Lipid-Based Formulations Containing Labrafil M2125-CS: A Deep Investigation on Nanosystem Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Labrafil M2125-Based Nanosystems
2.3. Physico–Chemical Characterization of Gained Nanosystems
2.4. Assessment of Storage, pH, and Temperature Influence on Stability
2.5. Freeze-Drying Studies
2.6. Statistical Analysis
3. Results and Discussion
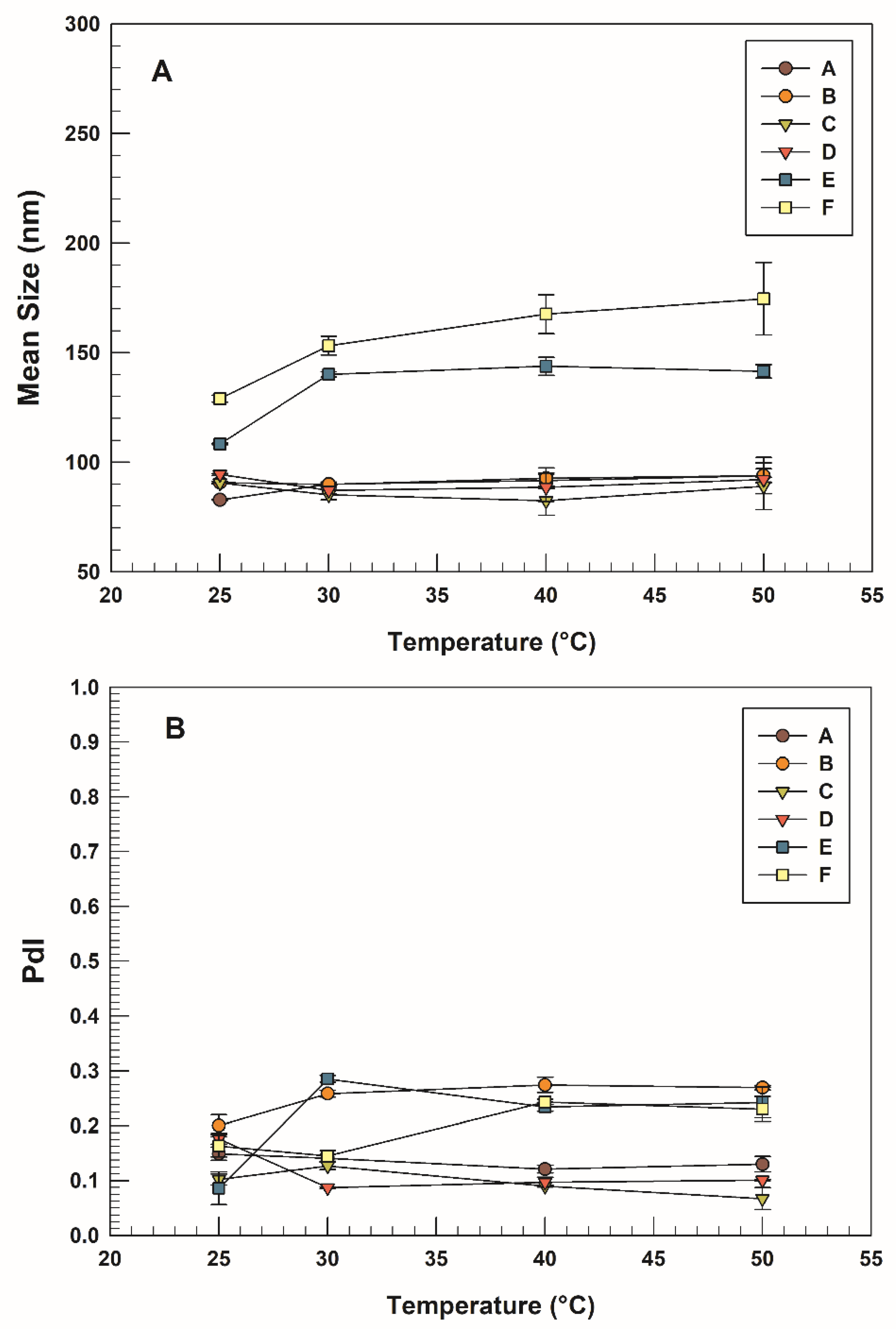
3.1. Physico–Chemical and Technological Characterization
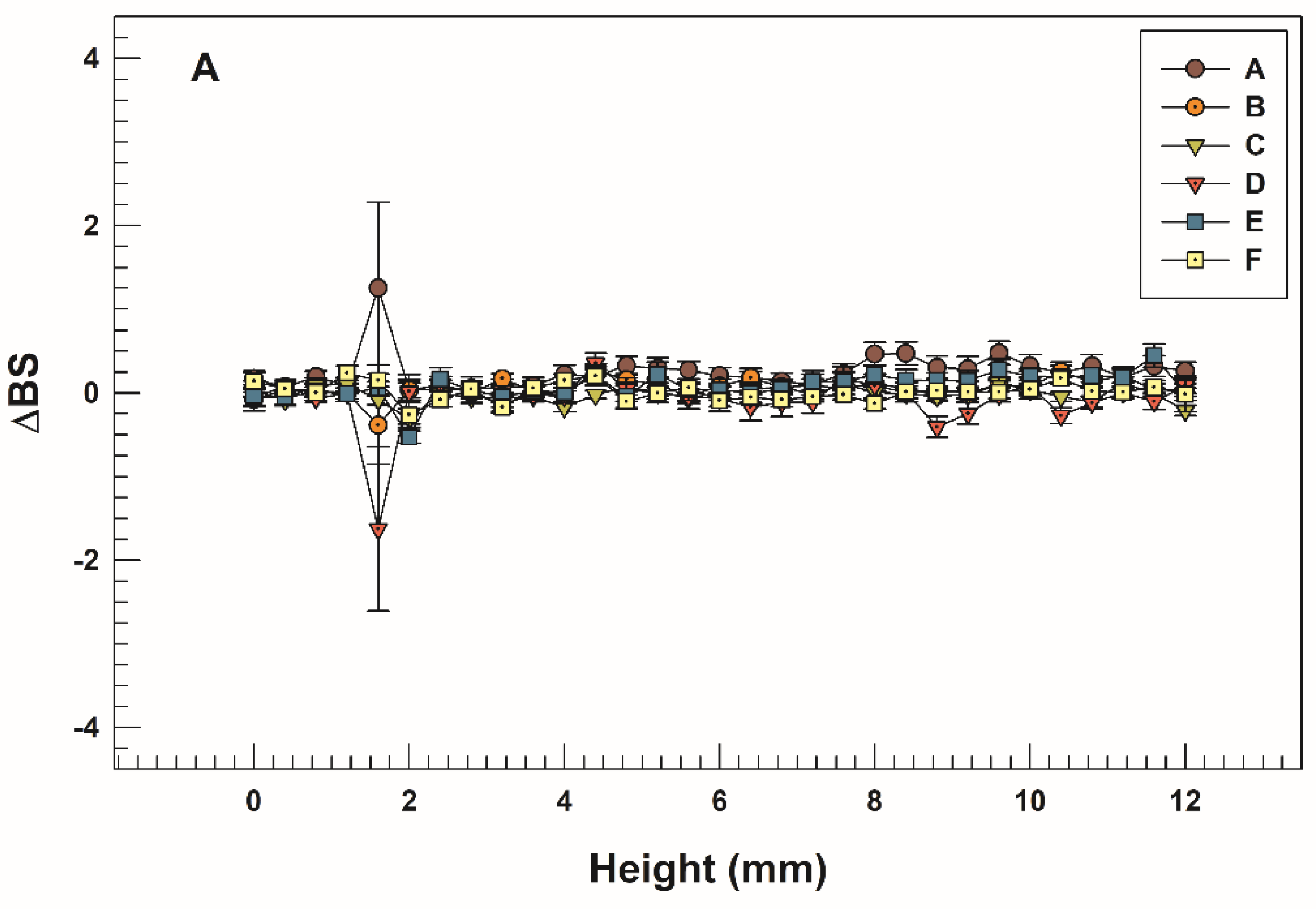
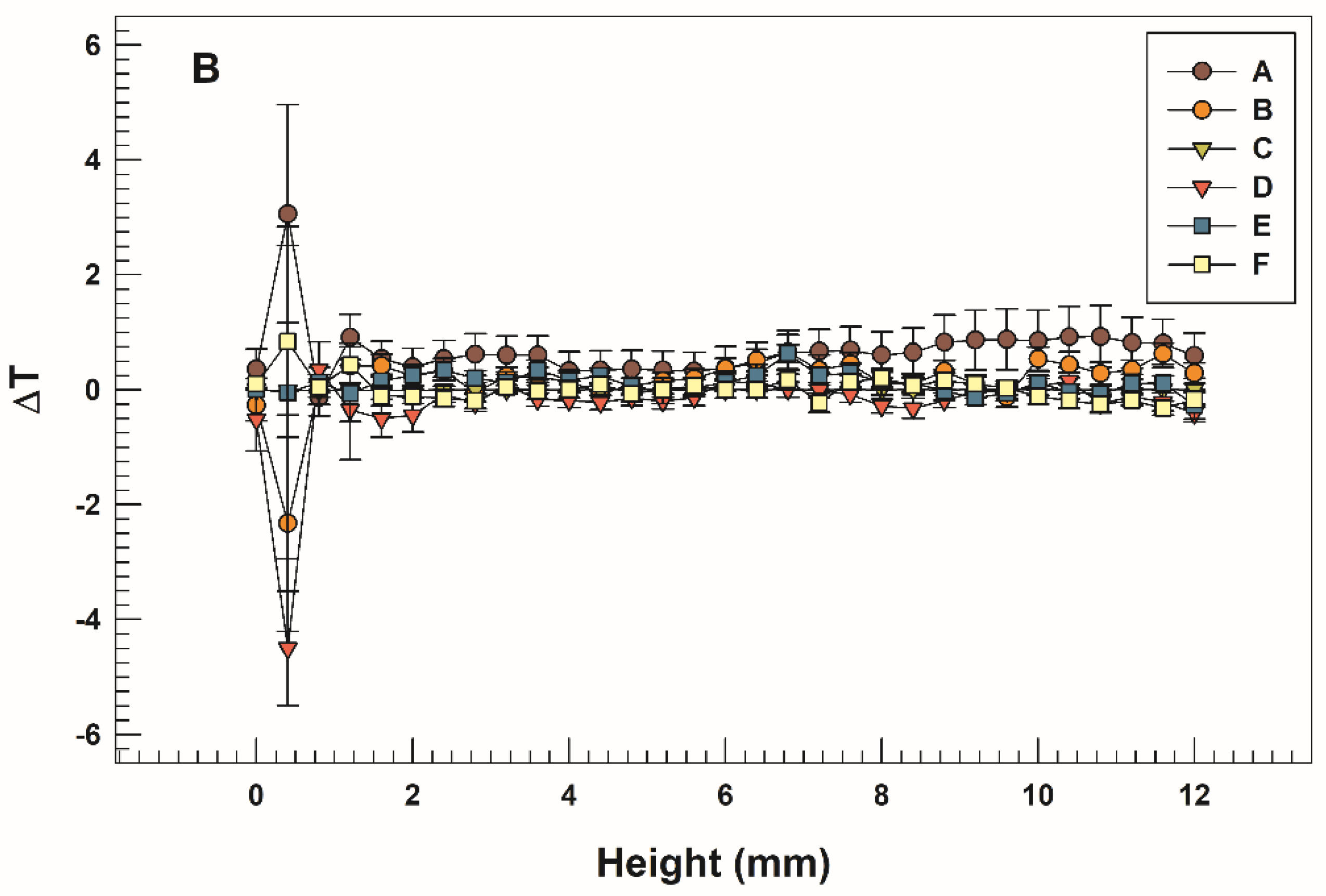
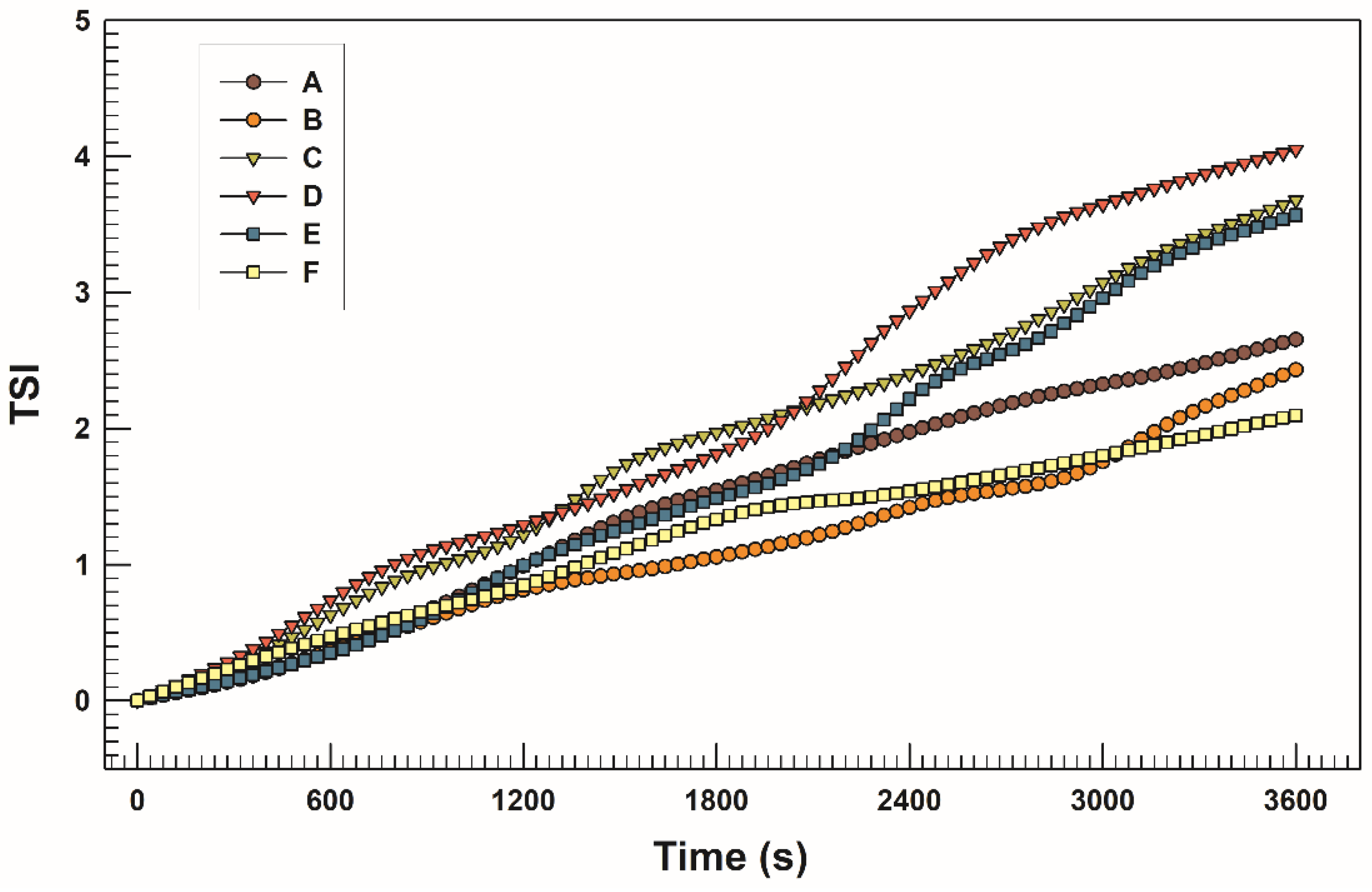
3.2. Storage, pH, and Temperature Influence on Stability
3.3. Freeze-Drying Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern drug delivery strategies applied to natural active compounds. Expert Opin. Drug Deliv. 2016, 14, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.-E.; Benoit, J.-P. Physicochemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Susa, F.; Bucca, G.; Limongi, T.; Cauda, V.; Pisano, R. Enhancing the preservation of liposomes: The role of cryoprotectants, lipid formulations and freezing approaches. Cryobiology 2021, 98, 46–56. [Google Scholar] [CrossRef]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Delongeas, J.-L.; de Conchard, G.V.; Beamonte, A.; Bertheux, H.; Spire, C.; Maisonneuve, C.; Becourt-Lhote, N.; Goldfain-Blanc, F.; Claude, N. Assessment of Labrasol®/Labrafil®/Transcutol® (4/4/2, v/v/v) as a non-clinical vehicle for poorly water-soluble compounds after 4-week oral toxicity study in Wistar rats. Regul. Toxicol. Pharmacol. 2010, 57, 284–290. [Google Scholar] [CrossRef]
