Abstract
Secondary metabolites such as flavonoids bring a range of biological properties to natural products, making them potential candidates for the pharmaceutical industry. Piptadenia stipulacea (Benth.) Ducke is well known in Brazil as Jurema Branca, and yet few studies have investigated its biological and phytochemical properties. This study aimed to characterize and evaluate the biological properties of ethanolic extract obtained from the bark of Jurema Branca. Characterization was performed by qualitative phytochemistry, HPLC, and mass spectroscopy. The antibacterial properties were investigated by microdilution method, cytotoxicity by MTT method, biocompatibility testing with human erythrocytes was performed, and antioxidant properties were investigated using DPPH and ABTS radical scavenging. The phytochemical tests demonstrated that rhamnetin and luteolin were the main constituents of the extract. This is the first report of these compounds in this species. The extract presented activity against Staphylococcus aureus (MIC = 500 µg/mL) and demonstrated activity against human colorectal adenocarcinoma (HCT-116), prostate adenocarcinoma (PC-3), and acute myeloid leukemia (HL-60) cell lines with IC50 of 37.96, 37.6, and 27.82 µg/mL, respectively, for this Piptadenia genus. Additionally, the extract presented excellent biocompatibility and antioxidant activity (IC50 = 956.7 and 147.2 µg/mL in DPPH and ABTS methods, respectively). These results are novel for the Piptadenia genus and pave the way for further evaluations regarding the biological importance of this species.
1. Introduction
Throughout human history, natural plant products have remained attractive alternatives for the prevention and treatment of disease, and they play an important role in the development of modern medicine. This is mainly because of the many chemical structures present in their compositions [1]. Plants belonging to the Fabaceae and Mimosoidae subfamilies are richly constituted with secondary metabolites such as tannins, flavonoids, alkaloids, and saponins [2]. The antimicrobial, antioxidant, antitumor, antiparasitic, and anti-inflammatory properties of these constituents are well-documented in the literature [3,4,5].
Piptadenia stipulacea (Benth.) Ducke, popularly known as Jurema Branca, belongs to the Fabaceae family and Mimosoideae subfamily. The plants or shrubs range from 2 to 4 m high, with light brown bark, alternating leaves, and white flowers [6]. P. stipulacea is endemic to the Caatinga and is widely found in northern and northeastern Brazil. The species has been used historically as a hallucinogen in indigenous rituals in mixtures such as Jurema wine and Ayuhasca. It is also used in agriculture to produce biomass [7], charcoal [8], for feeding goats, and as pasture [9].
The Brazilian northeast presents great biodiversity, harboring a wide variety of plant species with medicinal properties that are traditionally used by the local population. By analyzing chemical composition and pharmacological activity, it is possible to identify active substances and understand their therapeutic effects. This not only provides a scientific basis for the use of these plants in traditional medicine but also paves the way for the discovery of new bioactive compounds with pharmacological potential.
The literature reports that all parts of P. stipulacea present biological properties that can be attributed to the presence of secondary metabolites, principally tannins and flavonoids [10]. In popular medicine, the species is applied as a cicatrizing agent [6], as a sedative, and as an anti-inflammatory [11]. Due to its wide range of biological properties, and its antioxidant [12], antimicrobial [13], and antitumor potential [14], secondary metabolites obtained from P. stipulacea may be an alternative source for many biologically active agents.
Considering its regional prevalence and the lack of studies regarding the pharmacology of this plant, this study aimed to characterize and identify the major constituents of the ethanolic extract of P. stipulacea and investigate its antibacterial, cytotoxic, hemolytic, and antioxidant properties.
2. Materials and Methods
2.1. Collection and Identification of the Plant Material
The species P. stipulacea is represented in Figure 1. The plant was collected in April 2017 in the municipality of Poranga, in the state of Ceará, Brazil (coordinates: 4°45′25″ S//40°57′26″ W). The plant material was identified, and the exsiccate was archived at the Parnaíba Delta Herbarium (HDELTA) of the Parnaíba Delta Federal University—UFDPAR, under voucher specimen 4814. The genetic access was registered on the Sisgen platform, with registration number A11C0A7.

Figure 1.
Images of Piptadenia stipulacea (Benth.) Ducke (A), barks (B) and leaves (C).
2.2. Preparation of the P. stipulacea Ethanolic Extract (EEPs)
The P. stipulacea bark was washed, dried in an oven at 50 °C for 4 days, and ground in a manual mill (IKA A11, BIOVERA®, Ramos, Brazil). The ethanolic extract was obtained by cold extraction [15]. The grounded material was mixed in ethanol P.A. (1:10, w/v) and left under stirring for 3 days (5 min/day). Subsequently, the content was filtered using a qualitative QUALY® filter paper (80 g/m2) and dried in a water bath at 50 °C. The resulting solution was then placed in a vacuum centrifuge (Eppendorf Vacufuge Plus 5305 Concentrator Lab Centrifuge) to remove the excess solvent. The EEPS presented a 30% yield and was used for phytochemical characterization tests and evaluation of biological properties. The material was protected from light during all processes.
