Identification of Constituents and Evaluation of Biological Activity of Piptadenia stipulacea (Benth.) Ducke Ethanol Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of the Plant Material
2.2. Preparation of the P. stipulacea Ethanolic Extract (EEPs)
2.3. Characterization
2.3.1. Qualitative Phytochemistry
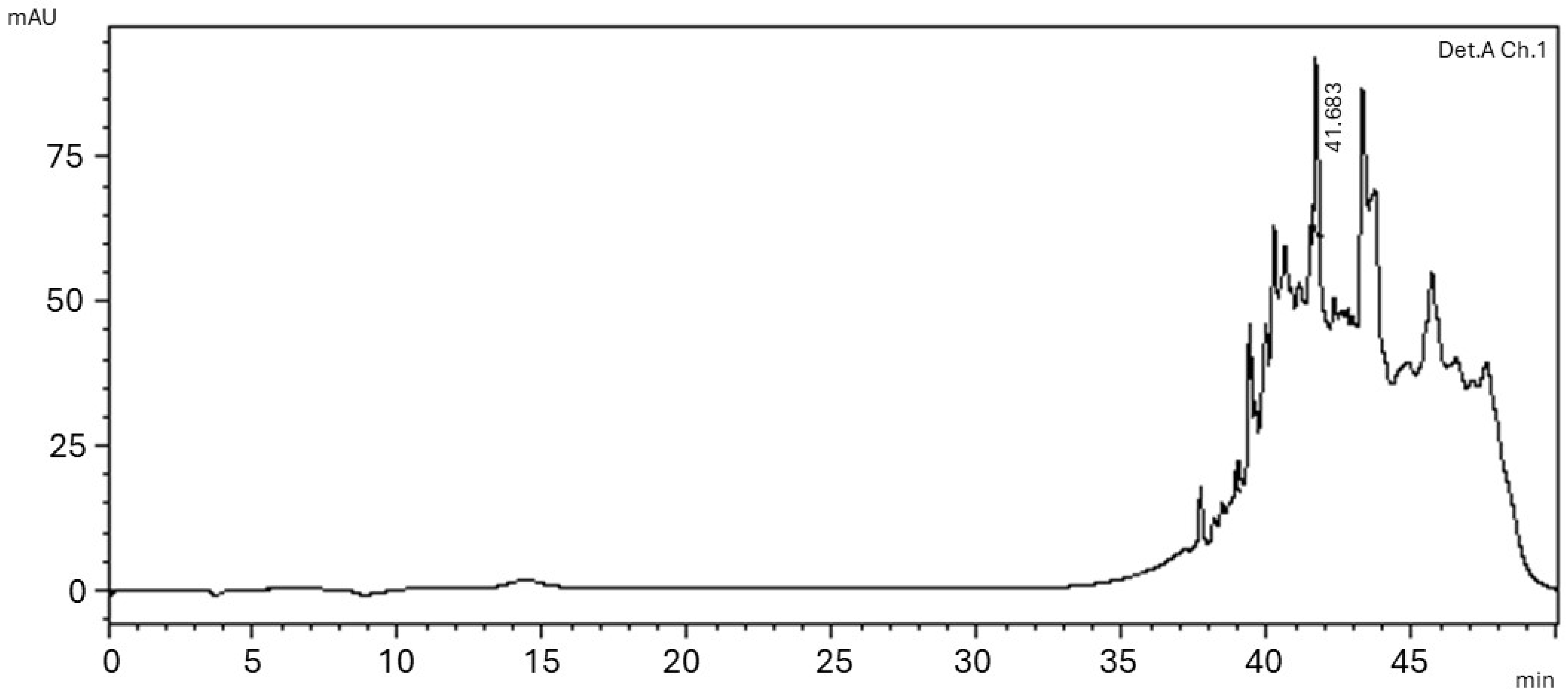
2.3.2. High Performance Liquid Chromatography (HPLC)
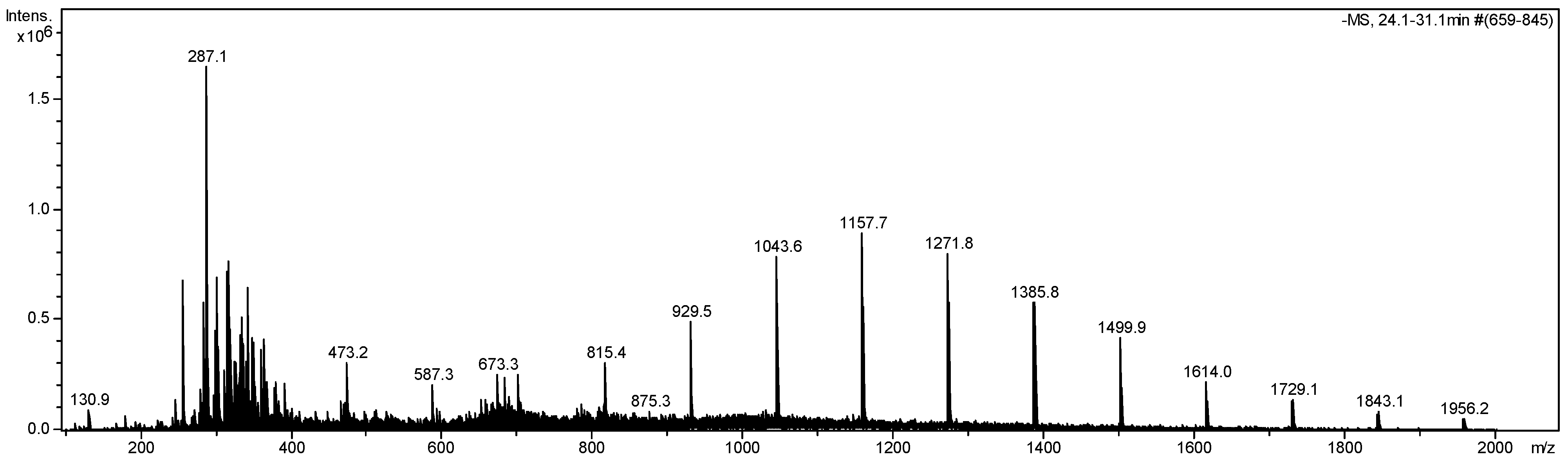
2.3.3. Mass Spectrometry
2.4. Biological Activity
2.4.1. Determination of Antibacterial Potential by Microdilution Broth
2.4.2. Evaluation of Cytotoxicity by MTT Method
2.4.3. Hemolysis Assay for Human Biocompatibility
2.4.4. In Vitro Antioxidant Activity
Antioxidant Potential—DPPH Radical Scavenging
Antioxidant Potential—ABTS Radical Scavenging
2.5. Statistical Analysis
2.6. Reagents
3. Results
3.1. Phytochemical Characterization
3.2. Biological Activities
3.2.1. Evaluation of Antibacterial Properties
3.2.2. Cytotoxic Activity of EEPS
3.2.3. EEPS Antioxidant Activity
4. Discussion
4.1. Phytochemical Characterization
4.2. Biological Activities
4.2.1. Evaluation of Antibacterial Properties
4.2.2. Cytotoxic Activity of EEPS
4.2.3. EEPS Antioxidant Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Franco, J.L.; Saldívar-González, F.I. Cheminformatics to Characterize Pharmacologically Active Natural Products. Biomolecules 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Awouafack, M.D.; Tane, P.; Spiteller, M.; Eloff, J.N. Eriosema (Fabaceae) Species Represent a Rich Source of Flavonoids with Interesting Pharmacological Activities. Nat. Prod. Commun. 2015, 10, 1325–1330. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Silva, S.A.d.N.M.; Barros, A.B.; Souza, J.M.T.; Moura, A.F.; de Araújo, A.R.; Mendes, M.G.A.; Daboit, T.C.; da Silva, D.A.; Araújo, A.J.; Filho, J.D.B.M. Phytochemical and biological prospection of Mimosa genus plants extracts from Brazilian northeast. Phytochem. Lett. 2020, 39, 173–181. [Google Scholar] [CrossRef]
