Abstract
Three-dimensional printing (3DP) technologies are characterized as a set of innovative manufacturing techniques that allow for the creation of complex and/or personalized three-dimensional physical objects on the work surface of a 3D printing machine (based on the computer-aided design (CAD) project designs of these parts). Three-dimensional printing techniques are widely used in various areas of knowledge, such as education, engineering, and biomedicine. Polymeric materials are widely used for these applications, mainly due to their desirable workability during part manufacturing, compatibility with other chemical materials, the wide range of polymers with different physical and chemical characteristics, and the possibility for recycling. The development of polymeric drug delivery systems (DDSs) by 3D printing is currently an active field of research, both in academia and industry, given the potential of this technique for medical purposes. In this context, this work reviews potential polymers for the production of drug delivery systems via 3D printing techniques. The demonstrations of the main 3DP techniques used for drug delivery applications include their working principles and advantages and how the technologies develop the final product. In addition, potential synthetic and natural polymers that are currently used in 3DP drug delivery devices are presented and discussed based on recent scientific studies.
1. Introduction
Three-dimensional printing (3DP) techniques are technologies for manufacturing three-dimensional objects based on computer-aided design (CAD) and the deposition of successive layers of a molten material of interest on the flat working surface of a three-dimensional printer [1,2].
Currently, 3D printing techniques such as fused deposition modeling (FDM), selective laser sintering (SLS), stereolithography (SLA), and bioprinting are among the 3DP technologies that are available for the development of devices in emerging materials science areas, such as in sensors, supercapacitors, flexible circuits, customized biomedical implants, and personalized drug delivery devices [3,4,5,6]. The choice of one of the 3DP techniques for producing a three-dimensional object of interest involves assessing the required properties for the final product and the mode of deposition that is suitable for the production of the designed part [7]. Polymers are the first choice of all materials used in these 3DP technologies, as they are thermoplastic matrices with good compatibility with other chemical materials. The low melting points of these materials increase the manufacturability of the parts, and flexible materials are needed for various applications [8,9,10].
The term drug delivery systems (DDSs) can be understood as a set of methods for transporting drugs within an organism for a specific therapeutic objective [11]. An increasing number of publications on the application of 3DP techniques in combination with polymers for the production of drug delivery systems (DDSs) of active ingredients were published between 2012 and 2022 (Figure 1). The evolution of this research area followed the temporal progress of publications in the 3DP area but still involved a much smaller number of works (compared to the total number of publications: 40,000 works in the same period). This is an indication that 3DP techniques are widely used in various areas of knowledge, from education to engineering, and that the development of polymeric DDSs by 3DP is currently an active field of research, both in academia and industry (given the potential of this technique for biomedical purposes).
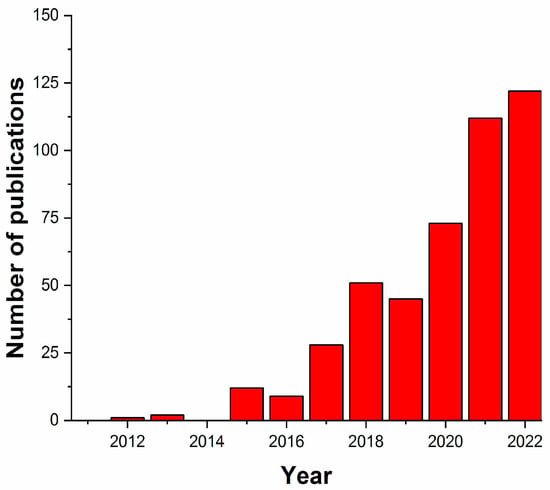
Figure 1.
Number of publications on 3DP techniques using polymers for the production of DDSs (from 2012 to 2022). The search considered works containing the terms “3D printing”, “polymer”, and “drug delivery”. Source: scopus.com., (accessed on 11 January 2024)
For example, the first successful commercial product used in medical printing was Spritam (used in epilepsy treatment), which was authorized in 2015 by the Food and Drug Administration (FDA) of the USA. With the emergence of 3DP medicines, pharmacies can now shift from mass production to the specific dosage forms that are available for patients [12].
In the drug delivery system (DDS) area, the different 3DP techniques allow flexibility in the manufacturing of drug dosage forms (tablets, dermal patches, capsules, and suppositories, among others), which would be difficult to achieve using other forms of pharmaceutical production techniques [13]. These technologies have evolved as novel alternatives to provide personalized DDSs to users, with the aim of facilitating the administration of active substances and obtaining the greatest desirable therapeutic effect [12,14].
Polymers are considered base materials in the production of personalized dosage forms, as these materials can be chosen to modify the release rate of the drug into the medium and provide physical stability for the incorporated drug. The biocompatibility of the polymer is also required for improving pharmacokinetics, reducing side effect interactions with the host tissue, and degrading the device after its action [15]. A biopolymer is preferable for biocompatibility and biodegradability because it does not cause inflammatory reactions in user tissues and does not produce toxic byproducts resulting from degradation [16].
Numerous polymers of synthetic and natural origins are available for application in DDS 3D printing. Synthetic polymers such as polylactic acid (PLA), polyvinyl pyrrolidone (PVP), polyethylene glycol (PEG), polyurethane (PU), and polyvinyl alcohol (PVA) have recently gained attention in this area because of their excellent physicochemical characteristics, cost–benefits, and known interactions with drug molecules [17]. In addition, natural polymers such as chitosan, alginate, gelatin, and collagen are also utilized for the development of novel DDSs by 3DP techniques.
Novel drug delivery systems are being developed using a 3D-printed polymer matrix. Solid dosage forms, implantable drug delivery vehicles, and topical drug delivery systems are the most common applications. Other potential dosage forms include microcapsules, antibiotic micropatterns, synthetic extracellular matrices, mesoporous bioactive glass scaffolds, nanosuspensions, and multilayered DDSs. For example, the development of caplets containing internal gaps, termed gaplets, is intended to increase or decrease the medication release velocity. Owing to their higher surface-area-to-mass ratio, smaller tablets exhibit faster drug release [18]. Similarly, the change in the geometrical shape of a 3DP tablet influences its drug release behavior. In vitro dissolution studies have shown that the ratio of the surface area relative to the volume of 3DP tablets alters the time that is needed for complete drug release [19]. Polymeric gels have also been widely used as materials for 3DP personalized DDSs. Recently, hydrogels have been used as biomaterials in 3D printing, because they can be easily modified without complex synthesis steps to replicate the physicochemical properties of most biological tissues. Hydrogels can exhibit up to 40-fold changes in volume as they swell or shrink in the presence or absence of water, respectively, and can modify the response to various physical and biological stimuli, such as temperature, light, pH, ions, and biochemical signals [20,21].
The development of 3D-printed DDSs has been based on the constant search for new materials/methods, designs, and formulations with the potential for the production of personalized dosage forms. This has resulted in increased interest in drug bioavailability, programmed/controlled action with greater precision in specific regions of the body, and increased drug administration intervals for given medications to improve users’ quality of life [22,23,24].
Based on these aspects, this work presents a bibliographic review of the main 3D printing techniques and polymers that are currently used for the production of novel personalized DDSs for biomedical applications. The theme is presented in detail in the following sections.
2. Three-Dimensional (3D) Printing Techniques
2.1. Stereolithography (SLA)
This 3D printing technique involves converting liquid resins into solid parts through the photopolymerization process [25]. The final printed part is produced with successive layers of the material on the flat surface of a stereolithography apparatus [26]. This technique uses an electron beam of UV light to cause a chain reaction in each layer of resin (or also epoxy or acrylic), which is capable of converting the initial material into polymer chains in the solid state. Other processes, such as photocuring, can be used to mechanically improve printed objects [18].
The printing process starts when the equipment work surface is directed to the resin stock compartment with a layer of distance between them. This layer is then cured with UV light, and a new layer of fresh resin is released above the previous layer so that the process is repeated until the last layer of object formation is activated. Generally, the printed part is washed with isopropyl alcohol to remove surface excesses. Finally, the object photocuring process is established in a specific UV chamber. For this process, the choice of resin must take into account the appropriate curing rate and its approval by the FDA for use in pharmaceutical products [26].
SLA technology also stands out for enabling the deposition of extremely thin layers under the work surface. This means that the technique is capable of faithfully reproducing submillimeter details of the designed part, which provides greater quality and precision to the final product [26]. Moreover, objects are printed in a relatively short time compared to other 3D printing techniques. Like with other 3DP technologies, the duration of the printing process is directly determined by the dimensions of the part, the thickness of the deposition layers, and the complexity of the part details [25]. Stereolithography is also superior to other free-form solid fabrication (FFF) techniques in regard to resolution and accuracy (up to 10 μm) [18]. Several types of resins have been developed in recent years with the aim of providing wide variations in final mechanical properties [26]. On the other hand, a limitation of the use of this technique in DDSs is the reduced quantity of resins that are biocompatible and biodegradable for the required applications [18].
Polylactic acid (PLA) and poly(vinyl alcohol) (PVA) are alternative polymers that have been used in SLA technology [27]. Other authors [28] have used poly(methyl methacrylate) (PMMA) and polyvinylpyrrolidone (PVP) for the production of DDSs by stereolithography for applications in dermal wound healing devices and in the area of dentistry. In another study, Healy et al. produced customized controlled-release tablets through stereolithography. The results showed that the incorporation of different drugs (such as aspirin and paracetamol) into the polymeric matrix can directly impact the dimensions of the printed pharmaceutical form [13].
2.2. Selective Laser Sintering (SLS)
Selective laser sintering (SLS) is a 3D printing technology dating back to the late 1980s. Objects printed by the SLS technique are produced by depositing successive layers of thermoplastic polymer powder, which are sintered by a high-power laser to form structured three-dimensional parts [27].
Currently, SLS technology is widely used in industry due to its cost–benefit relationship and high productivity [28]. The printing process begins with the placement of a layer of powder on the printer work surface following the project design. This layer is preheated with the aim of improving the surface quality of the part that is in contact with the work surface and ensuring that a lower laser power is used for sintering (preventing polymer degradation). When this layer is sintered, a new layer of powder is made available. The layer deposition and sintering processes are repeated until the designed three-dimensional structure is finalized [28,29]. The quality of the final part is also dependent on the layer deposition process. For example, the distance between two subsequent laser scanning paths over the layer and the layer thickness are important parameters for obtaining a part without apparent structural defects [30].
SLS involves high-resolution printing due to the particle size of the material used, which is approximately 50 to 80 µm and allows for the production of objects with more complex geometries [29]. It also has the ability to carry out medicinal printing without the need for a solvent [28]. This printing technique permits the use of polymers or combinations of polymers. It is still possible to recycle the powdered material that is not used during the process to produce new parts [30,31]. In addition, SLS is the least suitable method for oral drug delivery, as the raw surface after printing requires more complex postprocessing finishing. This technique is limited by the use of thermoplastic polymers, such as polylactic acid (PLA) and polycaprolactone (PCL), combined with pharmaceutical molecules that are stable at sintering temperatures [31,32].
Trenfield et al. mentioned that SLS printing has been used recently for manufacturing drug release capsules with more complex geometries [32]. The aim of this study was to achieve significant changes in the drug release profile. Fina et al. also suggested that SLS 3D printing is suitable for the production of solid pharmaceutical solutions for oral administration using polymers with a fast- or slow-release profile [33].
2.3. Fused Deposition Modeling (FDM)
The fused deposition modeling (FDM) technology originated in 1988 as an invention by the American Scott Crump [19]. This technology is also known by the term fused filament fabrication (FFF). In an FDM printer, a cylindrical solid filament with a millimeter diameter (generally 1.75 or 3.00 mm) of thermoplastic material (polymer or polymer composite) is heated to a temperature that is greater than its glass transition temperature in the extrusion nozzle with the aim of making the material fluid. By keeping the material flow and extrusion temperature constant, it is possible to form a layer of material on the flat working surface of the printer through the programmed movement of the extruder nozzle in the horizontal plane. With the completion of the first layer, the process is repeated for the other overlapping layers until the three-dimensional part formation project is completed [18,28].
In FDM technology, the thermoplastic characteristic of the filament material is essential for part printing, since each layer of molten material returns to the solid state when the temperature decreases, structuring the formation of the object with dimensional accuracy. Some important parameters for the quality of the final part include the part filling method, diameter of the printer extruder nozzle, thickness of the layers, and extrusion temperature [18]. The extrusion temperature will depend on the polymer/composite material, considering its glass transition and melting temperatures. Generally, these temperatures for pure polymer filaments are indicated by manufacturers, while the use of new composite filaments containing additives of interest on an experimental scale may require additional tests to define the optimized temperature for the process [27].
The main reason for the vast use of FDM technology for 3D printing in drug delivery devices is the low cost of production, including the technology itself, and the wide range of available polymers. The cost-effective characteristics of FDM include preventing material waste and reducing the cost per unit of drug delivery device [34]. Recently, advances in multiple-extrusion FDM printers have demonstrated the potential of this technology for dispersing multiple drugs with different release profiles in the same printed pharmaceutical solid solution [11].
PLA, PCL, and polyvinyl alcohol (PVA) are polymers that stand out in FDM for the production of printed DDSs, as they melt at temperatures that are compatible with the thermal stability range of several drugs, maintaining their bioactivity [28]. Furthermore, when PCL and PVA are used to produce oral formulations, they undergo hydrolysis and are eliminated from the body via excretory routes [34].
