1. Introduction
The transition toward a low-carbon energy future has significantly accelerated the development of sustainable energy technologies, with Proton Exchange Membrane Fuel Cells (PEMFCs) and Proton Exchange Membrane Water Electrolyzers (PEMWEs) playing pivotal roles [
1,
2]. PEMFCs convert chemical energy stored in hydrogen into electricity, producing only water and heat as byproducts. Depending on the operating temperature, fuel cells are categorized into Low-Temperature PEMFCs (LT-PEMFCs, which operate below 100 °C) and High-Temperature PEMFCs (HT-PEMFCs, which operate above 120 °C), offering improved CO tolerance and electrode kinetics as well as eliminating the need for complex humidification systems [
3]. In contrast, PEMWEs utilize electrical energy, ideally sourced from renewable energy systems, to drive the electrolysis of water, generating hydrogen and oxygen gases. When powered by renewable electricity, the resulting hydrogen is classified as “green hydrogen”.
Proton Exchange Membrane (PEM) technologies are integral to clean hydrogen production and efficient energy conversion, supporting the global shift toward decarbonization and energy resilience. Central to their performance are highly active and durable electrocatalysts, which drive the key reactions of hydrogen evolution, hydrogen oxidation, oxygen evolution and oxygen reduction. These catalysts are conventionally supported on porous carbon materials and are deposited onto both sides of a PEM, typically a sulfonated tetrafluoroethylene-based fluoropolymer–copolymer (Nafion
TM) for LT-PEMFCs and polybenzimidazole (PBI) for HT-PEMFCs, to form the anode and cathode catalyst layers within the Membrane Electrode Assembly (MEA). Traditionally, electrocatalysts used in PEMFCs and PEMWEs rely on Platinum Group Metals (PGMs), such as platinum (Pt) and iridium oxide (IrO
2), due to their exceptional catalytic activity and stability [
4,
5,
6,
7]. However, the limited natural abundance, high cost and environmentally intensive extraction processes of PGMs present significant economic and sustainability challenges, hindering large-scale commercialization and raising concerns about long-term supply security [
8]. In parallel, the rapid deployment of PEM technologies has led to the growing accumulation of End-of-Life (EoL) MEAs, creating both a waste management concern and a valuable opportunity for resource recovery.
Beyond hydrogen technologies, electrocatalysis plays a broader role across the energy sector. For example, the electrochemical two-electron Oxygen Reduction Reaction (2e
− ORR) provides a sustainable pathway for decentralized H
2O
2 production, with carbon-based and metal-free catalysts emerging as green alternatives to the anthraquinone process [
9]. Similarly, dual-function oxygen electrocatalysts, such as Cobalt/Neodymium-doped Nitrogen-doped carbon derived from Metal Organic Frameworks (MOFs), are showing high performance in rechargeable Zink-air batteries, outperforming traditional Pt/C and IrO
2 systems [
10]. In parallel, biomass-derived carbons from sources like Camellia shells have demonstrated excellent electrochemical properties in sodium-ion energy storage systems, offering sustainable and low-cost solutions for large-scale applications [
11]. Given these challenges and the increasing demand for sustainable catalyst solutions, the recycling and direct reuse of PGMs from EoL MEAs of PEM devices have emerged as a promising strategy to mitigate resource scarcity, reduce environmental impact and promote circular economy [
12,
13]. Electrode ink, which constitutes approximately 46% of the total cost associated with the mass production of PEMFCs, relies on costly and limited PGMs, making efficient recycling strategies for EoL MEAs of PEMFCs and PEMWEs both economically and environmentally imperative [
14]. However, recycling the functional components of PEM systems, such as MEAs, presents several technical challenges. More specifically, the recovery of PGMs from spent catalysts involves intricate dissolution, separation and purification steps, necessitating the development of advanced recycling processes and novel hydrometallurgical or hybrid technologies to maximize recovery efficiency and to ensure their reuse [
15]. Considering these material-specific constraints, recycling strategies must be technically efficient, economically viable, sustainable and scalable, ensuring the secure supply of PGMs and reducing the environmental footprint of hydrogen technologies [
16].
Current State-of-the-Art (SoA) methods for recovering PGMs from EoL MEAs are broadly categorized into pyrometallurgical and hydrometallurgical approaches (
Figure 1) [
17]. Pyrometallurgy involves the application of high temperatures to extract metals such as Pt and can process large quantities of waste with high recovery efficiencies (
Figure 1a). However, it also presents significant drawbacks such as high energy requirements and the emission of hazardous substances (e.g., fluorinated gases from Nafion
TM degradation) and destroys non-metallic components such as membranes, limiting the overall materials recovery and increasing environmental impact [
17].
Hydrometallurgical methods, particularly chloride-based leaching processes, have been identified as effective means for recovering PGMs from spent catalysts, achieving high recovery rates (up to 100% for Pt, 92% for Pd), using acidic chloride media with oxidants like hydrogen peroxide or chlorine gas (
Figure 1b) [
18,
19]. The use of pregnant leachate solutions presents an opportunity to utilize recycled metal precursors for the synthesis of electrocatalysts, thereby promoting a circular economy within the energy sector [
20]. Hydrometallurgical techniques generally consume less energy than pyrometallurgical methods and allow for more targeted metal recovery, often with reduced greenhouse gas emissions. However, hydrometallurgy also presents several limitations, such as the use of corrosive or toxic chemicals and the need for specialized equipment, multi-step purification and filtration processes and relatively slower reaction kinetics particularly when dealing with complex matrixes or residual catalysts in the membrane [
17]. Challenges include poor dissolution of PGMs due to fluoropolymer coatings in EoL MEAs and low selectivity, leading to impurity co-extraction. Similar to pyrometallurgical methods, hydrometallurgy also struggles with the effective recovery of polymeric membrane material, limiting overall sustainability.
To overcome these limitations, recent research has focused on developing innovative recycling strategies aimed at improving the recovery of both metallic and non-metallic components from EoL MEAs [
21,
22,
23]. These include approaches such as electrochemical dissolution techniques, hydrothermal treatments, acid-based leaching with optimized reagents and alcohol-solvent-assisted dissolution [
24,
25,
26]. These emerging methods aim to enhance safety, reduce processing costs, improve selectivity and recovery rates and enable the recovery of additional materials such as ionomer membranes. These innovations represent a promising direction for the development of efficient, environmentally sustainable and economically viable recycling pathways that support circularity in PEMFC and PEMWE technologies.