- Bezerra, D.A.C.; Rodrigues, F.F.G.; da Costa, J.G.M.; Pereira, A.V.; de Sousa, E.O.; Rodrigues, O.G. Abordagem fitoquímica, composição bromatológica e atividade antibacteriana de Mimosa tenuiflora (Wild) Poiret E Piptadenia stipulacea (Benth) Ducke. Acta Sci. Biol. Sci. 2011, 33, 99–106. [Google Scholar] [CrossRef]
- Souza, C.O.; Ramos, A.L.D.; Júnior, A.F.D.; Fernandes, M.M.; Marques, J.J. Potential for the use of coconut shell (Cocus nucifera) as an alternative fuel in the production of cassava flour. Res. Soc. Dev. 2021, 10, e250101119485. [Google Scholar] [CrossRef]
- de Carvalho, A.C.; dos Santos, R.C.; Castro, R.V.O.; de Sousa Santos, C.P.; de Lima Costa, S.E.; de Carvalho, A.J.E.; Pareyn, F.G.C.; Vidaurre, G.B.; Dias Júnior, A.F.; de Almeida, M.N.F. Produção de energia da madeira de espécies da Caatinga aliada ao manejo florestal sustentável. Sci. For. 2020, 48, e3086. [Google Scholar] [CrossRef]
- Nóbrega, J.S.; de Lucena Alcântara Bruno, R.; Gleide da Silva, L.; Lima, L.K.S.; Nunes de Medeiros, A.; Pereira de Andrade, A.; Magalhães, A.L.R.; Rufino de Lima, C. Seed recovery of native plant species of the Caatinga biome ingested by goats and its effect on seed germination, in Brazilian semiarid region. Arid. Land Res. Manag. 2023, 38, 109–121. [Google Scholar] [CrossRef]
- Pereira, A.V.; Santana, G.M.; Góis, M.B.; Sant’Ana, D.G. Tannins obtained from medicinal plants extracts against pathogens: Antimicrobial potential. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Formatex Research Center: Badajoz, Spain, 2015; pp. 228–235. [Google Scholar]
- de Albuquerque, U.P.; Andrade, L.H.C. Uso dos recursos vegetais da caatinga: O caso do agreste do estado de Pernambuco (Nordeste do Brasil). Interciência 2002, 27, 336–346. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Matos, F.J.A. Introdução à Fitoquimica Experimental, 3rd ed.; Edições UFC: Ceará, Brazil, 2009. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Approved Standard M02–A10; Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. CLSI—Clinical Laboratory Standards Institute: Wayne, PA, USA, 2016.