Matijašić et al. successfully applied FDM technology to produce 3D-printed capsules. The authors also highlighted the importance of FDM for the area of pharmaceutical technology, given the versatility of the technique for producing customized DDSs in geometry and for different release profiles, in addition to the possibility of administering more than one active ingredient in the same pharmaceutical form [35]. Krause et al. demonstrated the possibility of producing pressure-controlled dosage forms using FDM. This variation in technique was able to print a variety of pharmaceutical solid solutions with different geometries. In addition, using this technique, there was no need to use structural supports during the printing process, which favors the customization of pharmaceutical forms and a reduction in the device printing time [36].
2.4. Bioprinting
The three-dimensional (3D) bioprinting technology was first developed by Thomas Boland in the early 2000s and continues to grow in popularity in both academia and industry [37]. This technique involves engineering and biological concepts for printing functional biological materials [38].
Bioprinting technology is also an additive manufacturing technology with great potential for developing new personalized biomaterials [39]. This technique uses polymeric fluids/gels containing active cells to precisely manufacture microscale materials layer by layer for tissue engineering applications [40]. The main applications involve the production of tissues containing regenerative cells, similar to natural cells, and drug delivery systems with programmed release [41]. The bioprinting technique involves two well-known procedures, namely, extrusion- and inkjet-based bioprinting.
The technique most commonly used in industry is the extrusion-based bioprinting technique (or pressure-based bioprinting). This technique combines usual bioprinting methods (such as a fluid dispensing system or biological paste) with a robotic system for the material extrusion process. The fluid is inserted into a reservoir coupled to a robotic arm that moves three-dimensionally over the work surface to disperse the material and form the designed object [42]. According to Mobaraki et al., alginate, cellulose, chitosan, gelatin, and hyaluronic acid (HA) have been used for 3D printing of drug delivery devices in hydrogel forms that are usually produced using extrusion-based bioprinting [38]. For example, Kim et al. developed a new biogel for extrusion-based bioprinting. The authors showed that a mixture of alginate and carrageenan in an optimized proportion can be used to develop biomedical devices that satisfactorily inhibit inflammatory processes [40].
On the other hand, using the inkjet-based bioprinting technique, the ejection of droplets of biological fluid onto a flat printing table is established to form the object [43]. With a constant flow of biological gel from the reservoir to the tip of the printing nozzle (a millimetric metallic capillary), an electric field is established between the nozzle and the printing table, with sufficient electrical force to overcome the surface tension of the droplet at the capillary exit. This causes drops of the material to be ejected toward the flat collector to form the first layer of bioink on the work surface as a result of the continuous spraying of fluid drops. This process is repeated to form the second layer, and so on, until the required object is shaped as a junction of these layers [38].
Inkjet printing has been used to create DDSs that improve the bioavailability of poorly soluble molecules for the administration of low concentrations of drugs and on-demand medications [38]. Cellulose, chitosan, and hyaluronic acid are the most popular polymers for the production of drug delivery systems by the abovementioned technique [38,44]. The working principles, advantages, and applications of these 3D printing techniques for developing DDSs are summarized in Figure 2.
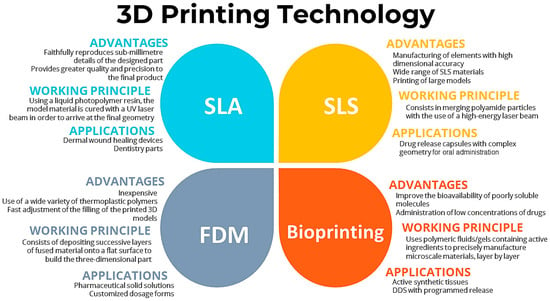
Figure 2.
Working principles, advantages, and applications of 3D printing techniques for developing DDSs.
3. Polymers
3.1. Synthetic Biopolymers
3.1.1. Polyvinyl Alcohol (PVA)
Hermann Staudinger was the first researcher to perform polyvinyl alcohol (PVA) synthesis in 1927 [45]. Since then, this polymer has been employed in many applications, with an emphasis on the production of food, industrial components, and pharmaceutical disposables [46]. PVA is a polymer that is soluble in water, while the opposite is the case in the majority of organic solvents and ethanol [47]. Moreover, it is a synthetic polymer with a semicrystalline molecular structure, thermoplastic properties, high temperature stability, and low reactivity. In terms of biocompatibility, PVA has a high biological degradation potential and very low toxicity. In terms of mechanical properties, this polymer exhibits high malleability and adherence and low mechanical strength. These characteristics can be modified by blending PVA with other polymers or additives [48].
The production of PVA occurs via the hydrolysis of polyvinyl acetate, which can be partial or total. The polymer is inodorous, and its thermoplasticity is not dependent on the additive [47]. This material is classified by the FDA as a generally recognized safe (GRAS) substance, as PVA is a suitable commercial polymer for FDM 3D printing [49]. The high solubility of this polymer in water is a relevant characteristic for its use, as its cleaning is effortless. Moreover, the PVA glass transition temperature (Tg) is 85 °C, while the temperature degradation is between 350 °C and 450 °C [18]. These characteristics enable the use of PVA 3D-printed filaments in drug delivery [50], even if passive diffusion for PVA drug loading is lacking, which is an important factor that limits the adoption of this polymer for the drug delivery of pharmaceutical ingredients only at low dosages [49].
PVA is a favorable choice for 3D inkjet printing, because its polymer malleability is adequate for the production of multiple layers. However, the polymer has a high molecular weight, which increases the polymer viscosity. As a result, the printing ink obstructs the printer nozzle, which negatively influences the printability. To avoid printer nozzle obstruction, the printing ink may be composed of glycerin or monopropylene glycol, which are feasible humectants for PVA aqueous solutions. As a solution for achieving satisfactory viscosity in 3D printing, high-molecular-weight PVA and low-molecular-weight PVA were used. Inks containing PVA with a high molecular weight preserve chemical stability for approximately six months without changing their color. On the other hand, previous research has demonstrated that PVA inks present Newtonian fluid characteristics at high shear rates but thixotropic and pseudoplastic characteristics at low shear rates [47].
The most effective technique for the production of PVA drug delivery filaments is hot-melt extrusion, which is the most favorable technique for the production of PVA drug delivery filaments, while the most favorable technique for 3D printing of drug delivery devices is fused deposition modeling (FDM). Oral disposables are the most commonly used formats for PVA drug delivery. Krause et al. mentioned that PVA wires can be used for drug delivery, but further analysis of the influence of PVA on the quality of FDM 3D-printed drug delivery devices is needed. This work identified different release profiles on PVA drug delivery devices, which may be dependent on the PVA quality. This release profile may be dependent on the quality control and synthesis processes of distinct manufacturers [36]. Using the FDM process, Matijašić et al. designed 3D-printed PVA capsules with multiple compartments for the storage and release of different drugs. The choice of PVA was due to its pharmaceutical acceptance and frequent use in the formulation of oral drug delivery devices. In addition, the commercial availability, biocompatibility, and water solubility of PVA are important factors for its use. The author mentioned that PVA controlled the drug release profile, which corroborates the feasibility of this polymer for the production of 3D-printed oral drug delivery devices [35].
More recently, Saviano et al. produced unloaded PVA capsules for drug delivery in the stomach using the fused deposition modeling (FDM) 3D printing technique. For drug loading, the capsules were immersed in a highly concentrated solution of anhydrous caffeine and subjected to microwave irradiation. To increase the loading process, the addition of 2% w/v polyethylene glycol (PEG) enhanced the microwave propagation. This research evaluated the impact of this process on the drug loading efficiency and stability and thermal and chemical characteristics of the drug release profile and printing morphology. The results demonstrated that drug impregnation into drug delivery devices is highly efficient, suggesting that this approach is an alternative technique for personalized drug delivery [51].
In addition, Junqueira et al. developed plain PVA oral tablets using FDM 3D printing. The tablets were jet-dispensed with a polymeric ink containing ethylcellulose, PVP, and Eudragit EPO. This tablet coating enables the personalization of the drug release profile according to the polymer that is used in the inks. The combination of these 3DP techniques enables the production of personalized pharmaceutical doses that could result in drug degradation via conventional 3DP techniques. The model drug was rosuvastatin calcium. The polymeric inks had a constant polymer-to-drug ratio of 3:1 (wt/wt) and were prepared using chloroform and methanol as the solution solvents [52].
Wei et al. developed a PVA-based suppository using 3D printing, which consisted of an external curved shell accompanied by an internal spring for structural reinforcement. The external shell contained mesalazine (drug), PVA, and polyethylene glycol (PEG). This shell production occurred with an FDM 3D printer. The springs were manufactured with thermoplastic urethane filaments (PLA, TPU, ABS, and PCL) using FDM 3D printing, followed by splitting. PEG and mesalazine (50 wt% of each) were used to prepare a homogenized paste via a water bath heating process. Furthermore, PVA particles were mixed with this homogenized solution until the external part of the particle was able to absorb the PEG/mesalazine solution [53].
Krueger et al. manufactured 3D-printed filaments composed of 74.4% PVA, 8.5% glycerol, 2.1% starch, and 15% caffeine using a hot melt extrusion device. The use of those filaments was satisfactory for the manufacturing of oral capsules using a fused deposition modeling (FDM) 3D printer. The choice of caffeine for this study was due to its side effect profile when the drug dose abruptly decreased, which depended on dose personalization. The choice of filament components was due to their mechanical strength, adhesion between layers, and flexibility, which resulted in the adequate use of FDM and HME techniques. Moreover, the decrease in PVA brittleness for 3D printing was a result of the use of glycerol as a plasticizer. The addition of starch improved the flowability of PVA filaments as an efficient lubricant and contributed to enhancing the plasticizer characteristics [54]. Table 1 shows some of the latest applications of PVA for 3D printing of drug delivery systems.

Table 1.
Recent applications of PVA in 3D-printed drug delivery systems.
3.1.2. Poly(vinyl pyrrolidone) (PVP)
Walter Reppe first described polyvinylpyrrolidone (PVP) as one of the acetylene chemistry products that he developed in the 1930s. The melting temperature of PVP is 150 °C [65]. PVP is soluble in many organic solvents and water. Moreover, PVP is atoxic, biocompatible, and chemically stable. These chemical and physical characteristics contribute significantly to the adequate use of PVP for the production of drug-loaded disposables [17].
PVP has extensive use for the development of nanoparticle devices, even as a bulky material. This is due to its nonionic nature and substantial hydrophilicity and hydrophobicity, which enhance the association of PVP with many substances. The solubility of PVP in many polar and nonpolar liquids, including water, is due to the presence of an amide group that is associated with the methane groups pyrrolidone ring and apolar methylene, which are present along the PVP backbone and the ring; this enables chemical interactions with substances of different polarities. The repulsive forces originating from the carbon chain extend to solvents, resulting in molecular interactions that are responsible for polymer solubility and the prevention of nanoparticle aggregation originating from repulsive forces. Consequently, PVP solubility does not result in chemical instability [66].
The multipolar solubility of PVP contributes significantly to the satisfactory performance of this polymer when combined with most of the available drugs, even when PVP is poorly soluble in water. This is due to the feasibility of PVP forming intermolecular cross-links with substances of distinct polarities. Moreover, PVP is capable of inhibiting the degradation and changing the crystalline microstructure of pharmaceutical ingredients, as long as the solution is in contact with water. As a biocompatible and commercial polymer, these PVP characteristics demonstrate the great adequacy of PVP for the production of drug delivery devices for a wide range of medical therapies [67].
Recent studies have demonstrated that PVP is adequate for formulating oral drug carrier devices with rapid release (approximately 30 min to release 85% of the drug loaded). The FDA classified PVP as a safe substance, as the complete excretion of this polymer occurs through the kidneys. Moreover, previous research has shown that the physical and chemical characteristics of PVP make it suitable for 3D printing via FDM, which is the most commonly used technique for producing drug delivery devices for oral intake [68].
Windolf et al. developed a geometric model of oral tablets that could represent variable drug release profiles with the same volume-to-surface area ratio [69]. The models were developed via FDM 3D printing using a formulation of PVA, pramipexole (active pharmaceutical ingredient, 5 wt%), mannitol (plasticizer), and fumed silica (glidant). A secondary formulation was developed with PVA and praziquantel (active pharmaceutical ingredient, 5 wt%). The results demonstrated that variable doses are able to personalize the medical response according to the requirements for patient therapy, using only one standard filament. The medical therapy did not affect the blood plasma profile after oral intake of the printed tablets, even with various combined drugs.
Jovanovic et al. [70] produced drug-loaded gelatin-PVP mucoadhesive patches loaded with propranolol hydrochloride (active pharmaceutical ingredient, 1.5 g per device) for buccal release. A polymer/drug semisolid solution feeds the extrusion-based 3D printer, resulting in patch production. The effects of the polymers on the drug release profiles and physical properties were analyzed. The results demonstrated that PVP is capable of prolonging drug release, enhancing mechanical and thermal properties, and enabling exceptional mucoadhesion. This is a requirement for the medical treatment of cardiovascular illnesses, such as arrhythmias and hypertension.
Than and Titapiwatanakun [71] characterized and developed extended-release oral tablets using an FDM 3D printer. The tablets contained PVA, PVP, hydroxypropyl cellulose, and indomethacin (active pharmaceutical ingredient, 10 wt%). The results demonstrated the feasibility of prolonging drug release for approximately 12 h, depending on the filament composition and tablet geometry.
3.1.3. Polyethylene Glycol
Since the 1950s, polyethylene glycol (PEG) has been widely used in many industrial processes, for example, as an additive for reducing the solidification temperature of fluids, as an additive for processed foods, as a lubricant for pharmaceutical disposables, for purifying and separating contaminants and embedding matrices, and for manufacturing incorporated matrices. In the pharmaceutical industry, the use of PEG includes the production of tablets, capsules, and pills for oral intake and the development of solutions and suppositories for intradermal or transdermal use [59].
