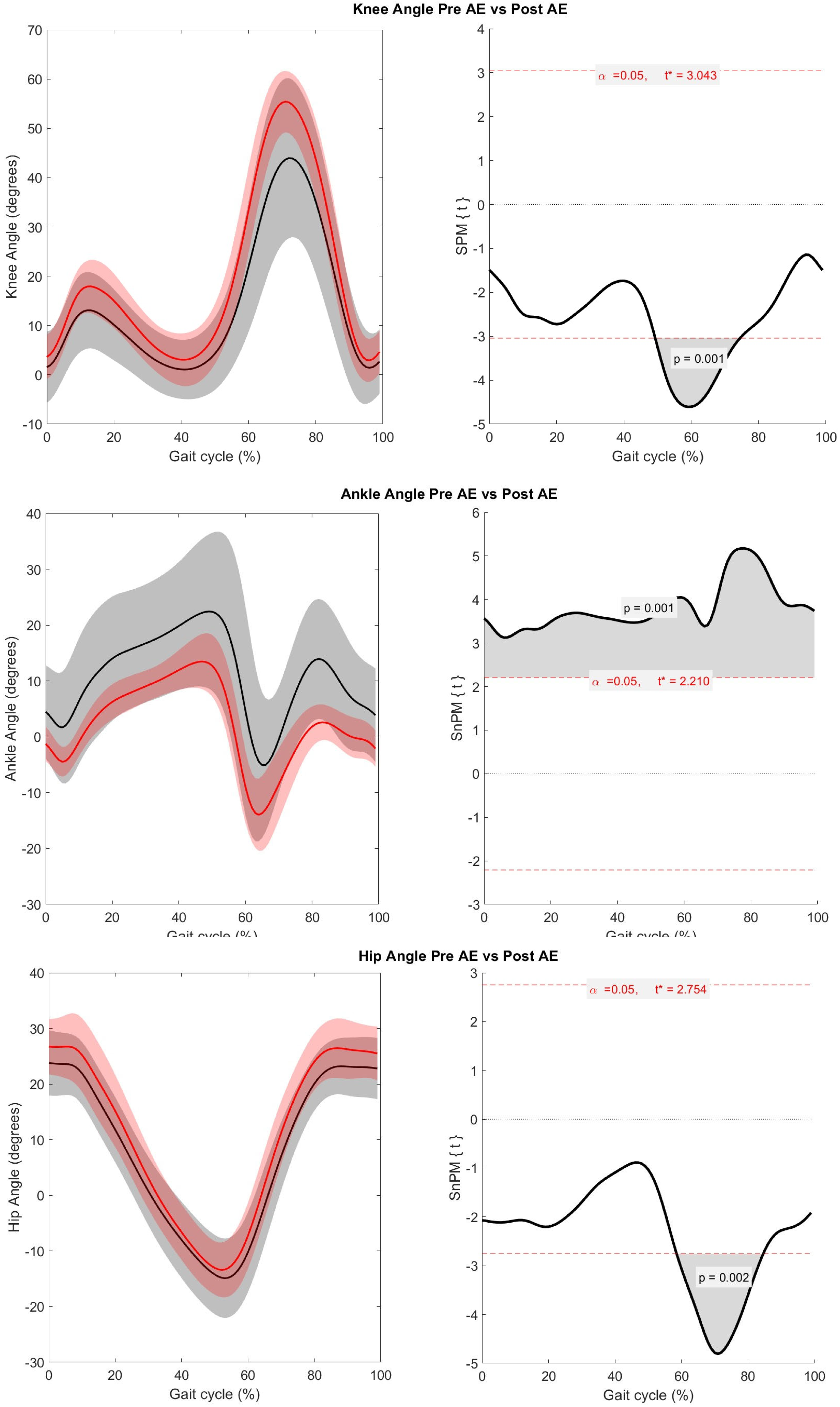
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| SPM | Statistical Parametric Mapping |
| AE | Aquatic Exercise |
| H&Y | Modified Hoehn and Yahr |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| LE | Land Exercise |
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Titova, N.; Padmakumar, C.; Lewis, S.J.G.; Chaudhuri, K.R. Parkinson’s: A syndrome rather than a disease? J. Neural Transm. 2017, 124, 907–914. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, A.P.J.; Silva, E.S.; Costa, R.R.; Passos-Monteiro, E.; Santos, I.O.; Kruel, L.F.M.; Peyré-Tartaruga, L.A. Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 752. [Google Scholar] [CrossRef]
- Spolaor, F.; Volpe, D.; Pavan, D.; Guiotto, A.; Fichera, F.; Torresin, P.; Fantinato, E.; Sawacha, Z. Surface EMG analysis in Parkinson disease patients before and after underwater gait training. Gait Posture 2017, 57, 185–191. [Google Scholar] [CrossRef]
- Volpe, D.; Spolaor, F.; Sawacha, Z.; Guiotto, A.; Pavan, D.; Bakdounes, L.; Urbani, V.; Frazzitta, G.; Iansek, R. Muscular activation changes in lower limbs after underwater gait training in Parkinson’s disease: A surface EMG pilot study. Gait Posture 2020, 80, 185–191. [Google Scholar] [CrossRef]
- Morris, M.E.; McGinley, J.; Huxham, F.; Collier, J.; Iansek, R. Constraints on the kinetic, kinematic and spatiotemporal parameters of gait in Parkinson’s disease. Hum. Mov. Sci. 1999, 18, 461–483. [Google Scholar] [CrossRef]
- Kuhman, D.; Hammond, K.G.; Hurt, C.P. Altered joint kinetic strategies of healthy older adults and individuals with Parkinson’s disease to walk at faster speeds. J. Biomech. 2018, 79, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Albani, G.; Cimolin, V.; Fasano, A.; Trotti, C.; Galli, M.; Mauro, A. “Masters and servants” in parkinsonian gait: A three-dimensional analysis of biomechanical changes sensitive to disease progression. Funct. Neurol. 2014, 29, 99–105. [Google Scholar]
- Sofuwa, O.; Nieuwboer, A.; Desloovere, K.; Willems, A.-M.; Chavret, F.; Jonkers, I. Quantitative gait analysis in Parkinson’s disease: Comparison with a healthy control group. Arch. Phys. Med. Rehabil. 2005, 86, 1007–1013. [Google Scholar] [CrossRef]
- Ernst, M.; Folkerts, A.K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M.; et al. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2023, 1, CD013856. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Hazzard, J.B.; Signorile, J.F.; Luca, C. Exercise Guidelines for Gait Function in Parkinson’s Disease: A Systematic Review and Meta-analysis. Neurorehabil. Neural Repair 2018, 32, 872–886. [Google Scholar] [CrossRef]