- Quelemes, P.V.; de Araújo, A.R.; Plácido, A.; Delerue-Matos, C.; Maciel, J.S.; Bessa, L.J.; Ombredane, A.S.; Joanitti, G.A.; Soares, M.J.d.S.; Eaton, P.; et al. Quaternized cashew gum: An anti-staphylococcal and biocompatible cationic polymer for biotechnological applications. Carbohydr. Polym. 2017, 157, 567–575. [Google Scholar] [CrossRef]
- Pires, J.; Torres, P.B.; dos Santos, D.Y.; Chow, F. Ensaio em Microplaca do Potencial Antioxidante Através do Método de Sequestro do Radical Livre DPPH para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; Available online: https://www.researchgate.net/publication/324452636_Ensaio_do_potencial_antioxidante_de_extratos_de_algas_atraves_do_sequestro_do_ABTS_em_microplaca (accessed on 28 March 2025). [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpúrea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal Plants, Human Health and Biodiversity: A Broad Review. In Advances in Biochemical Engineering/Biotechnology; Spring: Berlin/Heidelberg, Germany, 2014; pp. 59–110. [Google Scholar] [CrossRef]
- Cavalcanti-Dantas, V.D.M.; de Medeiros, K.L.; de Azevêdo, T.K.B.; Santana, G.M.; Pereira, A.V.; Goís, M.B.; Pereira, M.d.S.V.; Pereira, J.V. Taninos: Principal componente do extrato Piptadenia stipulacea (Benth) Ducke inibe o crescimento de cepas clínicas de Staphylococcus aureus de origem bovina. Biotemas 2016, 29, 109–114. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorganic Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Syllaios, A.; Schizas, D.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; Ntounis, T.; et al. Breast cancer apoptosis and the therapeutic role of luteolin. Chirurgia 2021, 116, 170–177. [Google Scholar] [CrossRef]