2.3. Characterization
2.3.1. Qualitative Phytochemistry
The presence of secondary metabolites in the EEPs was evaluated qualitatively [15]. Tests evaluated the presence of tannins and phenols by adding 1% FeCl3. The formation of a dark precipitate with a blue shade indicated the presence of hydrolysable (pyrogallic) tannins, while green tones suggested the presence of phlobaphenic (condensed) tannins. Additionally, anthocyanins, anthocyanidins, flavonoids, chalcones, and aurones were identified based on pH changes. Acidification was performed by adding 5% HCl to reach pH 3, while alkalization was achieved by adding 10% NaOH to reach pH 8.5 and pH 11, respectively. The presence or absence of flavonoids (by class) was expressed according to colorimetric change reactions [15]. Saponins constitution was observed by vigorous shaking of the extract solubilized in distillated water for 5 min in a hermetically sealed tube. The presence of saponins could then be observed by formation of a stable layer for more than half an hour. Alkaloids were detected by addition of a 5% HCl solution to the extract and adding Bouchardat and Dragendorff reagents, with the presence of alkaloids confirmed through respective formations of a reddish-orange precipitate and brick red precipitates. Polysaccharides and anthraquinones were detected using lugol and solutions of toluene and 10% NH4OH, respectively. The appearance of a blue color indicates the presence of polysaccharides, and a pink, red, or violet color indicates the presence of anthocyanidins.
2.3.2. High Performance Liquid Chromatography (HPLC)
The chromatographic profile of the EEPs was obtained with a Shimadzu liquid chromatograph (Shimadzu®, Tokyo, Japan), equipped with a UV-vis detector (SPD-20A), a binary pump system (LC-6AD), and an automatic sampler (SIL-10AF). Sample separation was performed with a Phenomenex-Luna C18 RP column (250 × 4.6 mm, 5 μm) at room temperature (25 ± 2 °C). The mobile phase consisted of acetonitrile + 0.05% trifluoroacetic acid (pump A) and ultrapure water + 0.1% formic acid (pump B). Samples were analyzed using a 0–100% gradient condition, with a 1 mL/min flow and 50 min runtime. UV–vis detection was performed at 218 nm and 269 nm. For the analysis, samples were dissolved in ethanol (1 mg/mL), filtered with 0.45 µm PTFE syringe filters, and injected (20 µL). All solvents used were of HPLC grade.
2.3.3. Mass Spectrometry
The mass spectra were obtained by the direct infusion of the sample (1 mg of EEPS to 10 mL of MeOH) into a mass spectrometer with an ion trap analyzer (Amazon X, Bruker Daltonics, Bremen, Germany) equipped with an electrospray ionization source (DI-ESI-IT-MSn) in the negative ion mode, an m/z range of 100–2000 Da, syringe flow of 3.0 μL min−1, capillary voltage of 4.5 kV, an 8.0 L min−1 drying gas flow (N2), nebulization pressure of 10.0 psi, and a source temperature of 220 °C.
2.4. Biological Activity
2.4.1. Determination of Antibacterial Potential by Microdilution Broth
Bacterial strains were maintained on Mueller–Hinton agar and incubated at 37 °C for 24 h. Antibacterial activity was determined against Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 25922) [16] using similar [5] concentrations and conditions. Oxacillin and Meropenem were used respectively as positive controls for the Gram-positive and Gram-negative strains. The plates were incubated under aerobic conditions, at 37 °C, for 24 h and the minimum inhibitory concentration capable of inhibiting visible bacterial growth (MIC) was determined.
2.4.2. Evaluation of Cytotoxicity by MTT Method
The cytotoxic potential of the EEPs was tested against four human tumor cell lines, obtained from the National Cancer Institute. HL-60 (Acute Myeloid Leukemia), HCT-116 (Colorectal Adenocarcinoma), PC-3 (Prostate Adenocarcinoma), and SF-295 (Glioblastoma) were cultivated in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO, USA) and supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin/streptomycin) at 37 °C and 5% CO2.
The MTT method [17] was performed under similar [5] concentrations and conditions. Briefly, a serial dilution (ratio 2 to 50 µg∕mL) EEPs was added to the human cell lines for 69 h. An MTT solution (0.5 mg∕mL) was then added for 3 h, totaling 72 h of treatment. Absorbance was read at 595 nm after solubilization of formazan crystals using DMSO, in a Beckman Coulter Inc., spectrophotometer—model DTX-880, and the OSoftware program 1.0 (Beckman Coulter Inc., Brea, CA, USA). Results were expressed in IC50 (half maximal inhibitory concentration). Doxorubicin was used as the positive control.
2.4.3. Hemolysis Assay for Human Biocompatibility
To determine the hemolytic potential of the EEPs, a hemolysis assay was performed according to [18] under the same concentrations and conditions as [5]. Triton X (0.1%) and saline (0.85%) were used as positive and negative controls, respectively. The EC50 (half-maximum effect) was calculated using GraphPad Prism 8. The investigations were performed following the rules of the Declaration of Helsinki of 1975, which was revised in 2013, and the procedure was approved by the research ethics committee of the Parnaíba Delta Federal University, under protocol number 5.114.527.
2.4.4. In Vitro Antioxidant Activity
Antioxidant Potential—DPPH Radical Scavenging
DPPH (1,1-diphenyl-β-picrylhydrazine, Sigma Aldrich) method was performed [19] under the same concentrations and conditions as [5]. Absorbance was measured at 517 nm using an ELISA reader (SPECTRAMAX 290). Trolox (6-hydroxy 2,5,7,8tetramethylchroman-2-carboxylic acid) was used as negative control and the IC50 values were calculated using GraphPad Prism 8, with 95% confidence.
Antioxidant Potential—ABTS Radical Scavenging
The ABTS radical (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) was prepared according to [19] and under the same concentrations and conditions as [5]. Absorbance was measured at 734 nm (SPECTRAMAX 290 ELISA plate). Trolox was used as the reference antioxidant. The IC50s values were calculated by GraphPad Prism 8, with 95% confidence.
