Bioactive Properties and Fatty Acid Profile of Seed Oil from Amomyrtus luma
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Seed and Extraction of Oil
2.2. Oil Extraction Techniques
2.2.1. Maceration
2.2.2. Ultrasound Extraction
2.2.3. Soxhlet Extraction
2.2.4. Oil Yield Determination
2.3. Bioactive Compound Extraction
2.3.1. Total Phenolic Content
2.3.2. Total Flavonoid
2.3.3. Total Tannin Content
2.4. Carotenoid Content
2.5. Determination of α-Tocopherol
2.6. Antioxidant Capacity Assays
2.7. Preparation of FAMEs and Determination of Fatty Acid Profile via GC-FID
2.8. Functional Quality and Oxidative Stability Indices
2.9. Measurement of Antimicrobial Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Seed Oil Extraction
3.2. Bioactive Compounds and Antioxidant Capacity
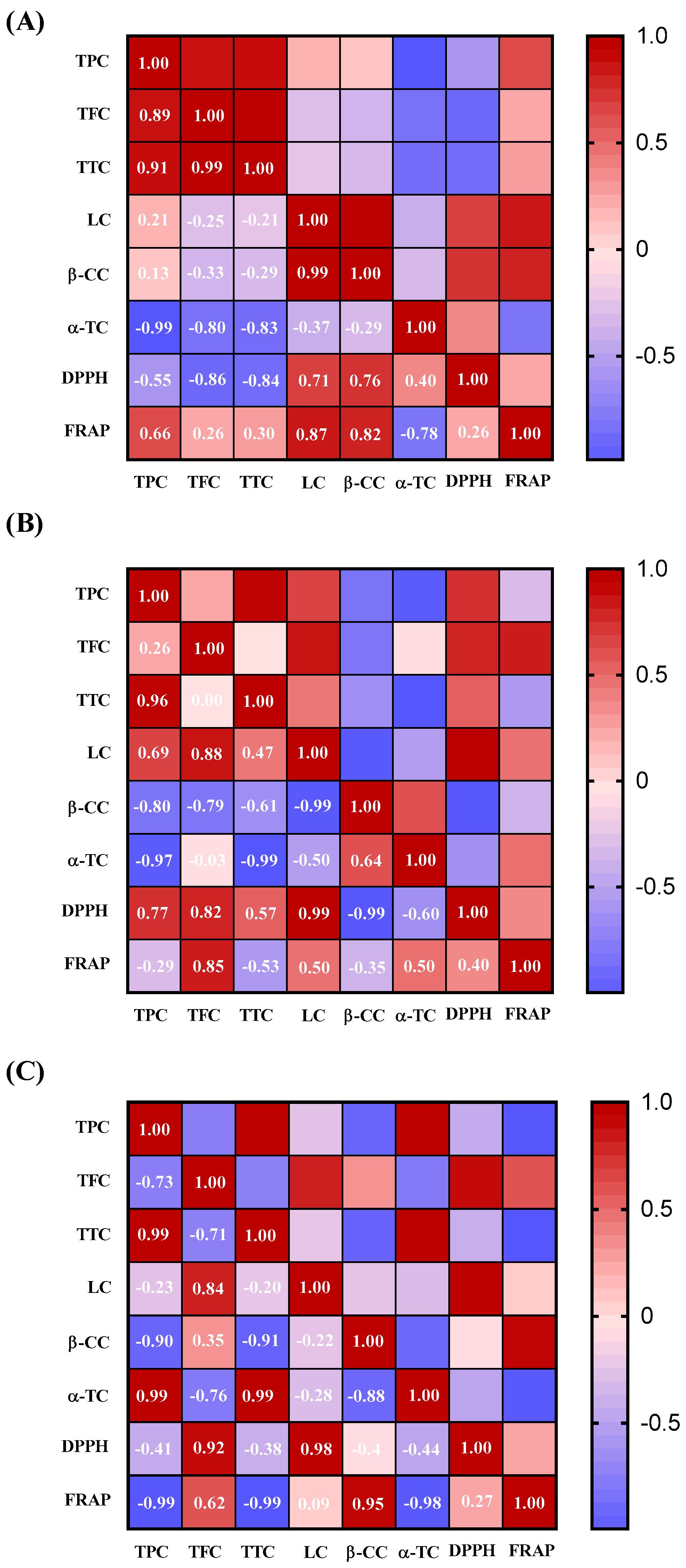
3.3. Effect of Extraction Methods on the Correlation Between Bioactive Compounds and Antioxidant Capacity
3.4. Fatty Acid Profile
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chenavaz, R.Y.; Dimitrov, S. From Waste to Wealth: Policies to Promote the Circular Economy. J. Clean. Prod. 2024, 443, 141086. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Ibarz, R.; Ferreira-Santos, P.; Teixeira, J.A.; Rocha, C.M.R.; Pérez-Fernández, M.; García-Juiz, S.; Osada, J.; Martín-Belloso, O.; et al. Valorization of Agro-Food by-Products and Their Potential Therapeutic Applications. Food Bioprod. Process. 2021, 128, 247–258. [Google Scholar] [CrossRef]
- García, N.; Ormazabal, C. Arboles Nativos de Chile; Enersis S.A.: Santiago, Chile, 2008. [Google Scholar]
- Maiolini, T.C.S.; Nicácio, K.J.; Rosa, W.; Miranda, D.O.; Santos, M.F.C.; Bueno, P.C.P.; Lago, J.H.G.; Sartorelli, P.; Dias, D.F.; Chagas de Paula, D.A.; et al. Potential Anti-Inflammatory Biomarkers from Myrtaceae Essential Oils Revealed by Untargeted Metabolomics. Nat. Prod. Res. 2023, 39, 985–992. [Google Scholar] [CrossRef]
- Velásquez, P.; Orellana, J.; Muñoz-Carvajal, E.; Faúndez, M.; Gómez, M.; Montenegro, G.; Giordano, A. Biological Activity of Native Myrtaceae Fruits from Chile as a Potential Functional Food. Nat. Prod. Res. 2021, 36, 3138–3142. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Landrum, L.R. Constituents of the Leaf Extract of Amomyrtus Meli (R. A. Philippi) Legrand et Kausel, Amomyrtus Luma (Molina) Legrand et Kausel and of Amomyrtella Guili (Speg.) Kausel. Flavour Fragr. J. 1992, 7, 247–251. [Google Scholar] [CrossRef]
