Chemical Compositions and Essential Fatty Acid Analysis of Selected Vegetable Oils and Fats
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Fatty Acid Methyl Esters (FAMEs)
2.3. GC-MS Analysis
2.4. Statistical Analysis
3. Results and Discussions
3.1. Fatty Acid Compositions
3.2. Uses of Vegetable Oils
3.2.1. Biofuel Production
3.2.2. Culinary Use
3.2.3. Cosmetic Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EI | Electron impact |
| eV | Electron volt |
| FA | Fatty acid |
| FAME | Fatty acid methyl ester |
| GC-MS | Gas chromatography–mass spectrometry |
| MCT | Medium-chain triglyceride |
| MSD | Mean ± standard deviation |
| RI | Retention index |
| USA | United States of America |
References
- Meijaard, E.; Abrams, J.F.; Slavin, J.L.; Sheil, D. Dietary fats, human nutrition and the environment: Balance and sustainability. Front. Nutr. 2022, 9, 878644. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-soluble vitamins: Updated review of their role and orchestration in human nutrition throughout life cycle with sex differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Sarnyai, F.; Somogyi, A.; Gór-Nagy, Z.; Zámbó, V.; Szelényi, P.; Mátyási, J.; Simon-Szabó, L.; Kereszturi, É.; Tóth, B.; Csala, M. Effect of cis- and trans-monounsaturated fatty acids on palmitate toxicity and on palmitate-induced accumulation of ceramides and diglycerides. Int. J. Mol. Sci. 2020, 21, 2626. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The effect of trans fatty acids on human health: Regulation and consumption patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. Clinical significance of polyunsaturated fatty acids in the prevention of cardiovascular diseases. Front. Nutr. 2022, 9, 998291. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Denke, M.A. Dietary fats, fatty acids, and their effects on lipoproteins. Curr. Atheroscler. Rep. 2006, 8, 466–471. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 fatty acids and their role in central nervous system—A review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, S.; Saeedan, A.S.; Ansari, M.N.; Singh, M. Polyunsaturated fatty acids mediated regulation of membrane biochemistry and tumor cell membrane integrity. Membranes 2021, 11, 479. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The importance of maintaining a low omega-6/omega-3 ratio for reducing the risk of autoimmune diseases, asthma, and allergies. Mo. Med. 2021, 118, 453–459. [Google Scholar]
- Dhaka, V.; Gulia, N.; Ahlawat, K.S.; Khatkar, B.S. Trans Fats—Sources, Health Risks and Alternative Approach—A Review. J. Food Sci. Technol. 2011, 48, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hygien. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- Ojha, P.K.; Poudel, D.K.; Dangol, S.; Rokaya, A.; Timsina, S.; Satyal, P.; Setzer, W.N. Volatile constituent analysis of wintergreen essential oil and comparison with synthetic methyl salicylate for authentication. Plants 2022, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Poudel, D.K.; Ojha, P.K.; Rokaya, A.; Satyal, R.; Satyal, P.; Setzer, W.N. Analysis of volatile constituents in Curcuma species, viz. C. aeruginosa, C. zedoaria, and C. longa, from Nepal. Plants 2022, 11, 1932. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Otgonbayar, C.; Matthaus, B.; Odonmajig, P. Fatty acid, tocopherol and sterol composition in sea buckthorn (Hippophae rhamnoides L.) of Mongolia. Mong. J. Chem. 2011, 12, 126–130. [Google Scholar] [CrossRef]
- Rahman, H.; Tursino; Sitompul, J.P.; Anggadiredja, K.; Gusdinar, T. The nutritional fatty acids profile and physicochemical properties of Canarium indicum nut oil. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1222–1226. [Google Scholar]
- Kakuda, Y.; Jahaniaval, F.; Marcone, M.F.; Montevirgen, L.; Montevirgen, Q.; Umali, J. Characterization of pili nut (Canarium ovatum) oil: Fatty acid and triacylglycerol composition and physicochemical properties. J. Am. Oil Chem. Soc. 2000, 77, 991–997. [Google Scholar] [CrossRef]
- Crane, S.; Aurore, G.; Joseph, H.; Mouloungui, Z.; Bourgeois, P. Composition of fatty acids triacylglycerols and unsaponifiable matter in Calophyllum calaba L. oil from Guadeloupe. Phytochemistry 2005, 66, 1825–1831. [Google Scholar] [CrossRef]
- Ferreira, M.D.C.C.; Neto, M.F.D.C.; De Melo, A.C.G.R.; Montero, I.F.; Chagas, E.A.; Ferraz, V.P.; Ribeiro, P.R.; De Melo Filho, A.A. Physical-chemical properties and chemical composition of Brazil nut oil, Bertholletia excelsa, from state of Roraima, Brazilian Amazon. Chem. Eng. Trans. 2019, 75, 391–396. [Google Scholar] [CrossRef]
- Garti, H.; Agbemafle, R.; Mahunu, G.K. Physicochemical properties and fatty acid composition of shea butter from Tamale, northern Ghana. UDS Int. J. Dev. 2019, 6, 35–40. [Google Scholar]
- Razafimamonjison, G.; Tsy, J.-M.; Randriamiarinarivo, M.; Ramanoelina, P.; Rasoarahona, J.; Fawbush, F.; Danthu, P. Fatty acid composition of baobab seed and its relationship with the genus Adansonia taxonomy. Chem. Biodivers. 2017, 14, e1600441. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; McClements, D.J.; Decker, E.A. Challenges of utilizing healthy fats in foods. Adv. Nutr. 2015, 6, 309S–317S. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in patients with inflammatory skin diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef] [PubMed]
- Prieto Vidal, N.; Adeseun Adigun, O.; Pham, T.H.; Mumtaz, A.; Manful, C.; Callahan, G.; Stewart, P.; Keough, D.; Thomas, R.H. The effects of cold saponification on the unsaponified fatty acid composition and sensory perception of commercial natural herbal soaps. Molecules 2018, 23, 2356. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Almiron-Roig, E. Human perceptions and preferences for fat-rich foods. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.-P., le Coutre, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 265–291. [Google Scholar]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fat as compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Alfekaik, D.F.; AL-Hilfi, S.A. Fatty acids composition by (GC-MS) and most important physical chemicals parameters of seed oil pomegranate and grape seeds. J. Biol. Agric. Healthc. 2016, 6, 25–32. [Google Scholar]
- Hosni, T.; Abbes, Z.; Abaza, L.; Medimagh, S.; Ben Salah, H.; Kharrat, M. Biochemical characterization of seed oil of Tunisian sunflower (Helianthus annuus L.) accessions with special reference to its fatty acid composition and oil content. J. Food Qual. 2022, 2022, e2875072. [Google Scholar] [CrossRef]
- Thilakarathna, C.; Madhusankha, M.; Navaratne, S. Determination of composition of fatty acid profile of Ethiopian and Indian black cumin oil (Nigella sativa). Int. J. Food Sci. Nutr. 2018, 3, 1–3. [Google Scholar]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. Fatty acid composition and tocopherol contents of some sesame seed oils. Iran. J. Chem. Chem. Eng. 2018, 37, 151–155. [Google Scholar]
- Dalibalta, S.; Majdalawieh, A.F.; Manjikian, H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J. 2020, 28, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Talebi, E.; Nasrollahi, I.; Nemati, Z. Study on Silybum marianum seed through fatty acids comparison, peroxide tests, refractive index and oil percentage. Pharmacogn. J. 2016, 8, 595–597. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H. Physicochemical, fatty acid and antioxidant properties of passion fruit (Passiflora species) seed oil. Pak. J. Nutr. 2019, 18, 421–429. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The role of moisturizers in addressing various kinds of dermatitis: A review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef]
- Ryu, H.S.; Jeong, J.; Lee, C.M.; Lee, K.S.; Lee, J.-N.; Park, S.-M.; Lee, Y.-M. Activation of hair cell growth factors by linoleic acid in Malva verticillata seed. Molecules 2021, 26, 2117. [Google Scholar] [CrossRef]
- Malcicka, M.; Visser, B.; Ellers, J. An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol. 2018, 45, 15–26. [Google Scholar] [CrossRef]
- Abad, A.; Shahidi, F. Compositional characteristics and oxidative stability of chia seed oil (Salvia hispanica L). Food Prod. Process. Nutr. 2020, 2, 9. [Google Scholar] [CrossRef]
- Cherif, A.; Slama, A. Stability and change in fatty acids composition of soybean, corn, and sunflower oils during the heating process. J. Food Qual. 2022, 2022, e6761029. [Google Scholar] [CrossRef]
- Güney, M. Determination of fatty acid profile and antioxidant activity of rosehip seeds from Turkey. Int. J. Agric. Env. Food Sci. 2020, 4, 114–118. [Google Scholar] [CrossRef]
