Abstract
Neglected diseases significantly impact the world, and there is a lack of effective treatments, requiring therapeutic alternatives. Thus, the study of the phytochemical and schistosomicidal activity evaluation of Copaifera oblongifolia leaves’ crude extract was conducted. The quercitrin (1) and afzelin (2) were isolated from the crude extract. In the in vitro schistosomicidal activity test, the isolated compounds demonstrated promising results, with 75% mortality at a concentration of 12.5 µM after 72 h. Molecular docking calculations indicated that compounds 1 and 2 could potentially interact with the amino acids of the FAD binding site in the TGR enzyme, a crucial enzyme for the survival of Schistosoma mansoni. These interactions could have binding energies comparable to praziquantel, a preferred drug for treating schistosomiasis. Therefore, in silico and in vitro investigations are crucial for developing new studies that can reveal the antiparasitic potential of compounds of plant origin.
1. Introduction
Neglected tropical diseases are a significant concern in developing countries, affecting millions [1]. These diseases lack innovative treatments and are not profitable for pharmaceutical companies, resulting in a lack of investment in research and development. They are responsible for over 35,000 daily deaths and generate more disability than mortality [2]. In Brazil, 19 tropical diseases are reported, including schistosomiasis. Schistosomiasis affects over 250 million people worldwide and is a significant cause of death [3,4]. The disease is caused by Schistosoma mansoni and is treated with oxamniquine or praziquantel (PZQ). Praziquantel is preferred due to its low cost; however, the development of new drugs is justified due to the potential emergence of resistance [5,6].
In Schistosoma, the redox systems depend on the thioredoxin glutathione reductase (TGR) enzyme [7]. Two schistosomiasis drugs used in the past, oltipraz and antimonyl potassium tartrate, promoted the inhibition of the TGR activity, indicating the importance of this enzyme to S. mansoni [8]. The fact that the thioredoxin glutathione reductase (TGR) enzyme appears to be an important protein in protecting S. mansoni against oxidative stress makes the TGR enzyme a relevant target for potential drugs [9].
Recent advances in the pharmacological targeting of thioredoxin glutathione reductase (TGR) have expanded the scope of schistosomiasis treatment. Notably, non-covalent inhibitors with demonstrated schistosomicidal activity in vivo have been identified, highlighting effective strategies for enzyme inhibition without permanent enzyme modification [10]. Structure-based discovery approaches have revealed phytocompounds from Azadirachta indica as promising TGR inhibitors, expanding the chemical diversity of potential therapeutic agents [11]. Additionally, the mechanism of ectopic suicide inhibition of TGR has been characterized, providing insights into irreversible enzyme inactivation [12]. Lead compounds targeting this selenoenzyme have also been extensively characterized, underscoring their potential for drug development [13]. Furthermore, fragment library screening combined with X-ray crystallography has elucidated detailed binding site features of TGR from Schistosoma mansoni, facilitating rational drug design [14]. Together, these recent studies demonstrate the dynamic progress in identifying and optimizing TGR inhibitors, supporting its validation as a critical drug target for schistosomiasis.
Thus, searching for new potential drugs from natural sources is urgently needed, as large pharmaceutical industries have neglected these diseases. Among the numerous medicinal plants used by the Brazilian population, the genus Copaifera, commonly known as “copaíbas”, stands out due to its long-established pharmacological applications in traditional medicine [15,16,17].
Therefore, this work aims to investigate the Schistosomicidal activity in vitro of quercitrin (1) and afzelin (2) isolated from the leaves of Copaifera oblongifolia. In addition, molecular docking calculations were performed to investigate the binding modes of these molecules to the TGR enzyme. The interactions between praziquantel (PZQ), a broad-spectrum anthelmintic drug employed to treat schistosomiasis, and the TGR enzyme were used as a reference in this study [18].
2. Results and Discussion
2.1. Compounds Isolated
The phytochemical analysis of the FD fraction obtained from Copaifera oblongifolia hydroalcoholic extract, using chromatographic techniques, allowed for us to isolate compounds 1 and 2 (Figure 1, Figure 2 and Figures S1–S12). The structures were identified as quercitrin (1) and afzelin (2). All isolated compounds were identified based on their structures using spectroscopic approaches (1H NMR, 13C NMR, and ESI-MS) and comparisons to published data [19].
