Abstract
Robotic cadaveric testing provides a controlled approach to studying knee ligament biomechanics under continuous motion, addressing limitations in static or mechanical loading testing. Our study describes an alternative method for soft-tissue strain measurement, followed by an investigation of this method on knee ligament strain and joint kinematics using a six-degree-of-freedom robotic system equipped with force and torque sensors. Six cadaveric knee specimens underwent controlled 90° flexion cycles, with uniaxial strain gauges sutured to the ACL, PCL, MCL, and LCL for strain measurement. Results indicate that the LCL exhibited the highest extension at 1.63 mm, while the ACL showed minimal extension at 0.09 mm. The MCL were at −0.76 mm and PCL at −1.76 mm contraction, suggesting a stabilizing function under flexion. Varus torque at 2.18 Nm at 90° flexion correlated with LCL strain, and PCL translation variability reflected its multi-planar engagement. These findings confirm ligament-specific strain responses under dynamic loading, highlighting that the LCL and PCL undergo the most significant length changes.
1. Introduction
The biomechanical evaluation of knee joint function, particularly in the context of ligament stability and injury mechanisms, has been extensively studied through in vivo and in vitro testing [1,2,3,4]. These studies provide critical insights into knee kinematics, ligament function, and post-injury biomechanical changes, which could influence surgical interventions, rehabilitation strategies, and the development of orthopedic devices [5]. Unlike in vivo studies, which are constrained by ethical and practical limitations, cadaveric testing allows precise simulation of physiological loading conditions to investigate ligament strain, joint stability, and kinematic alterations following injury or reconstruction [3].
Several methodologies have been employed to assess knee biomechanics in cadaveric models, including instrumented knee simulators, universal testing machines (UTMs), and robotic testing systems [3,6,7,8,9]. Instrumented knee simulators use electromechanical actuators to apply dynamic muscle forces to replicate movements such as jump landings and directional changes, guided by motion capture data from in vivo studies [10,11]. While UTMs and custom mechanical testing rigs can apply cyclic or static loads using linear actuators and load cells, and are often integrated with optical tracking or biplanar fluoroscopy for precise kinematic data, they remain limited in replicating multi-directional physiological motion [5,12].
The use of robotic testing systems has emerged as a sophisticated approach, providing six-degree-of-freedom (6-DOF) loading conditions to replicate physiological knee motion with high precision and reproducibility [3]. These robotic systems can simulate activities such as walking, jumping, and pivoting while precisely measuring joint kinematics and ligament forces under controlled loading conditions [5,9]. The ability to integrate real-time load measurement and computational modeling enhances the fidelity of cadaveric knee testing, allowing for a more comprehensive evaluation of ligament function. Strain gauges and differential variable reluctance transducers (DVRTs) have been widely utilized to measure ligament strain across these methodologies. Metal-foil strain gauges, used by Hinterwimmer et al. (2002) and France (1983), provided key insights into ligament elongation [13,14]. Zens et al. (2015) had applied capacitive strain gauges to assess strain in the anterolateral ligament (ALL), while Wilson et al. (2018) and Bate et al. (2017) implanted DVRTs in the ACL and medial collateral ligament (MCL) to capture high-resolution strain measurements under dynamic loading [9,15,16,17].
Challenges remain in replicating the complex interplay of muscle forces, joint compression, and soft tissue constraints observed in vivo. Existing methods often simplify knee kinematics to quasi-static or constrained loading scenarios, limiting their applicability to real-world conditions. In particular, DVRTs can provide high-resolution ligament strain data; however, their attachment methods may influence tissue mechanics, as the placement of these devices can interfere with muscles and tissue movement. Therefore, there is a need to enhance existing methodologies that integrate robotic control and real-time load measurement to improve the accuracy and reliability of cadaveric knee testing.
In parallel with cadaveric research, musculoskeletal modeling has emerged as a computational tool to simulate joint mechanics and estimate ligament strain under dynamic loading conditions. These models allow researchers to run repeatable simulations across a wide range of conditions, including complex muscle forces and ground reaction forces that are difficult to replicate experimentally. However, musculoskeletal models rely on simplifications such as idealized ligament paths, generic tissue properties, and static insertion points, which may not fully capture subject-specific or localized behaviors [18,19,20]. Conversely, cadaveric testing enables direct measurement of real anatomical structures and soft tissue responses. As such, combining insights from both approaches can advance our understanding of knee ligament function.