- Park, E.-S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.-E.; Kim, B.; Shin, H.-S. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef]
- Lan, L.; Wang, Y.; Pan, Z.; Wang, B.; Yue, Z.; Jiang, Z.; Li, L.; Wang, C.; Tang, H. Rhamnetin induces apoptosis in human breast cancer cells via the miR-34a/Notch-1 signaling pathway. Oncol. Lett. 2019, 17, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Pereira, A.V.; de Azevêdo, T.K.B.; Santana, G.M.; Trevisan, L.F.A.; Higino, S.S.d.S.; Macêdo-Costa, M.R.; Pereira, M.D.S.V.; Azevedo, S.S. Análise da atividade antimicrobiana de taninos totais de plantas aromáticas do Nordeste brasileiro. Agropecuária Técnica 2015, 36, 109–114. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Alves, M.d.J.; Moura, A.K.S.; Costa, L.M.; de Araújo, É.J.F.; de Sousa, G.M.; Costa, N.D.d.J.; Ferreira, P.M.P.; Silva, J.d.N.; Pessoa, C.; de Lima, S.G.; et al. Phenols, flavonoids and antioxidant and cytotoxic activity of leaves, fruits, peel of fruits and seeds of Piptadenia moniliformis Benth (Leguminosae-Mimosoideae). Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2014, 13, 466–476. [Google Scholar]
- Rosa, L.H.; Gonçalves, V.N.; Caligiorne, R.B.; Alves, T.M.A.; Rabello, A.; Sales, P.A.; Romanha, A.J.; Sobral, M.E.G.; Rosa, C.A.; Zani, C.L. Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Braz. J. Microbiol. 2010, 41, 420–430. [Google Scholar] [CrossRef]
- Yadegarynia, S.; Pham, A.; Ng, A.; Nguyen, D.; Lialiutska, T.; Bortolazzo, A.; Sivryuk, V.; Bremer, M.; White, J.B. Profiling Flavonoid Cytotoxicity in Human Breast Cancer Cell Lines: Determination of Structure-Function Relationships. Nat. Prod. Commun. 