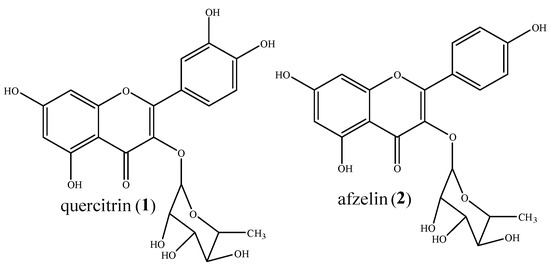
Figure 1.
Chemical structures of compounds 1 and 2.
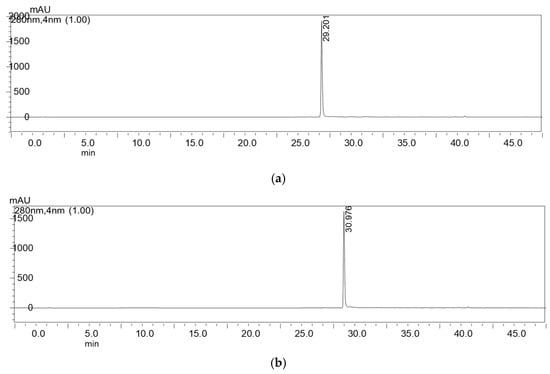
Figure 2.
Chromatograms of compounds 1 (a) and 2 (b) after isolation by preparative HPLC.
2.2. Schistosomicidal Activity
Table 1 presents the in vitro evaluation of the schistosomicidal activity of the crude extract of C. oblongifolia leaves against adult S.mansoni worms at 50 µg/mL and 100 µg/mL concentrations. The results indicate that there was no change at lower concentrations. However, the crude extract showed 75% mortality at a concentration of 100 µg/mL. Table 2 and Table 3 show the in vitro evaluation of the schistosomicidal activity of quercitrin (1) and afzelin (2) against adult S. mansoni worms. Compound 1 showed 75% mortality at concentrations of 12.5 and 25 µM after 48 h of incubation, with the most active concentration being 50 µM, which resulted in 100% mortality after 72 h. Compound 2 exhibited 75% mortality at concentrations of 12.5, 25, 50, and 100 µM after 72 h of incubation, with the most active concentration being 200 µM, which caused 75% mortality in 24 and 48 h and 100% mortality in 72 h.

Table 1.
Results of the in vitro schistosomicide assay of crude extract and fractions.

Table 2.
Results of the in vitro schistosomicide assay of compound 1.

Table 3.
Results of the in vitro schistosomicide assay of compound 2.
Reports on the schistosomicidal activity of compounds 1 and 2 are scarce. However, numerous reports have been published on various biological activities, primarily in investigations involving different types of cancer. Zhang et al. [20] explored the impact of afzelin, identified in Nymphaea odorata, on prostate cancer. Their investigation covered both androgen-sensitive LNCaP cells and androgen-independent PC-3 cells.
Furthermore, Shin et al. [21] highlighted afzelin’s antioxidant, DNA-protective, UV-absorbing, and anti-inflammatory attributes. Other studies, including that of Diantini et al. [22], corroborated the ability of afzelin to prevent the proliferation of breast cancer cells through the induction of apoptosis. In another study, afzelin demonstrated the potential to mitigate asthma in a murine model, as evidenced by Zhou and Nie [23]. Furthermore, Zhu et al. [24] demonstrated the inhibition of cell proliferation by afzelin in prostate cancer cell lines, primarily targeting the G phase of the cell cycle, as well as its modulation of several kinases involved in maintaining the actin cytoskeleton.
