Asymmetric Knee Joint Loading in Post-Stroke Gait: A Musculoskeletal Modeling Analysis of Medial and Lateral Compartment Forces
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Musculoskeletal Modeling
- is the activation level of muscle i.
- n is the total number of muscles in the model.
- 1.
- Moment equilibrium:
- ri(s) is the moment arm vector of muscle i at joint configuration s.
- Fi(s,ai) = ai·Fmax,i·fl(li)·fv(vi) is the force produced by muscle i.
- Fmax,i is the maximum isometric force of muscle i.
- fl(li) is the force–length relationship.
- fv(vi) is the force–velocity relationship.
- M is the net joint moment derived from inverse dynamics.
- 2.
- Activation bounds: 0 ≤ ai ≤ 1 for all i ∈ {1, 2, …, n}
2.4. Statistical Analysis
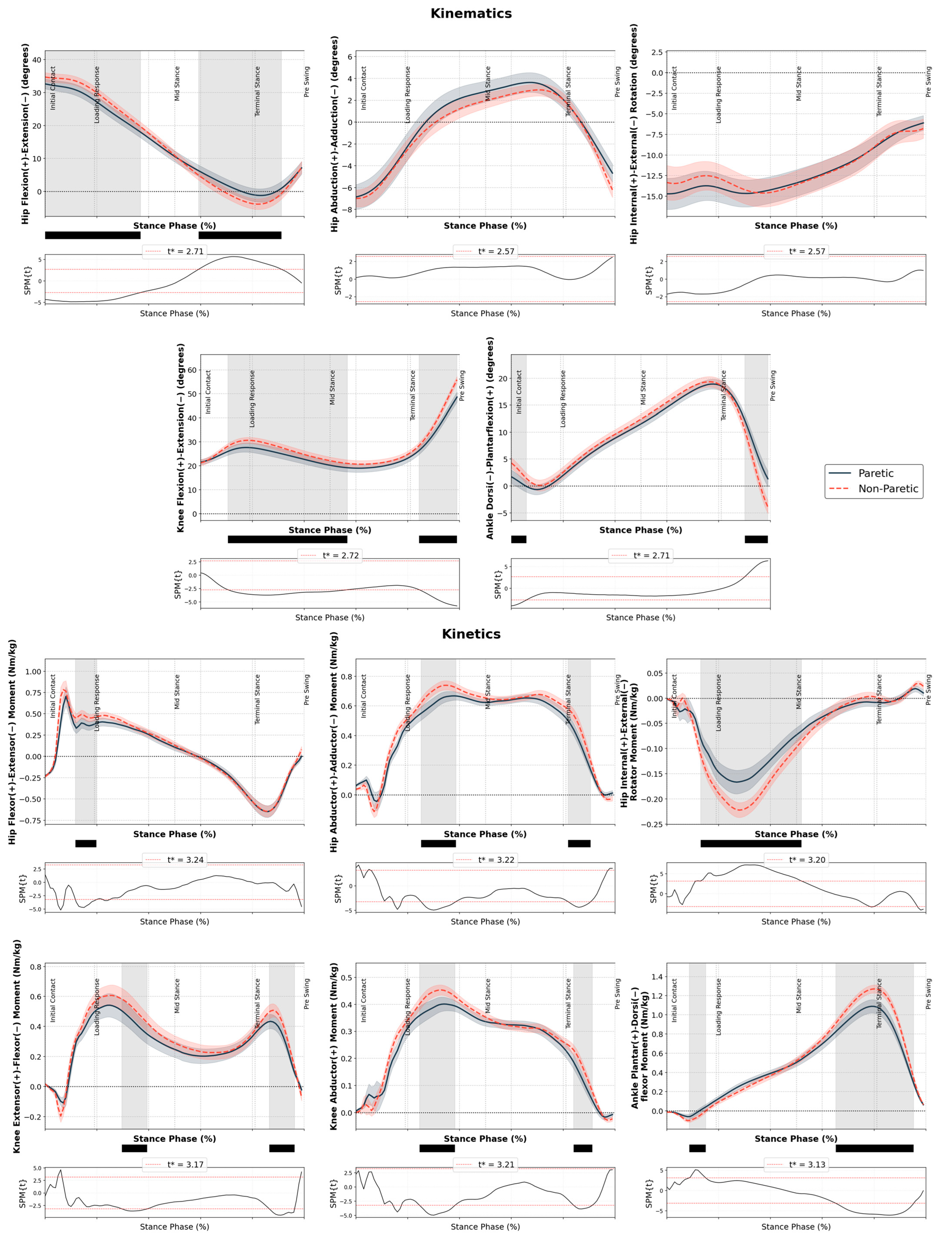
3. Results
3.1. Kinematics and Kinetics
Regression Analysis
4. Discussion
- Clinical understanding: The findings reveal biomechanical mechanisms underlying increased osteoarthritis risk in the non-paretic limb, moving beyond epidemiological observations to provide mechanistic insights into harmful joint loading patterns.