PEG is a polymer that is inert in biological organisms and has low cellular uptake, adhesion, and interaction. PEG metabolism is a result of alcohol group oxidation in the presence of alcohol dehydrogenase, which is a catalyzed enzyme. This chemical reaction results in the metabolization of hydroxil acid, diacids, and carboxylic acid, and their excessive development results in hypercalcemia and acidosis. PEG is also flexible, hydrophilic, and lipophilic and has a relevant capacity for water absorption. PEG also forms a monolayer between water and air, which is important for polymer permeation and solubilization. PEG absorption decreases up to 50% in the skin, stomach, and intestines as the molecular weight of the polymer increases. Moreover, PEG is soluble in a wide range of organic and inorganic solutions. This polymer’s hydrophilicity increases with increasing polarity, resulting in increased solubility in water. PEG has a low degree of inflammation and negligible protein adsorption, which emphasizes its high biocompatibility and suitability for medicinal use [72].
The synthesis of PEG is a result of the anionic polymerization of any type of hydroxyl initiator with ethylene oxide. Water, ethylene glycol, and all types of diols are the most commonly used hydroxyl groups for this purpose. The ring-opening polymerization is a consequence of the derivation of hydroxyl groups, which also results from epoxy ethane. PEG is commercially available with distinct activated functional groups and polymerization degrees, which may alter the physical and chemical characteristics of the polymer [73].
Due to its very low melting temperature (approximately 70 °C) and glass transition temperature (approximately 40 °C), PEG is frequently used as an additive (plasticizer) to improve the processability of other polymers [51], such as PCL, PLA, PLGA, PU, and PVA.
Using different concentrations of PEG, Salehi et al. prepared PLA scaffolds (20, 15, 10, 5, and 0 wt%) using FDM 3D printing technology. Research has evaluated printability optimization and the suitability of the mechanical and physical properties, including cellular behavior enhancement in bone tissue structures. The results demonstrated that the mechanical strength of the printed scaffolds was not considerably affected by the addition of 10 or 5 wt% PEG. Moreover, the inclusion of PEG in the polymeric solution enhanced 3D-printed scaffold degradation, and cell viability expansion occurred with the highest concentration of PEG (20 wt%) [73].
Digkas et al. produced oral tablets using a 3D printer, which customized the tablets to tubular and cylindrical formats. The ingredients included customized filaments containing PVA as the main polymer (75 wt%), PEG as the plasticizer (10 wt%), and diclofenac sodium (15 wt%) as the active pharmaceutical ingredient. The hot-melt extrusion process was used for filament manufacturing, which has adequate characteristics for FDM 3D printing. In this experiment, the release tests demonstrated satisfactory results, with a drug release of approximately 90% after 45 min [74].
Recently, Picco et al. [75] created subcutaneous implants loaded with a model drug using the robotic material extrusion 3D printing technique. This is a novel extrusion-based printing method in which a robotic device is able to deposit the molten polymer layer by layer with higher precision. The implants were prepared in a cylindrical shape and included PCL, PEG, and olanzapine (50 and 80 wt%). Drug release analyses demonstrated the accomplishment of a constant drug release profile in all the 3D-printed formulations, with 60% of the maximum drug release occurring within a period of more than 200 days [75].
3.1.4. Polyurethane (PU)
Otto Bayer first described polyurethane (PU) in 1947. Since then, PU has been applied in the product development industry in many different applications, such as automotive parts, construction buildings, and clinical, electrical, and medical devices [76]. PU is flexible, rigid, and lightweight [77]. Over the past forty years, PUs have also been employed for the production of biomedical disposables due to their mechanical elasticity, biocompatibility [76], biodegradability, and water sensitivity [16].
Usually, the synthesis of PUs includes polycondensations of di-isocyanates with alcohols and/or amines. The preparation of biodegradable PUs involves the incorporation of hydrolyzable moieties into the main polymer chain, which includes portions of degradable polycaprolactone. This process is mandatory for the use of PU to manufacture biomedical disposables for drug delivery and implants for tissue repair. PU’s chemical and physical properties are modified by modifying the water sensitivity and crystallinity, resulting in the desired profile for pharmaceutical ingredient release. The adjustment of cationic or anionic groups enhances electrostatic interactions with RNA, DNA, pharmaceutical ingredient molecules, and proteins [16]. Commercially available PU demonstrated great feasibility for the preparation of drug-loaded tablets via FDM 3D printing. The temperature for PU extrusion is in the range of 100–180 °C [17].
A variation of PU is the thermoplastic PU (TPU). The main difference between TPU and PU is that TPU has no cross-links in its structure, whereas conventional PU can have cross-links based on the type of polyols that are used during synthesis. The segmented blocks of TPU structures do not involve covalent bonding between their polymer chains, which contributes to the differences in their properties compared to those of PUs. Moreover, while TPU is made from polyester, polyether, or polycaprolactone soft segments, PUs are manufactured by reacting polyester or polyether, among other polyols, with isocyanates. The final materials have different characteristics based on the polyol that is used. TPUs consist of soft and hard segments within their molecular structure, which contributes to the versatility and unique properties of the material [78]. On the other hand, PUs have urethane linkages within their molecular systems that are formed through the reaction of isocyanates and polyols [79].
TPU enables material recyclability due to the possibility of material remodeling. This polymer also permits the incorporation of a high amount of pharmaceutical ingredients, which can reach up to 70% of the total mass, without problems in the hot-melt extrusion of filaments, consecutive 3D printing, or drug release. Furthermore, TPUs are capable of modifying their chemical composition to adapt their drug release profile to the desired medium with distinct aqueous solubility. TPU is a polymer that has the potential to produce release-retarding matrix devices, which emphasizes the potential of TPU for the manufacture of personalized drug delivery disposables for medical therapy [80].
Domínguez-Robles et al. [81] developed TPU vascular tubular grafts loaded with dipyridamole. The production of filaments occurred with the use of a hot-melt extruder followed by the use of an FDM 3D printer to manufacture the drug delivery devices. Vascular grafts are implants that bypass problematic blood vessels and are an alternative option for avoiding more invasive procedures, such as surgical procedures. Graft obstruction is the main undesirable outcome of tubular grafts due to the possibility of intimal hyperplasia or thrombosis. Olimition prevention occurs with graft drug loading, which prevents blood clot formation. The TPU was loaded with 5%, 10%, and 20% dipyridamole [81].
Zhang et al. [82] produced multifunctional microneedles (MNs) to accelerate skin wound healing, resulting in the prevention of infection, persistent pain, and systemic injury. Microneedle production occurred with the use of a customized extrusion process for 3D bioprinting. The primary material for the printing procedure was a hybrid emulsion containing spidroin, aloe vera gel (28% of total mass), polyurethane (60% of total mass), and eutectic cerium-indium (1% of total mass). The materials included materials with high flexibility, stretchability, biocompatibility, and self-healing characteristics, which are crucial for microneedle applications [82].
As a medical treatment strategy for achieving sustained release and redox response in solid cancer tissues, Martonana et al. developed 3D-printed nanocomposite scaffolds containing urea and polyurethane for the delivery of doxorubicin at specific locations. This therapy resulted in the prevention of bone cancer recurrence at specific sites after surgical resection. The successful production of scaffolds occurred via a solvent casting 3D printing process, enabling the simultaneous manufacture of both the polymer and the drug at room temperature. This was required due to the risk of thermal degradation that is associated with the high processing temperature of the molten polymer. The printing substrate contained the medication (doxorubicin, 0.1% of total mass), dimethylformamide, diethanolamine, 1,4-diisocyanatobutane, and oxidized L-glutathione [83]. Table 2 shows other recent applications of PU for 3D printing of drug release systems.

Table 2.
Recent applications of PU for 3D printing of drug release systems.
3.1.5. Poly(lactic acid)
The first synthesis of polylactide (PLA) or poly(lactic acid) was attributed to Wallace Hume Carothers in 1932. Later, Du Pont patented this method in 1954. Since then, PLA has been used in medical therapy for the development of biomedical devices and the production of drug delivery disposables. This usability is associated with favorable characteristics, such as biocompatibility and bioabsorbability, for those purposes [99].
Polylactide is a renewable and biodegradable polymer [17], whose synthesis may occur using natural resources, such as cornstarch or sugarcane. As an eco-friendly material, the energy required for its production is approximately half the energy that is usually used for the synthesis of polymers based on petroleum, such as polyamide (PA), polypropylene (PP), low-density polyethylene (LDPE), and polystyrene (PS). These attributes have increased interest in the use of PLA for academic and industrial purposes, especially for medical applications, such as drug delivery [100]. In addition, the melting temperature of PLA is approximately 210 °C [99], which makes it suitable for 3D printing.
PLA is atoxic and does not produce toxins in humans or activate toxic reactions. These aspects characterize the polymer as safe for human use by the FDA. It has been widely used in the production of controlled drug delivery materials with prolonged release times, ranging from weeks to months. Biocompatible PLA pellets or filaments for 3D printing are commercially available. Fused deposition modeling (FDM) and stereolithography (SLA) are the most suitable techniques for the 3D printing of PLA for drug delivery [47].
This polymer has many characteristics that enable the production of biomaterials. Hydrolysis can cleave the ester bonds that are located in the main polymeric chain, whose removal results from metabolic reactions. In comparison to other biocompatible polymers, PLA has a relevant mechanical strength and glass transition temperature, while it is semicrystalline and hydrophobic in nature [17].
Gowrav et al. developed nasal stents with a customized design containing PLA and the mometasone furoate (active pharmaceutical ingredient, 15.6 wt%) [101]. FDM 3D printing was the technology of choice for stent production. This technique enhances the customized format that enables mucosal tissue drug delivery in a regulated and adequate region. The personalization resulted in adequate assistance in wound healing and a decrease in inflammation, minimizing the formation of granulation tissue and scar tissue for 30 days before total disintegration [101].
As an approach for healing large infected bones, Gao et al. prepared bone tissue scaffolds containing polylactic acid (PLA) and nanohydroxyapatite using an extrusion-based 3D printing technique [102]. The hydrogels were subsequently reconstituted with 1 mg of vancomicin (an active pharmaceutical ingredient) per 40 mg of chitosan, after which the scaffolds were incorporated. As a result, the drug delivery devices achieved good biocompatibility and high porosity with cell conductivity due to the interconnected three-dimensional networks. Moreover, these devices demonstrated good mechanical properties, improved scaffold hydrophilicity, controlled release of antibiotics for more than 8 weeks, and antibacterial activity [102].
Asadi et al. developed a device for personalized drug release with a pH response using extrusion-based 3D printing to develop colon-specific tablet shells for oral drug administration [103]. Polylactic acid (PLA) and Eudragit® FS100 were used to construct the shells. The internal core was loaded with a hydrogel containing N-acetylglucosamine (active pharmaceutical ingredient, 30 mg/mL) and methyl cellulose. The most promising results of this research demonstrated improvements in processability and printability and a constant drug release profile without toxicity. The results demonstrated the possibility of personalized drug delivery via different drug combinations and the personalization of drug release profiles according to therapeutic requirements, with effective delivery to inflamed sites [103]. Table 3 shows some recent applications of PLA for the production of 3D-printed drug delivery disposables.

Table 3.
Recent applications of PLA for the production of 3D-printed drug delivery disposables.
3.1.6. Poly(lactide-co-glycolide)
Polylactide-co-glycolic acid (PLGA) is a copolymer of polyglycolic acid (PGA) and polylactic acid (PLA). The first synthesis of this polymer occurred in the 1970s with polymerization by the ring-opening of glycolide (GA) and lactide (LA) [120]. PLGA is a biomaterial with great feasibility and biocompatibility for the manufacture of drug delivery disposables. This polymer has European Medicine Agency (EMA) and FDA classifications and is safe for human body usage. In comparison to most natural polymers, PLGA has high reproducibility. As a synthetic material, this polymer is not dependent on natural environmental factors, resulting in better reproductivity and purity [121].
The biodegradability of PLGA in humans can occur via hydrolytic cleavage. Chemical processes cleave polymer molecules to glycolic acid and lactic acid, while metabolism through the Krebs cycle results in water and carbon dioxide. Moreover, PLGA has greater stability than PGA and PLA, which ensures a prolonged drug release that can last months [121].
Bassand et al. analyzed the significant controllability of drug release according to the filling density of mesh-shaped PLGA implants manufactured via 3D printing [122]. Ibuprofen (approximately 13.5 wt%, according to filling density) was included as the pharmaceutical ingredient for the treatment of inflammatory reactions. The implants with different configurations were prepared with the use of an Arburg plastic free-forming printer. In this type of printer, the polymer and drug, both in solid granular form, are inserted into the printer, melted, and discharged in individual droplets from the nozzle tip [122].
Bendicho-Lavilla et al. developed a 3D-printed porous intravitreal implant of PLGA using the stereolithography (SLA) technique. These implants incorporated dexamethasone (6.6 wt%) as a therapy for macular degeneration that is related to age. Current treatment strategies involve consecutive intravitreal injections, resulting in high costs and inconvenience for patients. The designed implants achieved the desired medical therapy, with an adjusted drug release that occurred in approximately four months [123].
Martínez-Perez et al. manufactured a 3D-printed drug delivery device based on gelatin hydrogels and PLGA nanoparticles using an extrusion-based printing technique. Rifampicin and vancomycin (3 to 10 wt%) were used as the active pharmaceutical ingredients to treat implant-associated infections. The results demonstrated that the drug delivery devices gradually released both antibiotics, which is suitable for preventing the development of antimicrobial resistance. Table 4 shows some technical specifications used in recent applications of PLGA as a 3DP drug delivery system [124].

Table 4.
Recent applications of PLGA for the production of 3D-printed drug delivery disposables.