In 2019, Sharma et al. developed an electrochemical approach for recycling spent Pt/C electrocatalysts from HT-PEMFCs’ electrodes [
27]. Their method utilizes potential cycling in an acidic medium (0.1–1 M HCl) to dissolve Pt from the spent catalyst (0.7 mgPt/mL), followed by its recovery through precipitation as ammonium hexachloroplatinate ((NH
4)
2PtCl
6). The recovered Pt precursor is then used to synthesize new Pt/C catalysts using a modified polyol process. This method demonstrated a Pt recovery efficiency exceeding 90%, and the resulting catalysts exhibited electrochemical performance comparable to or surpassing that of commercial 20 wt.% Pt/C catalyst.
A recent study demonstrated a selective electrochemical dissolution technique for leaching out Pt from spent HT-PEMFCs’ electrodes, followed by hot aqua regia treatment to dissolve the residual membrane and remaining Pt/C catalyst [
28]. The recovered Pt (as H
2PtCl
6 after solvent evaporation) was used to synthesize new Pt/C catalysts via the reverse microemulsion method. Electrodes fabricated with both commercial and recycled catalysts were evaluated, showing that those prepared from EoL MEAs maintained strong activity toward SO
2 oxidation and hydrogen evolution with particularly high stability.
In recent research, a hydrometallurgical approach was developed to effectively recover Pt and ruthenium (Ru) from spent LT-PEMFCs’ electrodes [
15]. Leaching experiments are performed at 75 °C using a solution of hydrochloric acid (4 M HCl) enhanced with aluminum chloride (1.5 M AlCl
3) as an alternative source of chloride ions. This method achieved high recovery rates, with up to 90% for Pt and 82% for Ru after four hours of treatment. The extracted precious metals were then processed into ammonium hexachloroplatinate ((NH
4)
2PtCl
6) and ammonium hexachlororuthenate ((NH
4)
2RuCl
6), which served directly as precursors for producing fresh PtRu electrocatalysts. These regenerated materials exhibited physical and chemical properties comparable to those of commercially available counterparts, demonstrating the potential for sustainable, circular use of materials in PEMFC technologies.
Bharti and Natarajan developed a simple, low-temperature hydrothermal process utilizing isopropanol (autoclave, 200 °C, 24 h), an environmentally benign solvent, for the recovery of both catalysts and Nafion membranes from EoL MEAs of LT-PEMFCs [
26]. The recovered Pt/C catalyst exhibited an Electrochemical Surface Area of 42 m
2/g (∼50% ECSA of the fresh catalyst) along with high stability, indicating its suitability for second-life applications. Furthermore, Fourier Transform Infrared (FT-IR) analysis of the recovered ionomer solution confirmed a molecular structure closely resembling that of commercial Nafion
TM dispersion.
In 2022, Chourashiya et al. reported a hydrometallurgical recycling method for Pt recovery from EoL LT-PEMFCs’ electrodes, utilizing low-concentration hydrochloric acid (1 M HCl) under reflux conditions (80 °C, 48 h) [
29]. The process involved leaching Pt from the spent electrodes, followed by solvent evaporation to obtain a solid Pt precursor, which was subsequently used for synthesizing Pt/C electrocatalysts via the polyol method. To assess industrial viability, gram-scale synthesis of Pt/C was performed, and the resulting catalyst demonstrated electrochemical performance comparable to that of commercial benchmark Pt/C material.
Carmo et al. demonstrated a straightforward and scalable recycling process for EoL MEAs from PEMWEs [
30]. The method employs ultrasonication in a water-based solution to recover both noble metal catalysts (Pt and Ir) and the fluoropolymer membrane, without the release of hazardous gases. Achieving recycling efficiencies above 90%, the process maintains catalyst integrity while measurable performance loss attributed to potential cross-contamination and reduced catalyst layer adhesion.
Sharma and Andersen proposed an environmentally friendly and cost-effective method for Pt recovery from spent LT-PEMFCs’ electrodes [
31]. The process involves dissolving Pt in hydrochloric acid (1 M HCl for 48 h at 80 °C or 1 M HCl/30%H
2O
2 for 2 h under reflux conditions) and directly using the resulting solution (0.75 mgPt/mL) as a precursor for synthesizing Pt/C catalysts via a microwave-assisted polyol method. The resulting Pt/C catalysts exhibited an Electrochemical Surface Area of approximately 58 m
2/g with a room for improvement and demonstrated superior durability compared to commercial counterparts, attributed to the formation of larger Pt nanoparticles.
As the hydrogen sector rapidly develops, the integration of circular economy practices by companies, such as Hensel Recycling GmbH and Johnson Matthey, is vital to conserve critical materials and reduce the environmental footprint. Hensel Recycling offers comprehensive fuel cells and electrolyzer recycling services, including the dismantling of complete fuel cell stacks, separation of individual components and recovery of PGMs and other critical raw materials to reduce CO
2 emissions [
32]. Johnson Matthey recently reported the successful lab-scale demonstration of its HyRefine technology, a chemical recycling process designed for recovering PGMs and ionomers from EoL MEAs, producing recycled catalysts with performance comparable to new ones, offering a sustainable alternative to traditional refining methods [
33].
Even though many novel recycling methods for critical materials recovery from EoL MEAs of PEMFCs and PEMWEs are reported in the literature, they still suffer from several limitations such as high costs, use of hazardous chemicals, multi-step and time-consuming purification procedures, incomplete recovery, low selectivity and challenges in scaling up. Although recent studies demonstrate the feasibility of a circular approach for Pt catalyst utilization in hydrogen devices and improved sustainability, practical large-scale implementation remains limited. The direct utilization of recycled metal precursors without purification for electrocatalyst synthesis presents a promising and sustainable alternative. This approach reduces chemical consumption and processing time (by over 70%), lowers operational costs (by up to 60%) and enables the use of highly concentrated Pt leachate, thereby improving process efficiency and reducing waste. These advancements can potentially reduce the demand for virgin Pt by 40–60% and decrease the carbon footprint of Pt recovery by up to 80%, making the process highly suitable for industrial-scale deployment. Innovative and efficient recycling processes that can maintain recycled catalyst performance comparable to commercial benchmarks and provide a commercially viable and resource-efficient pathway to sustainable hydrogen production and utilization at an industrial scale are critically needed. The recycling process proposed herein aims to address these challenges by offering an optimized strategy for the recovery and reintegration of Pt, thus advancing the circularity and economic feasibility of PEM hydrogen technologies. These benefits position the method as a viable path forward for sustainable fuel cell production, with applications in zero-emission transport, backup power and green hydrogen supporting economic and environmental goals. It is especially valuable for sectors like healthcare, where reliable and low-emission energy can reduce costs and environmental impact [
34]. Improving the availability of sustainable energy may also support public health by improving energy resilience for medical procedures [
35].