- Porto, C.D.; Decorti, D.; Natolino, A. Potential oil yield, fatty acid composition, and oxidation stability of the hempseed oil from four Cannabis sativa L. cultivars. J. Diet. Suppl. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, H.; Guo, Y.; Long, S.; Wang, Y.; Abbasi, A.M.; Guo, X.; Jarvis, D.I. Comparison of fatty acid composition, phytochemical profile and antioxidant activity in four flax (Linum usitatissimum L.) varieties. Oil Crop Sci. 2020, 5, 136–141. [Google Scholar] [CrossRef]
- Ako, H.; Kong, N.; Brown, A. Fatty acid profiles of kukui nut oils over time and from different sources. Ind. Crops Prod. 2005, 22, 169–174. [Google Scholar] [CrossRef]
- Abdul, D.A.; Majeed, S.N.; Aziz, N.M. Seed oil composition of red raspberry (Rubus ideaus) fruit in Sulaimani City. Middle East J. Intern. Med. 2012, 5, 39–43. [Google Scholar] [CrossRef]
- Bouabdallah, I.; Bouali, I.; Martinez-Force, E.; Albouchi, A.; Perez Camino, M.C.; Boukhchina, S. Composition of fatty acids, triacylglycerols and polar compounds of different walnut varieties (Juglans regia L.) from Tunisia. Nat. Prod. Res. 2014, 28, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; de Goede, J.; Brouwer, I.A. Alpha-linolenic acid: Is it essential to cardiovascular health? Curr. Atheroscler. Rep. 2010, 12, 359–367. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. The importance of marine omega-3s for brain development and the prevention and treatment of behavior, mood, and other brain disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef]
- Liu, L.; Feng, S.; Chen, T.; Zhou, L.; Yuan, M.; Liao, J.; Huang, Y.; Yang, H.; Yang, R.; Ding, C. Quality assessment of Camellia oleifera oil cultivated in southwest China. Separations 2021, 8, 144. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.; Mapel-Velazco, L.; Martín del Campo, S.; Chávez-Servia, J.L.; Martínez, A.; Verdalet, I. Fatty acids profile of oil from nine varieties of macadamia nut. Int. J. Food Prop. 2017, 20, 1263. [Google Scholar] [CrossRef]
- Gouta, H.; Ksia, E.; Laaribi, I.; Molino, F.; Estopañán, G.; Juan, T.; Ossama, K.; Martinez-Gomez, P.; Martínez-García, P.J. Evaluation of the chemical and nutritional properties of Tunisian almond cultivars. Ital. J. Food Sci. 2020, 32, 562–582. [Google Scholar]
- Revelou, P.-K.; Xagoraris, M.; Alexandropoulou, A.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Chemometric study of fatty acid composition of virgin olive oil from four widespread Greek cultivars. Molecules 2021, 26, 4151. [Google Scholar] [CrossRef] [PubMed]
- Kouidri, M.; Saadi, A.; Noui, A.; Medjahed, F. The chemical composition of argan oil. Int. J. Adv. Stud. Comput. Sci. Eng. 2015, 4, 24–28. [Google Scholar]
- Zhao, B.; Li, H.; Lan, T.; Wu, D.; Chen, Z. Characterization of the chemical composition of Chinese Moringa oleifera seed oil. J. Am. Oil Chem. Soc. 2019, 96, 523–533. [Google Scholar] [CrossRef]
- Mthiyane, D.M.N.; Mhlanga, B.S. The nutritive value of marula (Sclerocarya birrea) seed cake for broiler chickens: Nutritional composition, performance, carcass characteristics and oxidative and mycotoxin status. Trop. Anim. Health Prod. 2017, 49, 835–842. [Google Scholar] [CrossRef]
- Stryjecka, M.; Kiełtyka-Dadasiewicz, A.; Michalak, M.; Rachoń, L.; Głowacka, A. Chemical composition and antioxidant properties of oils from the seeds of five apricot (Prunus armeniaca L.) cultivars. J. Oleo Sci. 2019, 68, 729–738. [Google Scholar] [CrossRef]
- Nasri, C.; Halabi, Y.; Harhar, H.; Mohammed, F.; Bellaouchou, A.; Guenbour, A.; Tabyaoui, M. Chemical characterization of oil from four avocado varieties cultivated in Morocco. Oilseeds Fats Crops Lipids 2021, 28, 19. [Google Scholar] [CrossRef]
- Gao, F.; Yang, S.; Birch, J. Physicochemical characteristics, fatty acid positional distribution and triglyceride composition in oil extracted from carrot seeds using supercritical CO2. J. Food Compos. Anal. 2016, 45, 26–33. [Google Scholar] [CrossRef]
- Sagan, A.; Blicharz-Kania, A.; Szmigielski, M.; Andrejko, D.; Sobczak, P.; Zawiślak, K.; Starek, A. Assessment of the properties of rapeseed oil enriched with oils characterized by high content of α-linolenic acid. Sustainability 2019, 11, 5638. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; de Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini. Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Fl, H.; Zhu, G.; Chen, Y.; Meng, F.; Peng, M.; Chen, X.; He, Z.; Zhang, Z.; Chen, Y. Seed characteristics and fatty acid composition of castor (Ricinus communis L.) varieties in northeast China. Int. J. Exp. Bot. 2015, 84, 26–33. [Google Scholar] [CrossRef]
- Anjani, K. Castor genetic resources: A primary gene pool for exploitation. Ind. Crops Prod. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Huang, F.; Bao, C.; Peng, M.; Zhu, G.; He, Z.; Chen, X.; Luo, R.; Zhao, Y. Chromatographic analysis of fatty acid composition in differently sized seeds of castor accessions. Biotechnol. Biotechnol. Equip. 2015, 29, 892–900. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Cea, M.; Paneque, M.; González, M.E. Oil content and fatty acid composition in castor bean naturalized accessions under Mediterranean conditions in Chile. Agronomy 2020, 10, 1145. [Google Scholar] [CrossRef]
- Chaudhari, B.A. Oil content and fatty acid composition in castor (Ricinus communis L.) genotypes. Int. J. Agric. Environ. Biotechnol. 2021, 14, 319–324. [Google Scholar] [CrossRef]
- Omari, A.; Mgani, Q.A.; Mubofu, E.B. Fatty acid profile and physico-chemical parameters of castor oils in Tanzania. Green Sustain. Chem. 2015, 5, 154–163. [Google Scholar] [CrossRef]
- Poddar, K.H.; Sikand, G.; Kalra, D.; Wong, N.; Duell, P.B. Mustard oil and cardiovascular health: Why the controversy? J. Clin. Lipidol. 2022, 16, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Schwarzinger, B.; Feichtinger, M.; Blank-Landeshammer, B.; Weghuber, J.; Schwarzinger, C. Quick determination of erucic acid in mustard oils and seeds. J. Anal. Appl. Pyrolysis 2022, 164, 105523. [Google Scholar] [CrossRef]
- Ostrikov, A.N.; Kleymenova, N.L.; Bolgova, I.N.; Kopylov, M.V. Gas chromatographic analysis of the fatty acid composition of mustard oil obtained by cold pressing (method). Emir. J. Food Agric. 2020, 32, 391–396. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- Grygier, A. Mustard seeds as a bioactive component of food. Food Rev. Int. 2022, 39, 4088–4101. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R. Influence of food type, oil type and frying frequency on the formation of trans-fatty acids during repetitive deep-frying. Food Control 2023, 147, 109557. [Google Scholar] [CrossRef]
- Islam, M.K.; Rayhan, M.A.; Khatun, M.A.; Islam, D.; Rahman, M.N. Effect of raw and repeatedly fried mustard oil intake on metabolic and organ histological changes in Wistar rat. J. Food Biochem. 2020, 44, e13120. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Z.; Shi, L.; Son, J.H.; Li, L.; Wang, L.; Chen, J. Investigating aldehyde and ketone compounds produced from indoor cooking emissions and assessing their health risk to human beings. J. Environ. Sci. 2023, 127, 389–398. [Google Scholar] [CrossRef]
- Aslan, V. An overview of biodiesel produced from 2nd generation feedstock: Mustard seed types. Bioenerg. Res. 2023, 16, 1380–1400. [Google Scholar] [CrossRef]
- Wani, I.A.; ul Ashraf, Z.; Muzzaffar, S. Erucic acid. In Handbook of Plant and Animal Toxins in Food; Nayic, G.A., Kour, J., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 169–175. ISBN 978-1-00-317844-6. [Google Scholar]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Petersen, K.S.; Bowen, K.J.; Jones, P.J.H.; Taylor, C.G.; Zahradka, P.; Letourneau, K.; Perera, D.; Wilson, A.; Wagner, P.R.; et al. Effects of diets enriched with conventional or high-oleic canola oils on vascular endothelial function: A sub-study of the Canola Oil Multi-centre Intervention Trial 2 (COMIT-2), a randomized crossover controlled feeding study. Nutrients 2022, 14, 3404. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Sayyad, R. Comparison antioxidant activity of Tarom Mahali rice bran extracted from different extraction methods and its effect on canola oil stabilization. J. Food Sci. Technol. 2015, 52, 6385–6394. [Google Scholar] [CrossRef]
- Stupin, A.; Mihalj, M.; Kolobarić, N.; Šušnjara, P.; Kolar, L.; Mihaljević, Z.; Matić, A.; Stupin, M.; Jukić, I.; Kralik, Z.; et al. Anti-inflammatory potential of n-3 polyunsaturated fatty acids enriched hen eggs consumption in improving microvascular endothelial function of healthy individuals—Clinical trial. Int. J. Mol. Sci. 2020, 21, 4149. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Spencer, G.F.; Plattner, R.D.; Miwa, T. Jojoba oil analysis by high pressure liquid chromatography and gas chromatography/mass spectrometry. J. Am. Oil Chem. Soc. 1977, 54, 187–189. [Google Scholar] [CrossRef]