2.5. Statistical Analysis
The experiments were analyzed according to the mean ± standard deviation of the mean (SD) of percentage growth inhibition using the GraphPad Prism 8 program from two independent experiments, in triplicate.
2.6. Reagents
The following reagents were utilized: Ethyl alcohol P.A (C2H6O, Dinamica, Indaiatuba, São Paulo, Brazil), Iron(III) chloride solution (FeCl3, Dinamica), Hydrochloric acid (HCl, Dinamica), Sodium Hydroxide (NaOH, Dinamica), Bouchardat reagent, Dragendorf reagent, Lugol (Dinamica), Toluene (Dinamica), Ammonium Hydroxide (NH4OH, Êxodo Científica, Sumaré, São Paulo, Brazil), Acetonitrile HPLC grade (CH3CN, Êxodo Científica), Trifluoracetic Acid HPLC grade (CF3CO2H, Dinamica), Formic Acid (CH2O2, Sigma Aldrich), Methanol HPLC grade (MeOH, Sigma-Aldrich), MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide, Invitrogen, Waltham, MA, USA), DMSO (Dimethyl sufoxide, Êxodo Científica), Triton-X-100 (C14H22O(C2H4O)n(n = 9–10), Êxodo Científica), DPPH (1,1-diphenyl-β-picrylhydrazine, Sigma Aldrich), Trolox (6-hydroxy 2,5,7,8tetramethylchroman-2-carboxylic acid, Sigma Aldrich), ABTS radical (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, Sigma Aldrich)).
3. Results
3.1. Phytochemical Characterization
A qualitative phytochemical assay to determine the presence of secondary metabolites was performed and the results are represented in Table 1. It is possible to observe that flavonic compounds, such as flavones, flavonoids, and xanthones, are constituents present in the P. stipulacea ethanolic extract (EEPs). We did not observe the presence of tannins, phenols, antocyanins and antocyanidins, chalcones, aurones, saponins, alkaloids, polysaccharides, or anthraquinones, possibly because of the extraction method or some environmental factor.

Table 1.
Phytochemical characterization of Piptadenia stipulacea (Benth.) Ducke ethanol extract. (-) Not detected; (+) Detected.
The chromatographic profile of the EEPS was obtained by high-performance liquid chromatography (HPLC) and is represented in Figure 2. It is possible to observe the presence of different peaks with retention times from 38 to 48 min and different intensities, with a majority peak at 42 min. The difference in the intensity of the peaks probably indicates a difference in the concentration of the compounds.
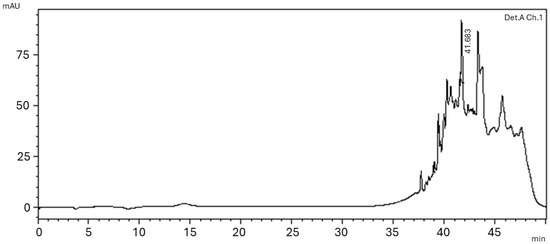
Figure 2.
Chromatographic profile of the Piptadenia stipulacea (Benth.) Ducke ethanolic extract. Mobile Phase: ACN-CF3CO2H (0.05%)/H2O-HCO2H (0.1%), gradient: 0–50 min, 0–100% ACN, injection volume 20 µL, flow rate 1 mL min−1 and λ = 218 nm.
We also performed a full Mass Spectroscopy scan with Ion Trap Analyzer and Electrospray Ionization Source (DI-ESI-IT-MS) to identify the compounds in the EEPs. The results are represented in Figure 3 and Table 2. The mass spectrum presented 20 peaks and intensities corresponding to 20 metabolites.
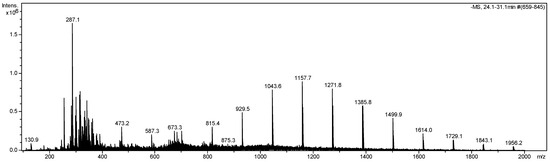
Figure 3.
Full scan DI-ESI-MS/MS [M−H]− from the Piptadenia stipulacea (Benth.) ethanolic extract, m/z 100–2000. Fingerprint obtained by DI-ESI-IT-MS/MS in negative mode.

Table 2.
Annotated chemical constituents of the ethanolic extract of the stem bark of P. stipulacea by DI-ESI-IT-MSn; N.I.: not identified.
Considering the fragmentation patterns, deprotonated molecule ions, and fragments, it was possible to observe five compounds, three being flavonoids (compounds 1–3) and two being caffeic acid derivatives (4–5). Based on the literature consulted, these compounds correspond to luteolin (1) [20]; rhamnetin (2) [20,21]; a luteolin derivative (3) [20]; caffeic acid hexoside-O-pentoside (4) [22]; and caffeic acid hexoside dimer (5) [23]. The spectra of the five noted compounds are shown in Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8.
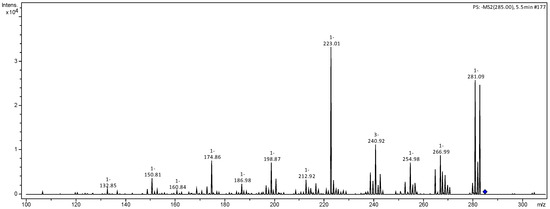
Figure 4.
MS2 mass spectrum of ion m/z 285 referring to luteolin (1).