A single-step, cost-effective, universal and environmentally friendly hydrometallurgical leaching process has been developed by MONOLITHOS (MON) for recycling PGMs from various spent catalysts streams, such as automotive catalysts (e.g., Three-Way Catalysts, Diesel Oxidation Catalysts), catalyzed Diesel Particulate Filters (c-DPFs) and electrocatalysts [
19,
36]. This method utilizes a low-acidity HCl-NaCl-H
2O
2 system, operates under mild conditions (70 °C, less than four hours) and results in high leaching efficiencies of >99% for Pt and 92% for Pd significantly reducing the use of toxic substances and minimizing energy consumption. By simplifying the recycling process and lowering chemical input, MON’s versatile leaching method decreases the environmental footprint of PGM recycling compared to conventional multi-step and energy-intensive methods while prioritizing sustainability by employing less hazardous chemicals and minimizing operational costs associated with the recovery of both PGMs and ionomers from EoL MEAs. The method has been patented at both national and European level (“Process of recovery of critical metals from electrochemical stack devices”, Hellenic Industrial Property Organization: GR1010390B, European Patent Office: EP4174196A1; “Process for Recovering and Reusing Electrodes, Electrocatalysts, Metals and Polymer Electrolyte from Polymer Electrolyte-Electrode Assemblies of Electrochemical Devices”, Hellenic Industrial Property Organization, application number: 20240100540). Notably, this approach aligns strongly with circular economy principles by not only recovering critical metals but also enabling the recovery and reuse of the Nafion electrolyte membrane, promoting material circularity and waste minimization in PEM systems.
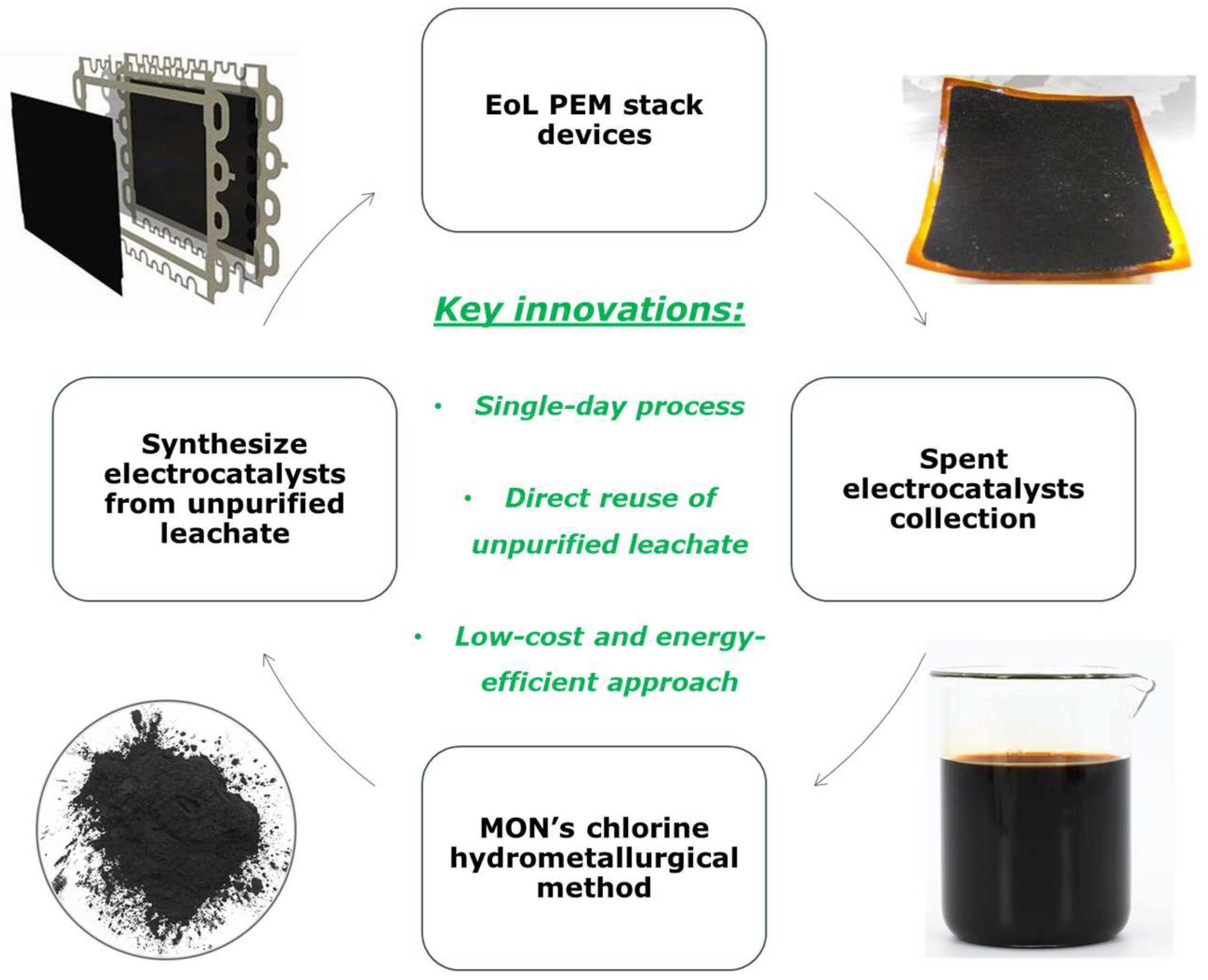
In the following study, an innovative approach on Pt/C catalyst preparation from unpurified Pt leachate solutions (recycled Pt precursor) recovered from EoL MEAs of PEMFCs with a potential industrial approach has been investigated. In particular, MON’s low-cost and environmentally friendly chlorine-based hydrometallurgical leaching method was employed to extract Pt-containing pregnant leachate solutions. These recycled Pt precursors without any purification were subsequently used to synthesize advanced Pt/C electrocatalyst through scalable, fast and sustainable chemical route. The recycled-metal-based Pt/C electrocatalyst was subjected to comprehensive physicochemical characterization and electrochemical testing toward the Oxygen Reduction Reaction (ORR), demonstrating performance comparable to that of commercial benchmark catalyst, highlighting its potential as a viable and lower-cost and sustainable alternative. The use of impure pregnant leachate solutions derived from MON’s leaching process presents an opportunity to directly utilize recycled metal precursors without any purification for the synthesis of sustainable and economically viable electrocatalysts. This approach promotes a circular economy strategy within the energy sector by reducing reliance on virgin materials, improving supply security through addressing the scarcity of PGMs, minimizing waste generation and supporting the broader commercialization of clean hydrogen energy technologies.