- Hvingelby, V.S.; Glud, A.N.; Sørensen, J.C.H.; Tai, Y.; Andersen, A.S.M.; Johnsen, E.; Moro, E.; Pavese, N. Interventions to improve gait in Parkinson’s disease: A systematic review of randomized controlled trials and network meta-analysis. J. Neurol. 2022, 269, 4068–4079. [Google Scholar] [CrossRef]
- Keus, S.H.; Munneke, M.; Graziano, M.; Paltamaa, J.; Pelosin, E.; Domingos, J.; Bloem, B. European Physiotherapy Guideline for Parkinson’s Disease; KNGF/ParkinsonNet: Nijmegen, The Netherlands, 2014. [Google Scholar]
- Osborne, J.A.; Botkin, R.; Colon-Semenza, C.; DeAngelis, T.R.; Gallardo, O.G.; Kosakowski, H.; Martello, J.; Pradhan, S.; Rafferty, M.; Readinger, J.L.; et al. Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys. Ther. 2022, 102, pzab302. [Google Scholar] [CrossRef]
- Rafferty, M.R.; Prodoehl, J.; Robichaud, J.A.; David, F.J.; Poon, C.; Goelz, L.C.; Vaillancourt, D.E.; Kohrt, W.M.; Comella, C.L.; Corcos, D.M. Effects of two years of exercise on gait impairment in people with Parkinson’s Disease: The PRET-PD randomized trial. J. Neurol. Phys. Ther. 2017, 41, 21–30. [Google Scholar] [CrossRef]
- Shen, X.; Mak, M.K. Balance and gait training with augmented feedback improves balance confidence in people with Parkinson’s disease: A randomized controlled trial. Neurorehabil. Neural Repair 2014, 28, 524–535. [Google Scholar] [CrossRef]
- Pinto, C.; Salazar, A.P.; Marchese, R.R.; Spector, N.D.; Mukherjee, D. The effects of hydrotherapy on balance, functional mobility, motor status, and quality of life in patients with Parkinson disease: A systematic review and meta-analysis. PMR 2019, 11, 278–291. [Google Scholar] [CrossRef]
- Gomes Neto, M.; Pontes, S.S.; Almeida, L.D.; da Silva, C.M.; Sena, C.; Saquetto, M.B. Effects of water-based exercise on functioning and quality of life in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.; Giantin, M.G.; Maestri, R.; Frazzitta, G. Comparing the effects of hydrotherapy and land-based therapy on balance in patients with Parkinson’s disease: A randomized controlled pilot study. Clin. Rehabil. 2014, 8, 1210–1217. [Google Scholar] [CrossRef]
- Volpe, D.; Pavan, D.; Morris, M.; Guiotto, A.; Iansek, R.; Fortuna, S.; Frazzitta, G.; Sawacha, Z. Underwater gait analysis in Parkinson’s disease. Gait Posture 2017, 52, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Orselli, M.I.; Duarte, M. Joint forces and torques when walking in shallow water. J. Biomech. 2011, 44, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Shirota, T.; Yamamoto, S.I.; Nakazawa, K.; Akai, M. Functional roles of lower-limb joint moments while walking in water. Clin. Biomech. 