2012, 7, 1295–1304. [Google Scholar] [CrossRef]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Ahmad, I.; Mathew, S.P.; Bhattacharjee, C.K.; Jaganathan, B.G.; Poddar, S.; Patel, H.; Subramaniyan, V.; Chinni, S.V.; et al. Rhamnetin, a nutraceutical flavonoid arrests cell cycle progression of human ovarian cancer (SKOV3) cells by inhibiting the histone deacetylase 2 protein. J. Biomol. Struct. Dyn. 2024, 42, 13421–13436. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Guedes, G.H.F.; Tenorio, B.M.; da Silva Gomes, J.A.; dos Santos, L.S.M.; da Costa, M.A.S.; de Oliveira, M.L.F.; Leite, M.C.B.; de Sobreira, R.C.B.; da Silva, T.M.S.; Tenório, F.d.C.A.M.; et al. Avaliação citotóxica das folhas de Piptadenia stipulacea. In As Ciências Biológicas e a Construção de Novos Paradigmas de Conhecimento; Atena Editora: Ponta Grossa, Brazil, 2019; pp. 1–388. [Google Scholar]
- Sobreira, R.C.B.; da Silva, W.A.; da Silva, T.M.S.; da Costa, M.A.S.; Bandeira, M.A.M.; dos Santos, T.E.M.; Lima, M.I.d.A.; da Silva, A.A.; Lima, S.M.d.A.; Leite, S.P. Toxicological evaluation and safety of the ethanol extract from leaves of Piptadenia stipulaceae. Res. Soc. Dev. 2022, 11, e46411326815. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Silva, J.D.N.; Monção, N.B.N.; de Farias, R.R.S.; Citó, A.M.d.G.L.; Chaves, M.H.; de Araújo, M.R.S.; Lima, D.J.B.; Pessoa, C.; de Lima, A.; Araújo, E.C.d.C.; et al. Toxicological, chemopreventive, and cytotoxic potentialities of rare vegetal species and supporting findings for the Brazilian Unified Health System (SUS). J. Toxicol. Environ. Health Part A 2020, 83, 525–545. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.-S.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar]
- Nisari, M.; Bozkurt, Ö.; Ertekin, T.; Ceylan, D.; İnanç, N.; Al, Ö.; Güler, H.; Unur, E. Rhamnetin improves antioxidant status in the liver of Ehrlich solid tumor bearing mice. Med. Sci. Discov. 2020, 7, 494–500. [Google Scholar]