2. Materials and Methods
2.1. Spent Electrocatalyst Collection and Characterization
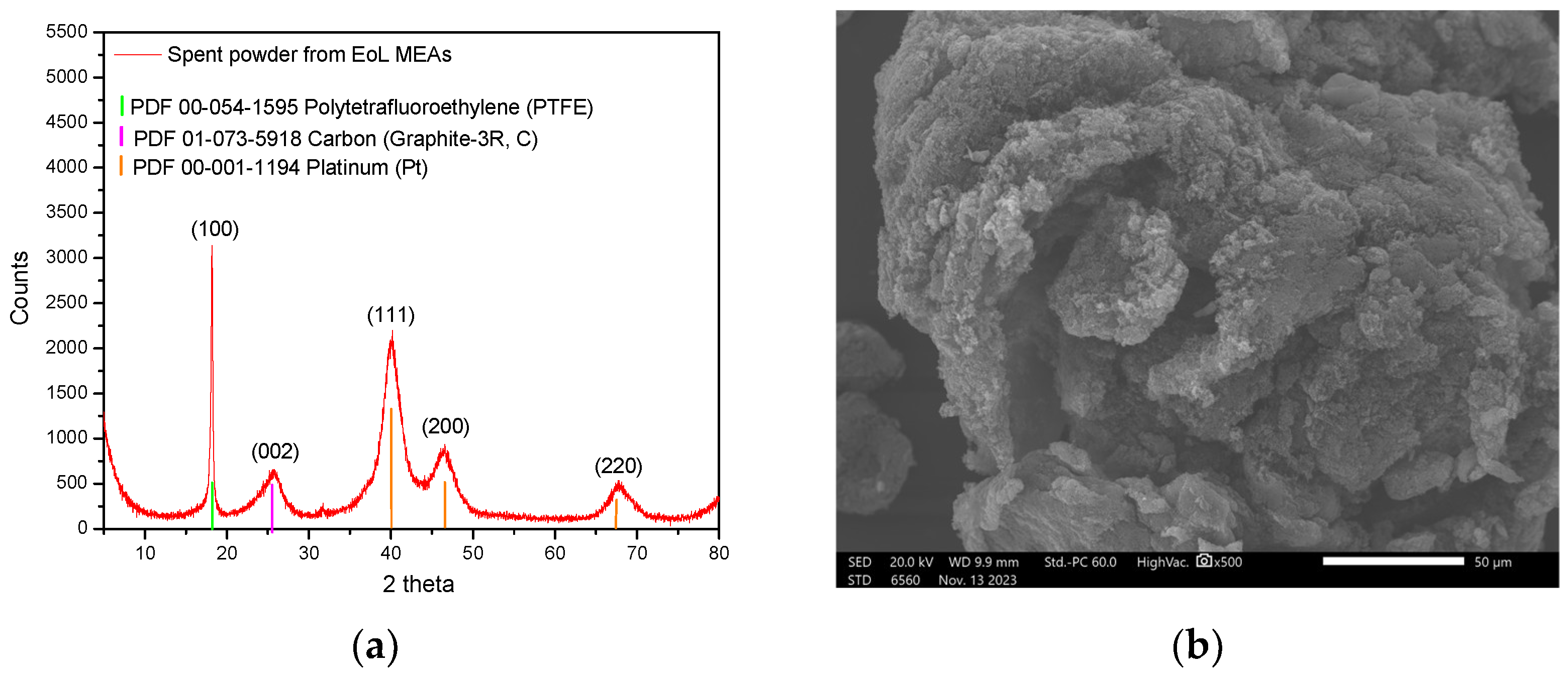
End-of-Life (EoL) Nafion and PBI MEAs from PEMFCs, which employed Pt/C catalysts at both the anode and cathode, have been used for spent electrocatalyst collection. For efficient dissolution of Pt, detachment of spent electrocatalysts from the polymeric membranes was carried out following a mechanical delamination process at room temperature for 5 to 30 min, depending on the applied cell compression and catalyst layers’ bonding strength. EoL MEAs were immersed in the aqueous solution under mild agitation to reduce interfacial adhesion between the catalyst layer and the polymeric membrane, thereby enabling efficient delamination while preserving the chemical integrity of the recovered materials. Recovered spent electrocatalytic powder was dried and physiochemically characterized using X-Ray Fluorescence (XRF), X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM) to support the development of a high-performance recycling method. More specifically, a Vanta Olympus XRF analyzer (2017, Waltman, MA, USA), specifically calibrated for accurate quantification of each metal as detailed in [
37], was used for the qualitative and quantitative analysis. Prior to analysis, the catalytic powders, having a particle size of less than 125 μm, were dried at 120 °C for 2 h. Approximately 5 g of the dried powder was then pressed into polyethylene cups to prepare the samples. Each sample was analyzed using 10 consecutive scans, with each scan lasting 90 s. X-Ray Diffraction (XRD) patterns were obtained using a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) fitted with a copper (Cu) radiation source (λ
CuKα = 1.54046 Å). The measurements were carried out over a 2θ range of 20° to 80°, with a scan rate of 2 s per step. Scanning Electron Microscopy (SEM) images were acquired using a Zeiss SUPRA 35VP-FEG instrument (Carl Zeiss AG, Oberkochen, Germany) operating at an accelerating voltage of 5–20 keV.