3.1.7. Polycaprolactone
The first synthesis of polycaprolactone (PCL) occurred in the 1930s, during which e-caprolactone ring-opening polymerization was performed [135]. PCL is a commercial polymer that has exceptional processability, biodegradability, and biocompatibility. PCL degradation in human organisms is slow because of ester linkage hydrolysis, which results in the degradation of water and carbon dioxide, which are atoxic substances. These characteristics corroborate the use of PCL for 3D printing of DDSs, which require a polymeric matrix for prolonged release of the incorporated substance [15]. PCL was also approved by the FDA for safe use in humans [17].
PCL is a convenient polymer when a long degradation period is needed. In comparison to other polymers, especially PLA, PCL requires approximately two to five years for complete degradation. PCL also has a high hydrophobicity, and its atomic microstructure has partial crystallinity [135], which is a relevant contributor to its chemical characteristics [17]. Moreover, the glass transition temperature of PCL is –60 °C, while its melting temperature is between 59 °C and 64 °C [135]. These temperatures are favorable for PCL use in 3D printing, as the polymer melting requires less energy. Some commercial drugs have low degradation temperatures. The use of a polymer with a low melting temperature is mandatory for these drugs, as ensuring drug integrity is important for achieving adequate medical therapy [136]. PCL also has good mechanical strength and flexibility, which are relevant factors for its use in the hot-melt extrusion of filaments [17]. Table 5 shows some recent applications of PCL for the production of 3D-printed drug delivery disposables.

Table 5.
Recent applications of PCL for the production of 3D-printed drug delivery disposables.
López-González et al. evaluated the antimicrobial efficacy of hybrid implantable scaffolds composed of chitosan hydrogel and PCL. The scaffolds were loaded with vancomicin at distinct concentrations (1, 5, 10, and 20 wt%). The scaffolds had controlled porosity and were produced with an FDM 3D printer. In this research, in vitro tests demonstrated antibacterial efficacy against Staphylococcus epidermidis and Staphylococcus aureus with high biocompatibility and bioactivity [151].
Utomo et al. proposed the manufacture of intravaginal devices with mucoadhesive properties as an alternative disposable device for the prolonged delivery of metronidazole (5 wt%). These devices were produced by extrusion-based printing of biodegradable and biocompatible solid PCLs associated with a copolymer of maleic anhydride and methyl vinyl ether. Multiple polymer formulations produced intravaginal devices in disc and mesh configurations. The results demonstrated clear antimicrobial properties, with the ability to provide sustained release of metronidazole while restraining the growth of Gardnerella vaginalis. These results demonstrated that patient compliance improved with a decreasing drug administration frequency [152].
Jeong et al. [153] analyzed controlled drug delivery and the incorporation of carbon nanofibers and gold particles as mechanical reinforcements for polycaprolactone (PCL) cardiovascular scaffolds. PCL and the reinforcement additives were processed using the FDM 3D printing technique, with doxorubicin hydrochloride (from 0 to 3.0 wt%) serving as the pharmaceutical ingredient. The results demonstrated that the manufactured cardiovascular scaffolds implemented the required feasibility for therapy at a specific location as an efficient method for the treatment of injured tissues, cancer, and atherosclerosis [153].
3.2. Natural Biopolymers
Natural biopolymers are polymers that originate from natural sources [15] and are versatile for distinct biomedical applications. Primarily, natural polymers are extracted from natural substances that are derived from microorganisms, plants, and animals. Compared to synthetic polymers, natural polymers have fewer side effects and produce fewer toxic reactions in humans. Natural polymers also have higher biocompatibility, biodegradability, availability, and capability for chemical modification. Among the main types of biopolymers used in the manufacturing of drug delivery systems produced by 3D printing, polysaccharides and proteins stand out [16]. The next subsections discuss the applications of these two polymer groups.
3.2.1. Polysaccharides
Polysaccharides comprise monosaccharide units in their backbone, generally joined by ether bond O-glycosidic linkages. These materials are very stable, hydrophilic in nature, nontoxic, biocompatible, biodegradable, and easy to modify, and they have a wide range of applications in drug delivery. Chitosan, hyaluronic acid, glycol chitosan, pullulan, dextrin, alginate, and cellulose are some of the polysaccharides that are used in the production of drug delivery systems developed by 3D printing [16].
Chitosan
Chitosan is a cationic polymer that is generated from a natural carbohydrate that is a polysaccharide of nonhuman origin. The extraction of chitosan usually comes from the crutician skeleton [16], which is a substance that is found in the shells of shrimp, lobsters, and crabs [121]. Asiri et al. reported the first description of chitosan nanoparticles in 1994. Since then, many approaches have demonstrated the use of chitosan for the production of drug delivery disposables [154]. This natural polymer is considered convenient for many medical applications because of its permeability to water and oxygen [16]. In addition, chitosan has favorable biocompatibility and degradability and low toxicity. Chitinases are the enzymes that are responsible for the degradation of chitosan. This enzyme is present in the human body. The solubility of chitosan occurs at room temperature in acidic aqueous solutions without requiring the use of heat or toxic solvents. The combination of chitosan with many existing additives or drugs, such as proteins, molecules, or polynucleotides, is possible [121].
Chitosan is hemostatic and highly permeable to oxygen and water. The blending or cross-linking of chitosan with other polymers enables the adoption of mechanical and drug release profiles according to the personalization demand. As an atoxic polymer, chitosan has FDA classification as safe for human body use. For this reason, this natural polymer has been used for the manufacturing of food additives and oral drug delivery devices [16].
The principal physical and chemical parameters for chitosan characterization are their deacetylation fraction, their crystalline atomic microstructure, and their density. Chitosan deacetylation is a very important factor for polymer use, because it determines the main physical, chemical, and biological parameters of this polymer. The most relevant parameters are the density, microstructural atomic crystallinity, degradation rate, cellular response, and hydrophilicity. An increase in the degree of deacetylation results in a reduction in inflammatory reactions and degradation rates. For this reason, chitosan with a higher deacetylation degree (approximately 90 to 95%) is the most recommended material for biomedical applications, even if this process is more sophisticated and increases the final polymer cost [155].
The development of suitable bioink formulations is the main application of chitosan in 3D printing. The filling material is in the form of hydrogels that are usually associated with other natural substances, such as cells, body fluids, drugs, or polymers. These hydrogels are capable of mimicking natural tissues, such as the extracellular matrix, bones, cartilage, neurons, skin, or organs. Moreover, chitosan is able to increase the attachment of supporting cells, proliferation, and differentiation for tissue repair. However, chitosan has a lower mechanical strength and high viscosity, which is inconvenient for the 3D printing process, because it promotes printer nozzle clogging. The higher degradation rate of chitosan is another drawback of this polymer, as the 3D printing process is associated with an increase in the temperature of the printed substances. For this reason, additional adjustments are required to assure easier extrusion, such as associations with PEG, pectin, and gelatin [156].
Baykara et al. designed and fabricated 3D-printed wound dressings for the production of implantable scaffolds. It contains chitosan, bismuth ferrite, and amoxicillin (active pharmaceutical ingredient, 0.45 mg/g bismuth ferrite), which are among the most commonly used medications for antibacterial treatment. Bismuth ferrite provided drug release via electrical triggering, which was intended to enhance skin regeneration. The scaffold was produced via inkjet-based 3D printing. The results also demonstrated that the addition of bismuth ferrite increased the porosity of the 3D-printed scaffold, which resulted in satisfactory cell attachment with solid biocompatibility and improved the drug release profile [157].
Koumentakou et al. indicated the feasibility and biocompatibility of a 3D-printed hydrogel that contained chitosan, PVA, gelatin, and levofloxacin (an active pharmaceutical ingredient, 10 wt%). This approach is intended for the manufacture of a potential implantable scaffold for tissue engineering with antimicrobial properties. The hydrogels presented good printability and were printed with the use of inkjet-based technology, enabling the production of smooth and uniform scaffolds. Moreover, research has demonstrated the outstanding incorporation of Levofloxacin, which can be continuously released for 48 h [158].
Ioannou et al. produced a 3D-printed implant intended to prevent conjunctival fibrosis in glaucoma patients. The implants were produced via hot-melt extrusion and were composed of PCL, chitosan, and 5-fluorouracil (active pharmaceutical ingredient, 1 wt%). The results demonstrated the biocompatibility and degradability required for the treatment. Moreover, sustained drug release has been demonstrated for more than two months without affecting the viability of conjunctival fibroblasts, which corroborates the efficacy of the medical therapy [159].
Hyaluronic Acid
John Palmer and Karl Meyer first described hyaluronic acid (HA), or hyaluronan, in 1934 [160]. This glycosaminoglycan polymer is natural in origin, biocompatible, and omnipresent in a wide range of natural tissues. It has been shown to be effective at regulating tissue functionality and cellular performance, including angiogenesis, cell expansion, differentiation, and location [161].
HA is synthesized by the addition of N-acetylglucosamine and glucuronic acid to the developing chain. This substance addition is alternated with the use of substrates, which are activated nucleotide sugars. The number of HA molecules can reach approximately 10,000 for repeated disaccharides [160].
HA composes the extracellular matrix of most human tissues, as this polymer is helpful for enhancing cellular performance and interactions with substances that are associated with HA [132]. For these reasons, HA is attractive for 3D printing, especially for bioinks based on this polymer.
However, HA has poor mechanical properties, as it exists as a viscous solution, which results in low mechanical strength and low shape retention immediately after 3D printing. Many studies are being carried out to find a solution for this drawback. The main solution is to associate HA with other polymers that have a higher mechanical strength, resulting in a copolymer with acceptable physical and chemical properties. HA is also an efficient additive that alters the viscosity of other polymers, resulting in bioinks that are suitable for inkjet-based printing. HA is a hydrophilic substance that works as a lubricant, even at lower concentrations, and is able to form hydrogels with high viscosity [162].
HA hydrogels are attracting increased recognition in the biomedical field because of their exceptional ability to respond to stimuli, adhere, and exert antibacterial effects. These characteristics improve drug release and therapeutic efficacy. The formation of HA hydrogels usually requires structural and chemical adjustments, which include the incorporation and cross-linking of other substances, which may lead to changes in HA properties. These changes may be favorable or not favorable [163].
Zhang et al. produced 3D-printed dual-drug delivery implantable PCL scaffolds using a micro-extrusion-based technique [164]. The incorporation of substances into the scaffolds occurred with biodegradable mesoporous silica nanoparticles (bMSNs) and fingolimod (an active pharmaceutical ingredient, 20 mg/380 mg of bMSN). A hydrogel containing hyaluronic acid, chitosan, and vancomycin (active pharmaceutical ingredient, 500 μg per scaffold) was incorporated into the scaffold’s pores. This approach resulted in an efficient alternative for the repair of bone defects and reduction in infection, as it provides the required structural support and promotes osteogenesis and angiogenesis. The results demonstrated rapid drug release in the initial stage with a consecutive sustained release profile, which is crucial for the control of infection in the first stage and sustained drug release for antibacterial therapy [164].
Hwang et al. successfully developed a hydrogel patch intended for spatiotemporally compartmentalized cerebral angiogenesis induction (SCAI) [165]. Inkjet-based 3D printing was performed using hybrid inks that contained HA and a decellularized extracellular matrix that was derived from vascular tissue. Hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) (0.05 g/L each) were used as model drugs. VEGF is a crucial factor for the development of the initial phase of angiogenesis, and endothelial cell propagation can activate sprouts in immature vessels. However, the use of HGF is required to reduce VEGF drawbacks, such as increased vascular permeability and a proinflammatory response. HGF promotes vascular endothelial cells and the maturation phase of angiogenesis. VEGF in combination with HGF prevented vessel regression and leaky vessel formation. The results demonstrated that the patches were an effective solution, as long-term drug delivery was a great challenge. It is a promising, efficient alternative therapy for the induction of neovascularization in most chronic cerebral ischemic diseases [165].
Hao et al. fabricated inkjet-based 3D-printed biomimetic meniscal scaffolds containing PCL, HA, and gelatin. The ink was loaded with meniscal extracellular matrix (0, 0.5, 1.0, and 2.0 wt%) as the active pharmaceutical ingredient. The results demonstrated the efficiency of drug release and the achievement of a biomimetic microarchitecture, which facilitates endogenous progenitor/stem cell migration, proliferation, and differentiation, which are crucial factors for meniscal regeneration [166].
Cellulose
Cellulose is the polysaccharide with the highest abundance in natural resources. This polymer is derived from bacteria, natural fibers, or plants [20]. Hermann Staudinger determined the polymeric structure of cellulose in 1920 [167]. The composition of cellulose includes glucose chains that are compressed into microfibrils and fibrils, whose orientation occurs at precise angles. This enables the formation of both amorphous and crystalline atomic microstructures in cellulose [168].
Cellulose is insoluble in water and most organic solvents. However, this polymer is hydrophilic. This is because of the existence of hydroxyl groups on cellulose chains with various intra- and intermolecular hydrogen bonds. This results in the strong holding of those molecules, which results in the formation of strong microfibrils [169].
The use of cellulose for the production of 3D-printed devices for biomedical applications is still an emerging active field with great potential. Research on the development and exploration of alternative materials is important for responding to global trends that require even more sustainable and biocompatible resources for biomedical personalized therapies [170].
Cellulose is a feasible material for the production of biomedical products. Due to their viscosity, cellulose hydrogels are the most commonly used for 3D printing. However, the maintenance of a printed shape is still a great challenge due to the low mechanical strength of cellulose [171]. The solution for this drawback is cross-linking with other polymers with higher mechanical strength [168].