| Secondary Metabolites | EEPS |
|---|---|
| Flobabenic Tannins | - |
| Pyrogalic Tannins | - |
| Phenols | - |
| Antocyanins and Antocyanidins | - |
| Flavones, Flavonols, Xanthones | + |
| Chalcones and Aurones | - |
| Saponins | - |
| Alkaloids | - |
| Polysaccharides | - |
| Anthraquinones | - |
| [M−H]− | MSn (m/z) | Identification | Reference |
|---|---|---|---|
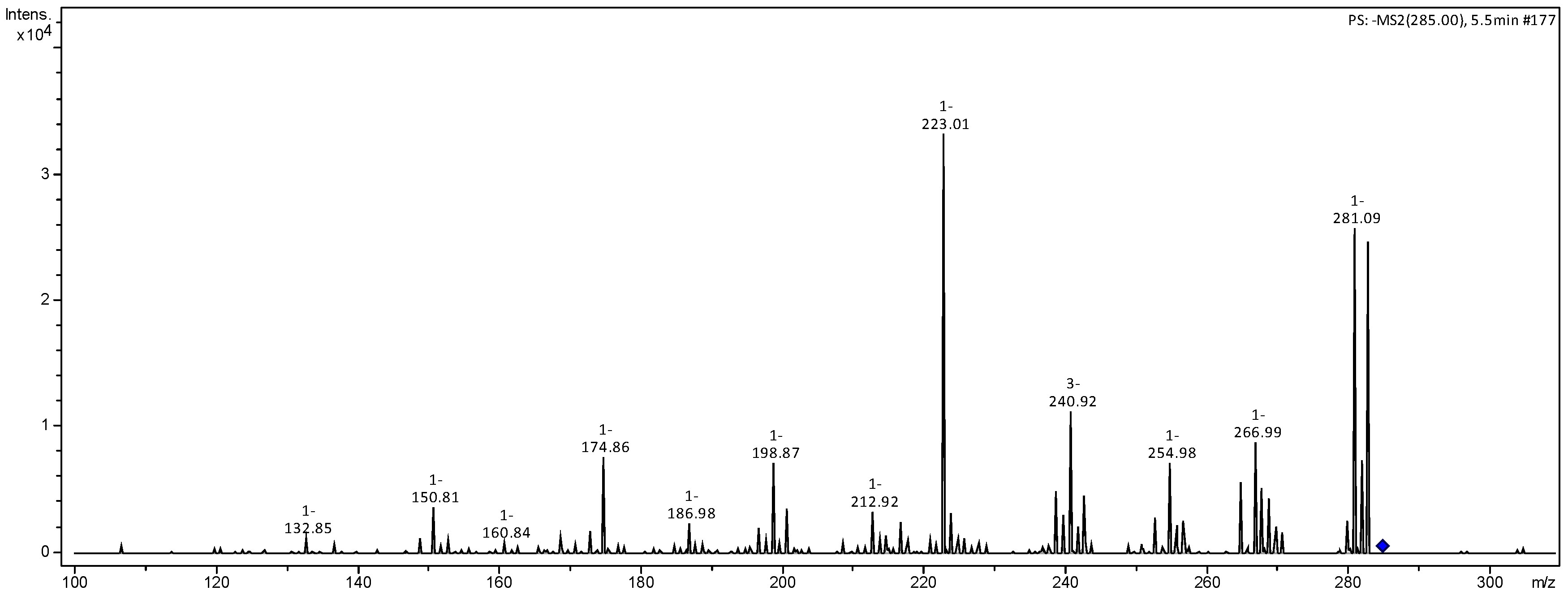
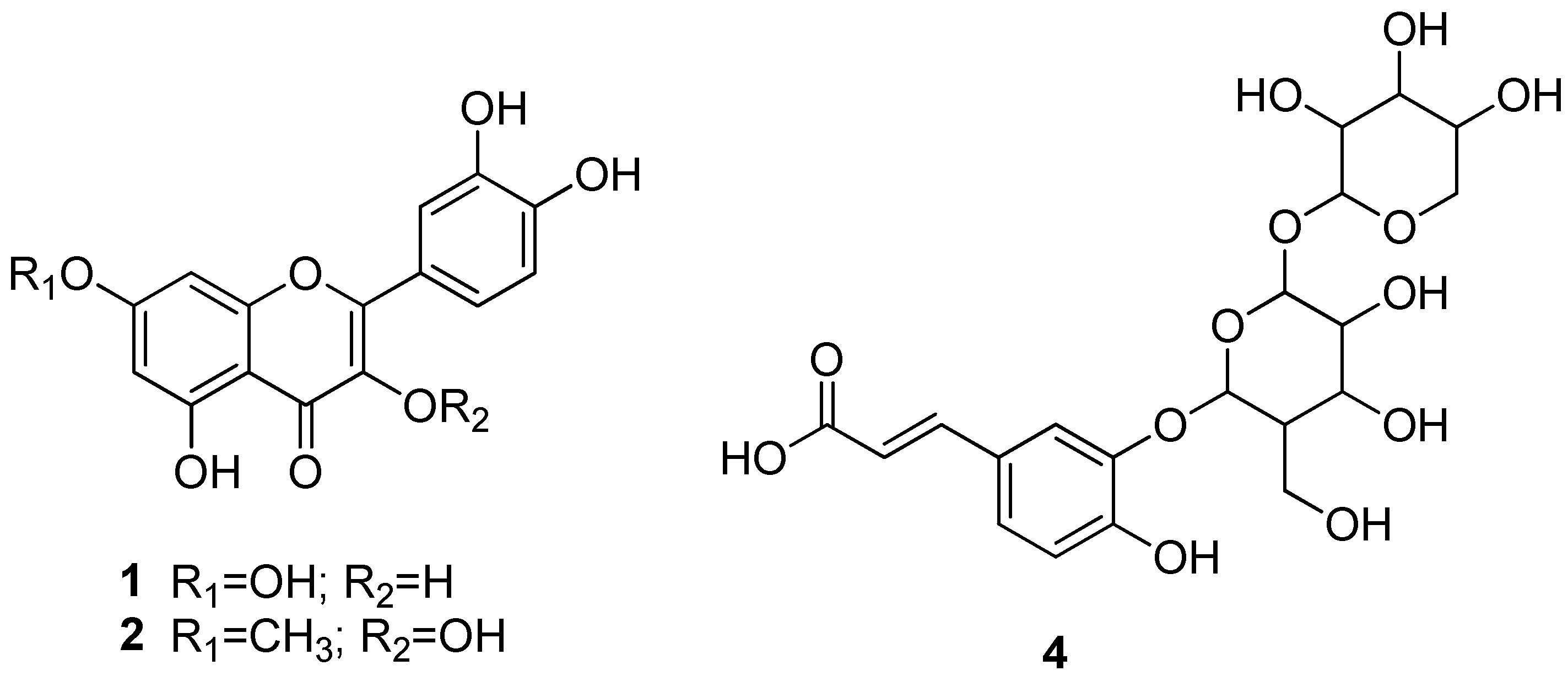
| 285 | 267 (30), 255 (24), 241 (33), 223 (100), 217 (19) 199 (13), 175 (19), 151 (16), 133 (8) | luteolin | [20] |
| 287 | 269 (24), 255 (16), 241 (35) 223 (100), 189 (9), 175 (37), 151 (11) | N.I. | |
| 301 | 284 (100), 271 (24), 257 (60) MS3 [257] 239 (77), 215 (34), 189 (83), 201 (35),175 (100) | N.I. | |
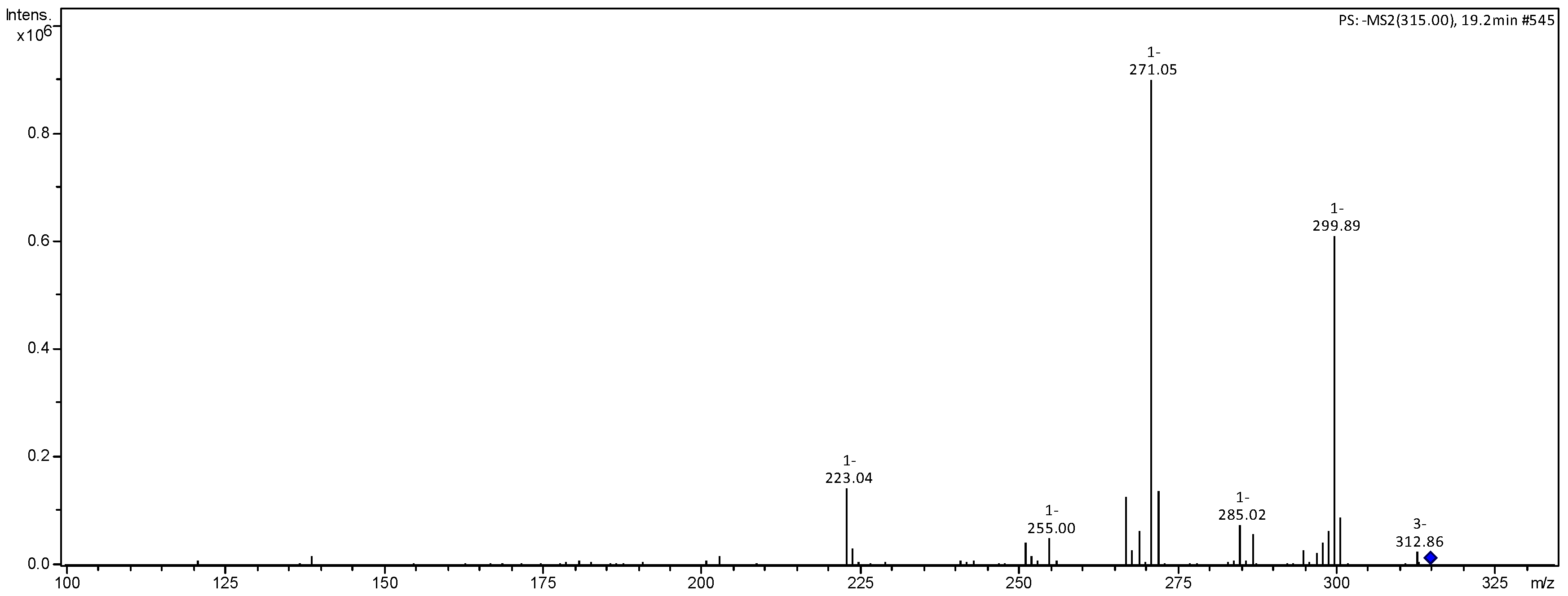
| 315 | 300 (59), 271 (100), 267 (19), 223 (15), 203 (3), 125 (4) | rhamnetin | [21] |
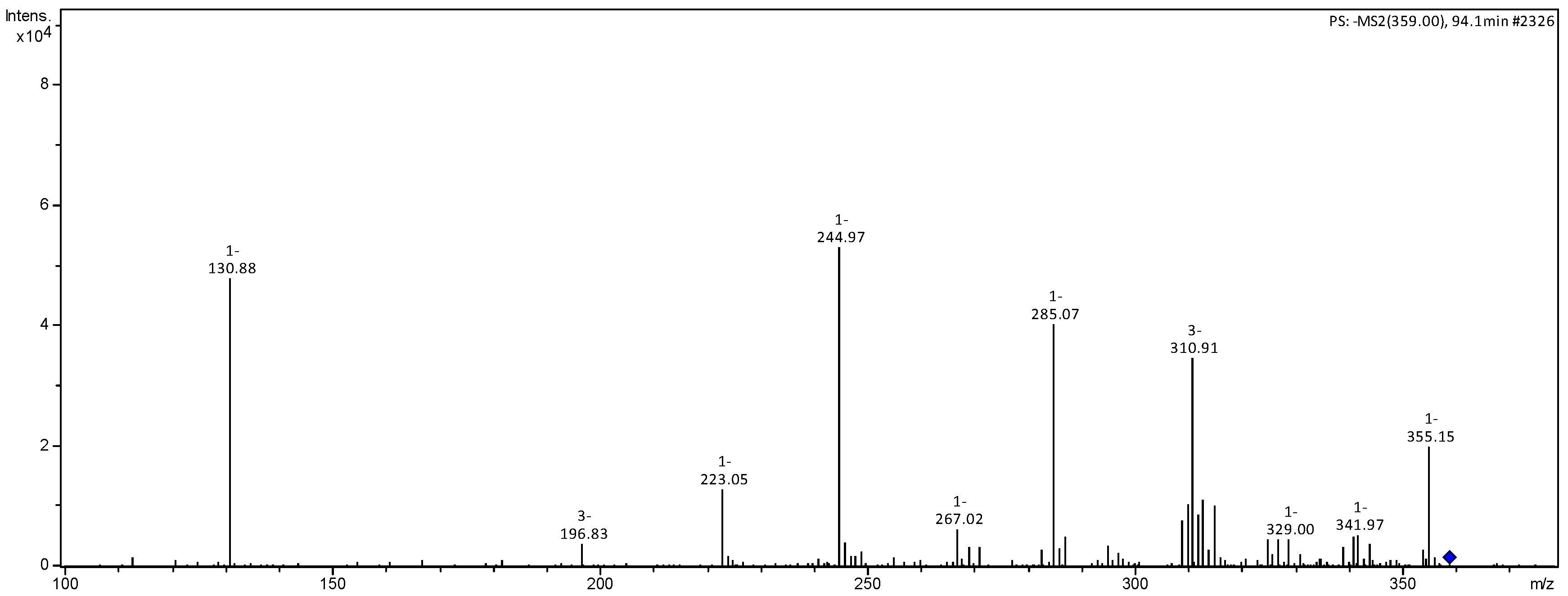
| 359 | 311 (48), 285 (79), 267 (15), 245 (100), 223 (18), 131 (91) MS3 [245] 131 (100), 113 (7) | luteolin derivative | |
| 447 | 415 (40), 401 (44), 347 (48), 293 (100), 285 (30), MS3 [415] 371 (21), 347 (100) | N.I. | |
| 473 | 397 (21), 359 (63), 341 (100), 245 (51) MS3 [341] 179 (100), 161 (23), 143 (20), 113 (24) MS4 [359] 245 (50), 131 (80) | caffeic acid hexoside-O-pentoside | [22] |
| 587 | 473 (42), 391 (23), 359 (42), 341 (100), 245 (50) MS3 [473] 359 (100), 245 (71) 131 (33) | N.I. | |
| 609 | 571 (29), 517 (16) 477 (100), 341 (51) 255 (82) MS3 [477] 293 (100) | N.I. | |
| 673 | 341 (22), 331 (25), 287 (100) MS3 [331] 301 (13), 287 (100) | N.I. | |
| 683 | 347 (18), 341 (100), 331 (28), 287 (51) MS3 [341] 179 (100), 161 (23), 143 (18), 113 (14) | caffeic acid hexoside dimer | [23] |