- Araiza-Lizarde, N.; Alcaraz-Meléndez, L.; Angulo-Escalante, M.A.; Reynoso-Granados, T.; Cruz-Hernández, P.; Calderón-Vázquez, C.L. Physicochemical composition of seed oil of wild jojoba populations in northwestern Mexico. J. Food Nutr. Res. 2017, 5, 443–450. [Google Scholar]
- Awad, N.A.; Eliraq, M.; El-Bassel, E.H.; Ismail, A.S.M.; Abd El-Aziz, Y.S.G.; Gawish, M.S.; Zewail, R.M.Y.; Sami, R.; Khojah, E.; Hilary, U.; et al. Evaluation of the effect of elite jojoba lines on the chemical properties of their seed oil. Molecules 2022, 27, 3904. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ma, S.; Tominaga, T.; Yokoyama, K.; Kitatani, K.; Horikawa, K.; Suzuki, K. Acute effects of transdermal administration of jojoba oil on lipid metabolism in mice. Medicina 2019, 55, 594. [Google Scholar] [CrossRef]
- Gad, H.A.; Roberts, A.; Hamzi, S.H.; Gad, H.A.; Touiss, I.; Altyar, A.E.; Kensara, O.A.; Ashour, M.L. Jojoba oil: An updated comprehensive review on chemistry, pharmaceutical uses, and toxicity. Polymers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Sánchez, M.; Avhad, M.R.; Marchetti, J.M.; Martínez, M.; Aracil, J. Jojoba oil: A state of the art review and future prospects. Energy Convers. Manag. 2016, 129, 293–304. [Google Scholar] [CrossRef]
- Bouaid, A.; Bajo, L.; Martinez, M.; Aracil, J. Optimization of biodiesel production from jojoba oil. Process Saf. Environ. Prot. 2007, 85, 378–382. [Google Scholar] [CrossRef]
- Selim, M.Y.E.; Ghannam, M.T.; Abdo, B.N.; Attai, Y.A.; Radwan, M.S. Raw jojoba oil as a sustainable fuel to diesel engines and comparison with diesel fuel. Energies 2022, 15, 5770. [Google Scholar] [CrossRef]
- Kýralan, M.; Gölükcü, M.; Tokgöz, H. Oil and conjugated linolenic acid contents of seeds from important pomegranate cultivars (Punica granatum L.) grown in Turkey. J. Am. Oil Chem. Soc. 2009, 86, 985–990. [Google Scholar] [CrossRef]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive components of pomegranate oil and their influence on mycotoxin secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.M.; Ramalhosa, E.; Casal, S. Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compos. Anal. 2015, 39, 13–22. [Google Scholar] [CrossRef]
- Hajib, A.; Nounah, I.; Harhar, H.; Gharby, S.; Kartah, B.; Matthäus, B.; Bougrin, K.; Charrouf, Z. Oil content, lipid profiling and oxidative stability of “Sefri” Moroccan pomegranate (Punica granatum L.) seed oil. Oilseeds Fats Crops Lipids 2021, 28, 5. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural by-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: London, UK, 2019; pp. 181–216. ISBN 978-0-12-814138-0. [Google Scholar]
- Loukhmas, S.; Kerak, E.; Elgadi, S.; Ettalibi, F.; El Antari, A.; Harrak, H. Oil content, fatty acid composition, physicochemical properties, and antioxidant activity of seed oils of ten Moroccan pomegranate cultivars. J. Food Qual. 2021, 2021, e6617863. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Zielińska, A.; Wójcicki, K.; Klensporf-Pawlik, D.; Marzec, M.; Lucarini, M.; Durazzo, A.; Fonseca, J.; Santini, A.; Nowak, I.; Souto, E.B. Cold-pressed pomegranate seed oil: Study of punicic acid properties by coupling of GC/FID and FTIR. Molecules 2022, 27, 5863. [Google Scholar] [CrossRef]
- de Melo, I.L.P.; de Carvalho, E.B.T.; de Oliveira e Silva, A.M.; Yoshime, L.T.; Sattler, J.A.G.; Pavan, R.T.; Mancini-Filho, J. Characterization of constituents, quality and stability of pomegranate seed oil (Punica granatum L.). Food Sci. Technol. 2016, 36, 132–139. [Google Scholar] [CrossRef]
- Çavdar, H.K.; Yanık, D.K.; Gök, U.; Göğüş, F. Optimisation of microwave-assisted extraction of pomegranate (Punica granatum L.) seed oil and evaluation of its physicochemical and bioactive properties. Food Technol. Biotechnol. 2017, 55, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Yayla, M.; Cetin, D.; Adali, Y.; Kilicle, P.A.; Toktay, E. Potential therapeutic effect of pomegranate seed oil on ovarian ischemia/reperfusion injury in rats. Iran J. Basic Med. Sci. 2018, 21, 1262–1268. [Google Scholar] [CrossRef]
- Matthäus, B.; Guillaume, D.; Gharby, S.; Haddad, A.; Harhar, H.; Charrouf, Z. Effect of processing on the quality of edible argan oil. Food Chem. 2010, 120, 426–432. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.A.; Soudagar, M.E.M.; Fattah, I.M.R. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Pham, T.L.-B.; Thi, T.T.; Nguyen, H.T.-T.; Lao, T.D.; Binh, N.T.; Nguyen, Q.D. Anti-aging effects of a serum based on coconut oil combined with deer antler stem cell extract on a mouse model of skin aging. Cells 2022, 11, 597. [Google Scholar] [CrossRef]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Watanabe, S.; Tsujino, S. Applications of medium-chain triglycerides in foods. Front. Nutr. 2022, 9, 802805. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, C. Determination of penetration and protection of fatty acids in bleached hair according to the fatty acid chain length and the application to understanding the protective effects of MCT oil and coconut oil. Fash. Text. 2023, 10, 10. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- Kumalasari, I.D.; Santosa, I.; Sulistiawati, E. Coconut oil production with various roasting temperatures and dried grated coconut as a by-product. Earth Environ. Sci. 2020, 515, 012026. [Google Scholar] [CrossRef]
- Sandupama, P.; Munasinghe, D.; Jayasinghe, M. Coconut oil as a therapeutic treatment for Alzheimer’s Disease: A review. J. Future Foods 2022, 2, 41–52. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Suzihaque, M.U.H.; Alwi, H.; Kalthum Ibrahim, U.; Abdullah, S.; Haron, N. Biodiesel production from waste cooking oil: A brief review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Colombo, K.; Ender, L.; Santos, M.M.; Chivanga Barros, A.A. Production of biodiesel from soybean oil and methanol, catalyzed by calcium oxide in a recycle reactor. S. Afr. J. Chem. Eng. 2019, 28, 19–25. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using canola oil biodiesel as an alternative fuel in diesel engines: A review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Farobie, O.; Hartulistiyoso, E. Palm oil biodiesel as a renewable energy resource in Indonesia: Current status and challenges. Bioenerg. Res. 2022, 15, 93–111. [Google Scholar] [CrossRef]
- Zhao, X.; Xiang, X.; Huang, J.; Ma, Y.; Sun, J.; Zhu, D. Studying the evaluation model of the nutritional quality of edible vegetable oil based on dietary nutrient reference intake. ACS Omega 2021, 6, 6691–6698. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega−3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Baig, A.; Zubair, M.; Sumrra, S.H.; Nazar, M.F.; Zafar, M.N.; Jabeen, K.; Hassan, M.B.; Rashid, U. Heating effect on quality characteristics of mixed canola cooking oils. BMC Chem. 2022, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.P. Trans fatty acids—A risk factor for cardiovascular disease. Pak. J. Med. Sci. 2014, 30, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.-B.; Kersten, S. Mechanisms of action of trans fatty acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Archambault, J.C.; Bonté, F. Vegetable fats in cosmeticology. Rev. Bol. Quim. 2021, 38, 80–94. [Google Scholar] [CrossRef]
- Orchard, A.; Kamatou, G.; Viljoen, A.M.; Patel, N.; Mawela, P.; van Vuuren, S.F. The influence of carrier oils on the antimicrobial activity and cytotoxicity of essential oils. Evid. Based Complement. Alternat. Med. 2019, 2019, 6981305. [Google Scholar] [CrossRef]
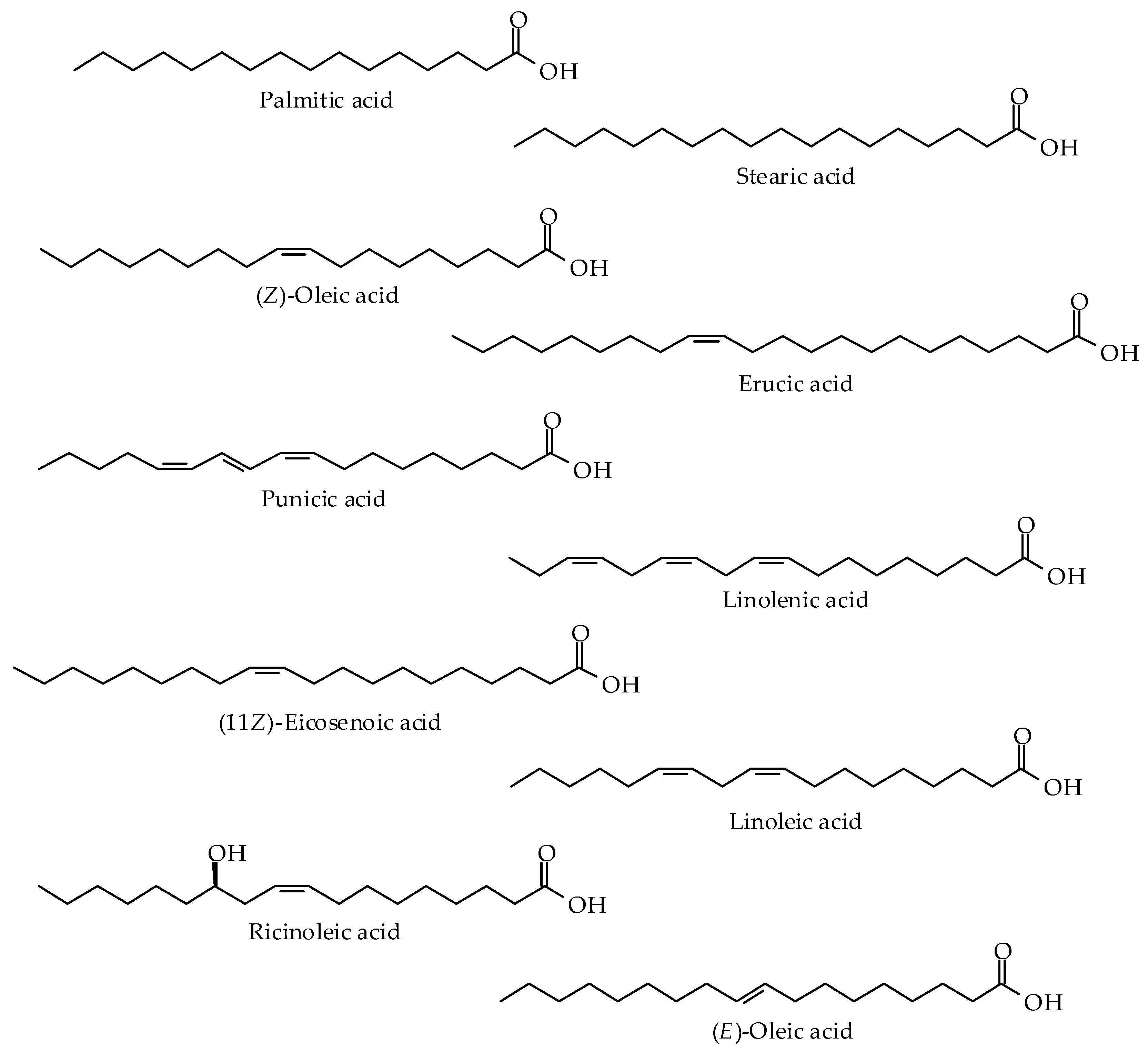
| Oils and Fats | Origin |
|---|---|
| Nangai nut, pili nut, shea butter, baobab, passion fruit, sesame, kukui, avocado, moringa, marula, and pomegranate seed | Kenya |
| Tamanu oil | Madagascar |
| Sea buckthorn berry, grape seed, pumpkin seed, flax seed, hemp seed, castor, and roasted coconut | India |
| Brazil nut, sunflower, and macadamia | Brazil |
| Black seed | Egypt |
| Milk thistle, rosehip, and carrot seed | Bulgaria |
| Walnut, almond, canola, evening primrose, soybean, red raspberry seed, and chia seed | USA |
| Olive | Italy |
| Argan | Morocco |
| Jojoba | Mexico |
| Medium-chain triglyceride (MCT) coconut | Philippines |
| Mustard | Nepal |
| Apricot kernel | France |
| Camellia seed | Japan |
| Synonyms | RI | Compound Name | Nangai Nut | Pili Nut | Shea Butter | Tamanu | Baobab | Sea Buckthorn Berry | Brazil Nut | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MSD (n = 6) | Range | MSD (n = 5) | Range | MSD (n = 5) | Range | MSD (n = 9) | Range | MSD (n = 5) | Range | MSD (n = 5) | Range | MSD (n = 8) | |||
| 1389 | (E)-Cinnamic acid | 0–0.04 | 0.02 ± 0.02 | 0–0.15 | 0.05 ± 0.05 | |||||||||||
| C12:0 | 1523 | Lauric acid | 0–0.01 | 0.01 ± 0.01 | 0.01–0.12 | 0.05 ± 0.04 | ||||||||||