- Methodological advancement: The study establishes musculoskeletal modeling as a valuable clinical tool providing insights unavailable through traditional external moment analysis alone.
- Clinical applications: Results demonstrate that interventions should target both limbs simultaneously rather than focusing exclusively on paretic limb restoration, as compensatory loading patterns may lead to long-term consequences. These findings provide a foundation for developing interventions that address both functional recovery and long-term joint health preservation, potentially reducing secondary musculoskeletal complications in stroke survivors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Belda-Lois, J.-M.; Horno, S.M.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of Gait after Stroke: A Top down Approach. J. Neuroeng. Rehabil. 2011, 8, 66. [Google Scholar] [CrossRef]
- Raghavan, P. Emerging Therapies for Spastic Movement Disorders. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 633–644. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Ferragut-Garcías, A.; Rodríguez-Blanco, C.; Heredia-Rizo, A.M.; Oliva-Pascual-Vaca, J.; Kiper, P.; Oliva-Pascual-Vaca, Á. A Decade of Progress Using Virtual Reality for Poststroke Lower Extremity Rehabilitation: Systematic Review of the Intervention Methods. BioMed Res. Int. 2015, 2015, 342529. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.H.; Lewek, M.D.; Dhaher, Y.Y. Biomechanical Impairments and Gait Adaptations Post-Stroke: Multi-Factorial Associations. J. Biomech. 2009, 42, 1673–1677. [Google Scholar] [CrossRef]
- Shakoor, N.; Foucher, K.C.; Wimmer, M.A.; Mikolaitis-Preuss, R.A.; Fogg, L.F.; Block, J.A. Asymmetries and Relationships between Dynamic Loading, Muscle Strength, and Proprioceptive Acuity at the Knees in Symptomatic Unilateral Hip Osteoarthritis. Arthritis Res. Ther. 2014, 16, 455. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Mündermann, A. The Role of Ambulatory Mechanics in the Initiation and Progression of Knee Osteoarthritis. Curr. Opin. Rheumatol. 2006, 18, 514–518. [Google Scholar] [CrossRef]
- Robbins, C.B.; Vreeman, D.J.; Sothmann, M.S.; Wilson, S.L.; Oidridge, N.B. A Review of the Long-Term Health Outcomes Associated With War-Related Amputation. Mil. Med. 2009, 174, 588–592. [Google Scholar] [CrossRef]
- Holder, J.; van Drongelen, S.; Uhlrich, S.D.; Herrmann, E.; Meurer, A.; Stief, F. Peak Knee Joint Moments Accurately Predict Medial and Lateral Knee Contact Forces in Patients with Valgus Malalignment. Sci. Rep. 2023, 13, 2870. [Google Scholar] [CrossRef]
- Richards, R.E.; Andersen, M.S.; Harlaar, J.; van den Noort, J.C. Relationship between Knee Joint Contact Forces and External Knee Joint Moments in Patients with Medial Knee Osteoarthritis: Effects of Gait Modifications. Osteoarthr. Cartil. 2018, 26, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.P.; D’Lima, D.D.; Colwell, C.W.; Fregly, B.J. Decreased Knee Adduction Moment Does Not Guarantee Decreased Medial Contact Force during Gait. J. Orthop. Res. 2010, 28, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Baert, I.A.C.; Jonkers, I.; Staes, F.; Luyten, F.P.; Truijen, S.; Verschueren, S.M.P. Gait Characteristics and Lower Limb Muscle Strength in Women with Early and Established Knee Osteoarthritis. Clin. Biomech. 2013, 28, 40–47. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait Differences between Individuals with Post-Stroke Hemiparesis and Non-Disabled Controls at Matched Speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef]