| 701 | 655 (16), 587 (52), 473 (45), 359 (100), 341 (53), 287 (59) MS3 [587] 473 (100), 359 (65), 245 (39) | N.I. | |
| 815 | 701 (47), 683 (26), 587 (39), 473 (100), 359 (46) MS3 [701] 587 (100), 473 (42), 359 (49), 245 (71) | N.I. | |
| 929 | 815 (63), 701 (53), 587 (100), 473 (64), 359 (59) MS3 [815] 701 (100), 587 (34), 473 (48), 359 (98), 245 (26) | N.I. | |
| 1043 | 929 (69), 815 (68), 701 (100), 587 (77), 473 (82), 359 (74) MS3 [929] 815 (63), 701 (27), 587 (100), 473 (99), 359 (40) | N.I. | |
| 1157 | 1043 (75), 929 (65), 815 (93), 701 (92), 587 (87), 473 (100), 359 (70) MS3 [1043] 929 (57), 815 (100), 701 (47), 587 (49), 473 (88), 359 (32) | N.I. | |
| 1271 | 1157 (55), 1043 (63), 929 (89), 815 (64), 587 (77), 473 (100), 359 (35) MS3 [1157] 929 (37), 815 (28), 473 (100) | N.I. | |
| 1385 | 1271 (73), 1157 (43), 1043 (100), 929 (65), 815 (77), 701 (84), 587 (75), 473 (63) MS3 [1043] 929 (38), 587 (100) | N.I. | |
| 1501 | 1385 (39),1271 (34), 1157 (100), 1043 (39), 929 (40), 815 (57), 701 (45), 587 (44), 473 (31) | N.I. | |
| 1615 | 1501 (33), 1385 (22),1271 (100), 1157 (30), 1043 (21), 929 (39), 815 (40), 701 (53), 587 (35), 473 (23) MS3 [1271] 815 (100), 701 (26) | N.I. |
| MIC (μg/mL) | ||
|---|---|---|
| Bacterial Strains | EEPS | Positive Control |
| S. aureus (ATCC 29213) | 500 | <0.25 a |
| E. coli (ATCC 25922) | >2000 | <0.5 b |
| IC50 (μg/mL) 95% Confidence Interval | ||
|---|---|---|
| Cell Lines | EEPS | Doxorubicin |
| HCT-116 | 37.96 (35.10–41.04) | 0.12 (0.09–0.16) |
| PC-3 | 37.6 (33.72–41.92) | 0.44 (0.34–0.54) |
| SF-295 | >50 | 0.23 (0.12–0.27) |
| HL-60 | 27.82 (24.16–32.04) | 0.02 (0.005–0.01) |
| IC50 (μg/mL) 95% Confidence Interval | ||
|---|---|---|