This technical study aims to build upon established methodologies by implementing an optimized cadaveric testing framework that integrates robotic control and high-resolution strain measurement. By leveraging insights from prior research, we seek to refine the accuracy of ligament strain quantification and joint stability assessment. Measuring how ligaments change in length under load can inform surgical repair, optimize rehabilitation, and guide injury prevention by revealing strain patterns that contribute to instability or tissue failure. As the focus is on presenting a technical advancement in experimental setup and strain acquisition—rather than hypothesis testing or generalizable biological outcomes—this work is appropriately formatted as a technical note.
2. Materials and Methods
Six unpaired male frozen cadaveric knee specimens (three left, three right, mean age: 64 ± 5.4 years) were obtained from an accredited tissue donation center, ensuring the absence of ligament laxity, prior surgical interventions, or structural defects. The tissue bank screened all specimens to exclude prior surgical interventions or major trauma. During dissection, the ACL, PCL, MCL, and LCL were observed to be grossly intact and free from signs of rupture, ensuring suitability for biomechanical testing. The specimens were preserved at −20 °C and thawed for 24 h at 4 °C before testing to maintain the soft tissue integrity. To standardize the setup, the tibia, femur, and fibula were dissected 200 mm from the joint line, while the fibula was dissected another 20 mm more distal to the proximal tibiofibular joint and rigidly fixed to the tibia using the bicortical screw. To facilitate mounting, the cut ends of the femur and tibia were embedded into cylindrical aluminum pits at 100 mm from the joint line and secured with poly-methyl methacrylate (PMMA) cement [21].
Figure 1a,b illustrate the dissection process used to access the primary knee ligament. A lateral incision along the joint line was performed to expose the lateral collateral ligament (LCL), while a medial incision was made to access the medial collateral ligament (MCL). To expose the anterior cruciate ligament (ACL), a longitudinal incision was created superior to the patella, with the knee maintained at approximately 90° of flexion. The posterior cruciate ligament (PCL) was accessed through the posterior approach, requiring careful separation of the gastrocnemius muscle to reach the posterior joint capsule.
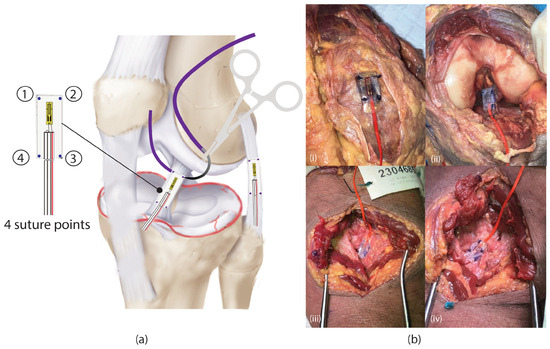
Figure 1.
(a) Graphical illustration on how the strain gauges were sutured on the ligaments, (b) an example of an actual photo of the strain gauge being sutured on the four ligaments (i) MCL, (ii) ACL, (iii) PCL, and (iv) LCL.
Once the ligaments were exposed, the uniaxial waterproof strain gauge sensors (Tokyo Measuring Instruments Lab, Shinagawa City, Tokyo 140-0013, Japan) were sutured onto each ligament using Ethicon 3-0 Vicryl sutures, ensuring secure attachment while minimizing disruption to native tissue properties. To maintain consistency with strain measurement, the strain gauges were aligned along the visually central portion of each ligament, and the sutures were anchored at four equidistant points of each ligament, ensuring uniform fixation and minimizing localized stress concentration.
Unlike previous studies that have used cyanoacrylate adhesives or epoxy to secure the strain gauges [6,14], our proposed technical method can avoid potential stiffening effects caused by adhesive application.