| C14:0 | 1758 | Myristic acid | 0.01–0.05 | 0.04 ± 0.03 | 0.08–0.16 | 0.11 ± 0.07 | 0–0.02 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0.1–0.19 | 0.15 ± 0.04 | 0.11–0.16 | 0.13 ± 0.02 | 0.02–0.16 | 0.08 ± 0.05 |
| C15:0 | 1821 | Pentadecanoic acid | 0–0.02 | 0.01 ± 0.01 | 0.01–0.04 | 0.02 ± 0.02 | 0.02–0.05 | 0.04 ± 0.01 | 0.04–0.06 | 0.05 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||||
| trans-7,10, C16:2 | 1887 | 7,10-Hexadecadienoic acid | 0–0.01 | 0.01 ± 0.01 | ||||||||||||
| cis-7, C16:1 | 1894 | (Z)-7-Hexadecenoic acid | 0.01–0.04 | 0.02 ± 0.02 | 0.76–1.06 | 0.97 ± 0.17 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0–0.32 | 0.05 ± 0.11 |
| cis-9, C16:1 | 1901 | Palmitoleic acid | 0.17–0.29 | 0.25 ± 0.12 | 0.24–0.36 | 0.31 ± 0.15 | 0.01–0.05 | 0.03 ± 0.02 | 0.08–0.25 | 0.14 ± 0.05 | 0.12–0.2 | 0.15 ± 0.03 | 28.96–31.1 | 29.87 ± 0.86 | 0–0.45 | 0.26 ± 0.19 |
| C16:0 | 1924 | Palmitic acid | 25.36–31.12 | 28.99 ± 3.96 | 26.47–33.65 | 29.81 ± 4.86 | 2.09–4.50 | 3.25 ± 1.13 | 11.14–16.03 | 13.08 ± 1.37 | 21.59–25.7 | 23.84 ± 1.8 | 23.73–27.13 | 25.2 ± 1.37 | 7.90–17.81 | 14.49 ± 3.33 |
| cis-9,12, C17:2 | 1990 | 9,12-Heptadecadienoic acid | 0.13–0.22 | 0.17 ± 0.03 | 0–0.04 | 0.01 ± 0.02 | 0–0.01 | 0.01 ± 0.01 | ||||||||
| cis-10, C17:1 | 1996 | (Z)-10-Heptadecenoic acid | 0–0.05 | 0.03 ± 0.03 | 0–0.04 | 0.02 ± 0.01 | 0.18–0.33 | 0.27 ± 0.06 | 0.05–0.07 | 0.06 ± 0.01 | 0.01–0.04 | 0.03 ± 0.01 | ||||
| C17:0 | 2026 | Heptadecanoic acid | 0.08–0.15 | 0.13 ± 0.06 | 0.11–0.16 | 0.14 ± 0.08 | 0.03–0.09 | 0.06 ± 0.03 | 0.06–0.19 | 0.12 ± 0.04 | 0.17–0.27 | 0.23 ± 0.04 | 0.03–0.05 | 0.04 ± 0.01 | 0.02–0.12 | 0.08 ± 0.03 |
| cis-9,12, C18:2 | 2087 | Linoleic acid | 10.04–15.23 | 13.63 ± 2.86 | 2.74–3.86 | 3.17 ± 0.88 | 2.51–6.55 | 4.14 ± 1.83 | 22.48–33.78 | 26.48 ± 3.48 | 25.29–29.6 | 27.34 ± 1.72 | 5.99–8.41 | 7.38 ± 1.2 | 29.44–50.34 | 34.62 ± 6.92 |
| cis-9,12,15, C18:3 | 2096 | Linolenic acid | 1.06–2.48 | 1.75 ± 0.65 | ||||||||||||
| cis-9, C18:1 | 2102 | (Z)-Oleic acid | 32.14–38.56 | 36.52 ± 5.63 | 36.62–43.28 | 39.82 ± 5.63 | 50.62–68.86 | 56.83 ± 7.15 | 28.58–45.97 | 39.03 ± 4.89 | 32.17–36.84 | 34.88 ± 2.17 | 18.97–20.87 | 19.86 ± 0.88 | 25.30–48.31 | 35.15 ± 6.96 |
| trans-9, C18:1 | 2109 | (E)-Oleic acid | 0.78–2.41 | 1.39 ± 0.86 | 18.14–23.68 | 20.62 ± 4.21 | 0.08–0.84 | 0.39 ± 0.28 | 0.53–1.14 | 0.85 ± 0.17 | 0.91–1.3 | 1.08 ± 0.14 | 8.53–10.38 | 9.6 ± 0.7 | 0.05–1.41 | 0.93 ± 0.47 |
| C18:0 | 2126 | Stearic acid | 12.84–17.95 | 16.62 ± 2.47 | 3.21–4.93 | 4.12 ± 0.83 | 21.69–39.01 | 32.1 ± 6.47 | 13.75–18.09 | 16.79 ± 1.38 | 4.42–5.82 | 5.02 ± 0.65 | 1.57–1.95 | 1.78 ± 0.15 | 7.87–14.15 | 12.73 ± 2.77 |
| C20:2 | 2183 | Ethyl linoleate | 0–0.04 | 0.01 ± 0.02 | 0–0.07 | 0.02 ± 0.03 | 0.01 ± 0.03 | 0–0.05 | 0.02 ± 0.02 | 0.01–0.14 | 0.05 ± 0.04 | |||||
| cis-10, C19:1 | 2193 | (Z)-10-Nonadecenoic acid | 0–0.04 | 0.02 ± 0.02 | 0–0.05 | 0.01 ± 0.02 | 0.05–0.1 | 0.07 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | ||||||
| trans-10, C19:1 | 2205 | 10-Nonadecenoic acid | 1.93–3.04 | 2.45 ± 0.4 | 0–0.01 | 0.01 ± 0.01 | ||||||||||
| C19:0 | 2222 | Nonadecanoic acid | 0.02–0.06 | 0.04 ± 0.02 | 0–0.05 | 0.02 ± 0.02 | 0–0.01 | 0 ± 0.01 | ||||||||
| 2267 | (Z)-9-Tricosene | 0–0.02 | 0.01 ± 0.01 | |||||||||||||
| cis-11, C20:1 | 2296 | (Z)-11-Eicosenoic acid | 0.11–0.23 | 0.16 ± 0.08 | 0.08–0.15 | 0.13 ± 0.08 | 0.14–0.4 | 0.28 ± 0.12 | 0.22–0.42 | 0.32 ± 0.08 | 0.27–0.39 | 0.34 ± 0.05 | 0.12–0.29 | 0.22 ± 0.07 | 0.02–0.32 | 0.12 ± 0.09 |
| C20:0 | 2322 | Arachidic acid | 0.58–0.75 | 0.69 ± 0.17 | 0.25–0.34 | 0.31 ± 0.09 | 1.02–2.01 | 1.41 ± 0.44 | 0.81–1.60 | 1.20 ± 0.34 | 1.06–2.16 | 1.4 ± 0.45 | 0.28–0.5 | 0.39 ± 0.11 | 0.14–0.66 | 0.49 ± 0.16 |
| C21:0 | 2422 | Heneicosanoic acid | 0.01–0.02 | 0.01 ± 0 | 0–0.06 | 0.03 ± 0.03 | 0.03–0.07 | 0.04 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | ||||
| 2467 | 1-Heneicosyl formate | 0–0.04 | 0.02 ± 0.02 | |||||||||||||
| 2498 | Pentacosane | 0.05–0.19 | 0.11 ± 0.05 | |||||||||||||
| cis-13, C22:1 | 2499 | Erucic acid | 0–0.01 | 0 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||||||||||
| C22:0 | 2522 | Behenic acid | 0.09–0.15 | 0.13 ± 0.05 | 0.04–0.09 | 0.07 ± 0.04 | 0.17–0.32 | 0.22 ± 0.06 | 0.21–0.76 | 0.47 ± 0.22 | 0.46–1.15 | 0.64 ± 0.29 | 0.11–0.34 | 0.21 ± 0.1 | 0.04–1.54 | 0.31 ± 0.5 |
| 2600 | Hexacosane | 0–0.01 | 0 ± 0.01 | |||||||||||||
| C23:0 | 2622 | Tricosanoic acid | 0.02–0.09 | 0.06 ± 0.04 | 0.02–0.04 | 0.03 ± 0.01 | 0–0.09 | 0.04 ± 0.04 | 0.08–0.19 | 0.11 ± 0.05 | 0–0.06 | 0.03 ± 0.02 | 0–0.05 | 0.03 ± 0.02 | ||
| 2698 | Heptacosane | 0.06–0.2 | 0.11 ± 0.06 | |||||||||||||
| cis-15, C24:1 | 2699 | (Z)-15-Tetracosenoic acid | 0–0.07 | 0.04 ± 0.04 | ||||||||||||
| C24:0 | 2722 | Lignoceric acid | 0.14–0.26 | 0.23 ± 0.11 | 0.02–0.08 | 0.06 ± 0.03 | 0.18–0.3 | 0.22 ± 0.05 | 0–0.33 | 0.17 ± 0.12 | 0.36–1.04 | 0.58 ± 0.28 | 0.12–0.48 | 0.29 ± 0.15 | 0.03–0.54 | 0.17 ± 0.17 |
| 2791 | Octacosane | 0–0.01 | 0 ± 0.01 | |||||||||||||
| 2803 | (E)-Squalene | 0.06–0.10 | 0.08 ± 0.03 | 0–0.03 | 0.02 ± 0.01 | 0.02–3.15 | 0.89 ± 1.24 | 0.01–0.04 | 0.02 ± 0.01 | 0–0.05 | 0.02 ± 0.02 | 0–0.35 | 0.16 ± 0.12 | |||
| C25:0 | 2830 | Pentacosanoic acid | 0.04–0.08 | 0.06 ± 0.03 | 0–0.03 | 0.01 ± 0.02 | 0.02–0.03 | 0.02 ± 0.01 | 0.07–0.17 | 0.1 ± 0.05 | 0–0.07 | 0.03 ± 0.03 | 0–0.03 | 0.02 ± 0.01 | ||
| 2899 | Nonacosane | 0–0.02 | 0.01 ± 0.01 | 0.1–0.48 | 0.27 ± 0.14 | |||||||||||
| C26:0 | 2926 | Hexacosanoic acid | 0.01–0.08 | 0.06 ± 0.04 | 0–0.03 | 0.02 ± 0.01 | 0.06–0.09 | 0.07 ± 0.02 | 0.09–0.23 | 0.14 ± 0.06 | 0.09–0.5 | 0.29 ± 0.16 | 0–0.04 | 0.02 ± 0.01 | ||
| 3091 | β-Sitosteryl acetate | 0–0.06 | 0.03 ± 0.02 | |||||||||||||
| 3096 | Untriacontane | 0–0.28 | 0.14 ± 0.11 | |||||||||||||
| C28:0 | 3130 | Octacosanoic acid | 0–0.01 | 0.01 ± 0 | 0.03–0.05 | 0.04 ± 0.01 | 0.22–0.81 | 0.53 ± 0.24 | ||||||||
| 3303 | γ-Sitosterol | 0.46–0.54 | 0.51 ± 0.12 | 0.01–0.06 | 0.04 ± 0.03 | 0.41–1.06 | 0.64 ± 0.26 | 0–1.24 | 0.69 ± 0.45 | |||||||
| C30:0 | 3328 | Triacontanoic acid | 0–0.18 | 0.04 ± 0.06 | 0–0.3 | 0.18 ± 0.12 | ||||||||||
| 3445 | Stigmast-4-en-3-one | 0–0.03 | 0.02 ± 0.02 | 0–0.04 | 0.01 ± 0.02 | |||||||||||
| 3496 | Lupeol acetate | 0.17–0.72 | 0.49 ± 0.22 | |||||||||||||
| C32:0 | 3522 | Lacceroic acid | 0–0.08 | 0.04 ± 0.04 | ||||||||||||
| Synonyms | RI | Compound Name | Grape Seed Oil | Black seed Oil | Evening Primrose Oil | Passion Fruit Oil | Milk Thistle Oil | Sunflower Oil | Pumpkin Seed Oil | Sesame Oil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MSD (n = 5) | Range | MSD (n = 9) | Range | MSD (n = 8) | Range | MSD (n = 10) | Range | MSD (n = 4) | Range | MSD (n = 10) | Range | MSD (n = 2) | Range | MSD (n = 10) | |||
| trans-2, C6:1 | 957 | (E)-2-Hexenoic acid | 0–0.02 | 0.01 ± 0.01 | ||||||||||||||
| trans-2, C8:1 | 1162 | (E)-2-Octenoic acid | 0–0.02 | 0.01 ± 0.01 | ||||||||||||||
| C12:0 | 1523 | Lauric acid | 0–0.26 | 0.04 ± 0.08 | 0–0.04 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | ||||||||||
| C14:0 | 1758 | Myristic acid | 0.01–0.07 | 0.04 ± 0.02 | 0.1–0.22 | 0.16 ± 0.04 | 0.02–0.05 | 0.03 ± 0.01 | 0.02–0.2 | 0.07 ± 0.06 | 0.03–0.09 | 0.06 ± 0.04 | 0.01–0.08 | 0.05 ± 0.02 | 0.05–0.12 | 0.08 ± 0.06 | 0–0.08 | 0.02 ± 0.02 |
| C15:0 | 1821 | Pentadecanoic acid | 0–0.01 | 0.01 ± 0 | 0.02–0.04 | 0.03 ± 0.01 | 0–0.01 | 0.01 ± 0 | 0–0.03 | 0.02 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | ||||
| trans-7,10, C16:2 | 1887 | 7,10-Hexadecadienoic acid | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0 ± 0.01 | 0–0.02 | 0.02 ± 0.01 | ||||||||||
| cis-7, C16:1 | 1894 | (Z)-7-Hexadecenoic acid | 0.01–0.03 | 0.02 ± 0.01 | 0.01–0.03 | 0.01 ± 0.01 | 0.01–0.03 | 0.02 ± 0.01 | 0.01–0.06 | 0.03 ± 0.02 | 0.01–0.02 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | 0.01–0.03 | 0.02 ± 0 | ||
| cis-9, C16:1 | 1901 | Palmitoleic acid | 0.02–0.16 | 0.08 ± 0.05 | 0.12–0.28 | 0.18 ± 0.05 | 0.01–0.06 | 0.04 ± 0.02 | 0.04–0.31 | 0.17 ± 0.1 | 0.04–0.08 | 0.06 ± 0.03 | 0.03–0.11 | 0.07 ± 0.02 | 0.03–0.14 | 0.12 ± 0.05 | 0.04–0.15 | 0.11 ± 0.03 |
| trans-9,12, C16:2 | 1905 | 9,12-Hexadecadienoic acid | 0–0.04 | 0.02 ± 0.01 | ||||||||||||||
| C16:0 | 1924 | Palmitic acid | 4.46–8.51 | 7.1 ± 1.61 | 12–14.32 | 12.86 ± 0.83 | 6.05–9.74 | 7.59 ± 1.2 | 7.74–17.5 | 11.51 ± 2.98 | 5.14–9.56 | 7.17 ± 1.86 | 4.47–8.35 | 6.39 ± 1.2 | 8.58–13.94 | 11.59 ± 2.75 | 8.21–11.54 | 9.69 ± 1.3 |
| cis-9,12, C17:2 | 1990 | 9,12-Heptadecadienoic acid | 0.01–0.02 | 0.01 ± 0.01 | 0.01–0.02 | 0.01 ± 0 | 0.02–0.04 | 0.03 ± 0.01 | 0.01–0.05 | 0.03 ± 0.02 | 0–0.01 | 0.01 ± 0.01 | 0–0.02 | 0.02 ± 0.01 | ||||
| cis-10, C17:1 | 1996 | (Z)-10-Heptadecenoic acid | 0.01–0.03 | 0.02 ± 0.01 | 0.03–0.05 | 0.04 ± 0.01 | 0.02–0.03 | 0.03 ± 0.01 | 0.01–0.06 | 0.03 ± 0.02 | 0–0.04 | 0.02 ± 0.01 | 0.01–0.04 | 0.03 ± 0.02 | 0.01–0.04 | 0.02 ± 0.01 | ||