2005, 20, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Kato, T.; Kaneko, N.; Kobayashi, H.; Hoshino, M.; Kokubun, T.; Nakazawa, K. Basic locomotor muscle synergies used in land walking are finely tuned during underwater walking. Sci. Rep. 2021, 11, 18480. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyarian, R.; Majlesi, M.; Azadian, E.; Ali, M.J. Examining virtual reality’s influence on kinetic variables for obstacle crossing in Parkinson’s disease. Gait Posture 2025, 121, 85–92. [Google Scholar] [CrossRef]
- Russo, M.; Amboni, M.; Pisani, N.; Volzone, A.; Calderone, D.; Barone, P.; Amato, F.; Ricciardi, C.; Romano, M. Biomechanics Parameters of Gait Analysis to Characterize Parkinson’s Disease: A Scoping Review. Sensors 2025, 25, 338. [Google Scholar] [CrossRef]
- Pataky, T.C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 2010, 43, 1976–1982. [Google Scholar] [CrossRef]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Schenkman, M.L.; Clark, K.; Xie, T.; Kuchibhatla, M.; Shinberg, M.; Ray, L. Spinal movement and performance of a standing reach task in participants with and without Parkinson disease. Phys. Ther. 2001, 81, 1400–1411. [Google Scholar] [CrossRef]
- Davis, R.B., III; Ounpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Carroll, L.M.; Morris, M.E.; O’Connor, W.T.; Volpe, D.; Salsberg, J.; Clifford, A.M. Evidence-Based Aquatic Therapy Guidelines for Parkinson’s Disease: An International Consensus Study. J. Parkinsons Dis. 2022, 12, 621–637. [Google Scholar] [CrossRef]
- Siega, J.; Iucksch, D.D.; Israel, V.L. Multicomponent Aquatic Training (MAT) Program for People with Parkinson’s Disease: A Protocol for a Controlled Study. Int. J. Environ. Res. Public Health 2022, 19, 1727. [Google Scholar] [CrossRef]
- Iucksch, D.D. Análise dos Efeitos de um Programa de Exercícios Físicos Multicomponentes em Ambiente Aquático E Terrestre na Funcionalidade, na Função Muscular, no Controle Postural e na Marcha de Pessoas Com Doença de Parkinson. Ph.D. Thesis, Federal University of Paraná, Curitiba, Brazil, 2022. [Google Scholar]
- Kanitz, A.C.; Delevatti, R.S.; Reichert, T.; Liedtke, G.V.; Ferrari, R.; Almada, B.P.; Pinto, S.S.; Alberton, C.L.; Kruel, L.F.M. Effects of two deep water training programs on cardiorespiratory and muscular strength responses in older adults. Exp. Gerontol. 2015, 64, 55–61. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S.; American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.O.; Scianni, A.; Rodrigues-De-Paula, F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson’s disease: A systematic review. J. Physiother. 2013, 59, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.C.B.; Alonso, A.C.; Silva, T.M.; de Raphael, A.C.B.; Mota, C.F. Ai Chi: Efeitos do relaxamento aquático no desempenho funcional e qualidade de vida em idosos. Fisioter. Em Mov. 2010, 23, 409–417. [Google Scholar] [CrossRef][Green Version]