Figure 2a shows the experimental setup utilizing a 6-DOF industrial robotic system (KR 210; KUKA Robotics, Augsburg, Germany), and Figure 2b,c define the direction of how forces and torque should be measured on the knee. The robot is integrated with a load cell sensor (ATI Omega 160, ATI Industrial Automation, Apex, NC, USA) that can measure the force and torque. All hardware components, which included the KUKA robotic system, load cell, and motion capture system, were controlled using simVITRO knee modules. To achieve precise three-dimensional (3D) tracking of the knee kinematics in space, an optical motion capture system comprising eight motion caputre cameras (Cortex motion analysis, Rohnert Park, California, United States) operating at 100 Hz was employed. Prior to testing, the load cell was calibrated to eliminate any residual forces, ensuring accurate force and torque measurements.

Figure 2.
Experimental setup for robotic cadaveric knee testing. (a) The robotic system (KR 210, KUKA Robotics) equipped with a load cell and uniaxial strain gauges attached to the ACL, PCL, MCL, and LCL for strain measurements, (b) the measurement of anterior and posterior forces by the robotic system to assess tibial translation, (c) measurement of torques (internal and external rotation, varus, and valgus) by the robotic system to evaluate ligament response and joint stability, (d) illustration of the robotic system moving the knee specimen from 0° (neutral) to 90° flexion.
A cadaveric knee specimen was firmly secured onto the robotic end effector with the tibia mounted distally toward the end effector, where the load cell was located. Key anatomical landmarks were then digitized to define the knee’s coordinate system for subsequent biomechanical analysis. The neutral (or initial) position was established by minimizing external forces and moments, with the exception of a constant 50 N compressive load applied along the Z-axis to maintain tibiofemoral contact [22]. This configuration served as the reference for measuring rotations and translations [21]. The testing protocol included three cycles of dynamic flexion–extension movement from 0° to 90°, as shown in Figure 2d.
During the experiment, knee joint kinetics were recorded using the load cell, while joint kinematics were captured by the robotic system and the optical motion capture system was used to accurately digitize the knee position in three-dimensional space. Strain gauge sensors were sutured onto the surface of each ligament, with their output wires connected to a Kyowa strain data logger (Model: PCD-300A, Kyowa eletronic instrument CO., Tokyo, Japan), which recorded ligament strain during testing.
The strain gauges were factory-calibrated by the manufacturer with a specified gauge factor of K = 5 and a confirmed linear operating range suitable for soft tissue strain measurement. Prior to testing, all sensors were zeroed using the Kyowa PCD-300A data logger to eliminate baseline drift and ensure measurement accuracy. The raw strain data were gathered as the robotic system flexed each specimen from 0° to 90°, capturing ligament strain responses throughout the motion. The raw data were then extracted for post-processing.
Analysis of ligament strain behavior was carried out using a Python (Version 3.14) workflow. First, raw data were gathered, capturing strain responses under a range of testing scenarios. A 5 Hz Butterworth low-pass filter was subsequently applied to minimize noise and short-duration signal fluctuations. Average strain values were then computed for each individual specimen and across all six specimens.
Following the filtering step, strain data were synchronized with the motion trajectory, covering 0° to 90° of knee flexion. These procedures enabled the evaluation of ligament behavior across varying angular positions. Next, the strain values were translated into changes in ligament length by means of Equation (1), in which K = 5 (the manufacturer-specified gauge factor), is the strain measurement, and L signifies the length change (mm). Ultimately, the strain/length relationship was represented as ligament length change plotted against angular displacement, providing a clear visualization of how ligament behavior varies with knee flexion. This descriptive approach illustrates mechanical responses without statistical analysis, focusing on the experimental capability of the setup.
Based on the gauge factor (K = 5) and the sensitivity of the Kyowa PCD-300A logger, the system can detect strain changes as small as 10–20, corresponding to a minimum detectable length change of approximately 0.0001–0.0002 mm across the strain gauge attachment region.
3. Results
Figure 3a–d displays the mean ligament deformation during dynamic knee flexion-extension. The LCL exhibited progressive elongation, reaching 1.63 ± 1.84 mm at higher flexion angles, corresponding with increased passive varus torque observed during mid-range flexion. This suggests a load-bearing role of the LCL in resisting varus torque during knee flexion. The ACL demonstrated minimal tension variations, with a peak increased tension of 0.09 ± 4.02 mm at approximately 80° flexion, suggesting relative stability across the motion cycle.