- Segale, L.; Giovannelli, L.; Bonda, A.F.; Pattarino, F.; Rinaldi, M. Effect of self-emulsifying phase composition on the characteristics of venlafaxine loaded alginate beads. J. Drug Deliv. Sci. Technol. 2020, 55, 101483. [Google Scholar] [CrossRef]
- Larsen, A.; Holm, R.; Pedersen, M.L.; Müllertz, A. Lipid-based Formulations for Danazol Containing a Digestible Surfactant, Labrafil M2125CS: In Vivo Bioavailability and Dynamic In Vitro Lipolysis. Pharm. Res. 2008, 25, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.C.; Gueutin, C.; Frebourg, G.; Burucoa, C.; Faivre, V. Erythromycin encapsulation in nanoemulsion-based delivery systems for treatment of Helicobacter pylori infection: Protection and synergy. Biochem. Biophys. Res. Commun. 2017, 493, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef]
- Cristiano, M.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.; Mancuso, A.; Fresta, M.; Torella, D.; De Gaetano, F.; Ventura, C.; Paolino, D. Topical Unsaturated Fatty Acid Vesicles Improve Antioxidant Activity of Ammonium Glycyrrhizinate. Pharmaceutics 2021, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Feng, N.-P.; Shen, L.-N.; Zhao, J.-H. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int. J. Nanomed. 2014, 9, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; De Gaetano, F.; Ventura, C.A.; Fresta, M.; Paolino, D. The Rheolaser Master™ and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel. Molecules 2020, 25, 1979. [Google Scholar] [CrossRef] [Green Version]