| Samples | DPPH | ABTS |
| EEPS | 956.7 (815.9–1122) | 147.2 (127.0–170.6) |
| TROLOX | 63.66 (57.80–69.93) | 73.91 (66.19–82.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.A.d.N.M.; Barros, A.B.; Souza, J.M.T.; Santiago, R.F.; Monção Filho, E.d.S.; Moura, A.F.; de Araújo, A.R.; da Silva, D.A.; Chaves, M.H.; Araújo, A.J.; et al. Identification of Constituents and Evaluation of Biological Activity of Piptadenia stipulacea (Benth.) Ducke Ethanol Extract. Compounds 2025, 5, 9. https://doi.org/10.3390/compounds5020009
Silva SAdNM, Barros AB, Souza JMT, Santiago RF, Monção Filho EdS, Moura AF, de Araújo AR, da Silva DA, Chaves MH, Araújo AJ, et al. Identification of Constituents and Evaluation of Biological Activity of Piptadenia stipulacea (Benth.) Ducke Ethanol Extract. Compounds. 2025; 5(2):9. https://doi.org/10.3390/compounds5020009
Chicago/Turabian StyleSilva, Stéphanie Aguiar de Negreiros Matos, Ayslan Batista Barros, Jessica Maria Teles Souza, Rodrigo Ferreira Santiago, Evaldo dos Santos Monção Filho, Andréa Felinto Moura, Alyne Rodrigues de Araújo, Durcilene Alves da Silva, Mariana Helena Chaves, Ana Jérsia Araújo, and et al. 2025. "Identification of Constituents and Evaluation of Biological Activity of Piptadenia stipulacea (Benth.) Ducke Ethanol Extract" Compounds 5, no. 2: 9. https://doi.org/10.3390/compounds5020009
APA StyleSilva, S. A. d. N. M., Barros, A. B., Souza, J. M. T., Santiago, R. F., Monção Filho, E. d. S., Moura, A. F., de Araújo, A. R., da Silva, D. A., Chaves, M. H., Araújo, A. J., & Marinho Filho, J. D. B. (2025). Identification of Constituents and Evaluation of Biological Activity of Piptadenia stipulacea (Benth.) Ducke Ethanol Extract. Compounds, 5(2), 9. https://doi.org/10.3390/compounds5020009