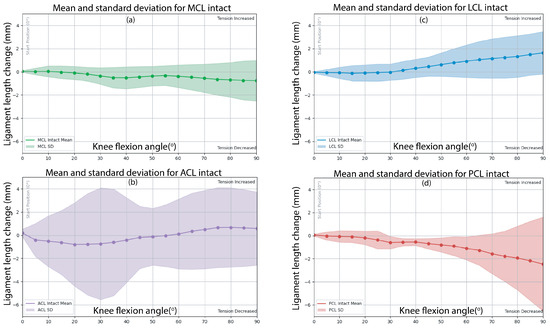
Figure 3.
Mean and standard deviation (SD) results of dynamic knee flexion from 0° to 90° for the following ligaments: (a) MCL, (b) ACL, (c) LCL, and (d) PCL.
In contrast, the MCL and PCL demonstrated decreased tension during the knee flexion of −0.76 ± 0.07 mm and −1.76 ± 0.10 mm, respectively, implying progressive shortening of the ligament as knee specimen flexion increased. During the dynamic flexions, varus torque was observed across all six specimens peaking at 90° of flexion at 2.18 ± 1.70 Nm, which may influence ligament strain. The LCL and PCL exhibited the most substantial length changes during passive knee flexion, indicating their increased engagement in resisting varus torque and posterior translation, respectively, under the specific motion tested. This observation does not imply a greater overall stabilizing role, as the ACL and MCL also contribute to knee stability in direction-specific ways.
Figure 4a,b also present the mean translation and rotations of the knee specimens during the robotic testing from 0° to 90°. The maximum flexion angle of the robot was approximately 83.94 ± 1.90°, while valgus and internal rotation angles exhibited variability, with peak values of −0.46 ± 8.46° and 1.91 ± 11.63°, respectively. Medial posterior and superior translations were observed during the flexion, and the values were 3.54 ± 3.89 mm, 0.54 ± 6.13 mm, and −0.24 ± 2.36 mm, respectively. The PCL exhibited the highest variability in translation, likely due to its multi-planar engagement during deep flexion. Unlike other ligaments, the PCL is influenced by both posterior tibial translation and compressive femoral rollback, leading to specimen-dependent strain behavior.
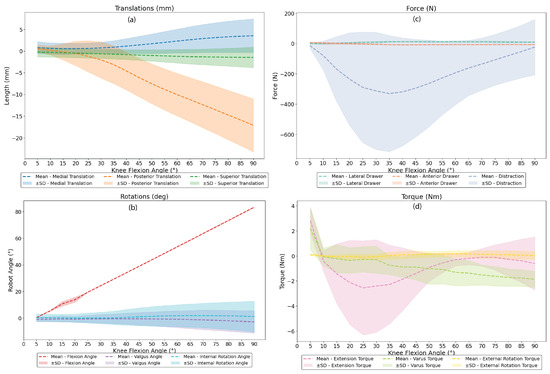
Figure 4.
Mean and standard deviation (SD) results of dynamic knee flexion from 0° to 90° for the following parameters: (a) translation, (b) rotation, (c) force, and (d) torque.
Figure 4c,d also illustrate the force and torque the knee specimens experience when the specimens flex along with the robotic system. The maximum recorded lateral drawer force was 12.68 ± 6.95 N, while the anterior drawer force peaked at 7.29 ± 6.97 N. Notably, distraction forces exhibited significant variation at −13.67 ± 387.44 N, reflecting interspecimen differences. During the knee flexion, varus torque was present across all specimens, peaking at 90° flexion at 2.18 ± 1.70 Nm, which may likely have influenced ligament strain behavior. The extension torque peaked at 2.81 ± 3.76 Nm, and the external rotation torque peaked at 0.16 ± 0.34 Nm, which remained relatively low and may have suggested limited rotational constraints during the dynamic conditions.