- Pereira, M.P.; Silva, N.A.; Matida, A.B.; Costa, J.N.A.; Gonçalves, C.D.; Safons, M.P.; Vianna, L.G. Protocolo de intervenção de Tai Chi Chuan para idosos. Lect. Educ. Física Y Deportes 2009, 14, 139. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Donnelly, C.J.; Alexander, C.; Pataky, T.C.; Stannage, K.; Reid, S.; Robinson, M. Vector-field statistics for the analysis of time varying clinical gait data. Clin. Biomech. 2017, 41, 87–91. [Google Scholar] [CrossRef][Green Version]
- Hass, C.J.; Bishop, M.; Moscovich, M.; Stegemöller, E.L.; Skinner, J.; Malaty, I.A.; Shukla, A.W.; McFarland, N.; Okun, M.S. Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J. Neurol. Phys. Ther. 2014, 38, 233–238. [Google Scholar] [CrossRef]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Fasano, A.; Bloem, B.R. Gait disorders. Continuum 2013, 19, 1344–1382. [Google Scholar] [CrossRef]
- Sloot, L.H.; van der Krogt, M.M. Interpreting Joint Moments and Powers in Gait. In Handbook of Human Motion; Müller, B., Wolf, S.I., Brueggemann, G.P., Deng, Z., McIntosh, A.S., Miller, F., Selbie, W.S., Eds.; Springer: Cham, Switzerland, 2018; pp. 625–643. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics of normal and pathological gait: Implications for understanding human locomotor control. J. Mot. Behav. 1989, 21, 337–355. [Google Scholar] [CrossRef]
- Skinner, J.W.; Lee, H.K.; Roemmich, R.T.; Amano, S.; Hass, C.J. Execution of activities of daily living in persons with Parkinson disease. Med. Sci. Sports Exerc. 2015, 47, 1906–1912. [Google Scholar] [CrossRef]
- Baudendistel, S.T.; Schmitt, A.C.; Roemmich, R.T.; Harrison, I.L.; Hass, C.J. Levodopa facilitates improvements in gait kinetics at the hip, not the ankle, in individuals with Parkinson’s disease. J. Biomech. 2021, 121, 110366. [Google Scholar] [CrossRef]
- Devita, P.; Hortobagyi, T. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Huxham, F.; McGinley, J.; Dodd, K.; Iansek, R. The biomechanics and motor control of gait in Parkinson disease. Clin. Biomech. 2001, 16, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Pistacchi, M.; Gioulis, M.; Sanson, F.; De Giovannini, E.; Filippi, G.; Rossetto, F.; Marsala, S.Z. Gait analysis and clinical correlations in early Parkinson’s disease. Funct. Neurol. 