The investigation conducted by Zhang et al. [25] demonstrated that quercitrin extracted from Toona sinensis leaves significantly reduced the viability of human colorectal cancer cells, leading to apoptosis. Several studies have documented the inhibitory effects of quercitrin against several protozoan parasites, including Toxoplasma, Babesia, Theileria, Trypanosoma, and Leishmania. Quercitrin has demonstrated efficacy in inhibiting the growth of various parasite species, including Trypanosoma brucei, Trypanosoma cruzi, and Leishmania donovani, both in vitro and in vivo [26]. In addition, compounds 1 and 2 have been reported in the literature to inhibit, through interactions with amino acids, enzymes responsible for the expression of various diseases [27,28]. Studies carried out by Awang et al. [29] showed that the Quercitrin-Rich fraction from Melastoma malabathricum leaves had antidiabetic properties, inhibiting the DPP-IV enzyme at a concentration of 100 µg/mL, where inhibition occurred through hydrogen bonding with the amino acids of the enzyme. Therefore, understanding enzyme inhibition is one of the current strategies for discovering treatments for diverse diseases.
2.3. Molecular Docking
Initially, a molecular docking calculation was performed between the native ligand, flavin adenine dinucleotide (FAD), and the TGR enzyme. Three FAD conformations exhibited root mean square deviation (RMSD) values below 2.0 Å relative to the crystal structure (1.449, 1.649, and 1.659 Å, respectively), demonstrating the robustness of the computational model employed in this study.
The PZQ and the TGR enzyme interact mainly from the amino acids Cys154, Cys159, Gly432, and Ala445 (Figure 3 and Table 4). The first three amino acids are related to the FAD active site in the TGR enzyme [18]. Structure 1 interacts with the TGR enzyme through the amino acids Ser117, Cys159, Lys162, Val297, Asp433, Thr442, and Pro443. Except for Val297, all other amino acids are associated with the FAD active site in the TGR enzyme [13]. Structure 2 interacts with the TGR enzyme via amino acids: Ser117, Cys154, Cys159, Lys162, Tyr296, Val297, Glu300, Leu441, and Pro443. The first five and the last two amino acids correlate to the FAD active site in the TGR enzyme [18]. Considering the ChemScore fitness dG, which represents the total free energy change that occurs on ligand binding (Table 5), it is observed that compounds 1 and 2 show values (−23.44 and −28.90 kcal mol−1, respectively) comparable to, or even more negative (thus more stable), than those obtained for PZQ (−22.03 kcal mol−1). It can be explained because compounds 1 and 2 establish a larger number of hydrophobic interactions (three and four, respectively) and hydrogen bonds (five to both) with the amino acids of the TGR enzyme than the PZQ structure (three hydrophobic interactions and two hydrogen bonds). In this sense, molecular docking results suggest that compounds 1 and 2 potentially inhibit the TGR, an important enzyme for the survival of Schistosoma worms [7]. Furthermore, Kuhn et al. [30] reported quercitrin as a selective inhibitor of the NAD+ catabolic enzyme of S. mansoni (SmNACE), located on the adult parasite’s outer surface (tegument). This enzyme is presumably crucial for the survival of S. mansoni, as it can influence the host’s immune regulatory pathways [30].
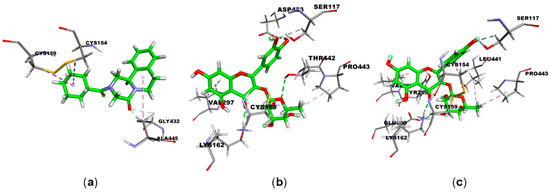
Figure 3.
Main interactions between the: (a) PZQ, (b) compound 1, and (c) compound 2; compounds and the amino acids present in the TGR enzyme. Ligands are shown in green; carbon (gray), oxygen (red), nitrogen (blue), and sulfur (yellow) atoms; hydrogen bonds are represented by purple dashed lines.

Table 4.
Main interactions between the compounds: PZQ, 1, and 2, and the amino acids present in the TGR enzyme.

Table 5.
Total free energy change that occurs on ligand (1, 2 or PZQ) binding to TGR enzyme.
Thus, the results presented in this work further highlight the potential of compounds 1 and 2 as promising candidates for developing new therapeutic strategies against parasitic diseases, such as Schistosomiasis.