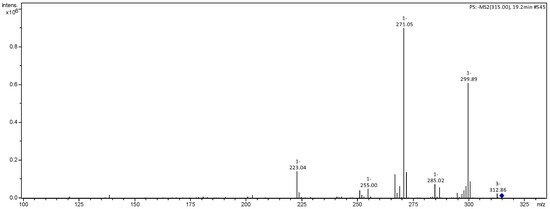
Figure 5.
MS2 mass spectrum of ion m/z 315 referring to rhamnetin (2).
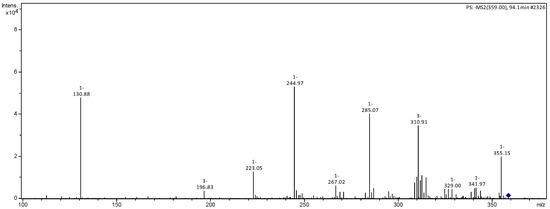
Figure 6.
MS2 mass spectrum of ion m/z 359 referring to luteolin derivative (3).
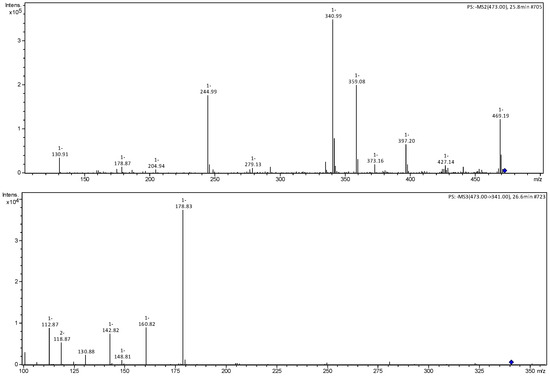
Figure 7.
MS2 and MS3 mass spectra of ions m/z 473 and 341 referring to hexoside-O-pentoside caffeic acid (4).
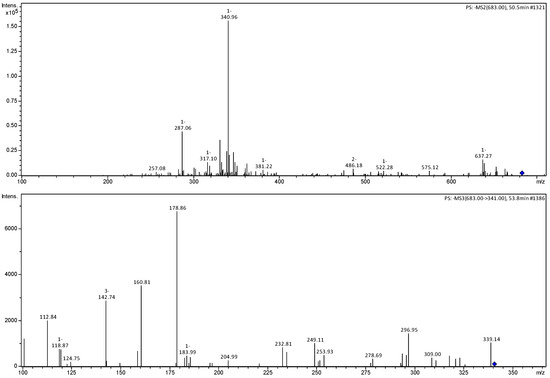
Figure 8.
MS2 and MS3 mass spectra of ions m/z 683 and 341 referring to hexoside caffeic acid dimer (5).
Compounds 1 and 2 present a fragmentation pattern characteristic of flavonoids belonging to the flavone class. Compound 1 presented as a deprotonated molecule with m/z 285 [M−H]− and fragmentation ions at m/z 151 and m/z 125, produced by RDA cleavage of the C ring. The neutral loss of H2O generating the ion m/z 267 was identified as luteolin (Figure 2). Compound 2 presented the ion m/z 359 [M−H]− and fragment ions m/z 285, 267, 241 and 223, similar to those of the previous compound, suggesting that this compound was derived from luteolin [20].
Compound 3 was identified as rhamnetin. Compound 3 produced m/z 315 [M−H]− with fragmentation ions m/z 300 [M−H−CH3]−, 271 [M−H−CO2]− and 125 from RDA cleavage of the C ring [20,21].
Compound 4 presented an ion of the deprotonated molecule at m/z 473 [M−H]−, with characteristic fragmentation ions at m/z 455 [M−H−H2O]−, 427 [M−H−CO2]− and 341 [M−H−pentose]−. The MS3 spectrum of the m/z 341 ion presented fragmentation ions m/z 179 [M−H−Hexose]−, corresponding to aglycone, and 161 [M−H−Hexose-H2O]−, corresponding to the loss of a water molecule, and being identified as caffeic acid hexoside-O-pentoside [22]. Compound 5 was identified as a dimer of caffeic acid hexoside by the m/z 683 [M−H]− ion and fragmentation ions m/z 341, corresponding to a unit of caffeic acid hexoside and 179 corresponding to the aglycone [22,23].
It was also possible to characterize the structure of compounds 1 (luteolin), 2 (rhamnetin), and 4 (hexoside-O-pentoside caffeic acid) (Figure 9), identified in the ethanolic extract, based on comparisons of DI-ESI-IT-MSn data with the corresponding fragmentation patterns and the pathways reported by [20,21,23].
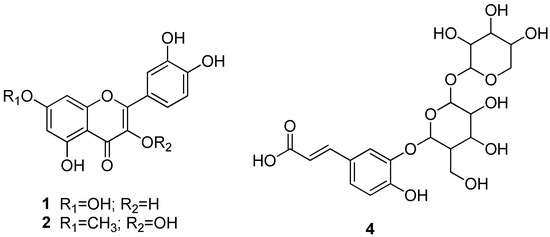
Figure 9.
Structure of compounds 1 (Luteolin), 2 (Rhamnetin), and 4 (hexoside-O-pentoside caffeic acid), identified in the ethanol extract of P. stipulacea stem bark.
We then conducted a comprehensive investigation into the antibacterial, cytotoxic, hemolytic, and antioxidant effects of EEPS. The objective was to gain insights into the broad and traditional utilization of P. stipulacea and facilitate in-depth studies on its properties, considering the dearth of reports in the existing literature.