- Strzałka, K.; Szymańska, R.; Ewa, Ś.; Skorupińska-Tudek, K.; Suwalsky, M. Tocochromanols, Plastoquinone and Polyprenols in Selected Plant Species from Chilean Patagonia. Acta Biol. Cracoviensia Ser. Bot. 2009, 51, 39–44. [Google Scholar]
- Falkenberg, S.S.; Tarnow, I.; Guzman, A.; Mølgaard, P.; Simonsen, H.T. Mapuche Herbal Medicine Inhibits Blood Platelet Aggregation. Evid.-Based Complement. Altern. Med. 2012, 2012, 647620. [Google Scholar] [CrossRef]
- Mølgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbæk, L.B.; Jebjerg, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial Evaluation of Huilliche Plant Medicine Used to Treat Wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Jiménez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian Berries as Native Food and Medicine. J. Ethnopharmacol. 2019, 241, 111979. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Islam, M.; Siger, A.; Fornal, E.; Tomaszewska-Gras, J. Oxidative Stability Assessment of Industrial and Laboratory-Pressed Fresh Raspberry Seed Oil (Rubus Idaeus L.) by Differential Scanning Calorimetry. Food Chem. Adv. 2023, 2, 100186. [Google Scholar] [CrossRef]
- de Menezes, M.L.; Johann, G.; Diório, A.; Schuelter Boeing, J.; Visentainer, J.V.; Raimundini Aranha, A.C.; Curvelo Pereira, N. Comparison of the Chemical Composition of Grape Seed Oil Extracted by Different Methods and Conditions. J. Chem. Technol. Biotechnol. 2023, 98, 1103–1113. [Google Scholar] [CrossRef]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing Technologies, Phytochemical Constituents, and Biological Activities of Grape Seed Oil (GSO): A Review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Liu, N.; Ren, G.; Faiza, M.; Li, D.; Cui, J.; Zhang, K.; Yao, X.; Zhao, M. Comparison of Conventional and Green Extraction Methods on Oil Yield, Physicochemical Properties, and Lipid Compositions of Pomegranate Seed Oil. J. Food Compos. Anal. 2022, 114, 104747. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Hanczné Lakatos, E.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Characterization of Fatty Acid, Antioxidant, and Polyphenol Content of Grape Seed Oil from Different Vitis Vinifera L. Varieties. OCL—Oilseeds Fats Crops Lipids 2021, 28, 30. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red Raspberry (Rubus Idaeus l.) Seed Oil: A Review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- Bahmani, M.; Shokri, S.; Akhtar, Z.N.; Abbaszadeh, S.; Manouchehri, A. The Effect of Pomegranate Seed Oil on Human Health, Especially Epidemiology of Polycystic Ovary Syndrome; a Systematic Review. JBRA Assist. Reprod. 2022, 26, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Rajesh, Y.; Khan, N.M.; Raziq Shaikh, A.; Mane, V.S.; Daware, G.; Dabhade, G. Investigation of Geranium Oil Extraction Performance by Using Soxhlet Extraction. In Proceedings of the Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; Volume 72, pp. 2610–2617. [Google Scholar]
- Ginocchio, R.; Muñoz-Carvajal, E.; Velásquez, P.; Giordano, A.; Montenegro, G.; Colque-Perez, G.; Sáez-Navarrete, C. Mayten Tree Seed Oil: Nutritional Value Evaluation According to Antioxidant Capacity and Bioactive Properties. Foods 2021, 10, 729. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric Determination of Tannins and Their Correlations with Chemical and Protein Precipitation Methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric Methods for Carotenoid Determination in Frequently Consumed Fruits and Vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid Extraction of Lycopene and Β-Carotene from Reconstituted Tomato Paste and Pink Grapefruit Homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of Drying Processes on Composition, Microstructure and Health Aspects from Maqui Berries. J. Food Sci. Technol. 2020, 57, 2241–2250. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. The National Research Council Od Canada a Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Fuentes, I.; Valenzuela, P.; Roco, T.; Pérez-Won, M.; Espinoza, J.; Bernal, G.; Bernal, C.; Martínez, R. Isolation and Characterization of Polyunsaturated Fatty Acid-Rich Oil from Jumbo Squid (Dosidicus gigas) Viscera: Antioxidant Potential and Anticancer Activity on Colorectal Cancer Cells. Waste Biomass Valorization 2025, 16, 3507–3517. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty Acid and Tocopherol Composition of Pomace and Seed Oil from Five Grape Varieties Southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef]
- CLSI Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Eleventh Edition, CLSI Document M02-A11; CLSI: Wayne, PA, USA, 2012.