3. Experimental
3.1. Instrument Specifications
Preparative analytical HPLC was carried out on a Shimadzu LC-6A chromatograph with UV–visible detector model SPD-6A, with SCL6B controller and C-R6A integrator. The NMR spectra were obtained on a Bruker spectrometer (400 MHz for 1H and 100 MHz for 13 C). The samples were dissolved in methanol-d4 and the mass spectra were obtained using an electrospray ionization quadrupole time-of-flight mass spectrometer (ESI-QTOF/MS, Waters Corp., Milford, CT, USA).
3.2. Collection and Extraction of Plant Material
Copaifera oblongifolia leaves were collected from a specimen located at Usina Santo Ângelo in Pirajuba-Minas Gerais (19°56.833′ S 48°33.504′ W). A copy was sent to Prof. Dr. Milton Groppo Júnior, responsible for the SPFR Herbarium, at the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto–USP (FFCLR-USP), being provisionally named Plant 3 pending final deposit. The collected leaves were dried in a circulating air oven (40 °C) and pulverized in a knife mill, producing 913.7 g of powder. This material was subjected to five successive extractions with ethanol/water (75:25 v/v), which occurred at an average interval of eight days. After drying, 64.84g of crude extract was obtained.
3.3. Isolation of Compounds 1 and 2 of Copaifera oblongifolia Hydroalcoholic Extract
A liquid–liquid partition was performed using 40 g of hydroalcoholic extract. The volumes of solvents used, the order in which they were applied, and the masses of each resulting fraction are shown in Table 6. The mass was dissolved in 300 mL of an ethanol/water solution (80:20 v/v) and placed in a separation flask. It was then partitioned with 1 L of dichloromethane, yielding 8.9 g of the FD fraction. The dichloromethane fraction was fractionated using a glass chromatographic column. Fractionation in a classic chromatographic column (CC) was performed in a glass column (5 cm × 60 cm). Silica gel 60 (0.063–0.200 mm, Merck, Darmstadt, Germany) was used as the stationary phase at a proportion of 20 g/g of sample. Organic solvents were used as eluents in an increasing polarity gradient, yielding 110 fractions of 45 mL each. The fractions obtained and the solvents used are summarized in Table 7.

Table 6.
Result of the partitioning of the crude extract of C. oblongifolia.

Table 7.
Fractionation of the dichloromethane fraction from the hydroalcoholic extract of C. oblongifolia.
Fractions 8 to 11 show chromatographic similarity with the presence of two constituents. The fractions were pooled and subjected to preparative HPLC using a Phenomenex® column, Gemini C-18 (250 × 21.20 mm equipped with pre-column), particle diameter equal to 5 µm, pore diameter 100 Å, being used as phase mobile CH3OH (B) and H2O +0.01% HAc (A) in the ratio 57:43 v/v in 50 min; the injection volume was 1mL, flow: 10.0 mL/min. It is possible to isolate the two constituents, compounds 1 (70 mg) and 2 (30 mg).
3.4. HPLC Analysis of the Compounds Obtained
The compounds obtained were analyzed by high-performance liquid chromatography (HPLC). For analytical HPLC, a Phenomenex® column, Gemini C-18 (250 × 4.6 mm equipped with pre-column), particle diameter equal to 5 µm, pore diameter 100 Å, was used as mobile phase CH3OH (B) and H2O + 0.01% HAc (A) in linear gradient, 5 min at 5% (B), (5%→100%) in 35 min, 10 min at 100% (B), 3 min of equilibrium, and 15 min to return to initial condition. Injection volume: 20 μL; flow: 1.0 mL/min.
3.5. Assessment of Schistosomicidal Activity
The biological cycle of Schistosoma mansoni, strain LE (Luiz Evangelista) is routinely maintained by serial passage in Biomphalaria glabrata molluscs, an invertebrate host, and in BALB/c mice as a vertebrate host at the Parasitology Research Laboratory of the University of Franca in the state of São Paulo, Brazil.