3.2. Biological Activities
3.2.1. Evaluation of Antibacterial Properties
Plants belonging to the Fabaceae family are known for antimicrobial effects, usually attributed to its phenolic compounds, which were also detected in our study. We evaluated the antibacterial potential of the EEPS tested against two bacterial strains of clinical relevance (S. aureus—ATCC29213 and E. coli—ATCC25922). The minimum inhibitory concentration (MIC) is shown in Table 3.

Table 3.
Minimum Inhibitory Concentration (MIC) of P. stipulacea (Benth.) Ducke ethanol extract against Gram-positive and Gram-negative bacterial strains. Positive control: Oxacillin a and Meropenem b.
These results demonstrated that EEPS presents antibacterial effects against Gram-positive strain S. aureus at 500 µg/mL. The tested concentrations had no effect against the Gram-negative strain E. coli.
3.2.2. Cytotoxic Activity of EEPS
To determine the cytotoxicity of EEPS, the MTT test was performed against four human cancer cell lines for 72 h: HCT-116 (Colorectal Adenocarcinoma), PC-3 (Prostate Adenocarcinoma), HL-60 (Acute Myeloid Leukemia), and SF-295 (Glioblastoma). The results are presented in Table 4.

Table 4.
Cytotoxic activity of P. stipulacea (Benth.) Ducke ethanol extract on human cancer cell lines and human erythrocytes. Doxorubicin was used as a positive control.
The MTT assay showed that the EEPS in concentrations ranging 0.39–50 µg∕mL caused cytotoxicity in three out of the four human cancer cell lines evaluated. All the cell lines presented similar IC50 values, but HL-60 was slightly more sensitive to the extract, with an IC50 of 27.82 μg/mL. The extract did not present activity against SF-295 at the tested concentrations.
The hemolytic potential of the EEPS against human erythrocytes was analyzed to determine the concentration that induces half the maximum effect (EC50). Hemolysis was not observed in any of the tested concentrations of the EEPS, presenting an EC50 greater than 1000 µg/mL.
3.2.3. EEPS Antioxidant Activity
The antioxidant potential of EEPS was evaluated using ABTS and DPPH radicals scavenging assays. The results are represented in Table 5.

Table 5.
Antioxidant potential of P. stipulacea (Benth.) Ducke ethanol extract in both DPPH and ABTS methods. Trolox was used as a positive control.
The results demonstrated that although EEPS did not show significant antioxidant activity in the DPPH assay (IC50 of 956.7 μg/mL), in the ABTS assay, the EEPS obtained an IC50 of 147.2 μg/mL, with a 95% confidence interval of 127–170.6 µg∕mL, an antioxidant effect close to the positive control Trolox, which presented IC50 of 73.91 µg∕mL.
4. Discussion
4.1. Phytochemical Characterization
Secondary metabolites play an important role in plant defense systems and have been used in popular medicine for therapeutic purposes due to their extensive biological activities. For this reason, secondary metabolites are one of the main sources of new drugs [24]. Phytochemical studies assess the constitution of these metabolites and certify their benefits as potential herbal medicines.
The presence of tannins, phenols, anthocyanidins, and saponins has been reported as being the main compounds obtained from P. stipulacea bark ethanolic extracts [6]. The presence of tannins in this type of extract [10,25] has also been reported. However, collection sites, seasonality, temperatures, soil fertility, and other factors directly influence the biosynthesis and thus the relative composition of secondary metabolites in plants [26].
Phytochemical components such as flavonoids, due to their numerous biological potentials [27], have value in medicine. Flavonoids from different plant parts (fruits, vegetables, stems, grains, and bark) are phenolic compounds recognized for having anticancer properties [28]; they are also antimicrobial, antiproliferative, and antioxidant agents [29], in addition to exerting neuroprotective effects [30]. Depending on their chemical structure, flavonoids are classified as flavones, flavonols, isoflavonoids, and chalcones [31].
Qualitative phytochemistry and HPLC indicated the presence of these flavonoid classes in the EEPS. Mass Spectroscopy determined that the extract was composed of the flavonoid luteolin (1), and derivatives (3) of rhamnetin (2), as well as hexoside caffeic acid-O-pentoside (4) and hexoside caffeic acid dimer (5). To our knowledge, this is the first report identifying these metabolites in Piptadenia stipulacea (Benth.) Ducke.
Luteolin is a natural flavone with neuroprotective [32] and antiviral properties [33]. It is important to anticancer therapies against various cell lines. In breast cancer, for example, luteolin inhibits proliferation, and protects against carcinogenic stimuli, by activating cell cycle arrest [34].
Rhamnetin is a distinguished category of flavonoids with recognized cardioprotective [35] and anti-inflammatory benefits. In cancer therapy, rhamnetin presents inhibitory effects on cell proliferation and epithelial-mesenchymal transition in lung tumor cell lines. It also induces apoptosis in breast tumor cell lines [36].
The HPLC chromatogram, shown in Figure 2, demonstrates the presence of peaks with differing intensities, and could indicate the presence of other compounds in varied concentrations. With the mass spectroscopy fingerprint, we determined the presence of caffeic acid (CA) derivatives and the flavonoids Luteolin and Rhamnetin, as previously mentioned.
Caffeic acid (CA) and its derivatives are phenolic compounds widely synthesized by plant species with known antimicrobial, antioxidant, anti-inflammatory, and anticancer properties. The anticancer effects of CA are related to the anti- and pro-oxidant capacities of its chemical structure [37].
Briefly, the methodologies employed in the current investigation confirmed the presence of phenolic compounds, particularly flavonoids, thus laying the groundwork for further biological evaluation.