- Patrice Didion, Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What Is next? The Greener Future of Solid Liquid Extraction of Biobased Compounds: Novel Techniques and Solvents Overpower Traditional Ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Yao, X.-H.; Shen, Y.-S.; Hu, R.-Z.; Xu, M.; Huang, J.-X.; He, C.-X.; Cao, F.-L.; Fu, Y.-J.; Zhang, D.-Y.; Zhao, W.-G.; et al. The Antioxidant Activity and Composition of the Seed Oil of Mulberry Cultivars. Food Biosci. 2020, 37, 100709. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, Characterization and Antioxidant Activity of Fenugreek (Trigonella-Foenum Graecum) Seed Oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical Profiling, Antioxidant, Anticholinesterase, and Antiprotozoal Potentials of Artemisia copa Phil. (Asteraceae). Front. Pharmacol. 2020, 11, 594174. [Google Scholar] [CrossRef] [PubMed]
- Banjanin, T.; Özcan, M.M.; Al Juhaimi, F.; Ranković-Vasić, Z.; Uslu, N.; Mohamed, I.A.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; et al. Effect of Varieties on Bioactive Compounds, Fatty Acids, and Mineral Contents in Different Grape Seed and Oils from Bosnia and Herzegovina. J. Food Process. Preserv. 2019, 43, e13981. [Google Scholar] [CrossRef]
- De Filette, M.; Schatteman, K.; Geuens, J. Characterization of Six Cold-Pressed Berry Seed Oils and Their Seed Meals. Appl. Sci. 2024, 14, 439. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Maraei, R.W.; El-Ansary, A.E.; Rezk, A.A.; Mansour, A.T.; Aly, A.A. Characterizing the Bioactive Ingredients in Sesame Oil Affected by Multiple Roasting Methods. Foods 2022, 11, 2261. [Google Scholar] [CrossRef]
- Sun, J.; Li, D.; Huyan, W.; Hong, X.; He, S.; Huo, J.; Jiang, L.; Zhang, Y. Blue Honeysuckle Seeds and Seed Oil: Composition, Physicochemical Properties, Fatty Acid Profile, Volatile Components, and Antioxidant Capacity. Food Chem. X 2024, 21, 101176. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Asgari Lajayer, B.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the Bulwark: Unravelling the Efficient Applications of Plant Phenolics and Tannins against Environmental Stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Holzapfel, N.; Holzapfel, B.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D. The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Md Norodin, N.S.; Sarkawi, N.S.; Hamzah, M.H.S.; Mohd Nasir, H.; Abang Zaidel, D.N.; Che Yunus, M.A.; Md Salleh, L. Valorisation of Plant Seed as Natural Bioactive Compounds by Various Extraction Methods: A Review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Langston, F.M.A.; Nash, G.R.; Bows, J.R. The Retention and Bioavailability of Phytochemicals in the Manufacturing of Baked Snacks. Crit. Rev. Food Sci. Nutr. 2023, 63, 2141–2177. [Google Scholar] [CrossRef]
- Chang, J.; Kang, X.; Yuan, J. Enhancing Emulsification and Antioxidant Ability of Egg Albumin by Moderately Acid Hydrolysis: Modulating an Emulsion-Based System for Mulberry Seed Oil. Food Res. Int. 2018, 109, 334–342. [Google Scholar] [CrossRef]
- Tao, X.-M.; Wang, J.; Wang, J.; Feng, Q.; Gao, S.; Zhang, L.-R.; Zhang, Q. Enhanced Anticancer Activity of Gemcitabine Coupling with Conjugated Linoleic Acid against Human Breast Cancer in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2012, 82, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial Effects of Dietary Supplementation with Olive Oil, Oleic Acid, or Hydroxytyrosol in Metabolic Syndrome: Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective Effects of Oleic Acid and Polyphenols in Extra Virgin Olive Oil on Cardiovascular Diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated Fatty Acids and Risk of Coronary Heart Disease: Modulation by Replacement Nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef]