2.2. Spent Electrocatalyst Recycling
The following chemicals were used for leaching experiments: HCl (37%, PanReac AppliChem/ITW Reagents, Castellar del Vallès, Spain, ACS, for analysis), H2O2 (35%, Solvay S.A., Neder-Over-Heembeek, Brussels, Belgium, food grade), NaCl (99.9%, Kali K&S GmbH, K+S AG, Kassel, Germany) and DI H2O (deionized water). The spent electrocatalyst powder recovered from EoL MEAs was subjected to a mild thermal pre-treatment at temperatures below 300 °C to enhance its leachability by increasing metal surface exposure and improving powder wettability to the lixiviant solution (evaporation of residual solvents and disruption of catalyst-support interactions). The final validation of the leachate solution revealed that this pre-treatment step did not affect the structural or physicochemical properties of the constituent metals and support material. Subsequently, the treated powder was introduced into the leaching reactor. Reagents were added sequentially under mechanical stirring in the following order: hydrochloric acid (HCl, serving as the chloride ion source), hydrogen peroxide (H2O2, to oxidize metallic species, thereby accelerating dissolution and enabling the use of lower acid concentrations), sodium chloride (NaCl, to stabilize metal ions through the formation of chloro-complexes and to moderate the overall acidity) and deionized water (DI H2O). To assess the effect of the Pt-to-lixiviant ratio, varying solid-to-liquid mass ratios were tested. The experiments were conducted using a stirring and heating plate (Medine Scientific Limited, MS300HS, Mtops Korea, Yangju, Republic of Korea). Maximum Pt leaching efficiency was obtained using a lixiviant composed of 3 M HCl, 1% v/v H2O2 and 4.5 M NaCl, with a Pt-to-lixiviant ratio of up to 20 mg Pt/mL and a reaction temperature of 70 °C. Upon completion of the leaching reaction (approximately four hours), the mixture is subjected to vacuum filtration to separate the solid residue from the pregnant leachate solution, which contains the dissolved Pt. The solid residue (low PGM content) is then dried at 80 °C and analyzed by XRF to assess the extent of Pt dissolution. Concurrently, the pregnant leachate solution (high PGM content) is analyzed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) to accurately quantify the leaching efficiency of Pt. Each experiment was performed at least twice to confirm the consistency and reproducibility of the results, with percentage differences between repeated measurements found to be within 0.5–1%, indicating high reliability.
2.3. Electrocatalyst Development
Carbon-supported catalyst based on Pt (nominal metal loading of 20 wt.%) was synthesized using commercial Pt(IV) chloride precursor (PtCl4, Heraeus Holding GmbH, Hanau, Germany, 58%Pt). Commercial Vulcan XC-72 carbon black was dispersed in aqueous solution to form a stable and uniform suspension. Separately, an aqueous solution of a commercial Pt(IV) chloride salt (PtCl4, Heraeus, 58%Pt) was prepared. This platinum precursor solution was then introduced into the carbon suspension under continuous stirring to ensure homogeneous distribution of the Pt ions on the carbon surface. A sodium borohydride (NaBH4) solution (Thermo Fisher Scientific, Waltham, MA, USA, 99%, VenPureTM SF powder) was subsequently added to the mixture, initiating the reduction of Pt(IV) ions to metallic Pt nanoparticles. The reduction process was carried out at room temperature under constant stirring for two hours. Upon completion, Pt/C catalyst powder was isolated by vacuum filtration, thoroughly washed with DI H2O to remove residual ions and reaction by-products and dried under ambient conditions overnight to ensure gentle solvent removal without compromising the dispersion and surface characteristics of the Pt/C catalysts.
Following the wet chemical reduction method described above, Pt/C catalyst (nominal metal loading of 20 wt.%) was synthesized using impure Pt-containing pregnant leachate solution, obtained via MON’s low-acidity HCl-based hydrometallurgical leaching process, as the metal precursor. In the step wherein the platinum precursor is introduced into the carbon suspension, the leachate solution was used in place of commercial precursor. This substitution aimed to evaluate the feasibility of utilizing recycled Pt from EoL PEMFC systems in electrocatalyst fabrication. Inductively coupled plasma (ICP) analysis was performed on the leachate solution prior to its use in order to determine the metal concentration and to calculate the appropriate volume required to achieve the targeted metal loading.
2.4. Physicochemical Characterization
Comprehensive characterization was conducted to evaluate the structural, compositional, morphological and electrochemical properties of the synthesized catalysts. Techniques such as X-ray Fluorescence (XRF), X-ray Diffraction (XRD), Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) were used to assess metal content, crystallinity, morphology and elemental distribution. A Vanta Olympus XRF analyzer (2017, Waltman, MA, USA), calibrated for precise quantification of individual metals as outlined in [
37], was employed for elemental analysis. Prior to measurement, the catalytic powders were dried at 80 °C for 5 h to remove residual moisture. Approximately 1 g of the dried material was then compacted into polyethylene sample cups. Each sample underwent 10 successive scans, with each scan lasting 90 s, to ensure analytical consistency. X-ray diffraction (XRD) analysis was performed using a Bruker D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with a Cu Kα radiation source (λ = 1.54046 Å). Data were collected over a 2θ range of 20–80° with a step scan rate of 2 s per increment. Transmission Electron Microscopy (TEM) analysis was performed using a JEOL instrument (JEOL Ltd., Akishima, Tokyo, Japan) operated at an accelerating voltage of 200 kV to examine the particle size and morphology at the nanoscale. Surface morphology and particle dispersion were examined via scanning electron microscopy (SEM) using a Zeiss SUPRA 35VP-FEG instrument operated at an accelerating voltage between 5 and 20 keV, depending on the resolution and contrast requirements. All measurements were performed under standardized laboratory conditions. For benchmarking purposes, a commercial 20 wt.% Pt/C catalyst (US Research Nanomaterials Inc., Houston, TX, USA) was also subjected to XRF, XRD and SEM analysis.