- Celia, C.; Locatelli, M.; Cilurzo, F.; Cosco, D.; Gentile, E.; Scalise, D.; Carafa, M.; Ventura, C.A.; Fleury, M.; Tisserand, C.; et al. Long Term Stability Evaluation of Prostacyclin Released from Biomedical Device through Turbiscan Lab Expert. Med. Chem. 2015, 11, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Munye, M.M.; Tagalakis, A.D.; Manunta, M.; Hart, S.L. The Role of the Helper Lipid on the DNA Transfection Efficiency of Lipopolyplex Formulations. Sci. Rep. 2014, 4, 7107. [Google Scholar] [CrossRef] [Green Version]
- Vanaja, K.; Wahl, M.; Bukarica, L.; Heinle, H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: Evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sci. 2013, 93, 917–923. [Google Scholar] [CrossRef]
- Li, J.; Shin, G.H.; Chen, X.; Park, H.J. Modified curcumin with hyaluronic acid: Combination of pro-drug and nano-micelle strategy to address the curcumin challenge. Food Res. Int. 2015, 69, 202–208. [Google Scholar] [CrossRef]
- Edwards, K.; Almgren, M. Surfactant-induced leakage and structural change of lecithin vesicles: Effect of surfactant headgroup size. Langmuir 1992, 8, 824–832. [Google Scholar] [CrossRef]
- Cole, M.L.; Whateley, T.L. Release rate profiles of theophylline and insulin from stable multiple w/o/w emulsions. J. Control. Release 1997, 49, 51–58. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Preparation and evaluation of uniformly sized solid lipid microcapsules using membrane emulsification. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 293, 87–94. [Google Scholar] [CrossRef]