| C17:0 | 2026 | Heptadecanoic acid | 0.03–0.06 | 0.05 ± 0.02 | 0.05–0.08 | 0.07 ± 0.01 | 0.06–0.09 | 0.07 ± 0.01 | 0.02–0.15 | 0.07 ± 0.04 | 0.01–0.06 | 0.04 ± 0.03 | 0.02–0.08 | 0.04 ± 0.02 | 0.04–0.10 | 0.09 ± 0.05 | 0.02–0.08 | 0.05 ± 0.02 |
| cis-6,9,12, C18:3 | 2077 | γ-Linolenic acid | 8.34–11.5 | 9.7 ± 1.08 | ||||||||||||||
| cis-9,12, C18:2 | 2087 | Linoleic acid | 51.06–72.89 | 60.96 ± 8.4 | 44.6–52.64 | 49.71 ± 2.63 | 60.8–70.59 | 65.93 ± 3.55 | 57.61–76.77 | 66.23 ± 6.72 | 45.74–52.16 | 49.01 ± 4.34 | 47.38–56.24 | 52.34 ± 2.99 | 38.96–44.74 | 44.56 ± 4.63 | 36.66–54.91 | 42.41 ± 4.91 |
| cis-9, C18:1 | 2102 | (Z)-Oleic acid | 15.98–34.37 | 23.84 ± 7.53 | 24.74–30.56 | 27.34 ± 2.07 | 8.44–10.34 | 9.45 ± 0.6 | 11.55–19.72 | 16.34 ± 2.59 | 27.16–33.12 | 31.59 ± 3.02 | 26.23–38.68 | 31.64 ± 4.18 | 28.69–35.88 | 33.5 ± 6.5 | 25.36–41.45 | 37.31 ± 4.65 |
| trans-9, C18:1 | 2109 | (E)-Oleic acid | 0.79–1.33 | 1.1 ± 0.21 | 1.05–2.15 | 1.45 ± 0.36 | 0.74–1.44 | 0.96 ± 0.22 | 0.01–1.46 | 0.6 ± 0.52 | 0.54–0.63 | 0.59 ± 0.14 | 0.13–1.66 | 0.97 ± 0.47 | 0.56–1.03 | 0.81 ± 0.69 | 0.8–1.97 | 1.23 ± 0.36 |
| trans-13, C18:1 | 2113 | (E)-13-Octadecenoic acid | 0–0.09 | 0.01 ± 0.03 | 0–0.01 | 0.01 ± 0.01 | ||||||||||||
| C18:0 | 2126 | Stearic acid | 3.79–6.86 | 5.1 ± 1.11 | 2.94–4.57 | 3.82 ± 0.55 | 2.35–3.58 | 3.05 ± 0.45 | 1.84–7.64 | 3.85 ± 1.69 | 4.10–6.89 | 5.13 ± 1.03 | 3.74–8.15 | 5.3 ± 1.59 | 3.56–8.19 | 5.28 ± 1.81 | 4.01–8.33 | 6.58 ± 1.4 |
| C20:2 | 2183 | Ethyl linoleic acid | 0.01–0.41 | 0.12 ± 0.17 | 0–0.03 | 0.01 ± 0.01 | 0.03–0.06 | 0.04 ± 0.01 | 0.01–0.07 | 0.03 ± 0.02 | 0.03–0.15 | 0.07 ± 0.04 | 0–0.01 | 0.01 ± 0.01 | 0–0.07 | 0.03 ± 0.02 | ||
| cis-10, C19:1 | 2193 | (Z)-10-Nonadecenoic acid | 0.01–0.02 | 0.01 ± 0 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.02 ± 0.01 | 0–0.03 | 0.01 ± 0.01 | 0.01–0.02 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||
| trans-10, C19:1 | 2205 | 10-Nonadecenoic acid | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | ||||||
| C19:0 | 2222 | Nonadecanoic acid | 0–0.01 | 0 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||||||||||
| cis-11,14, C20:2 | 2284 | (Z)-11,14-Eicosadienoic acid | 0–0.04 | 0.02 ± 0.02 | 2.09–4.8 | 2.92 ± 0.92 | 0.05–0.07 | 0.06 ± 0.01 | 0.01–0.06 | 0.03 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | ||||
| cis-11, C20:1 | 2296 | (Z)-11-Eicosenoic acid | 0.18–0.27 | 0.23 ± 0.04 | 0.35–0.87 | 0.52 ± 0.18 | 0.27–0.9 | 0.49 ± 0.2 | 0–0.35 | 0.15 ± 0.1 | 0.42–0.78 | 0.69 ± 0.19 | 0.15–0.35 | 0.23 ± 0.07 | 0.09–0.19 | 0.15 ± 0.06 | 0.17–0.57 | 0.26 ± 0.11 |
| C20:0 | 2322 | Arachidic acid | 0.21–0.42 | 0.28 ± 0.08 | 0.16–0.36 | 0.26 ± 0.06 | 0.35–0.62 | 0.49 ± 0.1 | 0.1–0.56 | 0.24 ± 0.13 | 1.74–3.40 | 2.42 ± 0.86 | 0.28–0.64 | 0.43 ± 0.14 | 0.45–0.69 | 0.56 ± 0.21 | 0.48–1.11 | 0.77 ± 0.17 |
| C21:0 | 2422 | Heneicosanoic acid | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0 | ||||
| cis-13–16, C22:2 | 2495 | (Z)-13,16-Docasadienoic acid | 0–0.08 | 0.03 ± 0.03 | ||||||||||||||
| cis-13, C22:1 | 2499 | Erucic acid | 0–0.05 | 0.02 ± 0.02 | 0–0.43 | 0.16 ± 0.13 | 0–0.06 | 0.02 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | ||||||||
| C22:0 | 2522 | Behenic acid | 0.04–1.28 | 0.44 ± 0.57 | 0.03–0.23 | 0.1 ± 0.07 | 0.16–0.39 | 0.25 ± 0.09 | 0.05–0.26 | 0.11 ± 0.06 | 1.42–2.59 | 2.05 ± 0.71 | 0.81–2.18 | 1.39 ± 0.46 | 0.10–0.21 | 0.16 ± 0.09 | 0.14–0.72 | 0.25 ± 0.17 |
| C23:0 | 2622 | Tricosanoic acid | 0.01–0.06 | 0.03 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0.02–0.05 | 0.03 ± 0.01 | 0–0.06 | 0.02 ± 0.02 | 0–0.04 | 0.03 ± 0.02 | 0.03–0.09 | 0.05 ± 0.02 | 0.01–0.03 | 0.02 ± 0.03 | 0–0.04 | 0.02 ± 0.01 |
| 2698 | Heptacosane | 0–0.08 | 0.02 ± 0.03 | |||||||||||||||
| cis-15, C24:1 | 2699 | (Z)-15-Tetracosenoic acid | 0–0.33 | 0.11 ± 0.1 | 0–0.02 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | ||||||||||
| C24:0 | 2722 | Lignoceric acid | 0.02–0.54 | 0.17 ± 0.23 | 0.02–0.1 | 0.06 ± 0.03 | 0.07–0.22 | 0.12 ± 0.05 | 0.05–0.28 | 0.12 ± 0.08 | 0.51–0.78 | 0.69 ± 0.17 | 0.27–0.83 | 0.53 ± 0.19 | 0.07–0.13 | 0.10 ± 0.07 | 0.09–0.35 | 0.17 ± 0.08 |
| 2791 | Octacosane | |||||||||||||||||
| 2803 | (E)-Squalene | 0–0.02 | 0.01 ± 0.01 | 0–0.04 | 0.01 ± 0.01 | 0–0.07 | 0.02 ± 0.02 | 0.01–0.08 | 0.04 ± 0.03 | 0.01–0.07 | 0.05 ± 0.04 | 0–0.02 | 0.01 ± 0.01 | 0.10–0.19 | 0.15 ± 0.08 | |||
| C25:0 | 2830 | Pentacosanoic acid | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | 0.01–0.05 | 0.02 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.02 | 0.01–0.05 | 0.03 ± 0.01 | ||||
| 2899 | Nonacosane | 0–0.01 | 0 ± 0.01 | 0–0.04 | 0.01 ± 0.01 | 0–0.05 | 0.01 ± 0.02 | 0.02–0.05 | 0.04 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | |||||||
| C26:0 | 2926 | Hexacosanoic acid | 0.01–0.04 | 0.02 ± 0.01 | 0–0.04 | 0.01 ± 0.01 | 0–0.04 | 0.02 ± 0.01 | 0.01–0.07 | 0.03 ± 0.02 | 0–0.03 | 0.02 ± 0.01 | 0.01–0.23 | 0.05 ± 0.07 | 0.02–0.04 | 0.03 ± 0.02 | 0.02–0.09 | 0.05 ± 0.03 |
| 3091 | β-Sitosteryl acetate | 0–0.06 | 0.02 ± 0.03 | 0.01–0.68 | 0.19 ± 0.29 | 0–0.05 | 0.01 ± 0.02 | 0–0.06 | 0.01 ± 0.02 | |||||||||
| 3096 | Untriacontane | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | |||||||||||
| C28:0 | 3130 | Octacosanoic acid | 0–0.03 | 0.01 ± 0.01 | 0–0.01 | 0 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||||||||
| 3163 | Sesamin | 0.02–0.81 | 0.25 ± 0.22 | |||||||||||||||
| 3227 | Methyl cholesterol | 0–0.03 | 0.01 ± 0.02 | 0–0.15 | 0.04 ± 0.06 | 0–0.04 | 0.01 ± 0.02 | 0–0.29 | 0.13 ± 0.09 | |||||||||
| 3246 | Stigmasterol | 0–0.04 | 0.02 ± 0.02 | 0–0.04 | 0.01 ± 0.02 | 0–0.1 | 0.02 ± 0.04 | 0–0.06 | 0.02 ± 0.02 | 0–0.1 | 0.02 ± 0.03 | |||||||
| 3253 | 3β-Methoxystigmast-5-ene | 0–0.14 | 0.04 ± 0.05 | |||||||||||||||
| 3303 | γ-Sitosterol | 0–0.41 | 0.22 ± 0.17 | 0–0.22 | 0.06 ± 0.09 | 0–1.93 | 0.83 ± 0.78 | 0–0.14 | 0.05 ± 0.06 | 0.25–0.33 | 0.28 ± 0.11 | 0–0.5 | 0.23 ± 0.14 | 0–0.9 | 0.46 ± 0.3 | |||
| C30:0 | 3328 | Triacontanoic acid | 0–0.07 | 0.02 ± 0.02 | ||||||||||||||
| 3445 | Stigmast-4-en-3-one | 0–0.02 | 0 ± 0.01 | 0.02–0.14 | 0.06 ± 0.04 | |||||||||||||
| Synonyms | RI | Compound Name | Soybean Oil | Flax Seed Oil | Kukui Oil | Red Raspberry Seed Oil | Walnut Oil | Chia Seed Oil | Hemp Seed Oil | Rosehip Oil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MSD (n = 8) | Range | MSD (n = 7) | Range | MSD (n = 5) | Range | MSD (n = 6) | Range | MSD (n = 7) | Range | MSD (n = 5) | Range | MSD (n = 4) | Range | MSD (n = 7) | |||
| C12:0 | 1523 | Lauric acid | 0–0.02 | 0.01 ± 0.02 | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | ||||||||||
| C14:0 | 1758 | Myristic acid | 0.01–0.08 | 0.06 ± 0.02 | 0.02–0.06 | 0.04 ± 0.03 | 0.05–0.09 | 0.07 ± 0.01 | 0.02–0.05 | 0.04 ± 0.03 | 0.01–0.04 | 0.03 ± 0.02 | 0.01–0.03 | 0.03 ± 0.01 | 0.02–0.06 | 0.04 ± 0.01 | ||
| 1817 | Hexadecanal | 0.71–1.06 | 0.92 ± 0.23 | |||||||||||||||
| C15:0 | 1821 | Pentadecanoic acid | 0–0.02 | 0.01 ± 0.01 | 0.01–0.03 | 0.02 ± 0.02 | 0.01–0.03 | 0.02 ± 0.01 | 0–0.04 | 0.02 ± 0.02 | 0.00–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.04 | 0.02 ± 0.01 | ||
| 1836 | Neophytadiene | 0.24–0.37 | 0.33 ± 0.12 | |||||||||||||||
| cis-7, C16:1 | 1894 | (Z)-7-Hexadecenoic acid | 0–0.02 | 0.01 ± 0.01 | 0.02–0.04 | 0.03 ± 0.02 | 0.01–0.04 | 0.02 ± 0.01 | 0.17–0.25 | 0.21 ± 0.09 | 0–0.05 | 0.03 ± 0.04 | 0.01–0.02 | 0.01 ± 0 | 0–0.03 | 0.01 ± 0.02 | 0.02–0.04 | 0.02 ± 0.01 |
| cis-9, C16:1 | 1901 | Palmitoleic acid | 0.01–0.09 | 0.07 ± 0.03 | 0.05–0.09 | 0.07 ± 0.05 | 0.08–0.12 | 0.11 ± 0.07 | 0.11–0.17 | 0.15 ± 0.06 | 0.08–0.13 | 0.10 ± 0.04 | 0.01–0.06 | 0.05 ± 0.03 | 0.03–0.12 | 0.09 ± 0.04 | 0–0.19 | 0.11 ± 0.06 |
| C16:0 | 1924 | Palmitic acid | 5.41–13.14 | 11.49 ± 2.53 | 4.23–7.87 | 5.46 ± 1.32 | 6.27–8.57 | 7.47 ± 1.02 | 3.21–6.32 | 4.88 ± 1.56 | 5.28–8.64 | 7.52 ± 1.23 | 5.04–8.10 | 7.45 ± 1.43 | 4.53–8.98 | 7.52 ± 2.07 | 3.35–6.0 | 4.51 ± 0.82 |
| cis-9,12, C17:2 | 1990 | 9,12-Heptadecadienoic acid | 0.01–0.04 | 0.03 ± 0.01 | 0.01–0.03 | 0.02 ± 0.01 | 0–0.15 | 0.06 ± 0.05 | ||||||||||
| 1992 | (Z)-9-Octadecenal | 0.86–2.35 | 1.72 ± 0.43 | |||||||||||||||
| cis-10, C17:1 | 1996 | (Z)-10-Heptadecenoic acid | 0.01–0.06 | 0.05 ± 0.02 | 0.01–0.04 | 0.02 ± 0.03 | 0.01–0.04 | 0.03 ± 0.02 | 0.01–0.06 | 0.03 ± 0.03 | 0.01–0.02 | 0.01 ± 0 | 0–0.04 | 0.03 ± 0.02 | 0.01–0.09 | 0.05 ± 0.02 | ||
| 2018 | Octadecanal | 2.01–4.51 | 3.28 ± 1.03 | |||||||||||||||
| C17:0 | 2026 | Heptadecanoic acid | 0.03–0.12 | 0.1 ± 0.03 | 0.03–0.06 | 0.04 ± 0.05 | 0.02–0.06 | 0.05 ± 0.01 | 0.02–0.06 | 0.04 ± 0.03 | 0.03–0.06 | 0.04 ± 0.02 | 0.02–0.07 | 0.06 ± 0.02 | 0.02–0.11 | 0.07 ± 0.03 | ||
| cis-6,9,12, C18:3 | 2077 | γ-Linolenic acid | 0.25–0.78 | 0.55 ± 0.22 | ||||||||||||||