4.2. Biological Activities
4.2.1. Evaluation of Antibacterial Properties
Plants belonging to the Fabaceae family are known for antimicrobial effects, usually attributed to phenolic compounds, which were also detected in our study. Refs. [6,10] demonstrated that the ethanolic extract of P. stipulacea bark had low antibacterial activity against Gram-negative and Gram-positive strains, such as E. coli, Proteus vulgaris, Pseudomonas aeruginosa, S. aureus, and Aeromonas caviae.
Tannins isolated from the bark of P. stipulacea showed a better antibacterial effect against the Gram-positive strain S. aureus [38]. In this aspect, it is possible to infer that the presence of tannins improves the antimicrobial effect of P. stipulacea. These secondary metabolites were not detected in the EEPS, which could explain the lower antibacterial activity detected in gram-negative bacteria. It is also important to emphasize that Gram-negative bacteria have a more complex outer membrane structure than Gram-positive bacteria; this characteristic provides more resistance against antimicrobial agents [39] and could also explain our results.
4.2.2. Cytotoxic Activity of EEPS
To the best of our knowledge, there are no reports of the antitumor potential of P. stipulacea on human cancer cell lines in literature. However, the cytotoxic potential of other species belonging to the Piptadenia genus has already been reported.
Hydroalcoholic and hexane extracts and dichloromethane and ethyl acetate fractions of Piptadenia moniliformes leaves, fruits, fruit peel, and seeds were evaluated against three human tumor cell lines, HCT-116 (colon), OVCAR-3 (ovary), and SF295 (glioblastoma), for cytotoxic activity. However, they did not present satisfactory toxicity [40]. Piptadenia adiantoides crude extract has also been shown to be ineffective against UACC-62, MCF-7, and TK-10 human cell lines to a concentration of 20 µg/mL [41].
The cytotoxic potential attributed to Fabaceae family is usually associated with flavonoids. These compounds have great cytotoxic potential against cancer cells, mainly by cell cycle arrest and inhibition of cell permeability [42]. In this study, the cytotoxic potential of EEPS is likely attributable to luteolin and rhamnetin, since these flavonoid compounds present cytotoxicity, as already described [43,44]. Our results indicate that among the Piptadenia genus, P. stipulacea presents promising cytotoxic activities and the application of the extract in cancer therapy is plausible.
Hemolysis assay is a preliminary trial that evaluates the cytotoxicity of many compounds. It is widely used to evaluate blood biocompatibility and the potential of samples to cause premature lysis or disruption of red blood cells of human or animal origin [45]. In the present study, hemolysis was not observed in any of the evaluated EEPS concentrations, with EC50 greater than 1000 µg/mL. This effect is probably related to the diverse pool of molecules presented in the secondary metabolites of EEPS [6]. The widely varied phytochemical composition highlighted in this study could influence the biological properties of the extract.
The hemolytic activity of P. stipulacea ethanolic extract leaves against sheep erythrocytes has been evaluated [46,47]. It was noted that the extract did not present lysis at any of the concentrations tested. These results, together with the present study, reveal that P. stipulacea is safe and biocompatible. It is also important to note that this is the first study to evaluate the hemolytic properties of P. stipulacea against human erythrocytes.
4.2.3. EEPS Antioxidant Activity
Flavonoids play an important role in oxidative stress in plants, besides exerting potential antioxidant activity and free radical scavenging capacity [48]. Previous studies by our group have indicated that plants of the Fabaceae family exert great antioxidant potential [5].
It has been observed that alcoholic and ethyl acetate fractions of Piptadenia moniliformis fruit peels have antioxidant potential [40], and the aqueous fraction of P. moniliformis also has antioxidant potential [49]. It is important to note that the present study is the first work demonstrating the antioxidant potential of P. stipulacea, and that these effects may be related to the presence of the flavonoids luteolin and rhamnetin.
The antioxidant potential of P. stipulacea can be linked to its cytotoxic activity. Flavonoids are natural compounds capable of promoting redox and ROS level alterations in cancer cells, and subsequently suppress signaling pathways responsible for tumor survival and tumorigenesis [50].
Some studies have reported that luteolin and rhamnetin exhibit antioxidant mechanisms in cancer cells, showing correlations with mitochondrial dysfunction, alterations in epithelial-to-mesenchymal transition (EMT), and changes in key biomarkers such as malondialdehyde (MDA), superoxide dismutase (SOD), and the antioxidant enzyme catalase (CAT) [51,52]. Based on these studies, it can be suggested that the flavonoids luteolin and rhamnetin, present in the ethanol extract of Piptadenia stipulacea (EEPS), may target redox imbalances in tumor cells. However, further studies are needed to confirm these potential mechanisms.
5. Conclusions
In the present study, we obtained an ethanolic extract of P. stipulacea primarily composed of phenolic compounds. Mass spectrometry identified three flavonoids (rhamnetin, luteolin, and a derivative) as well two caffeic acid derivatives, the latter identified for the first time in this species.
The biological assays demonstrated firsthand a desirable cytotoxic effect against three human cancer cell lines (HCT-116, PC-3, and HL-60) with good antioxidant activity, antibacterial activity against gram-positive bacteria S. aureus, and with no observed hemolytic effects. These findings may be related to the presence of the identified flavonoids. This study opens the way for further evaluations to deepen our knowledge concerning the use of this plant in biomedicine.