- Jorgensen, L.; Moeller, E.; van de Weert, M.; Nielsen, H.; Frokjaer, S. Preparing and evaluating delivery systems for proteins. Eur. J. Pharm. Sci. 2006, 29, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.-F.; Lu, L.-J.; Li, M.-X.; Xu, J.-C.; Deng, H.-P. Turbiscan Lab® Expert analysis of the biological demulsification of a water-in-oil emulsion by two biodemulsifiers. J. Hazard. Mater. 2011, 190, 214–221. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Kaombe, D.D.; Lenes, M.; Toven, K.; Glomm, W.R. Turbiscan as a Tool for Studying the Phase Separation Tendency of Pyrolysis Oil. Energy Fuels 2013, 27, 1446–1452. [Google Scholar] [CrossRef]
- Qi, X.; Dong, Y.; Wang, H.; Wang, C.; Li, F. Application of Turbiscan in the homoaggregation and heteroaggregation of copper nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 535, 96–104. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Iannone, M.; Fresta, M.; Fiorito, S.; Celia, C.; Paolino, D. In vitro and in vivo trans-epidermal water loss evaluation following topical drug delivery systems application for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2020, 186, 113295. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Optimization of Orange Oil Nanoemulsion Formation by Isothermal Low-Energy Methods: Influence of the Oil Phase, Surfactant, and Temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Sagalowicz, L.; Leser, M.E. Delivery systems for liquid food products. Curr. Opin. Colloid Interface Sci. 2010, 15, 61–72. [Google Scholar] [CrossRef]