| cis-9,12, C18:2 | 2087 | Linoleic acid | 46.35–55.97 | 48.26 ± 3.18 | 28.54–32.14 | 30.24 ± 3.26 | 40.35–45.75 | 43.02 ± 3.86 | 37.86–44.25 | 41.63 ± 4.66 | 52.61–56.28 | 54.48 ± 4.27 | 19.89–23.19 | 22.09 ± 1.65 | 51.03–59.63 | 53.85 ± 3.91 | 41.38–56.70 | 47.81 ± 4.65 |
| cis-9,12,15, C18:3 | 2096 | Linolenic acid | 0–6.91 | 2.55 ± 3.22 | 25.78–35.26 | 29.89 ± 6.87 | 20.27–24.18 | 22.08 ± 2.75 | 8.63–13.41 | 10.15 ± 2.15 | 3.12–6.44 | 4.92 ± 1.45 | 56.25–60.73 | 58.25 ± 3.98 | 15.7–19.4 | 17.85 ± 1.62 | 20.45–29.44 | 26.09 ± 3.06 |
| cis-9, C18:1 | 2102 | (Z)-Oleic acid | 22.42–30.63 | 27.11 ± 3.14 | 20.27–25.63 | 23.19 ± 3.47 | 15.20–19.84 | 17.99 ± 1.96 | 17.47–23.72 | 19.45 ± 3.02 | 24.74–29.21 | 26.27 ± 2.89 | 0.80–1.46 | 1.02 ± 0.82 | 10.34–14.64 | 11.95 ± 2.03 | 6.25–23.24 | 13.24 ± 5.54 |
| trans-9, C18:1 | 2109 | (E)-Oleic acid | 0.78–2.31 | 1.9 ± 0.48 | 0.84–1.06 | 0.96 ± 0.65 | 0.44–0.76 | 0.61 ± 0.32 | 0.56–0.77 | 0.69 ± 0.27 | 0.78–1.24 | 1.16 ± 0.69 | 0.55–1.66 | 1.02 ± 0.46 | 0.17–1.52 | 0.89 ± 0.44 | ||
| trans-13, C18:1 | 2113 | (E)-13-Octadecenoic acid | ||||||||||||||||
| C18:0 | 2126 | Stearic acid | 3.09–7.52 | 5.77 ± 1.44 | 4.41–6.58 | 5.14 ± 1.68 | 3.87–7.21 | 5.40 ± 1.12 | 1.86–3.42 | 2.55 ± 0.56 | 2.14–5.12 | 3.73 ± 1.09 | 3.28–5.26 | 4.56 ± 0.58 | 2.78–4.37 | 3.75 ± 0.71 | 2.37–4.96 | 3.29 ± 0.86 |
| 2163 | (2E)-Nonadecenal | 0–0.1 | 0.01 ± 0 | |||||||||||||||
| C20:2 | 2183 | Ethyl linoleate | 0.04–0.1 | 0.07 ± 0.02 | 0.16–0.28 | 0.24 ± 0.17 | 0.04–0.08 | 0.06 ± 0.03 | 0–0.02 | 0.01 ± 0.01 | 0.01–0.05 | 0.03 ± 0.02 | 0.02–0.21 | 0.09 ± 0.07 | ||||
| cis-10, C19:1 | 2193 | (Z)-10-Nonadecenoic acid | 0.01–0.02 | 0.02 ± 0.01 | 0.09–0.14 | 0.12 ± 0.08 | 0.01–0.04 | 0.03 ± 0.02 | 0–0.04 | 0.02 ± 0.02 | ||||||||
| trans-10, C19:1 | 2205 | 10-Nonadecenoic acid | 0.01–0.03 | 0.02 ± 0.01 | 0.06–0.10 | 0.07 ± 0.05 | 0–0.02 | 0–0.01 | 0.01 ± 0.01 | 0–0.05 | 0.02 ± 0.02 | |||||||
| C19:0 | 2222 | Nonadecanoic acid | 0.01–0.2 | 0.01 ± 0.01 | 0–0.01 | 0 ± 0.01 | 0–0.12 | 0.03 ± 0.04 | ||||||||||
| cis-11,14, C20:2 | 2284 | (Z)-11,14-Eicosadienoic acid | 0.02–0.06 | 0.04 ± 0.01 | 0.06–0.09 | 0.07 ± 0.04 | 0.05–0.07 | 0.06 ± 0.01 | 0–0.05 | 0.03 ± 0.03 | 0.01–0.04 | 0.03 ± 0.02 | 0.04–0.13 | 0.09 ± 0.04 | 0.01–0.27 | 0.17 ± 0.09 | ||
| cis-11, C20:1 | 2296 | (Z)-11-Eicosenoic acid | 0.09–0.34 | 0.28 ± 0.09 | 0.21–0.32 | 0.26 ± 0.19 | 0.32–0.36 | 0.34 ± 0.12 | 0.19–0.27 | 0.24 ± 0.11 | 0.16–0.28 | 0.25 ± 0.12 | 0.25–0.43 | 0.40 ± 0.21 | 0.37–0.77 | 0.56 ± 0.18 | 0.13–0.87 | 0.59 ± 0.26 |
| C20:0 | 2322 | Arachidic acid | 0.23–0.73 | 0.56 ± 0.16 | 0.22–0.27 | 0.25 ± 0.20 | 0.35–0.39 | 0.37 ± 0.13 | 0.51–0.76 | 0.68 ± 0.19 | 0.19–0.24 | 0.22 ± 0.09 | 0.21–0.41 | 0.35 ± 0.15 | 0.8–1.34 | 1.02 ± 0.25 | 0.2–1.93 | 1.14 ± 0.54 |
| C21:0 | 2422 | Heneicosanoic acid | 0.02–0.05 | 0.04 ± 0.01 | 0.01–0.06 | 0.05 ± 0.03 | 0–0.02 | 0.01 ± 0.01 | 0–0.08 | 0.04 ± 0.02 | ||||||||
| cis-13, C22:1 | 2499 | Erucic acid | 0.02–0.03 | 0.03 ± 0.01 | ||||||||||||||
| 2498 | Pentacosane | 0.14–0.29 | 0.22 ± 0.12 | |||||||||||||||
| C22:0 | 2522 | Behenic acid | 0.38–0.88 | 0.68 ± 0.16 | 0.48–0.54 | 0.51 ± 0.41 | 0.95–1.05 | 1.01 ± 0.08 | 0.74–1.08 | 0.97 ± 0.18 | 0.61–0.76 | 0.68 ± 0.21 | 0.08–0.12 | 0.10 ± 0.02 | 0.27–0.64 | 0.43 ± 0.16 | 0.13–0.49 | 0.31 ± 0.13 |
| C23:0 | 2622 | Tricosanoic acid | 0.04–0.1 | 0.07 ± 0.02 | 0.03–0.05 | 0.04 ± 0.02 | 0.01–0.06 | 0.04 ± 0.03 | 0.01–0.04 | 0.03 ± 0.01 | 0.03–0.06 | 0.05 ± 0.02 | 0.02–0.12 | 0.06 ± 0.04 | ||||
| 2698 | Heptacosane | 3.64–6.89 | 5.26 ± 1.78 | 0–0.03 | 0.01 ± 0.01 | |||||||||||||
| cis-15, C24:1 | 2699 | (Z)-15-Tetracosenoic acid | 0–0.03 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | ||||||||||||
| C24:0 | 2722 | Lignoceric acid | 0.16–0.42 | 0.28 ± 0.1 | 0.19–0.26 | 0.23 ± 0.14 | 0.62–0.67 | 0.64 ± 0.15 | 0.32–0.49 | 0.44 ± 0.14 | 0.20–0.62 | 0.40 ± 0.24 | 0.09–0.15 | 0.12 ± 0.05 | 0.11–0.36 | 0.23 ± 0.12 | 0.06–0.25 | 0.15 ± 0.06 |
| 2791 | Octacosane | 0.27–0.33 | 0.31 ± 0.07 | |||||||||||||||
| 2803 | (E)-Squalene | 0–0.01 | 0.01 ± 0.01 | 0.01–0.04 | 0.02 ± 0.03 | 0.74–0.95 | 0.88 ± 0.09 | |||||||||||
| C25:0 | 2830 | Pentacosanoic acid | 0.01–0.05 | 0.03 ± 0.01 | 0.01–0.05 | 0.03 ± 0.04 | 0.03–0.07 | 0.05 ± 0.02 | 0–0.05 | 0.04 ± 0.02 | 0–0.04 | 0.02 ± 0.02 | 0–0.04 | 0.02 ± 0.02 | ||||
| 2871 | Cyclotetracosane | 0–0.03 | 0.01 ± 0.01 | |||||||||||||||
| 2886 | Nonacos-1-ene | 0.54–0.71 | 0.65 ± 0.18 | 0–0.27 | 0.18 ± 0.1 | |||||||||||||
| 2899 | Nonacosane | 0.02–0.06 | 0.04 ± 0.03 | 2.14–4.65 | 3.94 ± 1.05 | 0–0.02 | 0.01 ± 0.01 | 0–0.19 | 0.1 ± 0.06 | |||||||||
| C26:0 | 2926 | Hexacosanoic acid | 0.01–0.06 | 0.03 ± 0.02 | 0.01–0.04 | 0.03 ± 0.02 | 0.08–0.12 | 0.10 ± 0.05 | 0.01–0.06 | 0.04 ± 0.03 | 0.01–0.05 | 0.03 ± 0.01 | 0–0.04 | 0.02 ± 0.02 | 0–0.03 | 0.02 ± 0.01 | ||
| 3091 | β-Sitosteryl acetate | 0.02–0.11 | 0.08 ± 0.04 | |||||||||||||||
| 3096 | Untriacontane | 0–0.03 | 0.01 ± 0.02 | 0.49–0.58 | 0.55 ± 0.07 | 0–0.02 | 0.01 ± 0.01 | |||||||||||
| 3087 | Ethyl octacosyl ether | 0–0.21 | 0.1 ± 0.07 | |||||||||||||||
| C28:0 | 3130 | Octacosanoic acid | 0–0.03 | 0.02 ± 0.01 | ||||||||||||||
| 3227 | Methyl cholesterol | 0–0.16 | 0.03 ± 0.06 | 0.01–0.15 | 0.08 ± 0.04 | 0–0.17 | 0.06 ± 0.08 | |||||||||||
| 3246 | Stigmasterol | 0–0.15 | 0.03 ± 0.05 | 0.06–0.11 | 0.08 ± 0.04 | 0.01–0.05 | 0.04 ± 0.02 | 0–1.18 | 0.66 ± 0.52 | |||||||||
| 3253 | 3β-Methoxystigmast-5-ene | 0–0.03 | 0.01 ± 00.01 | 0–0.06 | 0.01 ± 0.02 | |||||||||||||
| 3303 | γ-Sitosterol | 0–0.48 | 0.27 ± 0.18 | 0.21–0.26 | 0.23 ± 0.18 | 0.22–0.27 | 0.25 ± 0.13 | 0.15–1.02 | 0.56 ± 0.39 | 0–0.93 | 0.59 ± 0.41 | |||||||
| 3445 | Stigmast-4-en-3-one | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | |||||||||||||
| Synonyms | RI | Compound Name | Almond Oil | Avocado Oil | Carrot Seed Oil | Moringa Oil | Apricot Kernel Oil | Camellia Seed Oil | Macadamia Oil | Olive Oil | Marula Crude Oil | Argan Oil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MSD (n = 10) | Range | MSD (n = 10) | Range | MSD (N = 9) | Range | MSD (n = 7) | Range | MSD (n = 5) | Range | MSD (n = 5) | Range | MSD (n = 5) | Range | MSD (n = 6) | Range | MSD (n = 5) | Range | MSD (n = 8) | |||
| 1371 | Isoledene | 0–1.2 | 0.22 ± 0.37 | |||||||||||||||||||
| 1376 | β-Cubebene | 0–0.13 | 0.02 ± 0.04 | |||||||||||||||||||
| 1380 | Daucene | 0.25–3.23 | 1.26 ± 1.21 | |||||||||||||||||||
| 1443 | Acora-2,4(15)-diene | 0.23–1.64 | 0.76 ± 0.63 | |||||||||||||||||||
| 1492 | trans-Muurola-4(14),5-diene | 0–0.09 | 0.02 ± 0.03 | |||||||||||||||||||
| 1500 | α-Muurolene | 0–0.18 | 0.03 ± 0.06 | |||||||||||||||||||
| 1520 | trans-Calamenene | 0–0.17 | 0.05 ± 0.05 | |||||||||||||||||||
| 1611 | 1-[1-Methoxy-3,3-dimethyl-2-(3-methylbuta-1,3-dienyl)cyclopentyl]ethanone | 0–0.36 | 0.06 ± 0.12 | |||||||||||||||||||
| C12:0 | 1523 | Lauric acid | 0–0.05 | 0.01 ± 0.02 | 0.01–0.03 | 0.01 ± 0.01 | 0.01–0.06 | 0.04 ± 0.02 | ||||||||||||||
| C14:0 | 1758 | Myristic acid | 0–0.05 | 0.03 ± 0.01 | 0.02–0.27 | 0.08 ± 0.10 | 0.02–0.06 | 0.03 ± 0.01 | 0.06–0.13 | 0.08 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0–0.03 | 0.02 ± 0.02 | 0.40–0.76 | 0.6 ± 0.02 | 0–0.04 | 0.01 ± 0.01 | 0.02–0.07 | 0.05 ± 0.03 | 0.05–0.14 | 0.11 ± 0.03 |
| C15:0 | 1821 | Pentadecanoic acid | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | 0–0.03 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0.01–0.05 | 0.04 ± 0.01 | ||
| cis-7, C16:1 | 1894 | (Z)-7-Hexadecenoic acid | 0–0.03 | 0.02 ± 0.01 | 0.02–0.11 | 0.08 ± 0.03 | 0.02–0.12 | 0.06 ± 0.05 | 0.04–0.11 | 0.07 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0.01–0.02 | 0.01 ± 0.01 | 0.05–0.09 | 0.07 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0.01–0.02 | 0.01 ± 0 |
| cis-9, C16:1 | 1901 | Palmitoleic acid | 0.08–0.65 | 0.42 ± 0.17 | 1.22–10.65 | 4.78 ± 3.12 | 0.07–0.29 | 0.17 ± 0.1 | 1.08–1.68 | 1.37 ± 0.26 | 0.49–0.71 | 0.56 ± 0.23 | 0.03–0.08 | 0.06 ± 0.03 | 16.23–21.70 | 19.65 ± 1.95 | 0.53–2.58 | 1.62 ± 0.7 | 0.07–0.12 | 0.09 ± 0.06 | 0.01–0.09 | 0.08 ± 0.01 |
| C16:0 | 1924 | Palmitic acid | 4.95–9.83 | 7.1 ± 1.43 | 11.79–21.68 | 15.85 ± 3.31 | 3.88–4.86 | 4.28 ± 0.34 | 5.01–7.33 | 6.14 ± 0.84 | 3.78–6.09 | 4.86 ± 1.02 | 5.45–9.28 | 7.60 ± 1.05 | 5.57–9.5 | 8.90 ± 1.08 | 14.34–19.33 | 17.31 ± 1.95 | 11.86–15.75 | 13.65 ± 2.54 | 6.02–14.91 | 12.65 ± 2.85 |
| cis-9,12, C17:2 | 1990 | 9,12-Heptadecadienoic acid | 0–0.02 | 0.01 ± 0.01 | ||||||||||||||||||
| cis-10, C17:1 | 1996 | (Z)-10-Heptadecenoic acid | 0.03–0.17 | 0.11 ± 0.04 | 0.03–0.11 | 0.08 ± 0.03 | 0.03–0.06 | 0.04 ± 0.01 | 0.02–0.07 | 0.04 ± 0.02 | 0.10–0.17 | 0.14 ± 0.07 | 0.01–0.06 | 0.02 ± 0.01 | 0.02–0.07 | 0.04 ± 0.02 | 0.07–0.32 | 0.18 ± 0.11 | 0.03–0.06 | 0.04 ± 0.03 | 0.01–0.04 | 0.02 ± 0.01 |