- Buurke, J.H.; Nene, A.V.; Kwakkel, G.; Erren-Wolters, V.; Ijzerman, M.J.; Hermens, H.J. Recovery of Gait after Stroke: What Changes? Neurorehabil. Neural Repair 2008, 22, 676–683. [Google Scholar] [CrossRef]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Forward Propulsion Asymmetry Is Indicative of Changes in Plantarflexor Coordination during Walking in Individuals with Post-Stroke Hemiparesis. Clin. Biomech. 2014, 29, 780–786. [Google Scholar] [CrossRef]
- Robbins, S.M.K.; Maly, M.R. The Effect of Gait Speed on the Knee Adduction Moment Depends on Waveform Summary Measures. Gait Posture 2009, 30, 543–546. [Google Scholar] [CrossRef]
- Yang, C.P.; Lee, C.L.; Chen, T.W.; Lee, S.; Weng, M.C.; Huang, M.H. Ultrasonographic Findings in Hemiplegic Knees of Stroke Patients. Kaohsiung J. Med. Sci. 2005, 21, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, A.S.; Famurewa, O.C.; Komolafe, M.A.; Omisore, A.D.; Adetiloye, V.A. Sonographic Soft Tissue Arthritic Changes Associated with Post-Stroke Hemiplegic Knee Pain: Utility of Musculoskeletal Ultrasound in a Resource-Limited Setting. Polish J. Radiol. 2020, 85, e45. [Google Scholar] [CrossRef]
- Tunç, H.; Öken, Ö.; Kara, M.; Tiftik, T.; Doǧu, B.; Ünlü, Z.; Özçakar, L. Ultrasonographic Measurement of the Femoral Cartilage Thickness in Hemiparetic Patients after Stroke. Int. J. Rehabil. Res. 2012, 35, 203–207. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Lee, H.J. Prevalence of Knee Osteoarthritis and Health-Related Quality of Life in Stroke Patients over 60 Years Old: A Cross-Sectional Study Using Korean National Health and Nutrition Examination Survey V. Ann. Geriatr. Med. Res. 2021, 25, 178. [Google Scholar] [CrossRef]
- Fournier, J.; Finestone, H.; Lauzon, J.; Campbell, T.M. Prevalence, Impact, and Treatment of Co-Occurring Osteoarthritis in Patients With Stroke Undergoing Rehabilitation: A Review. Stroke 2021, 52, E618–E621. [Google Scholar] [CrossRef]
- Marrocco, S.; Crosby, L.D.; Jones, I.C.; Moyer, R.F.; Birmingham, T.B.; Patterson, K.K. Knee Loading Patterns of the Non-Paretic and Paretic Legs during Post-Stroke Gait. Gait Posture 2016, 49, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Prajapati, S.K.; Borrelli, J.; Gray, V.L.; Westlake, K.P.; Rogers, M.W.; Hsiao, H.Y. Neuromechanical Control of Impact Absorption during Induced Lower Limb Loading in Individuals Post-Stroke. Sci. Rep. 2022, 12, 19104. [Google Scholar] [CrossRef]
- Holder, J.; Trinler, U.; Meurer, A.; Stief, F. A Systematic Review of the Associations Between Inverse Dynamics and Musculoskeletal Modeling to Investigate Joint Loading in a Clinical Environment. Front. Bioeng. Biotechnol. 2020, 8, 1382. [Google Scholar] [CrossRef] [PubMed]