- Franks, F. Freeze-drying of bioproducts: Putting principles into practice. Eur. J. Pharm. Biopharm. 1998, 45, 221–229. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- do Vale Morais, A.R.; do Nascimento Alencar, É.; Júnior, F.H.X.; De Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze drying of nanosuspensions, 2: The role of the critical formulation temperature on stability of drug nanosuspensions and its practical implication on process design. J. Pharm. Sci. 2011, 100, 4471–4481. [Google Scholar] [CrossRef]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Qian, Y.; Chen, Y. The effects of cryoprotectants on the freeze-drying of ibuprofen-loaded solid lipid microparticles (SLM). Eur. J. Pharm. Biopharm. 2008, 69, 750–759. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Pohlmann, A.R.; Dalla-Costa, T.; Guterres, S.S. Freeze-drying polymeric colloidal suspensions: Nanocapsules, nanospheres and nanodispersion. A comparative study. Eur. J. Pharm. Biopharm. 2003, 56, 501–505. [Google Scholar] [CrossRef]
| t = 0 | |||
|---|---|---|---|
| Samples | Molar Ratio PL-90G®/LBF | Mean Sizes (nm) | PdI 1 |
| A | 0.24:0.76 | 83 ± 1 | 0.15 ± 0.01 |
| B | 0.32:0.68 | 91 ± 1 | 0.28 ± 0.02 |
| C | 0.48:0.52 | 91 ± 1 | 0.10 ± 0.01 |
| D | 0.44:0.56 | 94 ± 1 | 0.18 ± 0.01 |
| E | 0.64:0.36 | 109 ± 1 | 0.09 ± 0.03 |
| F | 0.76:0.24 | 129 ± 1 | 0.16 ± 0.02 |
| t = 24 h | t = 1 Week | t = 2 Weeks | t = 1 Month | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Mean Size (nm) | PdI | Mean Size (nm) | PdI | Mean Size (nm) | PdI | Mean Size (nm) | PdI |
| A | 84 ± 1 | 0.14 ± 0.02 | 89 ± 1 | 0.12 ± 0.01 | 89 ± 1 | 0.10 ± 0.01 | 86 ± 1 | 0.08 ± 0.02 |
| B | 84 ± 1 | 0.14 ± 0.01 | 84 ± 1 | 0.14 ± 0.01 | 84 ± 1 | 0.14 ± 0.10 | 92 ± 1 | 0.15 ± 0.01 |