2017, 32, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Truijen, S.; Abdullahi, A.; Bijsterbosch, D.; van Zoest, E.; Conijn, M.; Wang, Y.; Struyf, N.; Saeys, W. Effect of home-based virtual reality training and telerehabilitation on balance in individuals with Parkinson disease, multiple sclerosis, and stroke: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 2995–3006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uhlrich, S.D.; Falisse, A.; Kidziński, Ł.; Muccini, J.; Ko, M.; Chaudhari, A.S.; Hicks, J.L.; Delp, S.L.; Marsden, A.L. OpenCap: Human movement dynamics from smartphone videos. PLoS Comput. Biol. 2023, 19, e1011462. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Quan, W.; Ma, X.; Chon, T.-E.; Fernandez, J.; Gusztav, F.; Kovács, A.; Baker, J.S.; Gu, Y. New Insights Optimize Landing Strategies to Reduce Lower Limb Injury Risk. Cyborg Bionic Syst. 2024, 5, 0126. [Google Scholar] [CrossRef]

| Condition | Aquatic Exercise Program | Land Exercise Program | Whole Program (AE, Follow Up, LE) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre AE | Post AE | p-Value Cohen’s d | Pre LE | Post LE | p-Value Cohen’s d | Pre AE | Post LE | p-Value Cohen’s d | |
| Gait Speed (m/s) | 1.0 ± 0.2 | 1.1 ± 0.1 | p = 0.30 | 1.1 ± 0.2 | 1.2 ± 0.2 | p = 0.11 | 1.0 ± 0.2 | 1.2 ± 0.2 | p = 0.02 * d = 0.71 |
| Cadence (steps/ min) | 108.7 ± 10.3 | 108.1 ± 6.3 | p = 0.78 | 110.4 ± 8.5 | 114.9±10.0 | p < 0.01 * d = 0.48 | 108.7 ± 10.3 | 114.9 ± 10.0 | p < 0.01 * d = 0.61 |
| Stride Length (cm) | 1.1 ± 0.2 | 1.2 ± 0.1 | p = 0.97 | 1.2 ± 0.1 | 1.2 ± 0.2 | p = 0.87 | 1.13±0.16 | 1.21±0.16 | p = 0.07 |
| Swing Time (%) | 38.0 ± 2.7 | 38.0 ± 2.4 | p = 0.97 | 37.7 ± 2.5 | 38.7 ± 2.9 | p = 0.03 * d = 0.37 | 38.0 ± 2.7 | 38.7 ± 2.9 | p = 0.21 |
| Double Support (%) | 25.0 ± 3.5 | 23.9 ± 3.1 | p = 0.20 | 24.0 ± 3.5 | 23.0 ± 3.6 | p = 0.03 * d = 0.29 | 25.0 ± 3.5 | 23.0 ± 3.6 | p < 0.01 * d = 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iucksch, D.D.; Manffra, E.F.; Israel, V.L. Evolution of Gait Biomechanics During a Nine-Month Exercise Program for Parkinson’s Disease: An Interventional Cohort Study. Biomechanics 2025, 5, 53. https://doi.org/10.3390/biomechanics5030053
Iucksch DD, Manffra EF, Israel VL. Evolution of Gait Biomechanics During a Nine-Month Exercise Program for Parkinson’s Disease: An Interventional Cohort Study. Biomechanics. 2025; 5(3):53. https://doi.org/10.3390/biomechanics5030053
Chicago/Turabian StyleIucksch, Dielise Debona, Elisangela Ferretti Manffra, and Vera Lucia Israel. 2025. "Evolution of Gait Biomechanics During a Nine-Month Exercise Program for Parkinson’s Disease: An Interventional Cohort Study" Biomechanics 5, no. 3: 53. https://doi.org/10.3390/biomechanics5030053
APA StyleIucksch, D. D., Manffra, E. F., & Israel, V. L. (2025). Evolution of Gait Biomechanics During a Nine-Month Exercise Program for Parkinson’s Disease: An Interventional Cohort Study. Biomechanics, 5(3), 53. https://doi.org/10.3390/biomechanics5030053