S. mansoni eggs present in the feces of mice previously infected with the parasite were collected using the Hoffmann method [31]. Spontaneous sedimentation in the mixture of feces with water was exposed for approximately 1 h under light to release the miracidia. Miracidia were used to infect the intermediate host, which after 38 to 43 days released the infective form of the parasite, the Cercariae, which in turn infected the vertebrate host. Approximately 200 Cercariae are inoculated subcutaneously into mice, and after 21 or 58 days, young liver flukes or adult flukes are recovered from the hepatic portal system and mesenteric veins by perfusion [32]. After collection, the parasites were maintained in RPMI 1640 medium (Invitrogen) until use.
Adult worm pairs were recovered from BALB/c mice via perfusion of the hepatic portal system under aseptic conditions after 58 days of infection, as previously described. Then, the parasites were washed in RPMI 1640 buffered with HEPES20µM, pH 7.5, supplemented with penicillin (100U/mL), streptomycin (100 µg/mL), and 10% fetal bovine serum. Subsequently, one adult worm pair was transferred per well into a 24-well culture plate containing the same medium described previously and incubated in a humidifying atmosphere at 37 °C in the presence of 5% CO2. After 24 h of incubation, the crude extract and fractions were previously dissolved in dimethyl sulfoxide (DMSO) and added to the RPMI 1640 medium in a concentration range ranging 12.5, 25, 50, 100, and 200 μg/mL. The parasites were incubated under the same conditions described previously for 72 h. They monitored the parasites every 24 h using an inverted microscope (Leitz Diavert) to evaluate their general conditions, including mortality rates and motor activity. To confirm mortality, after the absence of movement for more than 2 min, the parasites were washed with RPMI medium and transferred to culture plates containing the same medium without the sample and monitored as previously described [33]. Adult worms maintained in RPMI 1640 medium served as negative controls, while adult worms incubated with 12.5 µg/mL of Praziquantel (Sigma Aldrich) were used as a positive control. Three independent experiments evaluated eight culture wells per concentration [34].
3.6. Theoretical Methods
The target used in the present study, based on molecular docking calculations, was the TGR enzyme. The coordinates of this enzyme were obtained from the protein data bank (PDB) ID: 2V6O [35]. The binding site was identified using the Discovery Studio 2020 software package [36] considering a 10 Å docking sphere from the centroid of the ligands associated with the enzyme found in the protein data bank.
The enzyme and ligand were prepared, and the docking simulations were realized using the GOLD 2020.2.0 software package [37,38]. The ranking of the best poses was achieved from the GoldScore fitness function, where the selection of the pose is accompanied by the use of a scoring function, which includes the following components: hydrogen bond energy of the complex, internal energy of the ligand, and torsional energy [39,40]. The rescore was made using the ChemScore fitness function for the calculation of the parameter dG, which represents the total free energy change that occurs on ligand binding. Keeping the conformations of the enzyme fixed, the conformations of the compound were allowed to change during the docking. The best poses and their respective molecular interactions were analyzed using the Discovery Studio 2020 software package.
4. Conclusions
Molecular docking calculations revealed that compounds 1 and 2 could interact with the amino acids of the FAD binding site in the TGR enzyme, a vital enzyme for S. mansoni, with binding energies comparable to those exhibited by PZQ, a drug of choice for treating schistosomiasis. Thus, this study suggests that flavonoids may have a potential impact on the treatment of Schistosomiasis. However, conducting comprehensive studies to validate the in vitro and molecular docking pharmacological claims for pharmaceutical applications is necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/compounds5030030/s1, Figure S1: 1H-NMR spectrum of quercitrin (methanol-d4, 400 MHZ), Figure S2: 1H NMR spectrum expansion of quercitrin, Figure S3. 13C NMR spectrum of quercitrin (methanol-d4, 100 MHZ), Figure S4: Expansion of the 13C NMR spectrum of quercitrin, Figure S5: Expansion of the 13C NMR spectrum of quercitrin, Figure S6: Mass spectrum of quercitrin with MS/MS 447, Figure S7: 1H-NMR spectrum of afzelin (methanol-d4, 400 MHZ), Figure S8: 1H NMR spectrum expansion of afzelin, Figure S9: 13C NMR spectrum of afzelin (methanol-d4, 100 MHZ), Figure S10: Expansion of the 13C NMR spectrum of afzelin, Figure S11: Expansion of the 13C NMR spectrum of afzelin, Figure S12: Mass spectrum of afzelin with MS/MS 431.
