Investigation of Impregnation Approach of Zinc Oxide Nano-Dispersions for Potential UV Stabilization in Abies alba and Fagus sylvatica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Pretreatment
2.2. Dispersion Preparation
2.3. Impregnation and Penetration Behaviour
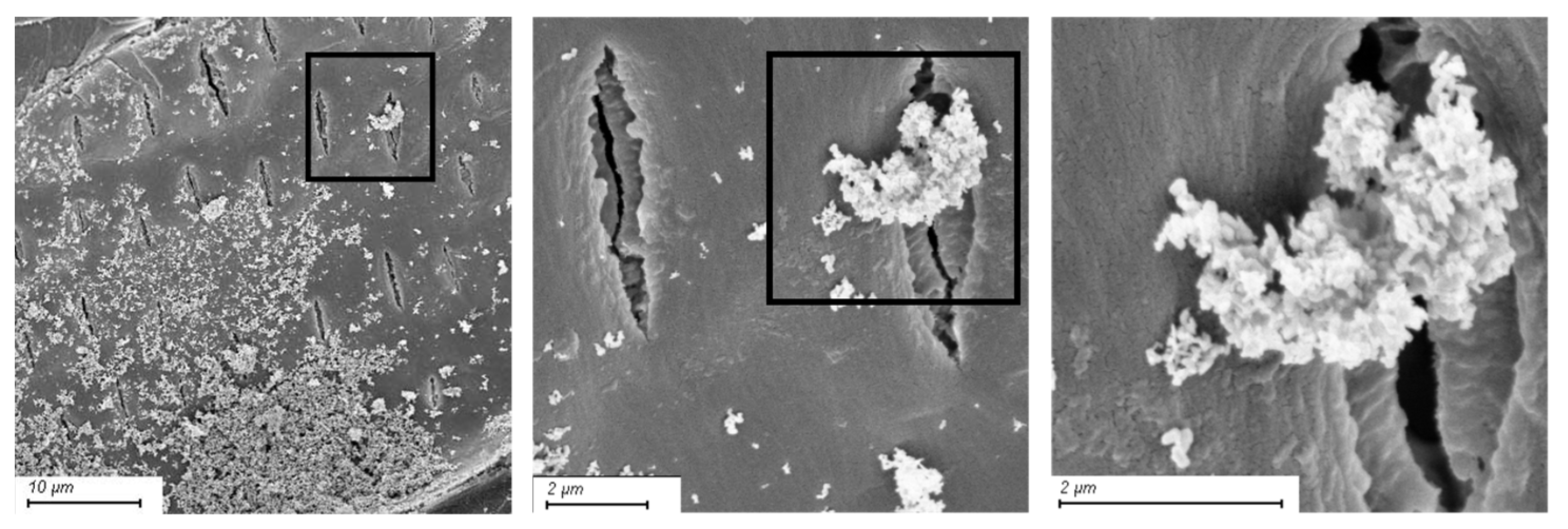
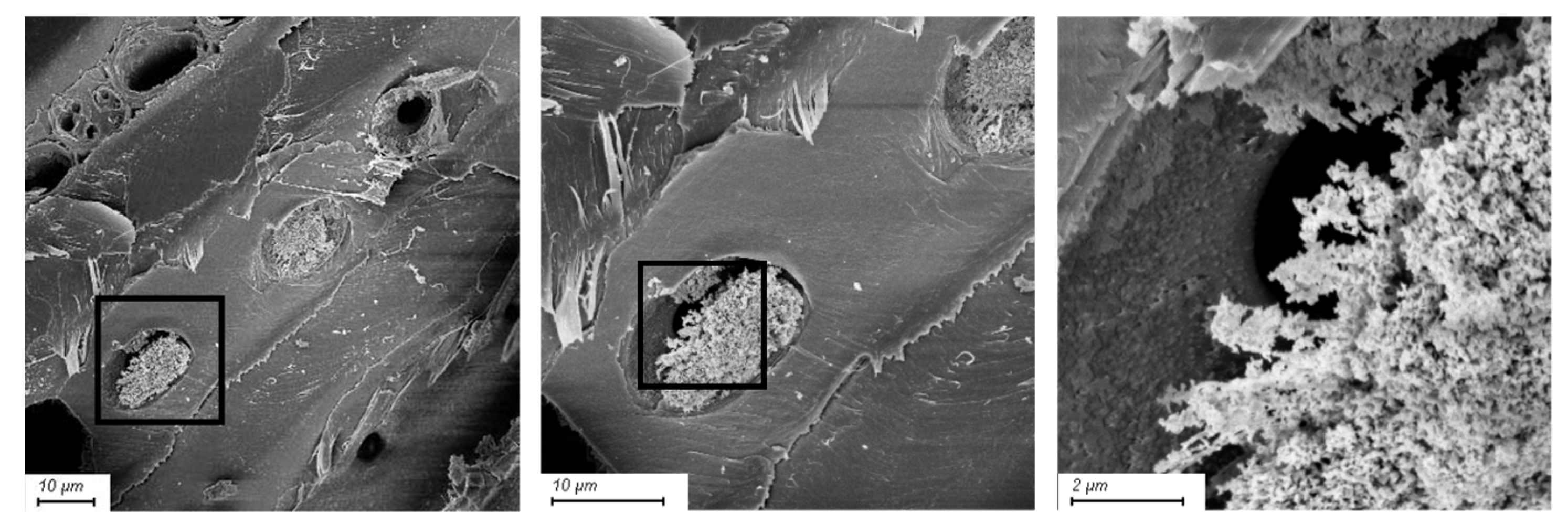
2.4. Scanning Electron Microscopy
2.5. Leaching Resistance
2.6. Short-Term Weathering and CIELab Color Measurement
2.7. Chemical Changes
2.8. Statistical Analysis and Data Processing
3. Results
3.1. Impregnation Behaviour
3.2. Scanning Electron Microscopy
3.3. Leaching Resistance
3.4. Short-Term Weathering and CIELab Color Measurement
3.5. Chemical Changes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zabel, R.A.; Morrell, J.J. Wood Microbiology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Schmidt, O. Indoor wood-decay basidiomycetes: Damage, causal fungi, physiology, identification and characterization, prevention and control. Mycol. Prog. 2007, 6, 261–279. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet; Landry, V. Wood degradation under UV irradiation: A lignin characterization. J. Photochem. Photobiol. B 2006, 158, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Vuorinen, T. Comparative study of photodegradation of wood by a UV laser and a xenon light source. Polym. Degrad. Stab. 2008, 93, 2138–2146. [Google Scholar] [CrossRef]
- Yu, H.X.; Pan, X.; Wang, Z.; Yang, W.M.; Zhang, W.F.; Zhuang, X.W. Effects of heat treatments on photoaging properties of Moso bamboo (Phyllostachys pubescens Mazel). Wood Sci. Technol. 2018, 52, 1671–1683. [Google Scholar] [CrossRef]
- Hon, D.N.-S.; Chang, S.-T. Surface degradation of wood by ultraviolet light. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 2227–2241. [Google Scholar] [CrossRef]
- Petrillo, M.; Sandak, J.; Grossi; Sandak, A. Chemical and appearance changes of wood due to artificial weathering—Dose–response model. J. Near Infrared Spectrosc. 2019, 27, 26–37. [Google Scholar] [CrossRef]
- Eaton, R.A.; Hale, M.D.C. Wood: Decay, Pests, and Protection. 1993. Available online: https://api.semanticscholar.org/CorpusID:82313434 (accessed on 12 October 2023).