The use of hydrogen bonding enables the preparation of cellulose hydrogels from native cellulose solution [20]. The multiple hydrogen bonds between the hydroxyl groups of the cellulose molecules are the reason for the hydrophilic nature of this polymer, even if it remains insoluble in water and other common organic solvents. The connections of cellulose backbone chains are strong, enabling the production of strong tensile strength microfibrils [169]. For this reason, the main inconvenience in the preparation of cellulose hydrogels is the search for an adequate solvent for this purpose. Hydroxy group etherification with methyl or ethyl units is the main process for synthesizing cellulose derivatives with water solubility. Some of the cellulose ethers are carboxyethylcellulose, hydroxypropyl methylcellulose, methylcellulose, hydroxyethylcellulose, ethylcellulose, and carboxymethylcellulose. The preparation of hydrogels involves chemical or physical cross-linking with these substances [20].
Cellulose and its derivatives have been the subject of extensive research for the production of 3D-printed personalized drug delivery systems with the ability to control drug release [168]. Despite extensive analysis, the cost efficiency of nanocellulose, associated with its chemical and structural properties, is still a drawback. This requires extensive research for a more adequate performance, especially in regard to lower weight functionalization percentages [170]. Recent studies have shown that cellulose can be feasibly combined with other natural substances for the development of bioinks for 3D printing while minimizing costs without compromising the requirements for biomedical applications [168].
Johannesson et al. [171] developed a formulation based on lipids without emulsification that were loaded with fenofibrate (active pharmaceutical ingredient, 4.22 wt%), which was incorporated into an emulsion via synergistic stabilization with silica nanoparticles, stearic acid, and cellulose. Oral tablet production was performed with the use of a 3D printer based on semisolid extrusion. The emulsion gel demonstrated feasible rheological characteristics for 3D printing. Compared to lipid-based self-emulsifying gels without emulsification, the developed gels demonstrated greater mechanical strength and greater viscosity, allowing for the conversion of gels without emulsification into solid forms for rapid drug release and digestion in the small intestine, although fenofibrate has poor solubility in water [171].
Asadi et al. [103] designed and produced tablet shells made of PLA and Eudragit® FS100 with a hydrogel core that contained N-acetylglucosamine (active pharmaceutical ingredient, 30 mg/mL) and methyl cellulose. The tablet shell was prepared with the use of an Arburg Plastic Freeforming 3D printer. This disposable drug delivery system is intended for the treatment of inflammatory bowel disease. The tablet’s thick shell was important for preventing rapid drug release and ensuring that the dissolution was effective in the dry colon environment. Moreover, the thermal decomposition of N-acetylglucosamine could occur upon its insertion into the 3D-printed device. The results demonstrated that methyl cellulose is responsible for the reduction in systemic exposure and enhanced biocompatibility [103].
Roche et al. [172] successfully developed a body-weight-adjusted dosage of caffeine as a medical treatment for premature apnea. The development of drug delivery routes for oral solid forms was achieved with a semisolid extrusion-based 3D printer that was flexible at various caffeine concentrations (25, 35, 50, and 75 wt%) through the testing of printing parameters and the use of distinct substances. Hydroxypropylmethyl cellulose and sodium alginate composed a hydrogel matrix with drug loading. Sodium croscarmellose and crospovidone, which are disintegrant substances, were also evaluated for their ability to accelerate the caffeine release. Three-dimensional models were designed with different diameters, thicknesses, infill patterns, and densities. The results demonstrated good printability, which enables the production of oral mucoadhesive devices. This work also demonstrated the achievement of adequate drug delivery, suggesting the use of water or milk dispersion in 3D-printed devices for neonatology [172].
3.2.2. Proteins
Biomaterials composed of proteins are recognized for their naturally regulated decomposition. These polymers break down through hydrolysis, which relates to the dissolution of phosphoester bonds in the main polymeric chain and the sided chains [16].
Since the chemical structure of collagen was first described in the mid-1930s, collagen has been the subject of research by many scientists [173]. This protein, which is naturally found in the connective tissue of animals, is a major component that is under exploration for numerous applications [16]. Animal tissues contain this dominant and omnipresent fibrillar protein, which corresponds to approximately 30% of all vertebrate proteins. This polymer can be extracted in large amounts. Many studies have explored its potential as a biomaterial [174], with ample use in the production of films, hydrogels, coatings, microparticles, sponges, pellets, and drug delivery systems [16].
When collagen is overexpressed in certain diseases, such as psoriasis, scleroderma, and lung and liver fibrosis, drug delivery targeting is feasible. The polypeptides that compose collagen usually include lysine, glycine, hydroxyproline, and proline. Extensive studies have evaluated the use of collagen for the localized administration of low-molecular-weight drugs, such as antibiotics [16].
Collagen is widely used for 3D bioprinting. However, this approach has several drawbacks. When this polymer is exposed to low temperatures, collagen becomes a liquid and generates a fibrous structure as the temperature increases. The total gelation of collagen may take approximately 30 min when the temperature is approximately 37 °C. This moderate gelation rate is adverse for 3D-bio-printed materials [38]. Collagen is soluble in an aqueous solution at a low pH, and it can be prepared in distinct forms, such as hydrogels, sponges, powders, sheets, and tubes [175].
Research performed by Song et al. targeted the development of tissue engineering scaffolds for bone defect repair. The scaffold mixture included sodium alginate, hydroxyapatite, collagen, and amoxicillin (the active pharmaceutical ingredient; 150, 300, and 600 mg/g hydroxyapatite). The scaffold was produced via extrusion-based 3D printing, followed by a freeze-drying composite procedure, which is required for the infection of bone defects. The antibacterial and drug release analyses indicated that the manufactured scaffolds demonstrated satisfactory long-term drug release with antibacterial efficiency and adequate cytocompatibility, which confirmed the feasibility of the produced scaffolds for infected bone defect repair [176].
Liu et al. developed innovative biological scaffolds containing silk fibroin, collagen, hydroxyapatite, and recombinant human erythropoietin as pharmaceutical ingredients (200 IU per scaffold), which were intended to be implantable for bone defect reconstruction. The scaffolds were produced using extrusion-based 3D printing technology at a low temperature. In vivo tests indicated that the biological scaffolds gradually degraded and improved the proliferation and accumulation of osteoblasts and collagen fiber development, which is crucial for the reconstruction of mandibular defects [175].
Biocompatible microneedle patches for transdermal drug delivery were successfully produced by Mutlu et al. using a stereolithography 3D printer [177]. The microneedles (MNs) were coated with collagen-gentamicin (1 wt%)-loaded nanoparticles, manufactured via the electrospray method. Collagen is a significant natural protein that is also associated with scar production and is a relevant factor during connective tissue repair. The release profiles of collagen-gentamicin-coated MNs demonstrated that complete drug release occurred in 9 h [177].
The collagen derivative gelatin is extracted from peptides and proteins and was first described by Denis Papin in 1682 [178]. This material has a low cost, water solubility, biodegradability, and biocompatibility because of the presence of multiple active components. Gelatin is not found in the natural environment, and there is no plant-based origin of gelatin [16]. Generally, gelatin is extracted from skin and bone collagen through acid or alkali processing [156].
This natural polymer is obtained from collagen by partial hydrolysis and heat denaturation procedures using acid [20], alkali, or enzymes [16]. Furthermore, during enzymatic degradation, gelatin does not produce any toxic substances. Since gelatin is denatured, it has moderate antigenicity compared to collagen, which has antigenicity. The presence of many functional groups in gelatin provides various possibilities for joining with ligands and cross-linkers for the creation of targeted biomaterials, such as drug delivery systems. Recent studies have shown that numerous delivery systems are being developed for the delivery of proteins, vaccines, and drugs [16].
Gelatin exhibits biodegradability and high biocompatibility and is compatible with enabling cell adherence with low antigenicity and mimicking the extracellular matrix. Due to these characteristics, gelatin is a prevalent natural polymer for the manufacture of 3D-printed hydrogels or scaffolds for tissue engineering. Nevertheless, the mechanical properties of gelatin-based printed objects are unsatisfactory and require the combination of gelatin with other substances, such as poly(lactic-co-glycolic acid) (PLGA), polyvinyl alcohol (PVA), carbon nanofibers, and alginate [157]. Moreover, gelatin is insoluble in cold water. Nonetheless, it is soluble in heated water (approximately over 35 °C) [178]. At lower temperatures (below 27 °C), gelatin can form a physical hydrogel. However, it is not stable at human body temperature because of its reversible thermal gelation. The use of various chemical modifications and covalent cross-linking strategies overcomes this drawback [20].
Zaer et al. successfully combined nanocarriers and 3D printing for drug delivery device production for breast cancer therapy. Inkjet-based 3D-printed alginate–gelatin nanocomposites, associated with niosomes that were loaded with doxorubicin (active pharmaceutical ingredient, 1 mg/mL), were developed and used as pH-dependent drug delivery devices. The results demonstrated that the designed nanocarriers presented a greater than 95% cytotoxicity against breast cancer cells and a greater than 90% cell viability. Moreover, the use of 3D printing for the production of smart drug delivery devices at a large scale is feasible, and the designed nanocarriers demonstrated efficacy for further research on cancer therapies [179].
Wei et al. developed a shell/core fibrous structure with a triple drug loading format, which was manufactured with the use of a coaxial inkjet-based hydrogel 3D printer [180]. The shell is composed of polydopamine and alginate, while the core is composed of gelatin. Doxorubicin (20 mg/g of gelatin) was chosen to load the core/shell fibers at three different positions: inside the core, on the external shell (which presented the fastest release), and in the channel part (which presented the slowest release) of the core/shell fibers. Furthermore, the drug release rate was controlled by the concentration of alginate gels, the cross-linking density, the helical structure design, the size, and the fiber porosity. These factors demonstrated the great feasibility of 3D printing for personalized release and the achievement of adequate medical treatment [181].
Inkjet-based 3D printing was used by Hao et al. to provide an innovative methodology for the manufacturing of biomimetic meniscal scaffolds. This therapy targets meniscal regeneration by providing bioactive components that contribute to an adequate biomechanical microenvironment and an anisotropic architecture. A 3D printing procedure was used to coencapsulate platelet-derived growth factor-BB (1.0 wt%) and kartogenin (1.0 wt%) as active pharmaceutical ingredients within biomimetic polycaprolactone–bioink scaffolds. These substances are intended to promote endogenous stem cell homing and target resident mesenchymal stem cell chondrogenesis. The bioink contains gelatin, hyaluronic acid, and meniscal extracellular matrix. The results for the 3D-printed scaffolds demonstrated that the combination of both pharmaceutical ingredients had combined advantages in enhancing cell migration and promoting mesenchymal stem cell chondrogenic differentiation, resulting in neomeniscal regeneration approximately three and six months after scaffold implantation [166].
Recently, emulsified gels have also been developed as new materials for the programmed release of antibodies for the treatment of cancer [180], with a potential application in 3D bioprinting of DDSs. In any case, the area of developing DDSs through 3D printing for cancer immunotherapy is currently an active field of research, with promising results for improving the health of patients who are affected by the disease.
In general, the 3D printing DDS manufacturing process primarily involves the incorporation of the active molecule into the polymeric matrix before the extrusion process and/or the deposition of the material on the working surface of the 3D printer [182]. Parameters such as the particle size, degree of crystallinity of the drug, melting point, thermal degradation of the polymer and active ingredient, molecular interactions between the polymer and drug, and hydrophilic/hydrophobic and/or pH-dependent nature of the matrix are essential factors for the choice of materials and study of the feasibility of a given demand [183].
In techniques that depend on the temperature to create a DDS, such as FDM of polymer/drug filaments (which generally works with hot extrusion at temperatures above 100 °C), microstructural characterization and the study of the thermal stability of the components in the final material are essential to ensure that the release device maintains its designed functionalities [182,183].
Given the numerous advantages that 3D printing using polymers provides for the development of DDSs, it is important to highlight some details that may represent limitations to the required applications. In terms of speed, some techniques, such as SLS and SLA, have relatively slow printing times compared to those of other 3D printing techniques and other techniques for manufacturing three-dimensional objects. Therefore, printing parts that combine large dimensions, high geometric complexity, and many submillimeter details may be unfeasible on a large scale.
A limitation of the bioprinting technique is related to the materials that are available for printing drug release devices. Currently, although there are a considerable number of functional hydrogels for specific applications in biotechnology, the majority of them are intended for applications in tissue cell incorporation. Furthermore, the cost of hydrogels and active substances for DDS applications can be high, and the administration of materials and printing equipment may require prior user training.
Although the FDM technique is the most popular 3D printing technique for DDS applications, unconventional polymeric filaments containing the active substance of interest are needed. These filaments are used for layered printing of pharmaceutical solid solutions. These polymer/drug filaments are generally unavailable on the market and need to be produced by the user. This previous stage of filament preparation requires microstructural, mechanical, and thermal characterization experiments to obtain functional and workable materials from available FDM 3D printers.
4. Conclusions
The current main 3DP technologies for producing customized DDSs are presented and discussed in detail. In summary, the described procedures of 3DP technologies have met potential demands in DDSs, with high reproducibility and precision. In addition, these technologies present themselves as having low costs associated with them, with the possibility of producing devices on a large scale.
Several synthetic and natural polymers have been characterized as viable for the production of DDSs by 3D printing, because they combine functionality, recognized biocompatibility, biodegradability, mechanical strength, and release kinetics. Bibliographical research has shown a wide variety of applications, encompassing the production of capsules, tablets for oral administration, controlled-release dermal devices, and suppositories for gynecological disease therapy.