Author Contributions
Conceptualization: S.A.d.N.M.S. and J.D.B.M.F.; Methodoloy: S.A.d.N.M.S., A.B.B., J.M.T.S., A.F.M., R.F.S. and E.d.S.M.F.; Validation: A.R.d.A., M.H.C., D.A.d.S., A.J.A. and J.D.B.M.F.; Formal analysis: J.D.B.M.F. and A.J.A.; Investigation: S.A.d.N.M.S.; Resources: A.R.d.A., M.H.C., D.A.d.S., A.J.A. and J.D.B.M.F.; Data curation: S.A.d.N.M.S., A.B.B., J.M.T.S., A.F.M., R.F.S. and E.d.S.M.F.; Writing-original draft: S.A.d.N.M.S.; Writing-review and editing: S.A.d.N.M.S., J.D.B.M.F., A.J.A. and J.M.T.S.; Visualization: S.A.d.N.M.S.; Supervision: J.D.B.M.F.; Project administration: J.D.B.M.F. and A.J.A.; Funding acquisition: J.D.B.M.F. and A.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ); Instituto Nacional de Ciência e Tecnologia em Biodiversidade e Produtos Naturais (INCT-Bionat) [CNPQ Grant Number 465637/2014-0, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Grant Number 2014/50926-0].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Instituto Nacional de Ciência e Tecnologia em Biodiversidade e Produtos Naturais (INCT-Bionat), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Piauí (FAPEPI) for the support of this work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Medina-Franco, J.L.; Saldívar-González, F.I. Cheminformatics to Characterize Pharmacologically Active Natural Products. Biomolecules 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Awouafack, M.D.; Tane, P.; Spiteller, M.; Eloff, J.N. Eriosema (Fabaceae) Species Represent a Rich Source of Flavonoids with Interesting Pharmacological Activities. Nat. Prod. Commun. 2015, 10, 1325–1330. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Silva, S.A.d.N.M.; Barros, A.B.; Souza, J.M.T.; Moura, A.F.; de Araújo, A.R.; Mendes, M.G.A.; Daboit, T.C.; da Silva, D.A.; Araújo, A.J.; Filho, J.D.B.M. Phytochemical and biological prospection of Mimosa genus plants extracts from Brazilian northeast. Phytochem. Lett. 2020, 39, 173–181. [Google Scholar] [CrossRef]
- Bezerra, D.A.C.; Rodrigues, F.F.G.; da Costa, J.G.M.; Pereira, A.V.; de Sousa, E.O.; Rodrigues, O.G. Abordagem fitoquímica, composição bromatológica e atividade antibacteriana de Mimosa tenuiflora (Wild) Poiret E Piptadenia stipulacea (Benth) Ducke. Acta Sci. Biol. Sci. 2011, 33, 99–106. [Google Scholar] [CrossRef]
- Souza, C.O.; Ramos, A.L.D.; Júnior, A.F.D.; Fernandes, M.M.; Marques, J.J. Potential for the use of coconut shell (Cocus nucifera) as an alternative fuel in the production of cassava flour. Res. Soc. Dev. 2021, 10, e250101119485. [Google Scholar] [CrossRef]
- de Carvalho, A.C.; dos Santos, R.C.; Castro, R.V.O.; de Sousa Santos, C.P.; de Lima Costa, S.E.; de Carvalho, A.J.E.; Pareyn, F.G.C.; Vidaurre, G.B.; Dias Júnior, A.F.; de Almeida, M.N.F. Produção de energia da madeira de espécies da Caatinga aliada ao manejo florestal sustentável. Sci. For. 2020, 48, e3086. [Google Scholar] [CrossRef]
- Nóbrega, J.S.; de Lucena Alcântara Bruno, R.; Gleide da Silva, L.; Lima, L.K.S.; Nunes de Medeiros, A.; Pereira de Andrade, A.; Magalhães, A.L.R.; Rufino de Lima, C. Seed recovery of native plant species of the Caatinga biome ingested by goats and its effect on seed germination, in Brazilian semiarid region. Arid. Land Res. Manag. 2023, 38, 109–121. [Google Scholar] [CrossRef]
- Pereira, A.V.; Santana, G.M.; Góis, M.B.; Sant’Ana, D.G. Tannins obtained from medicinal plants extracts against pathogens: Antimicrobial potential. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Formatex Research Center: Badajoz, Spain, 2015; pp. 228–235. [Google Scholar]
- de Albuquerque, U.P.; Andrade, L.H.C. Uso dos recursos vegetais da caatinga: O caso do agreste do estado de Pernambuco (Nordeste do Brasil). Interciência 2002, 27, 336–346. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Matos, F.J.A. Introdução à Fitoquimica Experimental, 3rd ed.; Edições UFC: Ceará, Brazil, 2009. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Approved Standard M02–A10; Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. CLSI—Clinical Laboratory Standards Institute: Wayne, PA, USA, 2016.