- Schoeman, M.; Dickinson, D. Growth of Aureobasidium pullulans on lignin breakdown products at weathered wood surfaces. Mycologist 1997, 11, 168–172. [Google Scholar] [CrossRef]
- Müller, U.; Rätzsch, M.; Schwanninger, M.; Steiner, M.; Zöbl, H. Yellowing and IR-changes of spruce wood as result of UV-irradiation. J. Photochem. Photobiol. B 2003, 69, 97–105. [Google Scholar] [CrossRef]
- Ayadi, N.; Lejeune, F.; Charrier, F.; Charrier, B.; Merlin, A. Color stability of heat-treated wood during artificial weathering. Holzals Rohund Werkst. 2003, 61, 221–226. [Google Scholar] [CrossRef]
- Mitsui, K.; Tsuchikawa, S. Low atmospheric temperature dependence on photodegradation of wood. J. Photochem. Photobiol. B 2005, 81, 84–88. [Google Scholar] [CrossRef]
- Deka, M.; Humar, M.; Rep, G.; Kričej, B.; Šentjurc, M.; Petrič, M. Effects of UV light irradiation on colour stability of thermally modified. copper ethanolamine treated and non-modified wood: EPR and DRIFT spectroscopic studies. Wood Sci. Technol. 2008, 42, 5–20. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Militz, H.; Mai, C. Natural weathering of scots pine (Pinus sylvestris L.) boards modified with functionalised commercial silicone emulsions. Bioresources 2009, 4, 659–673. [Google Scholar] [CrossRef]
- Aloui, F.; Ahajji, A.; Irmouli, Y.; George, B.; Charrier, B.; Merlin, A. Inorganic UV absorbers for the photostabilisation of wood-clearcoating systems: Comparison with organic UV absorbers. Appl. Surf. Sci. 2007, 253, 3737–3745. [Google Scholar] [CrossRef]
- Schaller, C.; Rogez, D.; Braig, A. Hindered amine light stabilizers in pigmented coatings. J. Coat. Technol. Res. 2009, 6, 81–88. [Google Scholar] [CrossRef]
- Yuan, B.; Ji, X.; Nguyen, T.T.; Huang, Z.; Guo, M. UV protection of wood surfaces by graphitic carbon nitride nanosheets. Appl. Surf. Sci. 2019, 467–468, 1070–1075. [Google Scholar] [CrossRef]