| C | 103 ± 1 | 0.07 ± 0.01 | 107 ± 1 | 0.06 ± 0.01 | 106 ± 1 | 0.06 ± 0.02 | 107 ± 1 | 0.09 ± 0.01 |
| D | 102 ± 1 | 0.18 ± 0.02 | 191 ± 2 | 0.30 ± 0.02 | 199 ± 1 | 0.08 ± 0.01 | 203 ± 1 | 0.03 ± 0.01 |
| E | 148 ± 1 | 0.33 ± 0.01 | 133 ± 1 | 0.18 ± 0.01 | 138 ± 2 | 0.11 ± 0.01 | ## | ## |
| F | 153 ± 1 | 0.25 ± 0.01 | 150 ± 2 | 0.20 ± 0.01 | 190 ± 2 | 0.18 ± 0.01 | ## | ## |
| T = 30 °C | T = 40 °C | T = 50 °C | ||||
|---|---|---|---|---|---|---|
| Sample | Mean Size (nm) | PdI | Mean Size (nm) | PdI | Mean Size (nm) | PdI |
| A | 90 ± 1 | 0.14 ± 0.01 | 91 ± 3 | 0.12 ± 0.01 | 91 ± 3 | 0.13 ± 0.01 |
| B | 90 ± 1 | 0.26 ± 0.01 | 93 ± 5 | 0.27 ± 0.01 | 94 ± 8 | 0.27 ± 0.01 |
| C | 85 ± 2 | 0.13 ± 0.01 | 82 ± 7 | 0.09 ± 0.01 | 89 ± 1 | 0.07 ± 0.02 |
| D | 87 ± 1 | 0.09 ± 0.01 | 89 ± 6 | 0.10 ± 0.01 | 92 ± 1 | 0.10 ± 0.01 |
| E | 140 ± 1 | 0.29 ± 0.01 | 144 ± 4 | 0.24 ± 0.01 | 142 ± 3 | 0.24 ± 0.03 |
| F | 153 ± 4 | 0.14 ± 0.01 | 168 ± 9 | 0.24 ± 0.01 | 175 ± 6 | 0.23 ± 0.02 |
| pH = 4 | pH = 7 | pH = 10 | ||||
|---|---|---|---|---|---|---|
| Sample | Mean Size (nm) | PdI | Mean Size (nm) | PdI | Mean Size (nm) | PdI |
| A | 131 ± 8 | 0.06 ± 0.02 | 87 ± 1 | 0.17 ± 0.01 | 92 ± 1 | 0.22 ± 0.01 |
| B | 130 ± 8 | 0.08 ± 0.01 | 94 ± 1 | 0.11 ± 0.01 | 103 ± 1 | 0.17 ± 0.01 |
| C | 130 ± 7 | 0.09 ± 0.02 | 96 ± 1 | 0.11 ± 0.01 | 113 ± 1 | 0.16 ± 0.02 |
| D | 131 ± 6 | 0.05 ± 0.01 | 107 ± 1 | 0.10 ± 0.01 | 116 ± 1 | 0.13 ± 0.01 |
| E | 128 ± 3 | 0.16 ± 0.01 | 123 ± 1 | 0.24 ± 0.01 | 131 ± 2 | 0.23 ± 0.02 |
| F | 146 ± 1 | 0.13 ± 0.02 | 155 ± 1 | 0.26 ± 0.01 | 164 ± 1 | 0.21 ± 0.01 |