Furthermore, recent research has led to future studies on 3D-printable polymeric DDSs that address the production of dual- or multimaterial 3D printing with different active ingredients and designs (side-by-side, core–shell, layer-by-layer, etc.). The effects of different geometric shapes and interior fill densities of the parts are also important, since these factors directly interfere with the release kinetics of the active ingredient in the action medium and the development of new pharmaceutical forms from smart hydrogels and polymers that respond quickly to the contact medium and that allow adjustments in their chemical structure (as in the case of cationic or anionic groups). The aim is to improve the bioavailability of active substances and provide greater interaction of the polymeric matrix with the molecules of interest (DNA, RNA, tissue cells, antibodies, and drugs, among others) in the applications for which they are needed.
Author Contributions
Writing—review and editing, P.H.N.C. and E.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by Brazilian research agencies (Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco—FACEPE, through APQ 1387-3.03/22 and IBPG-0849-3.03/20 Projects).
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T. Three-Dimensional Printing and 3D Scanning: Emerging Technologies Exhibiting High Potential in the Field of Cultural Heritage. Appl. Sci. 2023, 13, 4777. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Demidenko, A.V.; Sukovatyi, A.G.; Dudaev, A.E.; Mylnikov, A.V.; Kisterskij, K.A.; Volova, T.G. Three-Dimensional Printing of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)[P (3HB-co-3HV)] Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation. Int. J. Mol. Sci. 2023, 24, 12969. [Google Scholar] [CrossRef] [PubMed]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Surini, S.; Bimawanti, Y.; Kurniawan, A. The Application of Polymers in Fabricating 3D Printing Tablets by Fused Deposition Modeling (FDM) and The Impact on Drug Release Profile. Pharm. Sci. 2023, 29, 156–164. [Google Scholar] [CrossRef]
- Karanwad, T.; Lekurwale, S.; Banerjee, S. Chapter 4—Selective Laser Sintering (SLS) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals, 1st ed.; Banerjee, S., Ed.; Springer Nature: Singapore, 2023; pp. 125–169. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D bioprinting and nanotechnology in tissue engineering scaffolds. Biomimetics 2023, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single-and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. J. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Puza, F.; Lienkamp, K. 3D printing of polymer hydrogels—From basic techniques to programmable actuation. Adv. Funct. Mater. 2022, 32, 2205345. [Google Scholar] [CrossRef]
- Mohammed, A.; Elshaer, A.; Sareh, P.; Elsayed, M.; Hassanin, H. Additive Manufacturing Technologies for Drug Delivery Applications. Int. J. Pharm. 2020, 580, 119245. [Google Scholar] [CrossRef] [PubMed]
- Ramola, M.; Yadav, V.; Jain, R. On the adoption of additive manufacturing in healthcare: A literature review. J. Manuf. Technol. Manag. 2018, 30, 48–69. [Google Scholar] [CrossRef]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive manufacturing of personalized pharmaceutical dosage forms via stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Jain, A.; Bansal, K.K.; Tiwari, A.; Rosling, A.; Rosenholm, J.M. Role of Polymers in 3D Printing Technology for Drug Delivery—An Overview. Curr. Pharm. Des. 2018, 24, 4979–4990. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B. Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Curry, E.J.; Henoun, A.D.; Miller, A.N.; Nguyen, T.D. 3D nano-and micro-patterning of biomaterials for controlled drug delivery. Ther. Deliv. 2016, 8, 15–28. [Google Scholar] [CrossRef]
- Meng, F.; Meyer, C.M.; Joung, D.; Vallera, D.A.; McAlpine, M.C.; Panoskaltsis-Mortari, A. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 2019, 31, 1806899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Renata, J. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Małek, E.; Miedzińska, D.; Popławski, A.; Szymczyk, W. Application of 3D printing technology for mechanical properties study of the photopolymer resin used to print porous structures. Tech. Sci. 2019, 22, 183–194. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bashyal, S.; Keum, T.; Noh, G.; Seo, J.E.; Bastola, R.; Choi, J.; Sohn, D.H.; Lee, S. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 2019, 14, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Chapter 6—Three-dimensional printed drug delivery systems. In Engineering Drug Delivery Systems, 1st ed.; Seyfoddin, A., Dezfooli, S.M., Greene, C.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; Volume 1, pp. 147–162. [Google Scholar] [CrossRef]
- Wulf, K.; Riess, C.; Rekowska, N.D.; Eickner, T.; Seitz, H.; Grabow, N.; Teske, M. PMMA and PVP based polymers for stereolitho-graphic manufacture of tailored drug release. Trans. Addit. Manuf. Meets Med. 2019, 1, 1–2. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D printed sensors for biomedical applications: A review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H., Jr.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 519, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, G.; Gretić, M.; Vinčić, J.; Poropat, A.; Cuculić, L.; Rahelić, T. Design and 3D printing of multi-compartmental PVA capsules for drug delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 677–686. [Google Scholar] [CrossRef]
- Krause, J.; Bogdahn, M.; Schneider, F.; Koziolek, M.; Weitschies, W. Design and characterization of a novel 3D printed pressure-controlled drug delivery system. Eur. J. Pharm. Sci. 2019, 140, 105060. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.; Martinez, H.; Gatenholm, E. Chapter 1—History and trends of 3D bioprinting. In 3D Bioprinting: Principles and Protocols, 1st ed.; Crook, J.M., Ed.; Humana Press: New York, NY, USA, 2020; Volume 1, pp. 3–18. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Chapter 2—Three-dimensional bioprinting in drug delivery. In Materials for Biomedical Engineering: Nanomaterials-Based Drug Delivery, 1st ed.; Holban, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 19–40. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.-F.; Gao, G.; Yonezawa, T.; Cui, X. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017, 12, 1600734. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D printing processes for biomedical field: Opportunities and challenges. J. Polym. Environ. 2020, 28, 1345–1367. [Google Scholar] [CrossRef]
- Saygili, E.; Dogan-Gurbuz, A.A.; Yesil-Celiktas, O.; Draz, M.S. A powerful tool to leverage tissue engineering and microbial systems. Bioprinting 2020, 18, e00071. [Google Scholar] [CrossRef]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef]
- Miyasaka, K. Chapter 3—PVA-Iodine complexes: Formation, structure, and properties. In Structure in Polymers with Special Properties, 1st ed.; Zachmann, H.G., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 108, pp. 91–129. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Afsana; Jain, V.; Haider, N.; Jain, K. 3D printing in personalized drug delivery. Curr. Pharm. Des. 2018, 24, 5062–5071. [Google Scholar] [CrossRef]
- Musa, B.H.; Hameed, N.J. Study of the mechanical properties of polyvinyl alcohol/starch blends. Mater. Today Proc. 2020, 20, 439–442. [Google Scholar] [CrossRef]
- Curti, C.; Kirby, D.J.; Russell, C.A. Current formulation approaches in design and development of solid oral dosage forms through three-dimensional printing. Prog. Addit. Manuf. 2020, 5, 111–123. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Saviano, M.; Bowles, B.J.; Penny, M.R.; Ishaq, A.; Muwaffak, Z.; Falcone, G.; Russo, P.; Hilton, S.T. Development and analysis of a novel loading technique for FDM 3D printed systems: Microwave-assisted impregnation of gastro-retentive PVA capsular devices. Int. J. Pharm. 2022, 613, 121386. [Google Scholar] [CrossRef]
- Junqueira, L.A.; Tabriz, A.G.; Rousseau, F.; Raposo, N.R.B.; Brandão, M.A.F.; Douroumis, D. Development of printable inks for 3D printing of personalized dosage forms: Coupling of fused deposition modelling and jet dispensing. J. Drug Deliv. Sci. Technol. 2023, 80, 104108. [Google Scholar] [CrossRef]
- Wei, M.; Liu, D.; Sun, Y.; Xie, H.; Du, L.; Jin, Y. Mesalazine hollow suppositories based on 3D printing for treatment of ulcerative colitis. Int. J. Pharm. 2023, 642, 123196. [Google Scholar] [CrossRef]
- Krueger, L.; Cao, Y.; Zheng, Z.; Ward, J.; Miles, J.A.; Popat, A. D printing tablets for high-precision dose titration of caffeine. Int. J. Pharm. 2023, 642, 123132. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, A.; Midha, S.; Yuca, E.; Tukay, A.; Sasmazel, H.T.; Kalaskar, D.M.; Gunduz, O. Study on the cytocompatibility, mechanical and antimicrobial properties of 3D printed composite scaffolds based on PVA/Gold nanoparticles (AuNP)/Ampicillin (AMP) for bone tissue engineering. Mater. Today Commun. 2021, 28, 102458. [Google Scholar] [CrossRef]
- Pires, F.Q.; Gross, I.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Bao, S.N.; Cunha-Filho, M. In-situ formation of nanoparticles from drug-loaded 3D polymeric matrices. Eur. J. Pharm. Sci. 2023, 188, 106517. [Google Scholar] [CrossRef] [PubMed]
- Gaurkhede, S.G.; Osipitan, O.O.; Dromgoole, G.; Spencer, S.A.; Di Pasqua, A.J.; Deng, J. 3D printing and dissolution testing of novel capsule shells for use in delivering acetaminophen. J. Pharm. Sci. 2021, 110, 3829–3837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, P.; Chung, S.; Bandari, S.; Repka, M.A. Fabrication of bilayer tablets using hot melt extrusion-based dual-nozzle fused deposition modeling 3D printing. Int. J. Pharm. 2022, 624, 121972. [Google Scholar] [CrossRef] [PubMed]
- Manini, G.; Benali, S.; Mathew, A.; Napolitano, S.; Raquez, J.M.; Goole, J. Paliperidone palmitate as model of heat-sensitive drug for long-acting 3D printing application. Int. J. Pharm. 2022, 618, 121662. [Google Scholar] [CrossRef]
- Berger, V.; Buzhor, M.G.; Evstafeva, D.; Mügeli, L.; Leroux, J.C. 3D printing of a controlled urea delivery device for the prevention of tooth decay. Int. J. Pharm. 2023, 631, 122528. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Gong, Y.; Bu, T.; Wang, X.; Pan, H. Unidirectional drug release from 3D printed personalized buccal patches using FDM technology. Int. J. Pharm. 2023, 645, 123382. [Google Scholar] [CrossRef]
- Tagami, T.; Hayashi, N.; Sakai, N.; Ozeki, T. 3D printing of unique water-soluble polymer-based suppository shell for controlled drug release. Int. J. Pharm. 2019, 568, 118494. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Bandari, S.; Mandati, P.; Almotairy, A.; Almutairi, M.; Repka, M. Fabrication of a shell-core fixed-dose combination tablet using fused deposition modeling 3D printing. Eur. J. Pharm. Biopharm. 2022, 177, 211–223. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Li, L.; Fang, D.; Sun, H.; Qiao, S.; Li, X.; Pan, W. Exploration and preparation of patient-specific ciprofloxacin implants drug delivery system via 3D printing technologies. J. Pharm. Sci. 2021, 110, 3678–3689. [Google Scholar] [CrossRef]
- Chee, P.S.; Arsat, R.; He, X.; Kalantar-zadeh, K.; Arsat, M.; Wlodarski, W. Polyvinylpyrrolidone/Multiwall Carbon Nanotube Composite Based 36° YX LiTaO3 Surface Acoustic Wave For Hydrogen Gas Sensing Applications. In Proceedings of the International Conference on Enabling Science and Nanotechnology (ESciNano), Lumpur, Malaysia, 1–3 December 2010. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Yang, H.; Wang, J.; Li, Q.; Qu, R.; Wu, X. Nanocomplexes based polyvinylpyrrolidone K-17PF for ocular drug delivery of naringenin. Int. J. Pharm. 2020, 578, 119133. [Google Scholar] [CrossRef]
- Bianchi, M.; Pegoretti, A.; Fredi, G. An overview of poly (vinyl alcohol) and poly (vinyl pyrrolidone) in pharmaceutical additive manufacturing. J. Vinyl Addit. Technol. 2023, 29, 223–239. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Dose-independent drug release from 3D printed oral medicines for patient-specific dosing to improve therapy safety. Int. J. Pharm. 2022, 616, 121555. [Google Scholar] [CrossRef]
- Jovanović, M.; Petrović, M.; Cvijić, S.; Tomić, N.; Stojanović, D.; Ibrić, S.; Uskoković, P. 3d printed buccal films for prolonged-release of propranolol hydrochloride: Development, characterization and bioavailability prediction. Pharmaceutics 2021, 13, 2143. [Google Scholar] [CrossRef]