- Pánek, M.; Oberhofnerová, E.; Hýsek, Š.; Šedivka; Zeidler, A. Colour Stabilization of Oak. Spruce, Larch and Douglas Fir Heartwood Treated with Mixtures of Nanoparticle Dispersions and UV-Stabilizers after Exposure to UV and VIS-Radiation. Materials 2018, 11, 1653. [Google Scholar] [CrossRef]
- Grüll, G.; Tscherne, F.; Spitaler, I.; Forsthuber, B. Comparison of wood coating durability in natural weathering and artificial weathering using fluorescent UV-lamps and water. Eur. J. Wood Wood Prod. 2014, 72, 367–376. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Han, W.; Park, H.-H. Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomed. 2016, 12, 207–216. [Google Scholar] [CrossRef]
- Kookandeh, M.G.; Taghiyari, H.R.; Siahposht, H. Effects of heat treatment and impregnation with zinc-oxide nanoparticles on physical. mechanical, and biological properties of beech wood. Wood Sci. Technol. 2014, 48, 727–736. [Google Scholar] [CrossRef]
- Tshabalala, M.A.; Dejene, B.F.; Swart, H.C. Synthesis and characterization of ZnO nanoparticles using polyethylene glycol (PEG). Physica B Condens. Matter 2012, 407, 1668–1671. [Google Scholar] [CrossRef]
- Salla, J.; Pandey, K.K.; Srinivas, K. Improvement of UV resistance of wood surfaces by using ZnO nanoparticles. Polym. Degrad. Stab. 2012, 97, 592–596. [Google Scholar] [CrossRef]
- Nair, S.; Nagarajappa, G.B.; Pandey, K.K. UV stabilization of wood by nano metal oxides dispersed in propylene glycol. J. Photochem. Photobiol. B 2018, 183, 1–10. [Google Scholar] [CrossRef]
- Nair, S.; Pandey, K.K.; Giridhar, B.N.; Vijayalakshmi, G. Decay resistance of rubberwood (Hevea brasiliensis) impregnated with ZnO and CuO nanoparticles dispersed in propylene glycol. Int. Biodeterior. Biodegrad. 2017, 122, 100–106. [Google Scholar] [CrossRef]