Author Contributions
Conceptualization, M.F.C.S. and L.G.M.; methodology, R.C.R.; software, R.C.S.V.; validation, S.R.A., R.P.O. and R.L.T.P.; formal analysis, M.L.A.e.S.; investigation, J.K.B.; resources, M.d.O.S.; data curation, H.O.G.C.; writing—original draft preparation, A.C.R.R.; writing—review and editing, W.Z.C.; visualization, M.F.C.S.; project administration, R.C.R.; funding acquisition, supervision, W.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Animal Care and Use Committee of UNIFRAN (permit 6242260122, 10 August2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant # 2011/13630-7), Coordenadoria de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES, Finance Code 001), to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the fellowships and the FAPES.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PZQ | Oxamniquine or praziquantel |
| TGR | Thioredoxin glutathione |
| HPLC | High-performance liquid chromatography |
| NMR | Nuclear magnetic resonance |
| DMSO | Dimethyl sulfoxide |
| RPMI | Roswell Park Memorial Institute medium |
| FAD | Flavin adenine dinucleotide |
References
- Vaz, S.M.S.; Cristina, C.B.A.; Tozzati, M.G.; Magalhães, L.G.; Silva, M.L.A.E.; Januário, A.H.; Pauletti, P.M.; Crotti, A.E.M.; dos Passos, A.V.; de Jesus, E.G.; et al. Evaluation of the in vitro schistosomicidal, leishmanicidal, and trypanocidal activities of the capsaicin metabolite, Capsicum frutescens, and Capsicum baccatum extracts and of their analysis of the main constituents by HPLC/UV and CG/MS. Nat. Prod. Res. 2023, 38, 679–684. [Google Scholar] [CrossRef]
- Mitra, A.; Mawson, A. Neglected tropical diseases: Epidemiology and global burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef]
- Hotez, P.J.; Lo, N. Neglected tropical diseases. Elsevier eBooks 2014, 44, 597–603. [Google Scholar] [CrossRef]
- Santos, S.S.; de Araújo, R.V.; Giarolla, J.; Seoud, O.E.; Ferreira, E.I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: A review. Int. J. Antimicrob. Agents 2020, 55, 105906. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; da Costa, J.M.C. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017, 61, e01012–16. [Google Scholar] [CrossRef]
- Montagnini, D.L.; Katchborian-Neto, A.; Tahan, M.P.; Oliveira, N.D.; Magalhães, L.G.; Januário, A.H.; Pauletti, P.M.; Cavallari, P.S.S.R.; Cunha, W.R.; Araujo, O.P.; et al. The schistosomicidal activity of ethanolic extracts from branches, leaves, flowers and fruits of Handroanthus impetiginosus (Mart. ex DC.) Mattos (Bignoniaceae) plant and metabolic profile characterization by UPLC-ESI-QTOF analysis. Braz. J. Biol. 2023, 83, e275824. [Google Scholar] [CrossRef]
- Alger, H.M.; Williams, D.L. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol. Biochem. Parasitol. 2002, 121, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, A.N.; Davioud-Charvet, E.; Sayed, A.A.; Califf, L.L.; Dessolin, J.; Arnér, E.S.J.; Williams, D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: An essential parasite enzyme and a key drug target. PLoS Med. 2007, 4, e206. [Google Scholar] [CrossRef]
- Sharma, M.; Khanna, S.; Bulusu, G.; Mitra, A. Comparative modeling of thioredoxin glutathione reductase from Schistosoma mansoni: A multifunctional target for antischistosomal therapy. J. Mol. Graph. Model. 2009, 27, 665–675. [Google Scholar] [CrossRef]
- Petukhova, V.Z.; Aboagye, S.Y.; Ardini, M.; Lullo, R.P.; Fata, F.; Byrne, M.E.; Gabriele, F.; Martin, L.M.; Harding, L.N.M.; Gone, V.; et al. Non-covalent inhibitors of thioredoxin glutathione reductase with schistosomicidal activity in vivo. Nat. Commun. 2023, 14, 3737. [Google Scholar] [CrossRef] [PubMed]