| C17:0 | 2026 | Heptadecanoic acid | 0–0.08 | 0.05 ± 0.02 | 0.02–0.07 | 0.03 ± 0.02 | 0.02–0.04 | 0.03 ± 0.01 | 0.06–0.11 | 0.09 ± 0.02 | 0.01–0.05 | 0.03 ± 0.02 | 0.02–0.06 | 0.02 ± 0.01 | 0.01–0.03 | 0.02 ± 0.01 | 0.04–0.24 | 0.14 ± 0.10 | 0.10–0.14 | 0.12 ± 0.08 | 0.05–0.10 | 0.08 ± 0.02 |
| cis-9,12, C18:2 | 2087 | Linoleic acid | 23.26–37.72 | 27.81 ± 5.12 | 7.05–14.45 | 10.31 ± 2.08 | 7.54–12.85 | 9.75 ± 2.14 | 0.23–1.08 | 0.62 ± 0.27 | 24.26–28.87 | 26.54 ± 3.21 | 4.36–8.34 | 5.45 ± 1.01 | 1.09–2.99 | 2.53 ± 0.4 | 5.95–17.09 | 11.95 ± 4.59 | 4.26–8.74 | 6.33 ± 2.08 | 28.50–30.86 | 29.95 ± 0.69 |
| cis-9, C18:1 | 2102 | (Z)-Oleic acid | 49.74–67.3 | 60.32 ± 6.11 | 44.08–68.14 | 60.27 ± 7.70 | 72.89–80.86 | 77.56 ± 2.77 | 47.61–67.15 | 56.51 ± 7.39 | 61.49–67.61 | 64.97 ± 6.03 | 74.89–83.02 | 78.57 ± 8.01 | 42.89–47.53 | 45.56 ± 4.01 | 52.22–64.78 | 59.01 ± 5.44 | 65.47–72.29 | 68.86 ± 4.38 | 42.38–55.80 | 46.77 ± 4.11 |
| trans-9, C18:1 | 2109 | (E)-Oleic acid | 0.14–1.99 | 1.03 ± 0.83 | 1.14–7.08 | 4.51 ± 2.13 | 0.08–0.88 | 0.51 ± 0.33 | 5.33–6.89 | 6.09 ± 0.63 | 1.03–1.86 | 1.46 ± 0.89 | 0.10–1.06 | 0.34 ± 0.24 | 1.8–4.84 | 3.89 ± 0.75 | 2.08–4.16 | 3.33 ± 0.77 | 0.48–0.56 | 0.53 ± 0.22 | 0.20–1.28 | 0.59 ± 0.43 |
| trans-13, C18:1 | 2113 | (E)-13-Octadecenoic acid | 0.09–0.22 | 0.15 ± 0.04 | 0.01–0.03 | 0.02 ± 0.01 | ||||||||||||||||
| C18:0 | 2126 | Stearic acid | 1.52–4.31 | 2.34 ± 0.78 | 0.65–3.70 | 1.89 ± 1.26 | 0.82–4.4 | 2.57 ± 1.46 | 5.42–9.13 | 6.83 ± 1.17 | 1.42–2.69 | 1.78 ± 1.14 | 2.01–3.04 | 2.15 ± 0.40 | 1.56–4.29 | 3.50 ± 1.98 | 3.05–5.27 | 4.17 ± 0.91 | 6.23–10.41 | 8.58 ± 2.63 | 4.26–8.37 | 7.2 ± 1.4 |
| C20:2 | 2183 | Ethyl linoleate | 0–0.07 | 0.04 ± 0.02 | 0–0.06 | 0.03 ± 0.02 | 0–0.03 | 0.01 ± 0.01 | 0–0.13 | 0.04 ± 0.04 | 0.01–0.03 | 0.02 ± 0.01 | 0.01–0.07 | 0.04 ± 0.02 | 0.01–0.04 | 0.02 ± 0.03 | 0–0.03 | 0.02 ± 0.01 | 0–0.13 | 0.02 ± 0.05 | ||
| cis-10, C19:1 | 2193 | (Z)-10-Nonadecenoic acid | 0–0.03 | 0.02 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0.03–0.06 | 0.05 ± 0.01 | 0.02–0.04 | 0.03 ± 0.01 | 0–0.06 | 0.02 ± 0.02 | 0.02–0.06 | 0.04 ± 0.03 | 0.02–0.04 | 0.03 ± 0.01 | ||||||
| trans-10, C19:1 | 2205 | 10-Nonadecenoic acid | 0–0.01 | 0.01 ± 0 | 0–0.01 | 0.01 ± 0.01 | 0.01–0.01 | 0.01 ± 0 | 0.02–0.03 | 0.02 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||||||||
| C19:0 | 2222 | Nonadecanoic acid | 0–0.03 | 0.02 ± 0.01 | 0–0.03 | 0.01 ± 0.01 | 0–0.03 | 0.01 ± 0.02 | ||||||||||||||
| cis-11,14, C20:2 | 2284 | cis-11,14-Eicosadienoic acid | 0–0.04 | 0.01 ± 0.02 | ||||||||||||||||||
| cis-11, C20:1 | 2296 | (Z)-11-Eicosenoic acid | 0.03–0.26 | 0.12 ± 0.06 | 0.16–0.74 | 0.35 ± 0.21 | 0.13–0.35 | 0.25 ± 0.07 | 1.88–3.59 | 2.95 ± 0.57 | 0.09–0.21 | 0.15 ± 0.09 | 0.45–0.68 | 0.53 ± 0.23 | 1.45–3.09 | 2.41 ± 1.02 | 0.27–0.37 | 0.32 ± 0.04 | 0.23–0.56 | 0.41 ± 0.23 | 0.38–0.60 | 0.48 ± 0.08 |
| C20:0 | 2322 | Arachidic acid | 0.08–0.35 | 0.14 ± 0.08 | 0.08–0.86 | 0.30 ± 0.32 | 0.14–0.43 | 0.29 ± 0.1 | 3.63–7.03 | 4.9 ± 1.1 | 0.11–0.19 | 0.16 ± 0.08 | 0.05–0.08 | 0.04 ± 0.02 | 0.75–3.57 | 3.45 ± 1.13 | 0.55–0.83 | 0.71 ± 0.12 | 0.52–0.89 | 0.72 ± 0.31 | 0.42–0.70 | 0.55 ± 0.11 |
| C21:0 | 2422 | Heneicosanoic acid | 0–0.01 | 0.01 ± 0 | 0.04–0.11 | 0.07 ± 0.02 | 0–0.01 | 0.01 ± 0.01 | 0.01–0.03 | 0.02 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | ||||||||||
| 2464 | (Z)-Docos-13-en-1-ol | 0–0.02 | 0.01 ± 0.01 | |||||||||||||||||||
| 2498 | Pentacosane | 0–0.01 | 0 ± 0.01 | |||||||||||||||||||
| cis-13, C22:1 | 2499 | Erucic acid | 0–0.10 | 0.03 ± 0.04 | 0–0.04 | 0.01 ± 0.01 | 0.07–0.26 | 0.18 ± 0.07 | 0.09–0.11 | 0.10 ± 0.04 | 0.13–0.38 | 0.30 ± 0.12 | 0.01–0.04 | 0.02 ± 0.01 | ||||||||
| C22:0 | 2522 | Behenic acid | 0–1.27 | 0.15 ± 0.39 | 003–1.53 | 0.40 ± 0.61 | 0.07–1.36 | 0.69 ± 0.58 | 5.53–15.51 | 9.74 ± 3.58 | 0.05–0.09 | 0.07 ± 0.03 | 0.08–0.13 | 0.12 ± 0.05 | 0.75–1.29 | 0.95 ± 0.69 | 0.15–0.32 | 0.23 ± 0.07 | 0.12–0.16 | 0.15 ± 0.06 | 0.14–1.61 | 0.39 ± 0.49 |
| 2561 | Meadowlactone | 0–0.01 | 0.01 ± 0.01 | |||||||||||||||||||
| C23:0 | 2622 | Tricosanoic acid | 0–0.04 | 0.01 ± 0.01 | 0.01–0.07 | 0.03 ± 0.02 | 0–0.05 | 0.03 ± 0.02 | 0.06–0.2 | 0.14 ± 0.06 | 0.01–0.03 | 0.02 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0.02–0.06 | 0.04 ± 0.02 | 0–0.03 | 0.02 ± 0.01 | 0.02–0.08 | 0.03 ± 0.02 |
| 2698 | Heptacosane | 0–0.06 | 0.02 ± 0.02 | |||||||||||||||||||
| cis-15, C24:1 | 2699 | (Z)-15-Tetracosenoic acid | 0–0.04 | 0.01 ± 0.02 | 0.05–0.08 | 0.06 ± 0.03 | 0.01–0.02 | 0.01 ± 0 | 0.02–0.05 | 0.03 ± 0.01 | ||||||||||||
| C24:0 | 2722 | Lignoceric acid | 0–0.42 | 0.06 ± 0.13 | 0.06–0.80 | 0.25 ± 0.30 | 0.06–0.59 | 0.28 ± 0.2 | 1.31–3.82 | 2.68 ± 0.96 | 0.02–0.08 | 0.05 ± 0.06 | 0.02–0.07 | 0.05 ± 0.02 | 0.23–0.73 | 0.58 ± 0.25 | 0.08–0.22 | 0.15 ± 0.06 | 0.16–0.24 | 0.21 ± 0.09 | 0.07–0.77 | 0.22 ± 0.23 |
| 2803 | (E)-Squalene | 0.01–0.11 | 0.05 ± 0.04 | 0.01–0.04 | 0.03 ± 0.07 | 0.01–0.04 | 0.03 ± 0.02 | 0.02–0.47 | 0.24 ± 0.15 | 0.09–0.74 | 0.45 ± 0.26 | |||||||||||
| C25:0 | 2830 | Pentacosanoic acid | 0.02–0.07 | 0.04 ± 0.02 | 0.02–0.06 | 0.04 ± 0.02 | 0.01–0.03 | 0.02 ± 0.01 | 0–0.01 | 0.01 ± 0.01 | 0.01–0.03 | 0.02 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | 0–0.04 | 0.03 ± 0.01 | 0–0.02 | 0.02 ± 0.01 | ||||
| 2899 | Nonacosane | 0.01–0.06 | 0.03 ± 0.02 | 0–0.33 | 0.08 ± 0.11 | 0–0.03 | 0.01 ± 0.01 | |||||||||||||||
| C26:0 | 2926 | Hexacosanoic acid | 0.02–0.10 | 0.05 ± 0.02 | 0.01–0.06 | 0.03 ± 0.02 | 0.08–0.28 | 0.19 ± 0.08 | 0.01–0.04 | 0.03 ± 0.05 | 0–0.01 | 0.01 ± 0.01 | 0.09–0.16 | 0.12 ± 0.06 | 0.02–0.12 | 0.06 ± 0.04 | 0.04–0.09 | 0.07 ± 0.05 | 0.03–0.14 | 0.07 ± 0.04 | ||
| 3091 | β-Sitosteryl acetate | 0–0.02 | 0.01 ± 0.01 | 0–0.53 | 0.07 ± 0.16 | 0–0.04 | 0.01 ± 0.02 | 0–0.04 | 0.01 ± 0.02 | 0.01–0.05 | 0.03 ± 0.02 | 0–0.03 | 0.02 ± 0.01 | 0–0.03 | 0.02 ± 0.01 | |||||||
| 3096 | Untriacontane | 0–0.06 | 0.02 ± 0.02 | 0–0.09 | 0.02 ± 0.03 | |||||||||||||||||
| C28:0 | 3130 | Octacosanoic acid | 0–0.03 | 0.01 ± 0.01 | 0–0.11 | 0.03 ± 0.04 | 0–0.05 | 0.02 ± 0.02 | 0–0.02 | 0.01 ± 0.01 | 0–0.11 | 0.03 ± 0.04 | ||||||||||
| 3227 | Methyl cholesterol | 0–0.29 | 0.14 ± 0.11 | |||||||||||||||||||
| 3246 | Stigmasterol | 0–0.16 | 0.04 ± 0.07 | 0–0.38 | 0.17 ± 0.14 | |||||||||||||||||
| 3253 | 3β-Methoxystigmast-5-ene | 0–0.04 | 0.02 ± 0.02 | |||||||||||||||||||
| 3299 | Chondrillasterol | 0–0.11 | 0.03 ± 0.04 | |||||||||||||||||||
| 3303 | γ-Sitosterol | 0–0.55 | 0.14 ± 0.19 | 0–0.75 | 0.42 ± 0.25 | 0–0.44 | 0.09 ± 0.17 | 0–1.02 | 0.4 ± 0.4 | 0.22–0.35 | 0.27 ± 0.21 | 0.08–0.37 | 0.30 ± 0.13 | 0.13–0.63 | 0.31 ± 0.18 | 0–0.29 | 0.07 ± 0.12 | |||||
| C30:0 | 3328 | Triacontanoic acid | 0–0.14 | 0.02 ± 0.05 | 0–0.09 | 0.03 ± 0.03 | ||||||||||||||||
| 3445 | Stigmast-4-en-3-one | 0–0.02 | 0.01 ± 0.01 | 0–0.02 | 0.01 ± 0.01 | 0.01–0.06 | 0.04 ± 0.08 | |||||||||||||||
| Synonyms | RI | Compound Name | Castor Oil | |
|---|---|---|---|---|
| Range | MSD (n = 7) | |||
| C16:0 | 1924 | Palmitic acid | 0.32–1.51 | 0.87 ± 0.37 |
| C17:0 | 2026 | Heptadecanoic acid | 0.01–0.02 | 0.02 ± 0.01 |
| 2057 | Ricenalidic acid lactone | 0–0.31 | 0.05 ± 0.11 | |
| cis-9,12, C18:2 | 2087 | Linoleic acid | 1.33–5.48 | 3.58 ± 1.23 |
| cis-9,12,15, C18:3 | 2096 | Linolenic acid | 0.13–0.51 | 0.32 ± 0.12 |
| cis-9, C18:1 | 2102 | (Z)-Oleic acid | 0.93–4.0 | 2.74 ± 0.92 |
| trans-9, C18:1 | 2109 | (E)-Oleic acid | 0.15–0.79 | 0.47 ± 0.2 |
| C18:0 | 2126 | Stearic acid | 0.42–1.81 | 1.16 ± 0.44 |
| C20:2 | 2183 | Ethyl linoleate | 0–0.18 | 0.05 ± 0.07 |
| trans-10, C19:1 | 2205 | 10-Nonadecenoic acid | 0–0.08 | 0.04 ± 0.03 |
| cis-9, C18:1 | 2305 | Ricinoleic acid | 84.52–96.09 | 89.89 ± 3.51 |
| C20:0 | 2322 | Arachidic acid | 0–0.75 | 0.16 ± 0.26 |
| cis-13, C22:1 | 2499 | Erucic acid | 0–0.89 | 0.52 ± 0.36 |
| C22:0 | 2522 | Behenic acid | 0–0.02 | 0.01 ± 0.01 |
| C23:0 | 2622 | Tricosanoic acid | 0–0.01 | 0.01 ± 0.01 |
| C24:0 | 2722 | Lignoceric acid | 0–0.02 | 0.01 ± 0.01 |
| 3091 | γ-Sitosteryl acetate | 0–0.04 | 0.01 ± 0.01 | |
| 3246 | Stigmasterol | 0–0.03 | 0.03 ± 0.05 | |
| 3253 | 3β-Methoxystigmast-5-ene | 0–0.12 | 0.03 ± 0.05 | |
| Synonyms | RI | Compound Name | Canola Oil | Mustard Oil | ||
|---|---|---|---|---|---|---|
| Range | MSD (n = 4) | Range | MSD (n = 4) | |||
| C12:0 | 1523 | Lauric acid | 0–0.01 | 0.01 ± 0.01 | ||
| cis-9, C14:1 | 1702 | Myristoleic acid | 0–0.03 | 0.02 ± 0.02 | ||
| C14:0 | 1758 | Myristic acid | 0.02–0.07 | 0.05 ± 0.03 | 0.03–0.04 | 0.03 ± 0.01 |
| C15:0 | 1821 | Pentadecanoic acid | 0.03–0.05 | 0.04 ± 0.02 | 0.01–0.01 | 0.01 ± 0 |
| trans-7,10, C16:2 | 1887 | 7,10-Hexadecadienoic acid | 0.04–0.08 | 0.07 ± 0.03 | 0–0.01 | 0.01 ± 0.01 |
| cis-7, C16:1 | 1894 | (Z)-7-Hexadecenoic acid | 0.08–0.14 | 0.10 ± 0.04 | 0.03–0.11 | 0.05 ± 0.04 |