| Sample | Cryoprotectant (%w/v) | Mean Size (nm) | PdI |
|---|---|---|---|
| A | Glucose 5% | 71 ± 14 | 0.56 ± 0.06 |
| Glucose 10% | 178 ± 6 | 0.41 ± 0.06 | |
| Trehalose 5% | 238 ± 7 | 0.78 ± 0.08 | |
| Trehalose 10% | 357 ± 28 | 0.65 ± 0.03 | |
| Mannose 5% | 170 ± 6 | 0.46 ± 0.06 | |
| Mannose 10% | 400 ± 14 | 0.58 ± 0.06 | |
| Sucrose 5% | # | 0.96 ± 0.01 | |
| Sucrose 10% | 368 ± 9 | 0.54 ± 0.03 | |
| B | Glucose 5% | 112 ± 22 | 0.34 ± 0.02 |
| Glucose 10% | 139 ± 18 | 0.59 ± 0.13 | |
| Trehalose 5% | 383 ± 50 | 0.77 ± 0.08 | |
| Trehalose 10% | 215 ± 5 | 0.38 ± 0.01 | |
| Mannose 5% | # | 0.49 ± 0.08 | |
| Mannose 10% | 436 ± 21 | 0.63 ± 0.07 | |
| Sucrose 5% | # | 0.79 ± 0.06 | |
| Sucrose 10% | 241 ± 4 | 0.32 ± 0.02 | |
| C | Glucose 5% | 72 ± 50 | 0.41 ± 0.02 |
| Glucose 10% | 225 ± 39 | 0.15 ± 0.02 | |
| Trehalose 5% | # | 0.57 ± 0.04 | |
| Trehalose 10% | 271 ± 25 | 0.58 ± 0.14 | |
| Mannose 5% | # | 0.68 ± 0.08 | |
| Mannose 10% | # | 0.77 ± 0.01 | |
| Sucrose 5% | # | 0.88 ± 0.05 | |
| Sucrose 10% | 308 ± 13 | 0.50 ± 0.03 | |
| D | Glucose 5% | 209 ± 6 | 0.17 ± 0.03 |
| Glucose 10% | 214 ± 4 | 0.21 ± 0.01 | |
| Trehalose 5% | 124 ± 1 | 0.26 ± 0.02 | |
| Trehalose 10% | 155 ± 1 | 0.25 ± 0.01 | |
| Mannose 5% | 501 ± 13 | 0.66 ± 0.02 | |
| Mannose 10% | 218 ± 3 | 0.50 ± 0.01 | |
| Sucrose 5% | 304 ± 8 | 0.67 ± 0.05 | |
| Sucrose 10% | # | 0.43 ± 0.08 | |
| E | Glucose 5% | 82 ± 15 | 0.56 ± 0.01 |
| Glucose 10% | 100 ± 24 | 0.59 ± 0.04 | |
| Trehalose 5% | 213 ± 3 | 0.52 ± 0.06 | |
| Trehalose 10% | 239 ± 2 | 0.24 ± 0.01 | |
| Mannose 5% | 326 ± 4 | 0.43 ± 0.01 | |
| Mannose 10% | 203 ± 2 | 0.50 ± 0.01 | |