- Onile, O.S.; Raji, O.; Omoboyede, V.; Fadahunsi, A.I.; Onile, T.A.; Momoh, A.O.; Olukunle, S.; Nour, H.; Chtita, S. Structure-Based Discovery of Phytocompounds from Azadirachta indica as Potential Inhibitors of Thioredoxin Glutathione Reductase in Schistosoma mansoni. Cell Biochem. Biophys. 2025, 83, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, I.; Lyu, H.; Fata, F.; Banta, P.R.; Mattei, B.; Ippoliti, R.; Bellelli, A.; Pitari, G.; Ardini, M.; Petukhova, V.; et al. Ectopic suicide inhibition of thioredoxin glutathione reductase. Free Radic. Biol. Med. 2020, 147, 200–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyu, H.; Petukhov, P.A.; Banta, P.R.; Jadhav, A.; Lea, W.A.; Cheng, Q.; Arnér, E.S.J.; Simeonov, A.; Thatcher, G.R.J.; Angelucci, F.; et al. Characterization of Lead Compounds Targeting the Selenoprotein Thioredoxin Glutathione Reductase for Treatment of Schistosomiasis. ACS Infect. Dis. 2020, 6, 393–405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Souza, N.L.R.; Montoya, B.O.; Brandão-Neto, J.; Verma, A.; Bowyer, S.; Moreira-Filho, J.T.; Dantas, R.F.; Neves, B.J.; Andrade, C.H.; von Delft, F.; et al. Fragment library screening by X-ray crystallography and binding site analysis on thioredoxin glutathione reductase of Schistosoma mansoni. Sci. Rep. 2024, 14, 1582. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Porto, T.S.; Junior, A.H.C.; Santos, M.F.C.; Ramos, H.P.; Braun, G.H.; de Lima Paula, L.A.; Bastos, J.K.; Furtado, N.A.J.C.; Parreira, R.L.T.; et al. Schistosomicidal activity of kaurane, labdane and clerodane-type diterpenes obtained by fungal transformation. Process Biochem. 2020, 98, 34–40. [Google Scholar] [CrossRef]
- Carneiro, L.; Tasso, T.; Santos, M.; Goulart, M.; dos Santos, R.; Bastos, J.; da Silva, J.; Crotti, A.; Parreira, R.; Orenha, R.; et al. Copaifera multijuga, Copaifera pubiflora and Copaifera trapezifolia oleoresins: Chemical characterization and in vitro cytotoxic potential against tumoral cell lines. J. Braz. Chem. Soc. 2020, 31, 1679–1689. [Google Scholar] [CrossRef]
- Carneiro, L.J.; Bastos, J.K.; Veneziani, R.C.S.; Santos, M.F.C.; Ambrósio, S.R. A reliable validated high-performance liquid chromatography-photodiode array detection method for quantification of terpenes in Copaifera pubiflora, Copaifera trapezifolia, and Copaifera langsdorffii oleoresins. Nat. Prod. Res. 2022, 38, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Basso, A.; Bellelli, A.; Brunori, M.; Mattoccia, L.P.; Valle, C. The anti-schistosomal drug praziquantel is an adenosine antagonist. Parasitology 2007, 134, 1215–1221. [Google Scholar] [CrossRef]
- Nugroho, A.; Choi, J.K.; Park, J.H.; Lee, K.T.; Cha, B.C.; Park, H.J. Two new flavonol glycosides from Lamium amplexicaule L. and their in vitro free radical scavenging and tyrosinase inhibitory activities. Planta Med. 2009, 75, 364–366. [Google Scholar] [CrossRef]
- Zhang, Z.; Elsohly, H.N.; Li, X.C.; Khan, S.I.; Broedel, S.E.; Raulli, R.E.; Cihlar, R.L.; Burandt, C.; Walker, L.A. Phenolic compounds from Nymphaea odorata. J. Nat. Prod. 2003, 66, 548–550. [Google Scholar] [CrossRef]
- Diantini, A.; Subarnas, A.; Lestari, K.; Halimah, E.; Susilawati, Y.; Supriyatna, N.; Julaeha, E.; Achmad, T.H.; Suradji, E.W.; Yamazaki, C.; et al. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol. Lett. 2012, 3, 1069–1072. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, E.; Kim, S.; Kim, J.H.; Kim, E.G.; Lee, J.; Park, D. Antagonizing effects and mechanisms of afzelin against UVB-induced cell damage. PLoS ONE 2013, 8, e61971. [Google Scholar] [CrossRef]