- Than, Y.M.; Titapiwatanakun, V. Statistical design of experiment-based formulation development and optimization of 3D printed oral controlled release drug delivery with multi target product profile. J. Pharm. Investig. 2021, 51, 715–734. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Salehi, S.; Ghomi, H.; Hassanzadeh-Tabrizi, S.A.; Koupaei, N.; Khodaei, M. The effect of polyethylene glycol on printability, physical and mechanical properties and osteogenic potential of 3D-printed poly (l-lactic acid)/polyethylene glycol scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2022, 221, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Digkas, T.; Porfire, A.; Renterghem, J.V.; Samaro, A.; Borodi, G.; Vervaet, C.; Crisan, A.G.; Iurian, S.; Beer, T.; Tomuta, I. Development of Diclofenac Sodium 3D Printed Cylindrical and Tubular-Shaped Tablets through Hot Melt Extrusion and Fused Deposition Modelling Techniques. Pharmaceuticals 2023, 16, 1062. [Google Scholar] [CrossRef]
- Picco, C.J.; Utomo, E.; McClean, A.; Domínguez-Robles, J.; Anjani, Q.K.; Volpe-Zanutto, F.; McKenna, P.E.; Acheson, J.G.; Malinova, D.; Donnelly, R.F.; et al. Development of 3D-printed subcutaneous implants using concentrated polymer/drug solutions. Int. J. Pharm. 2023, 631, 122477. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef]
- Atiqah, A.; Mastura, M.T.; Ali, B.A.A.; Jawaid, M.; Sapuan, S.M. A Review on Polyurethane and its Polymer Composites. Curr. Org. Synth. 2017, 14, 233–248. [Google Scholar] [CrossRef]
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. 3D printing of polyurethane biomaterials. In Advances in Polyurethane Biomaterials, 1st ed.; Cooper, S.L., Guan, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 149–170. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.; Huang, S.; Wei, W.; Yang, A.; Zhao, H.; Wang, Y. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Snick, V.B.; Boone, M.N.; Hellemans, T.; Hoorebeke, L.V.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Utomo, E.; Cornelius, V.A.; Anjani, Q.K.; Korelidou, A.; Gonzalez, Z.; Donnelly, R.F.; Margariti, A.; Delgado-Aguilar, M.; Tarrés, Q.; et al. TPU-based antiplatelet cardiovascular prostheses prepared using fused deposition modelling. Mater. Des. 2022, 220, 110837. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Gao, B.; Li, J. Spidroin-based multifunctional microneedles with controlled drug release for efficient wound management. Eur. Polym. J. 2023, 198, 112429. [Google Scholar] [CrossRef]
- Martorana, A.; Fiorica, C.; Palumbo, F.S.; Federico, S.; Giammona, G.; Pitarresi, G. Redox responsive 3D-printed nanocomposite polyurethane-urea scaffold for Doxorubicin local delivery. J. Drug Deliv. Sci. Technol. 2023, 88, 104890. [Google Scholar] [CrossRef]
- Salimi, S.; Wu, Y.; Barreiros, M.I.E.; Natfji, A.A.; Khaled, S.; Wildman, R.; Hart, L.R.; Greco, F.; Clark, E.A.; Roberts, C.J.; et al. 3D printed drug delivery implant formed from a dynamic supramolecular polyurethane formulation. Polym. Chem. 2020, 11, 3453–3464. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Tang, L.; Liu, J.; Yang, J.; Lai, W.; Wu, T.; Liao, S.; Zhang, X.; Pan, H.; et al. A universally dispersible graphene-based ink modifier facilitates 3D printing of multi-functional tissue-engineered scaffolds. Mater. Des. 2022, 216, 110551. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Henry, S.; Samaro, A.; Marchesini, F.H.; Shaqour, B.; Macedo, J.; Vanhoorne, V.; Vervaet, C. Extrusion-based 3D printing of oral solid dosage forms: Material requirements and equipment dependencies. Int. J. Pharm. 2021, 598, 120361. [Google Scholar] [CrossRef] [PubMed]
- Linares, V.; Galdón, E.; Casas, M.; Caraballo, I. Critical points for predicting 3D printable filaments behaviour. J. Drug Deliv. Sci. Technol. 2021, 66, 102933. [Google Scholar] [CrossRef]
- Wen, Y.T.; Dai, N.T.; Hsu, S.H. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Campana, R.; Frangipani, E.; Casettari, L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int. J. Pharm. 2021, 596, 120290. [Google Scholar] [CrossRef]
- Bil, M.; Kijeńska-Gawrońska, E.; Głodkowska-Mrówka, E.; Manda-Handzlik, A.; Mrowka, P. Design and in vitro evaluation of electrospun shape memory polyurethanes for self-fitting tissue engineering grafts and drug delivery systems. Mater. Sci. Eng. C 2020, 110, 110675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Z.; Hua, C.; Ji, J.; Zhou, Z.; Fang, Y.; Weng, D.; Lu, L.; Pang, Y.; Sun, W. Design, modeling and 3D printing of a personalized cervix tissue implant with protein release function. Biomed. Mater. 2020, 15, 045005. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.; Domínguez-Robles, J.; Stewart, S.A.; Mancuso, E.; O’Donnell, K.; Larrañeta, E. Fused deposition modeling as an effective tool for anti-infective dialysis catheter fabrication. ACS Biomater. Sci. Eng. 2019, 5, 6300–6310. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, M.; Wang, G.; Peng, H.; Wahid, F.; Yang, Y.; Liang, L.; Liu, S.; Li, R.; Feng, S. Zein nanospheres assisting inorganic and organic drug combination to overcome stent implantation-induced thrombosis and infection. Sci. Total Environ. 2023, 873, 162438. [Google Scholar] [CrossRef] [PubMed]
- Eder, S.; Wiltschko, L.; Koutsamanis, I.; Urich, J.A.A.; Arbeiter, F.; Roblegg, E.; Spoerk, M. Toward a new generation of vaginal pessaries via 3D-printing: Concomitant mechanical support and drug delivery. Eur. J. Pharm. Biopharm. 2022, 174, 77–89. [Google Scholar] [CrossRef]
- Farmer, Z.L.; Utomo, E.; Domínguez-Robles, J.; Mancinelli, C.; Mathew, E.; Larrañeta, E.; Lamprou, D.A. 3D printed estradiol-eluting urogynecological mesh implants: Influence of material and mesh geometry on their mechanical properties. Int. J. Pharm. 2021, 593, 120145. [Google Scholar] [CrossRef]
- Martin, N.K.; Domínguez-Robles, J.; Stewart, S.A.; Cornelius, V.A.; Anjani, Q.K.; Utomo, E.; García-Romero, I.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; et al. Fused deposition modelling for the development of drug loaded cardiovascular prosthesis. Int. J. Pharm. 2021, 595, 120243. [Google Scholar] [CrossRef]
- Shao, Y.; Dong, K.; Lu, X.; Gao, B.; He, B. Bioinspired 3D-Printed MXene and Spidroin-Based Near-Infrared Light-Responsive Microneedle Scaffolds for Efficient Wound Management. ACS Appl. Mater. Interfaces 2022, 14, 56525–56534. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Chapter 1—PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications, 1st ed.; Jiménez, A., Peltzer, M.A., Ruseckaite, R.A., Eds.; The Royal Society of Chemistry: London, UK, 2015; Volume 12, pp. 3–36. [Google Scholar] [CrossRef]
- Vatansever, E.; Arslan, D.; Nofar, M. Polylactide cellulose-based nanocomposites. Int. J. Biol. Macromol. 2019, 137, 912–938. [Google Scholar] [CrossRef]
- Gowrav, M.P.; Siree, K.G.; Amulya, T.M.; Bharathi, M.B.; Ghazwani, M.; Alamri, A.; Alalkami, A.Y.; Kumar, T.M.P.; Ahmed, M.M.; Rahamathulla, M. Novel Rhinological Application of Polylactic Acid—An In Vitro Study. Polymers 2023, 15, 2521. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Z.; Li, S.; Cheng, L.; Xu, D.; Li, L.; Chen, L.; Xu, Y.; Liu, Z.; Liu, Y.; et al. Chitosan-vancomycin hydrogel incorporated bone repair scaffold based on staggered orthogonal structure: A viable dually controlled drug delivery system. RSC Adv. 2023, 13, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Salehi, Z.; Akrami, M.; Hosseinpour, M.; Jockenhövel, S.; Ghazanfari, S. 3D printed pH-responsive tablets containing N-acetylglucosamine-loaded methylcellulose hydrogel for colon drug delivery applications. Int. J. Pharm. 2023, 645, 123366. [Google Scholar] [CrossRef] [PubMed]
- Sri, K.H.; Ganapathy, D.; Nallasamy, D.; Venugopalan, S.; Sivaswamy, V.; Kamath, S.M. Sustained release of resveratrol from fused deposition modelling guided 3D porous scaffold for bone tissue engineering. Process Biochem. 2023, 131, 188–198. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Fang, D.; Sun, H.; Pan, W. 3D printed personalized amikacin sulfate local drug delivery system for bone defect therapy. J. Drug Deliv. Sci. Technol. 2022, 70, 103208. [Google Scholar] [CrossRef]
- Mei, Y.; He, C.; Gao, C.; Zhu, P.; Lu, G.; Li, H. 3D-printed degradable anti-tumor scaffolds for controllable drug delivery. Int. J. Bioprint. 2021, 7, 135–144. [Google Scholar] [CrossRef]
- Han, S.H.; Cha, M.; Jin, Y.Z.; Lee, K.M.; Lee, J.H. BMP-2 and hMSC dual delivery onto 3D printed PLA-Biogel scaffold for critical-size bone defect regeneration in rabbit tibia. Biomed. Mater. 2021, 16, 015019. [Google Scholar] [CrossRef]
- Cheng, L.; Xu, Z.; Liu, Y.; Zhou, D.; Sun, M.; Xu, Y.; Chen, L.; Sun, J. 3D-Printed Drug-Loaded Composite Scaffolds to Promote Osteogenesis and Antibacterial Activity. ACS Appl. Polym. Mater. 2022, 4, 4476–4485. [Google Scholar] [CrossRef]
- Khosraviboroujeni, A.; Mirdamadian, S.Z.; Minaiyan, M.; Taheri, A. Preparation and characterization of 3D printed PLA microneedle arrays for prolonged transdermal drug delivery of estradiol valerate. Drug Deliv. Transl. Res. 2021, 12, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, W.; Niu, R.; Li, Q.; Hu, C.; Jiang, S. 3D printed intragastric floating and sustained-release tablets with air chambers. J. Pharm. Sci. 2022, 111, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Park, J.; Kamaki, Y.; Kim, B. Optimization of the fused deposition modeling-based fabrication process for polylactic acid microneedles. Microsystems Nanoeng. 2021, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, K.; Chowdhury, N.; Coleman, E.; Ferdous, T.; Su, H.; Jennings, J.A.; Bumgardner, J.D.; Fujiwara, T. Long-Term Controlled Release of Simvastatin from Photoprinted Triple-Networked Hydrogels Composed of Modified Chitosan and PLA–PEG Micelles. Macromol. Biosci. 2021, 21, 2100123. [Google Scholar] [CrossRef]
- Basa, B.; Jakab, G.; Kállai-Szabó, N.; Borbás, B.; Fülöp, V.; Balogh, E.; Antal, I. Evaluation of biodegradable PVA-based 3D printed carriers during dissolution. Materials 2021, 14, 1350. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Sriamornsak, P.; Kittanaphon, T.; Suwanpitak, K.; Klinkesorn, N.; Sangnim, T. Development of a zero-order kinetics drug release floating tablet with anti–flip-up design fabricated by 3D-printing technique. J. Pharm. Investig. 2021, 51, 213–222. [Google Scholar] [CrossRef]
- Wu, L.; Shrestha, P.; Iapichino, M.; Cai, Y.; Kim, B.; Stoeber, B. Characterization method for calculating diffusion coefficient of drug from polylactic acid (PLA) microneedles into the skin. J. Drug Deliv. Sci. Technol. 2021, 61, 102192. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Gonzalez, Z.; Utomo, E.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Poly (caprolactone)-based coatings on 3D-printed biodegradable implants: A novel strategy to prolong delivery of hydrophilic drugs. Mol. Pharm. 2020, 17, 3487–3500. [Google Scholar] [CrossRef]
- Auvinen, V.V.; Virtanen, J.; Merivaara, A.; Virtanen, V.; Laurén, P.; Tuukkanen, S.; Laaksonen, T. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J. Drug Deliv. Technol. 2020, 57, 101625. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Niese, S.; Breitkreutz, J.; Quodbach, J. Development of a dosing device for individualized dosing of orodispersible warfarin films. Int. J. Pharm. 2019, 561, 314–323. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Cardoso, B.C.O.; Silva, M.E.S.R.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8–19. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bassand, C.; Siepmann, F.; Benabed, L.; Verin, J.; Freitag, J.; Charlon, S.; Soulestin, J.; Siepmann, J. 3D printed PLGA implants: How the filling density affects drug release. J. Control. Release 2023, 363, 1–11. [Google Scholar] [CrossRef]
- Bendicho-Lavilla, C.; Seoane-Viaño, I.; Santos-Rosales, V.; Díaz-Tomé, V.; Carracedo-Pérez, M.; Luzardo-Álvarez, A.M.; García-Gonzalez, C.A.; Otero-Espinar, F.J. Intravitreal implants manufactured by supercritical foaming for treating retinal diseases. J. Control. Release 2023, 362, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, D.; Guarch-Pérez, C.; Purbayanto, M.A.K.; Choińska, E.; Riool, M.; Zaat, S.A.J.; Święszkowski, W. 3D-printed dual drug delivery nanoparticle-loaded hydrogels to combat antibiotic-resistant bacteria. Int. J. Bioprint. 2023, 9, 683. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Huangfu, H.; Zhang, X.; Zhang, H.; Qin, Q.; Fu, L.; Wang, D.; Wang, C.; Wang, L.; et al. 3D printing of bone scaffolds for treating infected mandible bone defects through adjustable dual-release of chlorhexidine and osteogenic peptide. Mater. Des. 2022, 224, 111288. [Google Scholar] [CrossRef]
- Ji, J.; Wang, C.; Xiong, Z.; Pang, Y.; Sun, W. 3D-printed scaffold with halloysite nanotubes laden as a sequential drug delivery system regulates vascularized bone tissue healing. Mater. Today Adv. 2022, 15, 100259. [Google Scholar] [CrossRef]