- Mohout, I.; Elahi, S.A.; Esrafilian, A.; Killen, B.A.; Korhonen, R.K.; Verschueren, S.; Jonkers, I. Signatures of Disease Progression in Knee Osteoarthritis: Insights from an Integrated Multi-Scale Modeling Approach, a Proof of Concept. Front. Bioeng. Biotechnol. 2023, 11, 1214693. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Behzadipour, S.; Taghizadeh, G. A Planar Neuro-Musculoskeletal Arm Model in Post-Stroke Patients. Biol. Cybern. 2018, 112, 483–494. [Google Scholar] [CrossRef]
- Andersen, M.S. Introduction to Musculoskeletal Modelling. In Computational Modelling of Biomechanics and Biotribology in the Musculoskeletal System: Biomaterils and Tissues; Woodhead Publishing: Cambridge, UK, 2021; pp. 41–80. [Google Scholar]
- Giarmatzis, G.; Fotiadou, S.; Giannakou, E.; Kokkotis, C.; Fanaradelli, T.; Kordosi, S.; Vadikolias, K.; Aggelousis, N. Understanding Post-Stroke Movement by Means of Motion Capture and Musculoskeletal Modeling: A Scoping Review of Methods and Practices. BioMed 2022, 2, 409–421. [Google Scholar] [CrossRef]
- Curreli, C.; Di Puccio, F.; Davico, G.; Modenese, L.; Viceconti, M. Using Musculoskeletal Models to Estimate in Vivo Total Knee Replacement Kinematics and Loads: Effect of Differences Between Models. Front. Bioeng. Biotechnol. 2021, 9, 703508. [Google Scholar] [CrossRef]
- Tomasi, M.; Artoni, A.; Mattei, L.; Di Puccio, F. On the Estimation of Hip Joint Loads through Musculoskeletal Modeling. Biomech. Model. Mechanobiol. 2022, 22, 379–400. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Fregly, B.J.; Patil, S.; Steklov, N.; Colwell, C.W. Knee Joint Forces: Prediction, Measurement, and Significance. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 95–102. [Google Scholar] [CrossRef]
- Giarmatzis, G.; Fotiadou, S.; Giannakou, E.; Karakasis, E.; Vadikolias, K.; Aggelousis, N. Evaluating the Repeatability of Musculoskeletal Modelling Force Outcomes in Gait among Chronic Stroke Survivors: Implications for Contemporary Clinical Practice. Biomechanics 2024, 4, 333–345. [Google Scholar] [CrossRef]
- Lerner, Z.F.; DeMers, M.S.; Delp, S.L.; Browning, R.C. How Tibiofemoral Alignment and Contact Locations Affect Predictions of Medial and Lateral Tibiofemoral Contact Forces. J. Biomech. 2015, 48, 644–650. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C. One-Dimensional Statistical Parametric Mapping in Python. Comput. Methods Biomech. Biomed. Engin. 2012, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C. Www.Spm1d.Org. Available online: https://spm1d.org/ (accessed on 26 May 2025).
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar]
- Liu, T.; Xie, H.; Yan, S.; Zeng, J.; Zhang, K.; Liu, T.; Xie, H.; Yan, S.; Zeng, J.; Zhang, K. The Effect of Thigh Muscle Forces on Knee Contact Force in Female Patients with Severe Knee Osteoarthritis. Bioengineering 2024, 11, 1299. [Google Scholar] [CrossRef]
- Miller, R.H.; Krupenevich, R.L.; Pruziner, A.L.; Wolf, E.J.; Schnall, B.L. Medial Knee Joint Contact Force in the Intact Limb during Walking in Recently Ambulatory Service Members with Unilateral Limb Loss: A Cross-Sectional Study. PeerJ 2017, 2017, e2960. [Google Scholar] [CrossRef] [PubMed]
- Hak, L.; Houdijk, H.; Van Der Wurff, P.; Prins, M.R.; Mert, A.; Beek, P.J.; Van Dieën, J.H. Stepping Strategies Used by Post-Stroke Individuals to Maintain Margins of Stability during Walking. Clin. Biomech. 2013, 28, 1041–1048. [Google Scholar] [CrossRef]