- Bak, M. Possibilities of using nanotechnology in wood colour protection. Obuda Univ. e-Bull. 2018, 8, 29–33. [Google Scholar]
- de Peres, M.L.; de Avila Delucis, R.; Amico, S.C.; Gatto, D.A. Zinc oxide nanoparticles from microwave-assisted solvothermal process: Photocatalytic performance and use for wood protection against xylophagous fungus. Nanomater. Nanotechnol. 2019, 9, 184798041987620. [Google Scholar] [CrossRef]
- Soltani, M.; Najafi, A.; Yousefian, S.; Naji, H.R.; Bakar, E.S. Water repellent effect and dimension stability of beech wood impregnated with nano-zinc oxide. Bioresources 2013, 8, 6280–6287. [Google Scholar] [CrossRef]
- Clausen, C.A.; Green, F.; Kartal, S.N. Weatherability and Leach Resistance of Wood Impregnated with Nano-Zinc Oxide. Nanoscale Res. Lett. 2010, 5, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Weichelt, F.; Beyer, M.; Emmler, R.; Flyunt, R.; Beyer, E.; Buchmeiser, M. Zinc Oxide Based Coatings for the UV-Protection of Wood for Outdoor Applications. Macromol. Symp. 2011, 301, 23–30. [Google Scholar] [CrossRef]
- Sommerauer, L.; Thevenon, M.-F.; Petutschnigg, A.; Tondi, G. Effect of hardening parameters of wood preservatives based on tannin copolymers. Holzforschung 2019, 73, 457–467. [Google Scholar] [CrossRef]
- Chang, T.-C.; Chang, H.-T.; Wu, C.-L.; Lin, H.-Y.; Chang, S.-T. Stabilizing effect of extractives on the photo-oxidation of Acacia confusa wood. Polym. Degrad. Stab. 2010, 95, 1518–1522. [Google Scholar] [CrossRef]
- Chang, T.-C.; Chang, H.-T.; Wu, C.-L.; Chang, S.-T. Influences of extractives on the photodegradation of wood. Polym. Degrad. Stab. 2010, 95, 516–521. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Fan, Y.; Tshabalala, M.A.; Stark, N.M. Heat-induced chemical and color changes of extractive-free black locust (Robinia pseudoacacia) wood. Bioresources 2012, 7, 2. [Google Scholar] [CrossRef]