- Zheng, C.; Attarilar, S.; Li, K.; Wang, C.; Liu, J.; Wang, L.; Yang, J.; Tang, Y. 3D-printed HA15-loaded β-tricalcium phosphate/poly (Lactic-co-glycolic acid) bone tissue scaffold promotes bone regeneration in rabbit radial defects. Int. J. Bioprint. 2021, 7, 317. [Google Scholar] [CrossRef]
- Banche-Niclot, F.; Licini, C.; Montalbano, G.; Fiorilli, S.; Mattioli-Belmonte, M.; Vitale-Brovarone, C. 3D Printed Scaffold Based on Type I Collagen/PLGA_TGF-β1 Nanoparticles Mimicking the Growth Factor Footprint of Human Bone Tissue. Polymers 2022, 14, 857. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lo, T.S.; Lin, Y.T.; Chien, Y.H.; Lu, C.J.; Liu, S.J. Fabrication of drug-eluting polycaprolactone/poly (lactic-co-glycolic acid) prolapse mats using solution-extrusion 3D printing and coaxial electrospinning techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef] [PubMed]
- Serris, I.; Serris, P.; Frey, K.M.; Cho, H. Development of 3D-printed layered PLGA films for drug delivery and evaluation of drug release behaviors. AAPS Pharm. Sci. Tech. 2020, 21, 256. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Yang, X.; Yuan, M.; Xian, S.; Zhang, L.; Yang, D.; Liu, S.; Dai, F.; Tan, Z.; et al. The role of a drug-loaded poly (lactic co-glycolic acid)(PLGA) copolymer stent in the treatment of ovarian cancer. Cancer Biol. Med. 2020, 17, 237–250. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, S.; Zhu, T.; Tang, N.; Bai, X.; Zheng, C. Preparation of printable double-network hydrogels with rapid self-healing and high elasticity based on hyaluronic acid for controlled drug release. Polymer 2020, 186, 121994. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mirani, B.; Pagan, E.; Mirzaaghaei, S.; Nasimian, A.; Kawalec, P.; Rosa, S.C.S.; Hamadi, D.; Fernandez, N.P.; Toyota, B.D.; et al. A drug-eluting 3D-printed mesh (GlioMesh) for management of glioblastoma. Adv. Ther. 2019, 2, 1900113. [Google Scholar] [CrossRef]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.E.; Kamvari-Moghaddam, A. Chapter 5—Synthetic bioresorbable polymers. In Durability and Reliability of Medical Polymers, 1st ed.; Jenkins, A., Stamboulis, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; Volume 1, pp. 96–118. [Google Scholar]
- Chen, J.; Lee, D.; Yang, J.; Lin, S.; Lin, Y.; Liu, S. Solution extrusion additive manufacturing of biodegradable polycaprolactone. Appl. Sci. 2020, 10, 3189. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Hu, Y.; Liu, J.; Yuan, T.; Zhou, W.; Dong, X.; Wang, C.; Binks, B.P.; Yang, Z. Fabrication of Poly (ε-caprolactone)-embedded Lignin-Chitosan Nanocomposite Porous Scaffolds from Pickering Emulsions. Langmuir 2023, 39, 6947–6956. [Google Scholar] [CrossRef]
- Ghosh, S.; Yadav, A.; Rani, S.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. 3D Printed Hierarchical Porous Poly (ε-Caprolactone) Scaffolds from Pickering High Internal Phase Emulsion Templating. Langmuir 2023, 39, 1927–1946. [Google Scholar] [CrossRef]
- Mohsenifard, S.; Mashayekhan, S.; Safari, H. A hybrid cartilage extracellular matrix-based hydrogel/poly (ε-caprolactone) scaffold incorporated with Kartogenin for cartilage tissue engineering. J. Biomater. Appl. 2023, 37, 1243–1258. [Google Scholar] [CrossRef]
- Gong, W.; Li, M.; Zhao, L.; Wang, P.; Wang, X.; Wang, B.; Liu, X.; Tu, X. Sustained release of a highly specific GSK3β inhibitor SB216763 in the PCL scaffold creates an osteogenic niche for osteogenesis, anti-adipogenesis, and potential angiogenesis. Front. Bioeng. Biotechnol. 2023, 11, 1215233. [Google Scholar] [CrossRef] [PubMed]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-printed reservoir-type implants containing poly (lactic acid)/poly (caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef]
- Berger, V.; Luo, Z.; Leroux, J. 3D printing of a controlled fluoride delivery device for the prevention and treatment of tooth decay. J. Control. Release 2022, 348, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, Z.; Yang, Z.; Gao, C.; Fu, L.; Li, P.; Zhao, T.; Cao, F.; Chen, W.; Yuan, Z.; et al. 3D printed poly (ε-caprolactone)/meniscus extracellular matrix composite scaffold functionalized with kartogenin-releasing PLGA microspheres for meniscus tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 662381. [Google Scholar] [CrossRef]
- Muldoon, K.; Ahmad, Z.; Su, Y.; Tseng, F.; Chen, X.; McLaughlin, J.A.D.; Chang, M. A Refined Hot Melt Printing Technique with Real-Time CT Imaging Capability. Micromachines 2022, 13, 1794. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Gustaffson, T.; Gaisford, S.; Basit, A.W. 3D printing tablets: Predicting printability and drug dissolution from rheological data. Int. J. Pharm. 2020, 590, 119868. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a biodegradable subcutaneous implant for prolonged drug delivery using 3D printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.T.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef]
- Dang, H.P.; Hutmacher, D.W.; Tran, P.A. The use of 3D printed microporous-strut polycaprolactone scaffolds for targeted local delivery of chemotherapeutic agent for breast cancer application. In Proceedings of the 7th International Conference on the Development of Biomedical Engineering in Vietnam (BME7), Ho Chi Minh, Vietnam, 27–29 June 2018. [Google Scholar] [CrossRef]
- Harmanci, S.; Dutta, A.; Cesur, S.; Sahin, A.; Gunduz, O.; Kalaskar, D.M.; Ustundag, C.B. Production of 3D Printed Bi-Layer and Tri-Layer Sandwich Scaffolds with Polycaprolactone and Poly (vinyl alcohol)-Metformin towards Diabetic Wound Healing. Polymers 2022, 14, 5306. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.W.; Jung, W.K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- López-González, I.; Hernández-Heredia, A.B.; Rodríguez-López, M.I.; Auñón-Calles, D.; Boudifa, M.; Gabaldón, J.A.; Meseguer-Olmo, L. Evaluation of the In Vitro Antimicrobial Efficacy against Staphylococcus aureus and epidermidis of a Novel 3D-Printed Degradable Drug Delivery System Based on Polycaprolactone/Chitosan/Vancomycin—Preclinical Study. Pharmaceutics 2023, 15, 1763. [Google Scholar] [CrossRef]
- Utomo, E.; Domínguez-Robles, J.; Anjani, Q.K.; Picco, C.J.; Korelidou, A.; Magee, E.; Donnelly, R.F.; Larrañeta, E. Development of 3D-printed vaginal devices containing metronidazole for alternative bacterial vaginosis treatment. Int. J. Pharm. X 2023, 5, 100142. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Jeong, S.; Kim, S.; Kim, H.J.; Jo, J.; Shanmugasundaram, A.; Kim, H.; Choi, E.; Lee, D. 3D-printed cardiovascular polymer scaffold reinforced by functional nanofiber additives for tunable mechanical strength and controlled drug release. Chem. Eng. J. 2023, 454, 140118. [Google Scholar] [CrossRef]
- Asiri, S.M.; Khan, F.A.; Bozkurt, A. Synthesis of chitosan nanoparticles, chitosan-bulk, chitosan nanoparticles conjugated with glutaraldehyde with strong anti-cancer proliferative capabilities. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), 1152–1161. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Sabbatini, B.; Cambriani, A.; Cespi, M.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. An overview of natural polymers as reinforcing agents for 3D printing. ChemEngineering 2021, 5, 78. [Google Scholar] [CrossRef]
- Baykara, D.; Pilavci, E.; Ulag, S.; Okoro, O.V.; Nie, L.; Shavandi, A.; Koyuncu, A.C.; Ozakpinar, O.B.; Eroglu, M.; Gunduz, O. In vitro electrically controlled amoxicillin release from 3D-printed chitosan/bismuth ferrite scaffolds. Eur. Polym. J. 2023, 193, 112105. [Google Scholar] [CrossRef]
- Koumentakou, I.; Noordam, M.J.; Michopoulou, A.; Terzopoulou, Z.; Bikiaris, D.N. 3D-Printed Chitosan-Based Hydrogels Loaded with Levofloxacin for Tissue Engineering Applications. Biomacromolecules 2023, 24, 4019–4032. [Google Scholar] [CrossRef]
- Ioannou, N.; Luo, J.; Qin, M.; Di Luca, M.; Mathew, E.; Tagalakis, A.D.; Lamprou2, D.A.; Yu-Wai-Man, C. 3D-printed long-acting 5-fluorouracil implant to prevent conjunctival fibrosis in glaucoma. J. Pharm. Pharmacol. 2023, 75, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.; Duan, B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020, 233, 115803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, X.; Du, H.; Ha, Y.; Xu, Y.; Ao, R.; He, C. Bifunctional Hydrogel-Integrated 3D Printed Scaffold for Repairing Infected Bone Defects. ACS Biomater. Sci. Eng. 2023, 9, 4583–4596. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kim, J.; Heo, C.; Yoon, J.; Kim, H.; Lee, S.; Park, H.W.; Heo, M.S.; Moon, H.E.; Kim, C.; et al. 3D printed multi-growth factor delivery patches fabricated using dual-crosslinked decellularized extracellular matrix-based hybrid inks to promote cerebral angiogenesis. Acta Biomater. 2023, 157, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Tianyuan, Z.; Zhen, Y.; Fuyang, C.; Jiang, W.; Zineng, Y.; Zhengang, D.; Shuyun, L.; Chunxiang, H.; Zhiguo, Y.; et al. Biofabrication of cell-free dual drug-releasing biomimetic scaffolds for meniscal regeneration. Biofabrication 2022, 14, 015001. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Gupta, V.K. Substrate Analysis for Effective Biofuels Production, 1st ed.; Springer Nature: Singapore, 2020; pp. 149–168. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Sandler, N.; Willfor, S.; Xu, C. Three-dimensional printing of wood-derived biopolymers: A review focused on biomedical applications. ACS Sustain. Chem. Eng. 2018, 6, 5663–5680. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Durand, H.; Zeno, E.; Balsollier, C.; Watbled, B.; Sillard, C.; Fort, S.; Baussanne, I.; Belgacem, N.; Lee, D.; et al. The surface chemistry of a nanocellulose drug carrier unravelled by MAS-DNP. Chem. Sci. 2020, 11, 3868. [Google Scholar] [CrossRef]
- Johannesson, J.; Pathare, M.M.; Johansson, M.; Bergström, C.A.; Teleki, A. Synergistic stabilization of emulsion gel by nanoparticles and surfactant enables 3D printing of lipid-rich solid oral dosage forms. J. Colloid Interface Sci. 2023, 650, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Sanchez-Ballester, N.M.; Aubert, A.; Rossi, J.C.; Begu, S.; Soulairol, I. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS PharmSciTech 2023, 24, 122. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.N.; Dive, A.M.; Moharil, R.; Munde, P. Enigmatic insight into collagen. J. Oral Maxillofac. Pathol. 2016, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Nocera, A.D.; Salvatierra, N.A.; Cid, M.P. Printing Collagen 3D Structures. In Proceedings of the VI Latin American Congress on Biomedical Engineering, Paraná, Argentina, 29–31 October 2014. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Sun, X.; Zhan, C.; Li, Z.; Qiu, L.; Luo, R.; Liu, H.; Sun, X.; Li, R.; et al. Silk fibroin/collagen/hydroxyapatite scaffolds obtained by 3D printing technology and loaded with recombinant human erythropoietin in the reconstruction of alveolar bone defects. ACS Biomater. Sci. Eng. 2022, 8, 5245–5256. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Q.; Liu, Q.; Liu, S.; Wang, Y.; Zhang, H. Design and fabrication of drug-loaded alginate/hydroxyapatite/collagen composite scaffolds for repairing infected bone defects. J. Mater. Sci. 2023, 58, 911–926. [Google Scholar] [CrossRef]
- Mutlu, M.E.; Ulag, S.; Sengor, M.; Daglılar, S.; Narayan, R.; Gunduz, O. Electrosprayed Collagen/Gentamicin nanoparticles coated microneedle patches for skin treatment. Mater. Lett. 2021, 305, 130844. [Google Scholar] [CrossRef]
- Khan, S.A. Mini-Review: Opportunities and challenges in the techniques used for preparation of gelatin nanoparticles. Pak. J. Pharm. Sci. 2020, 33, 221–228. [Google Scholar] [PubMed]
- Zaer, M.; Moeinzadeh, A.; Abolhassani, H.; Rostami, N.; Yaraki, M.T.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Bashiri, Z.; Moeinabadi-Bidgoli, K.; Moradbeygi, F.; et al. Doxorubicin-loaded Niosomes functionalized with gelatine and alginate as pH-responsive drug delivery system: A 3D printing approach. Int. J. Biol. Macromol. 2023, 253, 126808. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, J.; Bao, X.; Guo, Y.; Jin, Y.; Yang, C.; Zhang, H.; Liu, L.; Gao, Y.; Ye, C.; et al. Induction of Tumor Ferroptosis-Dependent Immunity via an Injectable Attractive Pickering Emulsion Gel. Adv. Mater. 2023, 35, e2303542. [Google Scholar] [CrossRef]
- Wei, H.; Luo, Y.; Ma, R.; Li, Y. Three-Dimensional Printing Multi-Drug Delivery Core/Shell Fiber Systems with Designed Release Capability. Pharmaceutics 2023, 15, 2336. [Google Scholar] [CrossRef]
- Cardoso, P.H.N.; Oliveira, C.Y.B.; Nunes, M.; Tavares, G.F.; Faia, P.M.; Araújo, E.S. Eudragit E100/Hesperidin 3D Printing Filaments: Preparation, Characterization, and In Vitro Release Studies. Appl. Sci. 2023, 13, 11558. [Google Scholar] [CrossRef]
- Alhnan, M.A. Solid Dosage Form. Production. Patent EP3191084B1, 28 November 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).