| Sucrose 5% | # | 0.57 ± 0.01 | |
| Sucrose 10% | # | 0.72 ± 0.06 | |
| F | Glucose 5% | 350 ± 7 | 0.40 ± 0.01 |
| Glucose 10% | 148 ± 36 | 0.96 ± 0.08 | |
| Trehalose 5% | 148 ± 3 | 0.43 ± 0.04 | |
| Trehalose 10% | 152 ± 1 | 0.29 ± 0.01 | |
| Mannose 5% | 152 ± 2 | 0.18 ± 0.02 | |
| Mannose 10% | # | 0.58 ± 0.02 | |
| Sucrose 5% | 181 ± 1 | 0.16 ± 0.03 | |
| Sucrose 10% | 216 ± 2 | 0.18 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarsitano, M.; Cristiano, M.C.; Mancuso, A.; Barone, A.; Torella, D.; Paolino, D. Lipid-Based Formulations Containing Labrafil M2125-CS: A Deep Investigation on Nanosystem Stability. Nanomanufacturing 2022, 2, 41-52. https://doi.org/10.3390/nanomanufacturing2010003
Tarsitano M, Cristiano MC, Mancuso A, Barone A, Torella D, Paolino D. Lipid-Based Formulations Containing Labrafil M2125-CS: A Deep Investigation on Nanosystem Stability. Nanomanufacturing. 2022; 2(1):41-52. https://doi.org/10.3390/nanomanufacturing2010003
Chicago/Turabian StyleTarsitano, Martine, Maria Chiara Cristiano, Antonia Mancuso, Antonella Barone, Daniele Torella, and Donatella Paolino. 2022. "Lipid-Based Formulations Containing Labrafil M2125-CS: A Deep Investigation on Nanosystem Stability" Nanomanufacturing 2, no. 1: 41-52. https://doi.org/10.3390/nanomanufacturing2010003
APA StyleTarsitano, M., Cristiano, M. C., Mancuso, A., Barone, A., Torella, D., & Paolino, D. (2022). Lipid-Based Formulations Containing Labrafil M2125-CS: A Deep Investigation on Nanosystem Stability. Nanomanufacturing, 2(1), 41-52. https://doi.org/10.3390/nanomanufacturing2010003