4. Discussion
We introduced a suturing-based method for attaching strain gauges directly onto ligaments as an alternative to adhesive fixation or DVRT sensors. While adhesives are commonly used, they may introduce localized stiffening at the attachment site, potentially altering the ligament’s intrinsic mechanical properties and leading to non-physiological strain distributions. Similarly, although DVRTs are considered the gold standard for precise strain measurements, their application to deep structures such as the ACL and PCL presents challenges due to soft tissue decreased tension in artifacts, which can introduce measurement inaccuracies or compromise sensor integrity. Suturing with strain gauges, on the other hand, is an alternative attachment way that keeps the ligament’s natural biomechanics and reduces movement artifacts, which makes strain measurements more accurate. It is acknowledged that suturing strain gauges to the ligament surface may induce localized stress concentrations or tissue microdamage, which could influence strain readings at the attachment site. To mitigate this, fine sutures and minimal tension were used during sensor placement. Nevertheless, this remains a potential source of measurement variability. Although our study did not perform a direct comparative analysis between suturing, adhesive attachment, and DVRT sensors, we propose suturing as a viable alternative with distinct advantages. Future research should include direct comparisons among these methods to further validate their respective impacts on strain measurement accuracy.
Another key methodological advantage of this approach is the preservation of soft tissue integrity in knee specimens. Unlike prior studies that necessitated soft tissue removal to facilitate sensor placement [7,13,14,23], our method maintained the majority of periarticular structures. Although preserving soft tissue likely improves physiological relevance, we did not quantify how its presence may have influenced strain measurements compared to isolated ligament conditions. This remains a potential source of variability and should be investigated in future comparative studies. This methodological choice confers several biomechanical benefits. First, it ensures a more physiologically relevant load distribution, as soft tissues play a critical role in joint stability and ligament kinematics. Second, it helps maintain ligament hydration, preventing moisture loss that could otherwise lead to tissue desiccation and altered viscoelastic properties. Third, by preserving the surrounding soft tissues, our method minimizes the risk of artificial loading conditions that may arise when ligaments are studied in isolation. However, a limitation of this approach is that the true length of the ligament cannot be directly measured, as it remains attached to the bone with surrounding soft tissues, meaning that the strain values obtained represent an estimation along the ligament rather than a precise measurement of its absolute increased tension. Additionally, as strain distribution may vary along the ligament’s length, particularly under multi-directional loading, the reported values represent local deformation and may not reflect the total elongation across the full ligament span.
The results obtained using this alternative method align with existing literature on knee ligament behavior during dynamic flexion–extension, indicating that the ACL and PCL function synergistically to maintain knee stability. Specifically, the ACL restrains anterior tibial translation, while the PCL serves as the primary restraint against posterior tibial translation [1]. Additionally, our findings corroborate these principles, as the ACL exhibited minimal tension, while the PCL demonstrated shortening with increased knee flexion, a trend observed in prior in vivo studies [24,25]. Mancini et al. have reported that ACL strain increases under valgus loading, with peak tension occurring at full extension [8]. However, our results indicate a relatively stable ACL strain pattern throughout flexion, which may be attributed to differences in sensor placement or the ligament’s inherent resistance to dynamic loading. Furthermore, the literature suggests that the posterolateral (PL) bundle of the ACL plays a key role in controlling rotational instability, which may explain the observed strain trends [1].
Additionally, varus torque was consistently observed across all specimens during flexion, peaking at 83.94° with a maximum value of 2.18 ± 1.70 Nm. This coupled response, rather than externally applied varus loading, suggests increased LCL engagement resulting from joint mechanics during flexion. This finding aligns with previous reports indicating increased LCL engagement during varus loading, particularly at greater flexion angles [25]. Greater length changes were observed in the LCL and PCL during flexion–extension, indicating higher engagement of these ligaments under the tested motion. However, this does not imply a superior biomechanical role, as each ligament contributes to knee stability in a direction-specific manner depending on the type of joint loading. And our finding is consistent with prior research indicating that the PCL and LCL also contribute to knee stabilization during dynamic movements [26].
One limitation of this study is the relatively high standard deviation observed in several outcome measures, such as translations, torque, and strain values. This variability likely reflects natural anatomical differences, specimen-specific joint laxity, and the complex multi-planar mechanics of ligaments like the PCL during passive flexion. Furthermore, the small sample size (n = 6) limits statistical power and may amplify inter-specimen variation. As this work is intended as a technical note, the primary focus is on demonstrating the feasibility and resolution of the robotic testing and strain measurement approach, rather than drawing generalizable biological conclusions.