2.5. Electrochemical Characterization
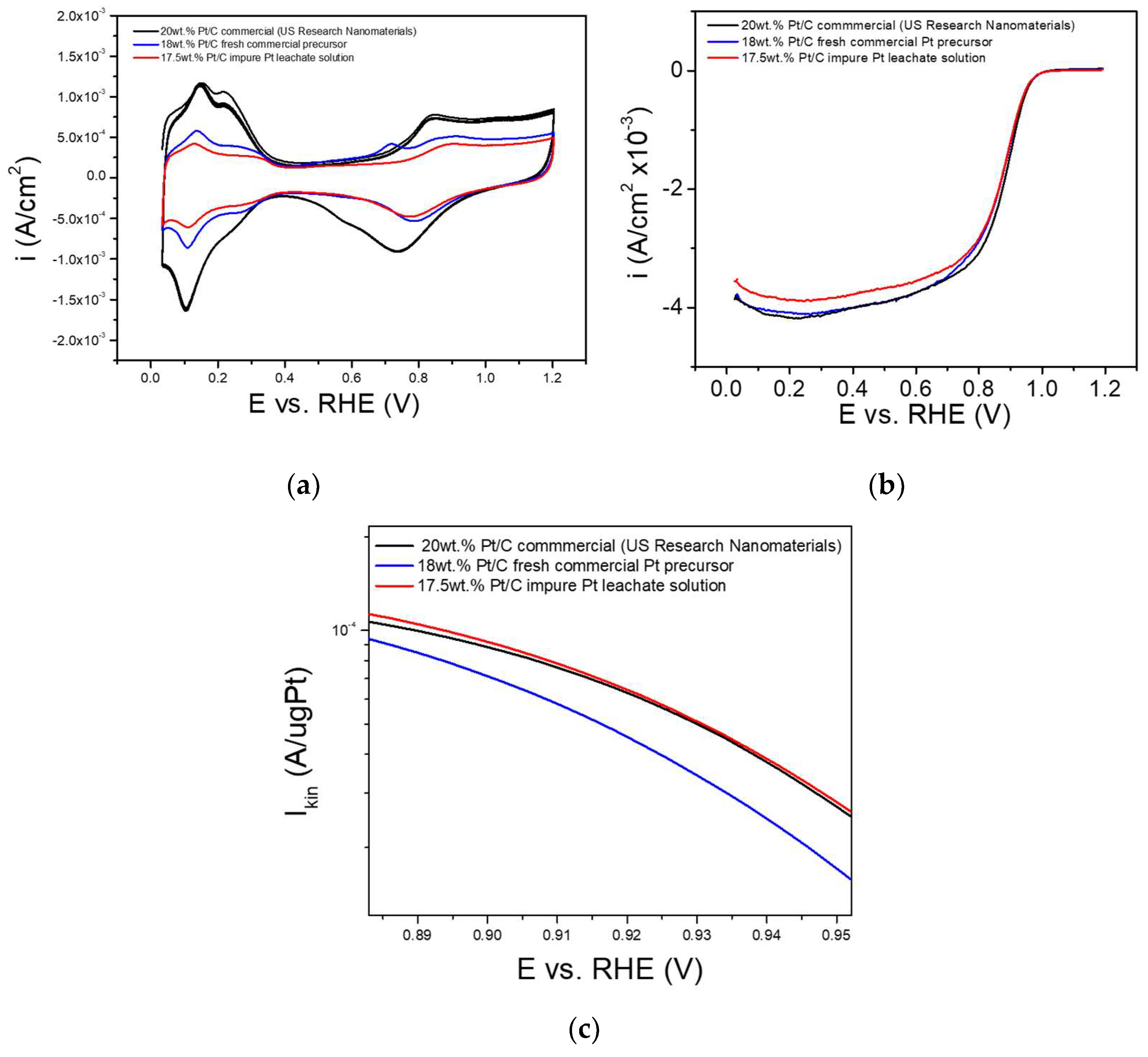
The electrochemical performance of the synthesized Pt/C catalysts was assessed using a Rotating Disk Electrode (RDE) system configured within a conventional three-electrode electrochemical cell. A platinum plate was used as the counter electrode, and a Reversible Hydrogen Electrode (RHE) served as the reference. The Pt/C Working Electrode (WE) was prepared by coating a Glassy Carbon (GC) disk electrode (diameter = 0.4 cm) with a Pt/C catalyst ink. Before catalyst deposition, the GC surface was polished to a mirror finish using a 0.5 μm alumina slurry in deionized water and then thoroughly rinsed with deionized water and dried in air. The catalyst ink was made by ultrasonically dispersing 10 mg of Pt/C catalyst in a 5 mL mixture of deionized water and alcohol. A specific volume of this ink was applied to the GC disk using a microsyringe, and the electrode was left to dry at room temperature. To ensure consistency and reliability of measurements, all electrodes were prepared with the same catalyst layer thickness, keeping the amount of carbon support constant across all samples.
A 0.1 M perchloric acid (HClO4) solution was used as the electrolyte. Prior to measurements, the electrolyte was purged with inert gas (e.g., N2) to eliminate any dissolved oxygen. All electrochemical measurements were conducted at room temperature using a potentiostat interfaced with the RDE setup. Cyclic Voltammetry (CV) was performed to evaluate the electrochemical activity and stability of the catalysts, as well as to estimate their Electrochemical Surface Area (ECSA). The ECSA was determined from the hydrogen underpotential deposition (Hupd) region (E < 0.4 V versus RHE) of the cyclic voltammograms at a scan rate of 100 mV/s by integrating the hydrogen adsorption and desorption peaks, using the double-layer charging current as the baseline. The calculation was based on a charge density of 210 μC/cm2 for polycrystalline platinum (standard charge required to oxidize a monolayer of hydrogen on Pt). Linear Sweep Voltammetry (LSV) was conducted in an oxygen-saturated environment to measure Oxygen Reduction Reaction (ORR) activity across a range of potentials and electrode rotation speeds to evaluate the diffusion and kinetic characteristics of the catalysts. For comparison, the commercial Pt/C catalyst (US Research Nanomaterials Inc.) was also subjected to identical electrochemical testing procedures. Mass activity was determined by dividing the measured catalytic current at 0.9 V versus RHE by the total mass of Pt loaded on the electrode, thereby providing an indicator of catalyst efficiency per unit mass. To verify the reproducibility of the data, all measurements were repeated in duplicate, with the percentage differences between duplicates being consistently within 0.5–2%.
4. Discussion
In this study, in order to enable efficient dissolution of Pt in the lixiviant solution, spent electrocatalysts were first detached from the polymeric membranes of EoL MEAs using a mild, aqueous-based treatment at room temperature. This pre-leaching step facilitates the selective separation of valuable components, allowing for the recovery of both PGM-based catalysts and functional polymer material [
22,
23]. Such an approach supports sustainable recycling by maximizing material recovery while minimizing environmental impact. The method selected for detaching spent electrodes from polymer membranes depends on the nature of the catalyst and the strength of its adhesion to these components. Techniques such as physical agitation, stirring or shaking the MEA components in a liquid medium can lead to catalyst recovery. Aqueous-based solutions are particularly useful for removing catalyst powders, offering benefits for cleaning, recycling and further analysis. For example, solvent-induced swelling of the membrane can help dislodge catalyst particles adhered to the electrolyte layer, without damaging the membrane itself [
30]. Preserving the structural integrity of both the membrane and the catalyst is essential for their effective reuse and performance. Although this approach is relatively simple, it may be less effective when dealing with strongly adhered catalyst layers. In the present work, a mild, aqueous-based mechanical delamination technique was employed under ambient conditions to detach the spent electrocatalysts from the polymer electrolyte membrane of EoL PEMFC MEAs. This low-cost and eco-friendly with scalable potential method is versatile and applicable to both Nafion- and PBI-based MEAs. This broad compatibility enhances its potential for industrial-scale recycling of diverse fuel cell components. This approach also enables the recovery of the Nafion membrane, which may be suitable for regeneration and potential reuse in second-life applications. Literature studies have shown that recycled Nafion
® membranes retain key physicochemical properties and exhibit fuel cell performance comparable to pristine membranes, though further purification is needed to enhance durability, demonstrating the feasibility of membrane regeneration and reuse [
45,
46]. A detailed assessment of recovered Nafion
® membrane regeneration and performance was outside the scope of the present study, which primarily focused on the recycling and reuse of spent PGMs from electrocatalysts. However, membrane reusability remains an important area for future research and will be explored in subsequent work.