- Popović, M.; Fuentes, J.B.; Papović, N.; Okuka, N.; Suručić, R.; Torović, L. Comparative Advantages of Fatty Acid Composition and Nutritional Indices of Specific Edible Plant Oils. Proceedings 2023, 91, 357. [Google Scholar]
- Fonseca, V.F.; Duarte, I.A.; Matos, A.R.; Reis-Santos, P.; Duarte, B. Fatty Acid Profiles as Natural Tracers of Provenance and Lipid Quality Indicators in Illegally Sourced Fish and Bivalves. Food Control 2022, 134, 108735. [Google Scholar] [CrossRef]


| Bioactive Compounds | Extraction Method | ||
|---|---|---|---|
| Maceration | Ultrasound | Soxhlet | |
| Polyphenols (mg GAE/100 g oil) | 112.13 ± 1.95 a | 113.12 ± 2.49 a | 66.93 ± 8.19 b |
| Flavonoids (mg QE/100 g oil) | 21.06 ± 0.29 a | 19.68 ± 0.22 a | 11.33 ± 0.06 b |
| Tannins (mg GAE/100 g oil) | 82.38 ± 2.76 a | 84.97 ± 2.00 a | 53.36 ± 8.79 b |
| Lycopene (mg/100 g oil) | 1.42 ± 0.03 a | 0.92 ± 0.05 b | 0.69 ± 0.04 c |
| β-Carotene (mg/100 oil g) | 1.29 ± 0.10 a | 1.04 ± 0.07 b | 0.19 ± 0.05 c |
| α-Tocopherol (mg/100 oil g) | 10.68± 0.10 a | 9.62 ± 0.11 b | 12.46 ± 0.26 c |
| Fatty Acid | Extraction Method | ||
|---|---|---|---|
| Maceration (%) | Ultrasound (%) | Soxhlet (%) | |
| 16:0 | 7.40 ± 0.13 a | 7.29 ± 0.05 a | 7.36 ± 0.07 a |
| 18:0 | 2.89 ± 0.06 a | 2.83 ± 0.02 a | 2.84 ± 0.03 a |
| 18:1n-9 | 9.18 ± 0.24 a | 9.15 ± 0.54 a | 8.89 ± 0.13 a |
| 18:2n-6 | 79.79 ± 0.44 a | 80.01 ± 0.55 a | 80.09 ± 0.15 a |
| 20:0 | 0.39 ± 0.01 a | 0.38 ± 0.01 a | 0.38 ± 0.01 a |
| 20:1n-9 | 0.35 ± 0.01 a | 0.34 ± 0.00 a | 0.36 ± 0.00 a |
| Saturated | 10.68 ± 0.20 a | 10.50 ± 0.04 a | 10.57 ± 0.06 a |
| Unsaturated | 89.32 ± 0.20 a | 89.50 ± 0.04 a | 89.43 ± 0.06 a |
| Index | Extraction Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Maceration | Ultrasound | Soxhlet | |||||||
| USFA/SFA | 8.68 | ± | 0.18 a | 8.85 | ± | 0.04 a | 8.77 | ± | 0.06 a |
| PUFA/SFA | 7.76 | ± | 0.18 a | 7.90 | ± | 0.09 a | 7.86 | ± | 0.05 a |
| AI | 0.08 | ± | 0.00 a | 0.08 | ± | 0.00 a | 0.08 | ± | 0.00 a |
| TI | 0.23 | ± | 0.00 a | 0.23 | ± | 0.00 a | 0.23 | ± | 0.00 a |
| H/H | 10.79 | ± | 0.24 a | 10.97 | ± | 0.14 a | 10.88 | ± | 0.12 a |
| COX | 8.22 | ± | 0.05 a | 8.24 | ± | 0.02 a | 8.25 | ± | 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovagnoli-Vicuña, C.; Viteri, R.; Aparicio, J.; Quispe-Fuentes, I.; Giordano, A. Bioactive Properties and Fatty Acid Profile of Seed Oil from Amomyrtus luma. Compounds 2025, 5, 31. https://doi.org/10.3390/compounds5030031
Giovagnoli-Vicuña C, Viteri R, Aparicio J, Quispe-Fuentes I, Giordano A. Bioactive Properties and Fatty Acid Profile of Seed Oil from Amomyrtus luma. Compounds. 2025; 5(3):31. https://doi.org/10.3390/compounds5030031
Chicago/Turabian StyleGiovagnoli-Vicuña, Claudia, Rafael Viteri, Javiera Aparicio, Issis Quispe-Fuentes, and Ady Giordano. 2025. "Bioactive Properties and Fatty Acid Profile of Seed Oil from Amomyrtus luma" Compounds 5, no. 3: 31. https://doi.org/10.3390/compounds5030031
APA StyleGiovagnoli-Vicuña, C., Viteri, R., Aparicio, J., Quispe-Fuentes, I., & Giordano, A. (2025). Bioactive Properties and Fatty Acid Profile of Seed Oil from Amomyrtus luma. Compounds, 5(3), 31. https://doi.org/10.3390/compounds5030031