| cis-9, C16:1 | 1901 | Palmitoleic acid | 0.34–0.62 | 0.42 ± 0.19 | 0.01–0.16 | 0.1 ± 0.06 |
| C16:0 | 1924 | Palmitic acid | 3.14–6.07 | 4.66 ± 1.86 | 1.77–1.97 | 1.84 ± 0.09 |
| cis-10, C17:1 | 1996 | (Z)-10-Heptadecenoic acid | 0.91–2.03 | 1.38 ± 0.72 | 0.01–0.01 | 0.01 ± 0 |
| C17:0 | 2026 | Heptadecanoic acid | 0.57–1.86 | 1.02 ± 0.59 | 0.01–0.02 | 0.02 ± 0.01 |
| cis-9,12, C18:2 | 2087 | Linoleic acid | 2.85–4.12 | 3.22 ± 1.05 | 10.65–13.57 | 11.81 ± 1.36 |
| cis-9,12,15, C18:3 | 2096 | Linolenic acid | 5.72–7.95 | 6.77 ± 1.19 | ||
| cis-9, C18:1 | 2102 | (Z)-Oleic acid | 67.08–73.84 | 71.66 ± 4.54 | 9.26–12.37 | 10.81 ± 1.69 |
| trans-9, C18:1 | 2109 | (E)-Oleic acid | 3.26–5.35 | 4.18 ± 1.19 | 0.80–1.24 | 0.97 ± 0.21 |
| C18:0 | 2126 | Stearic acid | 2.54–4.86 | 3.32 ± 0.86 | 1.01–1.16 | 1.10 ± 0.07 |
| C20:2 | 2183 | Ethyl linoleate | 0.06–0.12 | 0.09 ± 0.04 | ||
| cis-10, C19:1 | 2193 | (Z)-10-Nonadecenoic acid | 0.86–1.67 | 1.07 ± 0.47 | 0–0.01 | 0.01 ± 0.01 |
| trans-10, C19:1 | 2205 | 10-Nonadecenoic acid | 0.49–0.93 | 0.62 ± 0.23 | ||
| C19:0 | 2222 | Nonadecanoic acid | 0.06–0.23 | 0.12 ± 0.13 | 0–0.01 | 0.01 ± 0.01 |
| cis-11,14, C20:2 | 2284 | (Z)-11,14-Eicosadienoic acid | 0.02–0.18 | 0.11 ± 0.09 | 0.38–0.65 | 0.52 ± 0.15 |
| cis-11, C20:1 | 2296 | (Z)-11-Eicosenoic acid | 2.72–4.68 | 3.29 ± 1.26 | 5.69–7.52 | 6.44 ± 0.89 |
| C20:0 | 2322 | Arachidic acid | 0.96–2.23 | 1.56 ± 0.76 | 0.82–1.06 | 0.95 ± 0.1 |
| C21:0 | 2422 | Heneicosanoic acid | 0.09–0.26 | 0.13 ± 0.15 | 0–0.02 | 0.01 ± 0.01 |
| cis-13, C22:1 | 2499 | Erucic acid | 0.08–0.24 | 0.14 ± 0.07 | 51.99–56.14 | 54.32 ± 2.13 |
| C22:0 | 2522 | Behenic acid | 0.82–1.24 | 0.99 ± 0.21 | 0.94–1.55 | 1.26 ± 0.32 |
| C23:0 | 2622 | Tricosanoic acid | 0.11–0.17 | 0.14 ± 0.05 | 0.03–0.05 | 0.04 ± 0.01 |
| cis-15, C24:1 | 2699 | (Z)-15-Tetracosenoic acid | 0.24–0.39 | 0.37 ± 0.05 | 1.21–3.41 | 2.15 ± 0.97 |
| C24:0 | 2722 | Lignoceric acid | 0.63–0.89 | 0.84 ± 0.19 | 0.30–1.26 | 0.73 ± 0.45 |
| C25:0 | 2830 | Pentacosanoic acid | 0.01–0.07 | 0.05 ± 0.03 | 0–0.02 | 0.01 ± 0.01 |
| 2899 | Nonacosane | 0.01–0.06 | 0.04 ± 0.03 | |||
| C26:0 | 2926 | Hexacosanoic acid | 0–0.08 | 0.06 ± 0.05 | 0–0.08 | 0.04 ± 0.04 |
| 3091 | β-Sitosteryl acetate | 0–0.03 | 0.01 ± 0.01 | |||
| 3303 | γ-Sitosterol | 0.06–0.13 | 0.09 ± 0.07 | |||
| RI | Synonyms | Compound Name | Jojoba Oil | |
|---|---|---|---|---|
| Range | MSD (n = 8) | |||
| 1758 | C14:0 | Myristic acid | 0–0.01 | 0.01 ± 0.01 |
| 1894 | cis-7, C16:1 | (Z)-7-Hexadecenoic acid | 0.03–0.06 | 0.05 ± 0.01 |
| 1901 | cis-9, C16:1 | Palmitoleic acid | 0–0.01 | 0.01 ± 0 |
| 1924 | C16:0 | Palmitic acid | 0.17–0.33 | 0.26 ± 0.05 |
| 1996 | cis-10, C17:1 | (Z)-10-Heptadecenoic acid | 0–0.01 | 0.01 ± 0 |
| 2064 | (Z)-9-Octadecen-1-ol | 0–0.20 | 0.1 ± 0.06 | |
| 2087 | cis-9,12, C18:2 | Linoleic acid | 0.01–0.05 | 0.02 ± 0.01 |
| 2096 | cis-9,12,15, C18:3 | Linolenic acid | 0–0.03 | 0.02 ± 0.01 |
| 2102 | cis-9, C18:1 | (Z)-Oleic acid | 2.38–3.68 | 3.01 ± 0.39 |
| 2109 | trans-9, C18:1 | (E)-Oleic acid | 0.14–0.29 | 0.22 ± 0.05 |
| 2126 | C18:0 | Stearic acid | 0.01–0.02 | 0.01 ± 0 |
| 2205 | trans-10, C19:1 | 10-Nonadecenoic acid | 0–0.02 | 0.01 ± 0.01 |
| 2260 | (Z)-Eicos-9-en-1-ol | 15.41–25.48 | 18.45 ± 3.1 | |
| 2284 | cis-11,14, C20:2 | (Z)-11,14-Eicosadienoic acid | 0–0.10 | 0.04 ± 0.04 |
| 2296 | cis-11, C20:1 | (Z)-11-Eicosenoic acid | 23.88–35.41 | 29.55 ± 4.80 |
| 2322 | C20:0 | Arachidic acid | 0.04–0.07 | 0.05 ± 0.01 |
| 2464 | (Z)-Docos-13-en-1-ol | 24.7–31.94 | 27.96 ± 2.77 | |
| 2487 | n-Docosanol | 0–1.03 | 0.21 ± 0.37 | |
| 2499 | cis-13, C22:1 | Erucic acid | 8.97–13.26 | 10.6 ± 1.46 |
| 2522 | C22:0 | Behenic acid | 0.12–0.75 | 0.25 ± 0.20 |
| 2671 | (Z)-Tricos-14-enyl formate | 0–10.64 | 6.78 ± 3.75 | |
| 2699 | cis-15, C24:1 | (Z)-15-Tetracosenoic acid | 0.9–3.94 | 2.16 ± 0.85 |
| 2722 | C24:0 | Lignoceric acid | 0.03–0.43 | 0.1 ± 0.14 |
| 3091 | β-Sitosteryl acetate | 0–0.09 | 0.02 ± 0.03 | |
| 3303 | γ-Sitosterol | 0–0.28 | 0.07 ± 0.1 | |
| RI | Synonyms | Compound Name | Pomegranate Seed Oil | |
|---|---|---|---|---|
| Range | MSD (n = 10) | |||
| 1758 | C14:0 | Myristic acid | 0.01–0.02 | 0.02 ± 0.01 |
| 1821 | C15:0 | Pentadecanoic acid | 0–0.02 | 0.01 ± 0.01 |
| 1924 | C16:0 | Palmitic acid | 2.20–4.88 | 3.14 ± 0.83 |
| 2026 | C17:0 | Heptadecanoic acid | 0.04–0.08 | 0.05 ± 0.01 |
| 2087 | cis-9,12, C18:2 | Linoleic acid | 4.54–11.47 | 6.85 ± 2.36 |
| 2102 | cis-9, C18:1 | (Z)-Oleic acid | 5.11–10.73 | 6.77 ± 1.91 |
| 2109 | trans-9, C18:1 | (E)-Oleic acid | 0.38–1.02 | 0.74 ± 0.22 |
| 2126 | C18:0 | Stearic acid | 2.14–4.74 | 3.11 ± 0.88 |
| 2183 | C20:2 | Ethyl linoleic acid | 0–0.39 | 0.15 ± 0.18 |
| 2193 | cis-10, C19:1 | (Z)-10-Nonadecenoic acid | 0.02–0.1 | 0.05 ± 0.02 |
| 2241–2286 | cis-9,trans-11,cis-13, C18:3 | Punicic acid | 62.37–80.92 | 74.77 ± 6.35 |
| 2284 | cis-11,14, C20:2 | (Z)-11,14-Eicosadienoic acid | 0–0.13 | 0.06 ± 0.05 |
| 2296 | cis-11, C20:1 | (Z)-11-Eicosenoic acid | 0.49–1.64 | 1.02 ± 0.3 |
| 2322 | C20:0 | Arachidic acid | 0.52–1.12 | 0.79 ± 0.22 |
| 2422 | C21:0 | Heneicosanoic acid | 0–0.07 | 0.04 ± 0.02 |
| 2522 | C22:0 | Behenic acid | 0.13–0.38 | 0.23 ± 0.08 |
| 2622 | C23:0 | Tricosanoic acid | 0.03–0.10 | 0.06 ± 0.02 |
| 2722 | C24:0 | Lignoceric acid | 0.08–0.31 | 0.18 ± 0.07 |
| 2803 | (E)-Squalene | 0.06–1.01 | 0.39 ± 0.3 | |
| 2830 | C25:0 | Pentacosanoic acid | 0.03–0.14 | 0.09 ± 0.03 |
| 2926 | C26:0 | Hexacosanoic acid | 0.04–0.22 | 0.14 ± 0.05 |
| 3091 | β-Sitosteryl acetate | 0–0.27 | 0.06 ± 0.09 | |
| 3130 | C28:0 | Octacosanoic acid | 0–0.08 | 0.03 ± 0.02 |
| 3227 | Methyl cholesterol | 0–0.17 | 0.04 ± 0.06 | |
| 3303 | γ-Sitosterol | 0.23–1.37 | 0.81 ± 0.32 | |
| 3406 | Lupeol | 0–0.11 | 0.06 ± 0.04 | |
| RI | Synonyms | Compound Name | MCT Coconut Oil | Roasted Coconut Oil | ||
|---|---|---|---|---|---|---|
| Range | MSD (n = 3) | Range | MSD (n = 7) | |||
| 920 | C6:0 | Caproic acid | 0.03–0.17 | 0.10 ± 0.06 | ||
| Benzoic acid | 0.01–0.01 | 0.01 ± 0 | ||||
| 1124 | C8:0 | Caprylic acid | 43.41–47.94 | 46.41 ± 2.60 | 2.55–6.29 | 4.34 ± 1.35 |
| 1278 | C9:0 | Pelargonic acid | 0.04–0.07 | 0.06 ± 0.02 | ||
| 1320 | C10:0 | Capric acid | 51.91–56.37 | 53.42 ± 2.56 | 3.8–7.51 | 5.58 ± 1.29 |
| 1421 | C11:0 | Undecanoic acid | 0.02–0.04 | 0.03 ± 0.01 | 0.01–0.04 | 0.03 ± 0.01 |
| 1523 | C12:0 | Lauric acid | 0.05–0.12 | 0.07 ± 0.04 | 38.05–53.12 | 46.13 ± 4.75 |
| 1622 | C14:0 | 12-Methyl Tridecanoic acid | 0.03–0.06 | 0.04 ± 0.01 | ||
| 1758 | C14:0 | Myristic acid | 20.89–23.26 | 22.33 ± 0.90 | ||
| 1821 | C15:0 | Pentadecanoic acid | 0–0.01 | 0.01 ± 0.00 | ||
| 1901 | cis-9, C16:1 | Palmitoleic acid | 0–0.02 | 0.01 ± 0.01 | ||
| 1924 | C16:0 | Palmitic acid | 6.75–11.78 | 9.48 ± 1.80 | ||
| 2026 | C17:0 | Heptadecanoic acid | 0–0.01 | 0.01 ± 0.00 | ||
| 2087 | cis-9,12, C18:2 | Linoleic acid | 0.47–2.08 | 1.13 ± 0.54 | ||
| 2102 | cis-9, C18:1 | (Z)-Oleic acid | 3.39–10.69 | 6.76 ± 2.57 | ||
| 2109 | trans-9, C18:1 | (E)-Oleic acid | 0.03–0.16 | 0.07 ± 0.04 | ||
| 2126 | C18:0 | Stearic acid | 2.23–5.3 | 3.79 ± 1.20 | ||
| 2296 | cis-11, C20:1 | (Z)-11-Eicosenoic acid | 0.01–0.07 | 0.03 ± 0.02 | ||
| 2322 | C20:0 | Arachidic acid | 0.03–0.15 | 0.08 ± 0.04 | ||
| 2522 | C22:0 | Behenic acid | 0–0.03 | 0.01 ± 0.01 | ||
| 2622 | C23:0 | Tricosanoic acid | 0–0.04 | 0.01 ± 0.01 | ||
| 2722 | C24:0 | Lignoceric acid | 0–0.06 | 0.03 ± 0.02 | ||
| 2926 | C26:0 | Hexacosanoic acid | 0–0.01 | 0.01 ± 0.01 | ||
| 3091 | β-Sitosteryl acetate | 0–0.02 | 0.01 ± 0.01 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojha, P.K.; Poudel, D.K.; Rokaya, A.; Maharjan, S.; Timsina, S.; Poudel, A.; Satyal, R.; Satyal, P.; Setzer, W.N. Chemical Compositions and Essential Fatty Acid Analysis of Selected Vegetable Oils and Fats. Compounds 2024, 4, 37-70. https://doi.org/10.3390/compounds4010003
Ojha PK, Poudel DK, Rokaya A, Maharjan S, Timsina S, Poudel A, Satyal R, Satyal P, Setzer WN. Chemical Compositions and Essential Fatty Acid Analysis of Selected Vegetable Oils and Fats. Compounds. 2024; 4(1):37-70. https://doi.org/10.3390/compounds4010003
Chicago/Turabian StyleOjha, Pawan Kumar, Darbin Kumar Poudel, Anil Rokaya, Salina Maharjan, Sunita Timsina, Ambika Poudel, Rakesh Satyal, Prabodh Satyal, and William N. Setzer. 2024. "Chemical Compositions and Essential Fatty Acid Analysis of Selected Vegetable Oils and Fats" Compounds 4, no. 1: 37-70. https://doi.org/10.3390/compounds4010003
APA StyleOjha, P. K., Poudel, D. K., Rokaya, A., Maharjan, S., Timsina, S., Poudel, A., Satyal, R., Satyal, P., & Setzer, W. N. (2024). Chemical Compositions and Essential Fatty Acid Analysis of Selected Vegetable Oils and Fats. Compounds, 4(1), 37-70. https://doi.org/10.3390/compounds4010003