Overall, our findings align with existing literature while presenting suturing as an alternative technical approach. Although this study highlights its potential benefits, future research should directly compare suturing with adhesive attachment and DVRT sensors under controlled loading conditions to further validate its effectiveness.
5. Conclusions
This study provides quantitative insights into ligament mechanics during dynamic knee flexion using an alternative method, confirming that the LCL and PCL undergo the most significant length changes, while the ACL and MCL maintain relative stability. The method of suturing and testing with soft tissues supports established biomechanical principles but also reveals dynamic variations in ligament response, emphasizing the need for continued investigation into knee ligament function under physiological loading conditions.
Author Contributions
Experimentation and Robot Setup: P.P., J.L.L., J.L. and A.Y.; Suturing and Strain Gauge Attachment: A.Y. and S.L. (orthopedic surgeon); Mentorship, Guidance, and Grant Application: A.Y., S.M.C. and D.T.T.L.; Writing—Original Draft Preparation and Review: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by SingHealth Academic Medicine Research Grant Call FY2021 (AM/TP051/2021).
Institutional Review Board Statement
Ethical review and informed consent were not required for this study involving cadaveric specimens, in accordance with SingHealth institutional and national guidelines.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rao, Z.; Zhou, C.; Kernkamp, W.A.; Foster, T.E.; Bedair, H.S.; Li, G. In vivo kinematics and ligamentous function of the knee during weight-bearing flexion: An investigation on mid-range flexion of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 797–805. [Google Scholar] [CrossRef]
- Hosseini, A.; Qi, W.; Tsai, T.Y.; Liu, Y.; Rubash, H.; Li, G. In vivo length change patterns of the medial and lateral collateral ligaments along the flexion path of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 3055–3061. [Google Scholar] [CrossRef]
- Kanamori, A.; Woo, S.L.; Ma, C.B.; Zeminski, J.; Rudy, T.W.; Li, G.; Livesay, G.A. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: A human cadaveric study using robotic technology. Arthrosc. J. Arthrosc. Relat. Surg. 2000, 16, 633–639. [Google Scholar] [CrossRef]
- Fujie, H.; Mabuchi, K.; Woo, S.L.Y.; Livesay, G.A.; Arai, S.; Tsukamoto, Y. The use of robotics technology to study human joint kinematics: A new methodology. J. Biomech. Eng. 1993, 115, 211–217. [Google Scholar] [CrossRef]
- Belvedere, C.; Ensini, A.; Feliciangeli, A.; Cenni, F.; D’Angeli, V.; Giannini, S.; Leardini, A. Geometrical changes of knee ligaments and patellar tendon during passive flexion. J. Biomech. 2012, 45, 1886–1892. [Google Scholar] [CrossRef]
- Zens, M.; Ruhhammer, J.; Goldschmidtboeing, F.; Woias, P.; Feucht, M.J.; Mayr, H.O.; Niemeyer, P. A new approach to determine ligament strain using polydimethylsiloxane strain gauges: Exemplary measurements of the anterolateral ligament. J. Biomech. Eng. 2014, 136, 124504. [Google Scholar] [CrossRef]
- Jeffcote, B.; Nicholls, R.; Schirm, A.; Kuster, M.S. The variation in medial and lateral collateral ligament strain and tibiofemoral forces following changes in the flexion and extension gaps in total knee replacement: A laboratory experiment using cadaver knees. J. Bone Jt. Surg. Br. Vol. 2007, 89, 1528–1533. [Google Scholar] [CrossRef]
- Mancini, E.J.; Kohen, R.; Esquivel, A.O.; Cracchiolo, A.M.; Lemos, S.E. Comparison of ACL strain in the MCL-deficient and MCL-reconstructed knee during simulated landing in a cadaveric model. Am. J. Sport. Med. 2017, 45, 1090–1094. [Google Scholar] [CrossRef]
- Wilson, R.; Barhorst, A.A. Intercondylar notch impingement of the anterior cruciate ligament: A cadaveric in vitro study using robots. J. Healthc. Eng. 2018, 2018, 8698167. [Google Scholar] [CrossRef]