- Zhou, W.; Nie, X. Afzelin attenuates asthma phenotypes by downregulation of GATA3 in a murine model of asthma. Mol. Med. Rep. 2015, 12, 71–76. [Google Scholar] [CrossRef]
- Zhu, K.C.; Sun, J.M.; Shen, J.G.; Jin, J.Z.; Liu, F.; Xu, X.L.; Chen, L.; Liu, L.T.; Lv, J.J. Afzelin exhibits anti-cancer activity against androgen-sensitive LNCaP and androgen-independent PC-3 prostate cancer cells through the inhibition of LIM domain kinase 1. Oncol. Lett. 2015, 10, 2359–2365. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Wang, M.; Dong, H.; Zhang, J.; Zhang, L. Quercetrin from Toona sinensis leaves induces cell cycle arrest and apoptosis via enhancement of oxidative stress in human colorectal cancer SW620 cells. Oncol. Rep. 2017, 38, 3319–3326. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Johnson, J.L.; Rupasinghe, S.G.; Stefani, F.; Schuler, M.A.; de Mejia, G.E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3β enzymatic activity by lowering the interaction energy within the binding cavity. J. Med. Food 2011, 14, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Fauzi, M.; Khairul Ikram, N.K.; Mohd Gazzali, A.; Wahab, H.A. Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for anti-SARS-CoV-2. Molecules 2020, 25, 3980. [Google Scholar] [CrossRef] [PubMed]
- Awang, M.A.; Chua, L.S.; Abdullah, L.C. Solid-phase extraction and characterization of quercetrin-rich fraction from Melastoma malabathricum leaves. Separations 2022, 9, 373. [Google Scholar] [CrossRef]
- Kuhn, I.; Kellenberger, E.; Said-Hassane, F.; Villa, P.; Rognan, D.; Lobstein, A.; Haiech, J.; Hibert, M.; Schuber, F.; Muller-Steffner, H. Identification by high-throughput screening of inhibitors of Schistosoma mansoni NAD+ catabolizing enzyme. Bioorg. Med. Chem. 2010, 18, 7900–7910. [Google Scholar] [CrossRef]
- Hoffman, W.A.; Pons, J.A.; Janer, J.L. The Sedimentation Concentration Method in Schistosomiasis Mansoni. Puerto Rico J. Public Health Trop. Med. 1934, 9, 283–289. [Google Scholar]
- Smithers, S.R.; Terry, R.J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 1965, 55, 695–700. [Google Scholar] [CrossRef]
- Pica-Mattoccia, L.; Cioli, D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004, 34, 527–533. [Google Scholar] [CrossRef]
- Magalhães, L.G.; Machado, C.B.; Morais, E.R.; Bueno de Carvalho Moreira, É.; Soares, C.S.; da Silva, S.H.; da Silva Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2008, 104, 1197–1201. [Google Scholar] [CrossRef]
- Angelucci, F.; Miele, A.E.; Boumis, G.; Dimastrogiovanni, D.; Brunori, M.; Bellelli, A. Glutathione reductase and thioredoxin reductase at the crossroad: The structure of Schistosoma mansoni thioredoxin glutathione reductase. Proteins 2008, 72, 936–945. [Google Scholar] [CrossRef]
- BIOVIA Discovery Studio. Discovery Studio Modeling Environment; Version 4.0; Dassault Systemes: Woodland Hills, CA, USA, 2015; Available online: https://www.3ds.com/products/biovia/discovery-studio (accessed on 2 July 2023).
- Jones, G.; Willett, P.; Glen, R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Available online: https://www.ccdc.cam.ac.uk/solutions/software/gold/ (accessed on 16 December 2024).
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein–ligand docking using GOLD. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).