- Quelemes, P.V.; de Araújo, A.R.; Plácido, A.; Delerue-Matos, C.; Maciel, J.S.; Bessa, L.J.; Ombredane, A.S.; Joanitti, G.A.; Soares, M.J.d.S.; Eaton, P.; et al. Quaternized cashew gum: An anti-staphylococcal and biocompatible cationic polymer for biotechnological applications. Carbohydr. Polym. 2017, 157, 567–575. [Google Scholar] [CrossRef]
- Pires, J.; Torres, P.B.; dos Santos, D.Y.; Chow, F. Ensaio em Microplaca do Potencial Antioxidante Através do Método de Sequestro do Radical Livre DPPH para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; Available online: https://www.researchgate.net/publication/324452636_Ensaio_do_potencial_antioxidante_de_extratos_de_algas_atraves_do_sequestro_do_ABTS_em_microplaca (accessed on 28 March 2025). [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpúrea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal Plants, Human Health and Biodiversity: A Broad Review. In Advances in Biochemical Engineering/Biotechnology; Spring: Berlin/Heidelberg, Germany, 2014; pp. 59–110. [Google Scholar] [CrossRef]
- Cavalcanti-Dantas, V.D.M.; de Medeiros, K.L.; de Azevêdo, T.K.B.; Santana, G.M.; Pereira, A.V.; Goís, M.B.; Pereira, M.d.S.V.; Pereira, J.V. Taninos: Principal componente do extrato Piptadenia stipulacea (Benth) Ducke inibe o crescimento de cepas clínicas de Staphylococcus aureus de origem bovina. Biotemas 2016, 29, 109–114. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorganic Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Syllaios, A.; Schizas, D.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; Ntounis, T.; et al. Breast cancer apoptosis and the therapeutic role of luteolin. Chirurgia 2021, 116, 170–177. [Google Scholar] [CrossRef]
- Park, E.-S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.-E.; Kim, B.; Shin, H.-S. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef]
- Lan, L.; Wang, Y.; Pan, Z.; Wang, B.; Yue, Z.; Jiang, Z.; Li, L.; Wang, C.; Tang, H. Rhamnetin induces apoptosis in human breast cancer cells via the miR-34a/Notch-1 signaling pathway. Oncol. Lett. 2019, 17, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Pereira, A.V.; de Azevêdo, T.K.B.; Santana, G.M.; Trevisan, L.F.A.; Higino, S.S.d.S.; Macêdo-Costa, M.R.; Pereira, M.D.S.V.; Azevedo, S.S. Análise da atividade antimicrobiana de taninos totais de plantas aromáticas do Nordeste brasileiro. Agropecuária Técnica 2015, 36, 109–114. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Alves, M.d.J.; Moura, A.K.S.; Costa, L.M.; de Araújo, É.J.F.; de Sousa, G.M.; Costa, N.D.d.J.; Ferreira, P.M.P.; Silva, J.d.N.; Pessoa, C.; de Lima, S.G.; et al. Phenols, flavonoids and antioxidant and cytotoxic activity of leaves, fruits, peel of fruits and seeds of Piptadenia moniliformis Benth (Leguminosae-Mimosoideae). Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2014, 13, 466–476. [Google Scholar]
- Rosa, L.H.; Gonçalves, V.N.; Caligiorne, R.B.; Alves, T.M.A.; Rabello, A.; Sales, P.A.; Romanha, A.J.; Sobral, M.E.G.; Rosa, C.A.; Zani, C.L. Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Braz. J. Microbiol. 2010, 41, 420–430. [Google Scholar] [CrossRef]
- Yadegarynia, S.; Pham, A.; Ng, A.; Nguyen, D.; Lialiutska, T.; Bortolazzo, A.; Sivryuk, V.; Bremer, M.; White, J.B. Profiling Flavonoid Cytotoxicity in Human Breast Cancer Cell Lines: Determination of Structure-Function Relationships. Nat. Prod. Commun. 2012, 7, 1295–1304. [Google Scholar] [CrossRef]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Ahmad, I.; Mathew, S.P.; Bhattacharjee, C.K.; Jaganathan, B.G.; Poddar, S.; Patel, H.; Subramaniyan, V.; Chinni, S.V.; et al. Rhamnetin, a nutraceutical flavonoid arrests cell cycle progression of human ovarian cancer (SKOV3) cells by inhibiting the histone deacetylase 2 protein. J. Biomol. Struct. Dyn. 2024, 42, 13421–13436. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Guedes, G.H.F.; Tenorio, B.M.; da Silva Gomes, J.A.; dos Santos, L.S.M.; da Costa, M.A.S.; de Oliveira, M.L.F.; Leite, M.C.B.; de Sobreira, R.C.B.; da Silva, T.M.S.; Tenório, F.d.C.A.M.; et al. Avaliação citotóxica das folhas de Piptadenia stipulacea. In As Ciências Biológicas e a Construção de Novos Paradigmas de Conhecimento; Atena Editora: Ponta Grossa, Brazil, 2019; pp. 1–388. [Google Scholar]
- Sobreira, R.C.B.; da Silva, W.A.; da Silva, T.M.S.; da Costa, M.A.S.; Bandeira, M.A.M.; dos Santos, T.E.M.; Lima, M.I.d.A.; da Silva, A.A.; Lima, S.M.d.A.; Leite, S.P. Toxicological evaluation and safety of the ethanol extract from leaves of Piptadenia stipulaceae. Res. Soc. Dev. 2022, 11, e46411326815. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Silva, J.D.N.; Monção, N.B.N.; de Farias, R.R.S.; Citó, A.M.d.G.L.; Chaves, M.H.; de Araújo, M.R.S.; Lima, D.J.B.; Pessoa, C.; de Lima, A.; Araújo, E.C.d.C.; et al. Toxicological, chemopreventive, and cytotoxic potentialities of rare vegetal species and supporting findings for the Brazilian Unified Health System (SUS). J. Toxicol. Environ. Health Part A 2020, 83, 525–545. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.-S.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar]
- Nisari, M.; Bozkurt, Ö.; Ertekin, T.; Ceylan, D.; İnanç, N.; Al, Ö.; Güler, H.; Unur, E. Rhamnetin improves antioxidant status in the liver of Ehrlich solid tumor bearing mice. Med. Sci. Discov. 2020, 7, 494–500. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).