- Chen, Y.; Tshabalala, M.A.; Gao, J.; Stark, N.M.; Fan, Y. Color and surface chemistry changes of extracted wood flour after heating at 120 °C. Wood Sci. Technol. 2014, 48, 137–150. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Nogués, F. ; Garriga Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Pandey, K.K. Study of the effect of photo-irradiation on the surface chemistry of wood. Polym. Degrad. Stab. 2005, 90, 9–20. [Google Scholar] [CrossRef]
- Rosu, D.; Teaca, C.-A.; Bodirlau, R.; Rosu, L. FTIR and color change of the modified wood as a result of artificial light irradiation. J. Photochem. Photobiol. B 2010, 99, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Tolvaj, L.; Faix, O. Artificial Ageing of Wood Monitored by DRIFT Spectroscopy and CIE L*a*b* Color Measurements. 1. Effect of UV Light. Holzforschung 1995, 49, 397–404. [Google Scholar] [CrossRef]
- Ganne-Chédeville, C.; Jääskeläinen, A.-S.; Froidevaux, J.; Hughes, M.; Navi, P. Natural and artificial ageing of spruce wood as observed by FTIR-ATR and UVRR spectroscopy. Holzforschung 2011, 66, 163–170. [Google Scholar] [CrossRef]





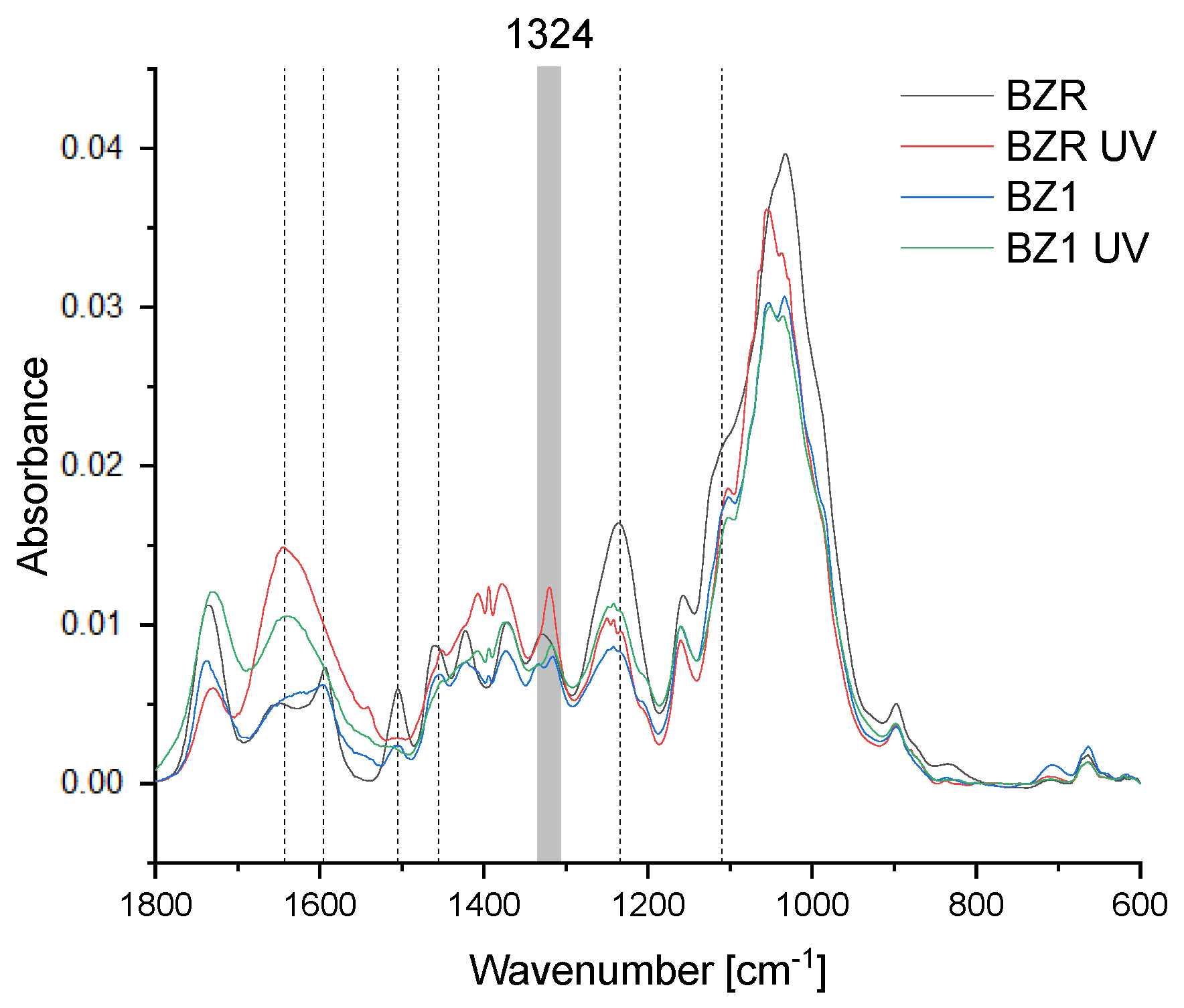
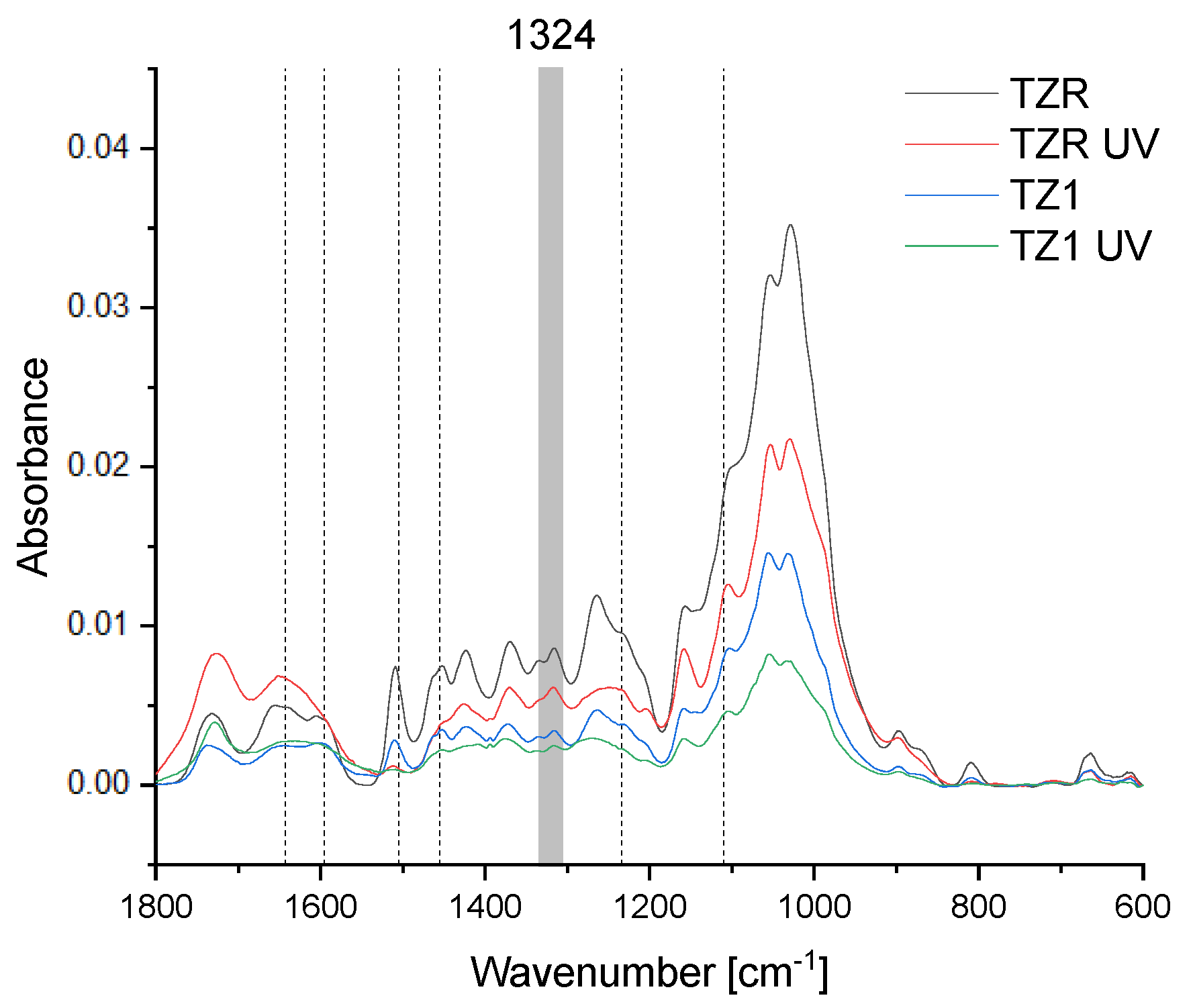
| Specimen | State | L* b | a* b | b* b | ΔE | |||
|---|---|---|---|---|---|---|---|---|
| BZR | beech untreated | 69.79 | (1.43) | 6.27 | (0.83) | 16.22 | (1.83) | - |
| BZR UV a | beech untreated | 60.70 | (2.04) | 13.25 | (0.93) | 31.70 | (1.21) | 19.26 |
| BZ1 | beech impr. ZnO 1% | 63.86 | (2.66) | 9.24 | (2.14) | 24.26 | (2.78) | - |
| BZ1 UV a | beech impr. ZnO 1% | 60.87 | (3.36) | 12.49 | (0.67) | 31.97 | (0.81) | 8.88 |
| BZ2 | beech impr. ZnO 2% | 66.55 | (6.32) | 6.21 | (1.69) | 17.19 | (3.04) | - |
| BZ2 UV a | beech impr. ZnO 2% | 54.13 | (3.08) | 10.94 | (1.23) | 25.95 | (2.29) | 15.92 |
| BZ3 | beech impr. ZnO 3% | 70.45 | (1.13) | 6.25 | (1.73) | 15.41 | (2.82) | - |