- Hak, L.; Blokland, I.; Houdijk, H. How Do People after Stroke Adapt Step Parameters and Margins of Stability at Different Walking Speeds? Gait Posture 2020, 81, 136–137. [Google Scholar] [CrossRef]
- Kumar, D.; Manal, K.T.; Rudolph, K.S. Knee Joint Loading during Gait in Healthy Controls and Individuals with Knee Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 298–305. [Google Scholar] [CrossRef]
- Giarmatzis, G.; Zacharaki, E.I.; Moustakas, K. Real-Time Prediction of Joint Forces by Motion Capture and Machine Learning. Sensors 2020, 20, 6933. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the Knee Joint during Activities of Daily Living Measured In Vivo in Five Subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.D.D.; Patil, S.; Steklov, N.; Slamin, J.E.; Jr, C.W.C. Tibial Forces Measured In Vivo After Total Knee Arthroplasty. J. Arthroplast. 2006, 21, 255–262. [Google Scholar] [CrossRef] [PubMed]


| Variable | Paretic Limb | Non-Paretic Limb |
|---|---|---|
| Medial Compartment | ||
| First peak contact force (BW) | 2.37 ± 0.51 | 2.42 ± 0.43 |
| Knee extensor moment at first peak (Nm/kg) | 0.57 ± 0.34 | 0.63 ± 0.34 |
| Knee abductor moment at first peak (Nm/kg) | 0.42 ± 0.11 | 0.44 ± 0.11 |
| Second peak contact force (BW) | 2.58 ± 0.60 | 2.72 ± 0.47 |
| Knee extensor moment at second peak (Nm/kg) | 0.30 ± 0.22 | 0.33 ± 0.23 |
| Knee abductor moment at second peak (Nm/kg) | 0.34 ± 0.10 | 0.32 ± 0.10 |
| Lateral Compartment | ||
| First peak contact force (BW) | 0.97 ± 0.41 | 0.88 ± 0.29 |
| Knee extensor moment at first peak (Nm/kg) | 0.39 ± 0.36 | 0.42 ± 0.33 |
| Knee abductor moment at first peak (Nm/kg) | 0.26 ± 0.28 | 0.21 ± 0.17 |
| Second peak contact force (BW) | 0.91 ± 0.41 | 1.03 ± 0.40 |
| Knee extensor moment at second peak (Nm/kg) | 0.41 ± 0.24 | 0.48 ± 0.26 |
| Knee abductor moment at second peak (Nm/kg) | 0.17 ± 0.13 | 0.20 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarmatzis, G.; Aggelousis, N.; Marinidis, M.; Fotiadou, S.; Giannakou, E.; Makri, E.; Liu, J.; Vadikolias, K. Asymmetric Knee Joint Loading in Post-Stroke Gait: A Musculoskeletal Modeling Analysis of Medial and Lateral Compartment Forces. Biomechanics 2025, 5, 39. https://doi.org/10.3390/biomechanics5020039
Giarmatzis G, Aggelousis N, Marinidis M, Fotiadou S, Giannakou E, Makri E, Liu J, Vadikolias K. Asymmetric Knee Joint Loading in Post-Stroke Gait: A Musculoskeletal Modeling Analysis of Medial and Lateral Compartment Forces. Biomechanics. 2025; 5(2):39. https://doi.org/10.3390/biomechanics5020039
Chicago/Turabian StyleGiarmatzis, Georgios, Nikolaos Aggelousis, Marinos Marinidis, Styliani Fotiadou, Erasmia Giannakou, Evangelia Makri, Junshi Liu, and Konstantinos Vadikolias. 2025. "Asymmetric Knee Joint Loading in Post-Stroke Gait: A Musculoskeletal Modeling Analysis of Medial and Lateral Compartment Forces" Biomechanics 5, no. 2: 39. https://doi.org/10.3390/biomechanics5020039
APA StyleGiarmatzis, G., Aggelousis, N., Marinidis, M., Fotiadou, S., Giannakou, E., Makri, E., Liu, J., & Vadikolias, K. (2025). Asymmetric Knee Joint Loading in Post-Stroke Gait: A Musculoskeletal Modeling Analysis of Medial and Lateral Compartment Forces. Biomechanics, 5(2), 39. https://doi.org/10.3390/biomechanics5020039