- Most, E. Development of a 6-DOF Robotic Test System for Studying the Biomechanics of Total Knee Replacement. Doctoral Dissertation, Massachusetts Institute of Technology, Cambridge, MA, USA, 2000. [Google Scholar]
- Herve, O.M.; Flanagan, W.; Kanetis, J.; Mooney, B.; Kremen, T.J.; McAllister, D.R.; Clites, T.R. A Robotic Clamped-Kinematic System to Study Knee Ligament Injury. Ann. Biomed. Eng. 2025, 53, 193–206. [Google Scholar] [CrossRef]
- Victor, J.; Wong, P.; Witvrouw, E.; Sloten, J.V.; Bellemans, J. How isometric are the medial patellofemoral, superficial medial collateral, and lateral collateral ligaments of the knee? Am. J. Sport. Med. 2009, 37, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Hinterwimmer, S.; Baumgart, R.; Plitz, W. Tension changes in the collateral ligaments of a cruciate ligament-deficient knee joint: An experimental biomechanical study. Arch. Orthop. Trauma Surg. 2002, 122, 454–458. [Google Scholar] [CrossRef] [PubMed]
- France, E.P.; Daniels, A.U.; Goble, E.M.; Dunn, H.K. Simultaneous quantitation of knee ligament forces. J. Biomech. 1983, 16, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Zens, M.; Ruhhammer, J.; Goldschmidtboeing, F.; Feucht, M.J.; Bernstein, A.; Niemeyer, P.; Mayr, H.O.; Woias, P. Polydimethylsiloxane strain gauges for biomedical applications. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 1763–1766. [Google Scholar]
- Bates, N.A.; Nesbitt, R.J.; Shearn, J.T.; Myer, G.D.; Hewett, T.E. Knee abduction affects greater magnitude of change in ACL and MCL strains than matched internal tibial rotation in vitro. Clin. Orthop. Relat. Res. 2017, 475, 2385–2396. [Google Scholar] [CrossRef]
- Sabharwal, P. A Combined In-Vivo/In-Vitro Approach to Study Knee Injury Mechanism. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2011. [Google Scholar]
- Frigo, C.A.; Donno, L. The effects of external loads and muscle forces on the knee joint ligaments during walking: A musculoskeletal model study. Appl. Sci. 2021, 11, 2356. [Google Scholar] [CrossRef]
- Adouni, M.; Faisal, T.R.; Dhaher, Y.Y. Computational frame of ligament in situ strain in a full knee model. Comput. Biol. Med. 2020, 126, 104012. [Google Scholar] [CrossRef]
- Pandy, M.G.; Shelburne, K.B. Dependence of cruciate-ligament loading on muscle forces and external load. J. Biomech. 1997, 30, 1015–1024. [Google Scholar] [CrossRef]
- Wierer, G.; Milinkovic, D.; Robinson, J.R.; Raschke, M.J.; Weiler, A.; Fink, C.; Herbort, M.; Kittl, C. The superficial medial collateral ligament is the major restraint to anteromedial instability of the knee. Knee Surgery Sport. Traumatol. Arthrosc. 2021, 29, 405–416. [Google Scholar] [CrossRef]
- Nagle, T.F.; Erdemir, A.; Colbrunn, R.W. A generalized framework for determination of functional musculoskeletal joint coordinate systems. J. Biomech. 2021, 127, 110664. [Google Scholar] [CrossRef]
- Matsueda, M.; Gengerke, T.R.; Murphy, M.; Lew, W.D.; Gustilo, R.B. Soft Tissue Release in Total Knee Arthroplasty: Cadaver Study Using Knees Without Deformities. Clin. Orthop. Relat. Res. 1999, 366, 264–273. [Google Scholar] [CrossRef]
- Li, G.; DeFrate, L.E.; Sun, H.; Gill, T.J. In vivo elongation of the anterior cruciate ligament and posterior cruciate ligament during knee flexion. Am. J. Sport. Med. 2004, 32, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, C.; Duthon, V.B.; Chagué, S.; Kolo, F.C.; Ménétrey, J. In vivo static and dynamic lengthening measurements of the posterior cruciate ligament at high knee flexion angles. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Ozada, N. Effect of six degrees of freedom knee kinematics on ligament length and moment arm in an intact knee model. Technol. Health Care 2015, 23, 485–494. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).