| BZ3 UV a | beech impr. ZnO 3% | 56.39 | (1.48) | 9.70 | (1.18) | 24.62 | (2.77) | 17.16 |
| TZR | fir untreated | 78.14 | (3.66) | 5.36 | (1.14) | 22.20 | (1.05) | - |
| TZR UV a | fir untreated | 66.61 | (2.12) | 14.00 | (0.39) | 39.05 | (0.77) | 22.17 |
| TZ1 | fir impr. ZnO 1% | 66.68 | (1.89) | 10.01 | (1.15) | 34.32 | (2.30) | - |
| TZ1 UV a | fir impr. ZnO 1% | 60.38 | (1.59) | 12.67 | (1.10) | 33.15 | (2.00) | 6.93 |
| TZ2 | fir impr. ZnO 2% | 79.06 | (3.04) | 3.77 | (0.84) | 22.69 | (2.63) | - |
| TZ2 UV a | fir impr. ZnO 2% | 61.70 | (2.65) | 12.04 | (0.73) | 32.08 | (1.89) | 21.40 |
| TZ3 | fir impr. ZnO 3% | 79.54 | (3.17) | 3.23 | (0.74) | 17.46 | (2.05) | - |
| TZ3 UV a | fir impr. ZnO 3% | 64.07 | (3.96) | 10.07 | (1.38) | 29.37 | (2.77) | 20.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerauer, L.; Petutschnigg, A.; Schnabel, T. Investigation of Impregnation Approach of Zinc Oxide Nano-Dispersions for Potential UV Stabilization in Abies alba and Fagus sylvatica. Compounds 2023, 3, 561-572. https://doi.org/10.3390/compounds3040040
Sommerauer L, Petutschnigg A, Schnabel T. Investigation of Impregnation Approach of Zinc Oxide Nano-Dispersions for Potential UV Stabilization in Abies alba and Fagus sylvatica. Compounds. 2023; 3(4):561-572. https://doi.org/10.3390/compounds3040040
Chicago/Turabian StyleSommerauer, Lukas, Alexander Petutschnigg, and Thomas Schnabel. 2023. "Investigation of Impregnation Approach of Zinc Oxide Nano-Dispersions for Potential UV Stabilization in Abies alba and Fagus sylvatica" Compounds 3, no. 4: 561-572. https://doi.org/10.3390/compounds3040040
APA StyleSommerauer, L., Petutschnigg, A., & Schnabel, T. (2023). Investigation of Impregnation Approach of Zinc Oxide Nano-Dispersions for Potential UV Stabilization in Abies alba and Fagus sylvatica. Compounds, 3(4), 561-572. https://doi.org/10.3390/compounds3040040