MON has developed a one-step, environmentally sustainable and versatile hydrometallurgical leaching process based on a HCl-H
2O
2-NaCl system for PGM recycling from spent PGM-based autocatalysts (TWC and DOC) [
19]. This method is specifically designed to enhance leaching efficiency and process flexibility under mild thermal conditions while simultaneously minimizing the formation of hazardous byproducts. The process has been adapted and optimized for application in PEM electrocatalyst recovery. The recycling and reuse approach presented in this study is characterized by its low cost, low energy consumption, environmental friendliness and overall sustainability. Furthermore, it features a short processing time with minimal procedural steps, reduced waste generation and high scalability, making it well suited for industrial implementation. Following MON’s leaching procedure, Pt demonstrated a leaching efficiency greater than 99 ± 0.5%, highlighting the excellent effectiveness of the proposed method for electrocatalyst recycling. The higher than 99 ± 0.5% Pt recovery indicates minimal material losses and highlights the potential for maximizing Pt recovery from EoL MEAs. In addition, the leaching method developed in this study produces a Pt-containing solution with higher metal concentration (up to 20 mg/mL) than those reported in the literature (approximately 0.7–0.75 mgPt/mL) [
27,
31], offering a distinct advantage by enabling more efficient downstream catalyst synthesis. The higher concentration of the pregnant leachate reduces the volume of solution required and improves overall process efficiency. Importantly, the recovered Pt can be reused in the synthesis of new high-performance Pt/C electrocatalysts, supporting a circular materials strategy for hydrogen energy devices such as PEMFCs and PEMWEs. Such an approach not only conserves critical raw materials but also contributes to the sustainability and economic viability of next-generation hydrogen technologies.
In the present work, Pt-containing pregnant leachate solutions, derived from EoL PEMFCs MEAs, were directly utilized as precursors for Pt/C catalyst synthesis. Rather than refining the leachates to high-purity metal precursors [
27,
29], this work explores their direct incorporation into catalyst synthesis. Conventional recovery techniques often involve resource-intensive purification, precipitation using ammonium hexachloroplatinate or solvent evaporation, which increases both cost and environmental burden [
29,
47]. In contrast, this study demonstrates a simplified and sustainable route for producing functional electrocatalysts from impure recycled Pt sources. The method avoids conventional resource-intensive steps by directly producing a high-concentration Pt solution suitable for catalyst synthesis, thereby offering a more efficient, cost-effective and eco-friendly alternative. By eliminating the need for intermediate purification steps, the process was significantly streamlined and highlighted the potential for converting industrial waste streams into valuable catalytic materials. Compared to previously reported methods [
15,
27,
28,
31], the proposed wet chemical synthetic approach offers several key advantages: it operates at low cost and low energy consumption, requires no thermal heating, follows a fast and straightforward procedure with minimal processing steps and does not involve organic solvents. Additionally, it generates low waste, is environmentally friendly and is readily scalable for industrial applications. The ability to repurpose these complex leachates into functional catalysts significantly supports the principles of resource efficiency and circular economy within the hydrogen energy sector (
Figure 7).
The successful synthesis of a uniform and high-performance Pt/C electrocatalyst using recycled Pt precursor (impure leachate solution) from EoL MEAs underscores the practical viability of this approach. Trace metals, ionic species and/or organic compounds in the leachate can arise from several components and degradation processes within the fuel cell, such as metallic corrosion of structural components (e.g., stainless steel or coated BPPs), impurities in humidification water or ambient air (e.g., sulfates) and leaching of organic additives and stabilizers from elastomers or polymers used for seals and gaskets (e.g., Viton
®) [
48]. The present study demonstrates that, despite the presence of possible impurities in the leachate, the Pt/C catalyst derived from recycled metal precursor (impure leachate solution) maintains comparable structural and morphological features to the one prepared from the high-purity commercial Pt precursor. This finding is a significant step toward sustainable electrocatalyst production, offering a low-cost and an environmentally friendly route for reintroducing critical raw materials into the clean energy value chain.
Despite a decrease in ECSA in the developed Pt/C, both catalysts synthesized from commercial and recycled Pt precursor showed comparable mass activity (169 ± 5 A/gPt and 170 ± 5 A/gPt, respectively) to the commercial benchmark (177 ± 5 A/gPt), indicating the influence of factors beyond surface area. The recycled Pt, derived from impure leachate, may possess unique properties, such as structural defects or dopants, that enhance catalytic performance. Further investigation into these effects is ongoing. Notably, Duclos et al. reported Pt recovery from Pt
3Co/C using solvent extraction and ion exchange (85% and 78% yields), but catalysts synthesized via a modified polyol method showed much lower ORR activity (10–25 A/gPt) [
49]. In contrast, the present approach uses impure Pt leachates directly, eliminating purification steps while achieving high performance (~170 A/gPt) using a fast, low-temperature and more energy-efficient NaBH
4-based synthesis. Compared to the method reported by Chourashiya et al. [
29], which required prolonged leaching (48 h at 80 °C) and additional solvent evaporation steps prior to catalyst synthesis (resulting in a mass activity of 154 A/gPt), the approach developed in this study is significantly less time-consuming and operationally simpler while achieving higher mass activity (~170 A/gPt), thereby enhancing both efficiency and performance. These findings demonstrate the technical feasibility and performance potential of utilizing recycled Pt directly from unpurified (impure) leachate solutions in the fabrication of high-performance Pt/C electrocatalysts, clearly distinguishing this work from existing recovery approaches. Importantly, this approach overcomes the need for multiple purification steps typically required in traditional catalyst manufacturing processes. As a result, it offers significant advantages in terms of cost-efficiency, process simplification and reduced environmental impact, thereby advancing the goals of sustainability and circularity in the production of critical materials for clean energy technologies.
This study gives the perspective for the utilization of impure Pt leachate recovered from EoL of LT- and HT-PEMFCs directly for fresh electrocatalysts preparation avoiding multi-step and time-consuming purification steps. This approach demonstrates novelty by expanding recycling strategies to less-studied and high-performance MEA types, such as PBI MEAs, contributing to more comprehensive and sustainable PGM recovery practices. Leaching and reusing impure Pt leachate from PBI-based MEAs present specific challenges compared to conventional approaches that focus on Nafion-derived leachates. These include potential interference from phosphoric acid residues, which can complicate downstream purification and stronger metal–polymer interactions that may hinder complete catalyst detachment. The recycling method described in this study is suitable to handle the challenges associated with Pt leachate from PBI-based MEAs. This innovative approach enables direct reuse of impure leachate, marking the first time in the literature that such streams have been successfully utilized to develop high-performing Pt/C electrocatalysts. The method demonstrates both novelty and practical value for advancing sustainable HT-PEMFC recycling. The same recycling and reuse approach could potentially be applied to EoL MEAs from PEMWEs. Given the similar structural and material compositions of PEMFCs and PEMWE MEAs, future research should investigate the adaptation of this approach to facilitate sustainable resource recovery and circularity in both technologies. In addition, MON’s recycling approach can be effectively applied at the stack level, enabling the sustainable recovery of critical materials from EoL units and supporting large-scale implementation in industrial hydrogen technologies. The complete optimized recycling workflow, from spent electrocatalyst recovery and leaching to Pt/C resynthesis, can be accomplished within a single day using the same equipment (e.g., chemical reactors and filtration units), enhancing the feasibility, efficiency and industrial relevance of catalyst recycling within a circular hydrogen economy framework.
To further validate the performance and durability of the synthesized catalysts, future work will focus on conducting Electrochemical Impedance Spectroscopy (EIS) and long-term cycling stability tests. Future work will also focus on the integration of these materials into full MEAs for in situ performance evaluation under realistic PEM system operating conditions. Such testing will provide critical insights into long-term durability, catalyst–membrane interface behavior and overall system efficiency. Further optimization of synthesis parameters and leachate treatment may also be explored to tailor electrocatalyst’s properties for specific applications. In parallel, the reuse of recovered delaminated Nafion membranes, remaining structurally intact after catalyst layer detachment, offers an additional opportunity to enhance material circularity and reduce system-level costs. The reusability of Proton Exchange Membranes represents a significant research direction and will be investigated in future studies. Ultimately, these efforts aim to bridge lab-scale advancements with industrial-scale implementation, reinforcing the viability of closed-loop manufacturing strategies, reinforcing the economic and environmental viability of sustainable hydrogen energy technologies.
5. Conclusions
The global transition to sustainable energy systems has highlighted the critical role of PEMFCs and PEMWEs in clean hydrogen production and efficient energy conversion. These technologies are central to decarbonization strategies, but their widespread adoption is constrained by the high cost and limited availability of PGMs, such as Pt, which are traditionally used as electrocatalyst. The scarcity of PGMs is further deteriorated by geopolitical factors and environmental concerns associated with their extraction processes. In parallel, with the increasing deployment of PEMFCs and PEMWEs, the volume of EoL systems containing PGMs is rising, underscoring the urgent need for eco-friendly and cost-effective recycling solutions. Such strategies are essential to support long-term resource security while minimizing waste generation and energy consumption. The recycling of PGMs from EoL MEAs offers a sustainable pathway to mitigate resource depletion and reduce the environmental footprint of PEM technologies.
This work supports the development of a closed-loop manufacturing model for PEM technologies in which critical raw materials like Pt are efficiently recovered and reintegrated into new catalytic materials. By reducing dependency on primary mining operations and enabling cost-effective electrocatalyst production, this approach contributes to the broader goals of technological affordability, environmental protection and sustainable growth in the hydrogen economy. More specifically, this study presents a sustainable and cost-efficient approach for fabricating advanced Pt/C electrocatalysts by utilizing impure Pt pregnant leachate solutions derived from EoL MEAs of PEMFCs. MON’s recycling strategy is suitable to manage the unique impurities present in Pt leachate from either Nafion- or PBI-based MEAs, such as phosphoric acid content and strong binding between metals and the polymer matrix. For the first time, this approach enables the direct reuse of such impure leachate solutions to synthesize high-performance Pt/C electrocatalysts, without additional purification steps. The leaching efficiency of Pt exceeded 99 ± 0.5%, demonstrating the high effectiveness of MONOLITHOS’s hydrometallurgical recycling method. Despite the decrease in the ECSA, the synthesized Pt/C catalysts derived from both commercial and recycled Pt precursors exhibited ORR mass activities of 169 ± 5 A/gPt and 170 ± 5 A/gPt, respectively, which are comparable to that of the commercial catalyst (177 ± 5 A/gPt), demonstrating the viability of using impure leachate solution (recycled metal precursor) in electrocatalyst production. The methodology demonstrated herein may also be applicable to EoL MEAs from PEMWEs. This represents a novel and impactful advancement in the field, contributing significantly to more sustainable and efficient fuel cell recycling practices.
The presented recovery process is designed to be scalable, environmentally sustainable and compatible with existing recycling infrastructures, emphasizing its suitability for industrial deployment. By combining MON’s innovative, single-step and eco-friendly chloride hydrometallurgical leaching method for recycling Pt with a scalable wet chemical synthesis method, a Pt/C electrocatalyst exhibiting almost identical electrochemical activity toward the ORR compared to commercial Pt/C electrocatalyst was successfully produced. Direct synthesis of advanced Pt/C electrocatalyst using the resulting impure pregnant leachate solution, eliminating the need for extensive purification typically required in conventional recycling processes, was performed. This method combines low energy requirements, minimal procedural steps, rapid execution and low environmental impact, which not only reduces energy consumption and costs but also enhances material circularity by repurposing complex waste streams directly into functional materials. Comparative assessment demonstrates that the use of impure (unpurified) recycled Pt precursor does not compromise essential catalytic performance parameters, validating its suitability for PEMFCs. This strategy effectively decreases reliance on primary raw materials, lowers catalyst production costs and promotes circular economy principles, thereby contributing to a more sustainable hydrogen energy landscape. The findings underscore the potential for industrial-scale adoption, providing a practical and environmentally friendly route for advancing affordable and high-performance PEMFC and PEMWE technologies.