Influence of the Physico-Chemical Properties of Model Compounds on the Mean Sizes and Retention Rate of Gliadin Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gliadin-Based Nanoparticles Containing Model Compounds
2.3. Physico-Chemical Characterization
2.4. Entrapment Efficiency and Loading Capacity of Model Drugs
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Palma, E.; Costa, N.; Molinaro, R.; Francardi, M.; Paolino, D.; Cosco, D.; Fresta, M. Improvement of the therapeutic treatment of inflammatory bowel diseases following rectal administration of mesalazine-loaded chitosan microparticles vs Asamax®. Carbohydr. Polym. 2019, 212, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Antitumor Features of Vegetal Protein-Based Nanotherapeutics. Pharmaceutics 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voci, S.; Gagliardi, A.; Molinaro, R.; Fresta, M.; Cosco, D. Recent Advances of Taxol-Loaded Biocompatible Nanocarriers Embedded in Natural Polymer-Based Hydrogels. Gels 2021, 7, 33. [Google Scholar] [CrossRef]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.M. Nanotechnology: Current uses and future applications in the food industry. Biotech 2018, 8, 74. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Eeda, V.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 17. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Helmy, M.W.; Samy, W.M.; Elgindy, N.A. Novel ionically crosslinked casein nanoparticles for flutamide delivery: Formulation, characterization, and in vivo pharmacokinetics. Int. J. Nanomed. 2013, 8, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein nanoparticles: Promising platforms for drug delivery applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Pangua, C.; Reboredo, C.; Campión, R.; Morales-Gracia, J.; Irache, J.M. Protein-based nanoparticles for drug delivery purposes. Int. J. Pharm. 2020, 581, 119–289. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Bonacci, S.; Iriti, G.; Procopio, A.; Fresta, M.; Cosco, D. SCLAREIN (SCLAREol contained in zeIN) nanoparticles: Development and characterization of an innovative natural nanoformulation. Int. J. Biol. 2021, 193, 713–720. [Google Scholar] [CrossRef]
- Irache, J.M.; González-Navarro, C.J. Zein nanoparticles as vehicles for oral delivery purposes. Nanomedicine 2017, 12, 1209–1211. [Google Scholar] [CrossRef]
- Reboredo, C.; González-Navarro, C.J.; Martínez-Oharriz, C.; Martínez-López, A.L.; Irache, J.M. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int. J. Pharm. 2021, 597, 120–287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Y.; Hu, Y.; Han, Y.; Xu, J.; Zhao, Y.; Li, B. Tuning the molecular interactions between gliadin and tannic acid to prepare Pickering stabilizers with improved emulsifying properties. Food Hydrocoll. 2021, 111, 106–179. [Google Scholar] [CrossRef]
- Caron, G.; Vallaro, M.; Ermondi, G. Log P as a tool in intramolecular hydrogen bond considerations. Drug Discov. Today Technol. 2018, 27, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Vrignaud, S.; Benoit, J.P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef] [Green Version]
- Bharate, S.S.; Vishwakarma, R.A. Impact of preformulation on drug development. Expert Opin. Drug Deliv. 2013, 10, 1239–1257. [Google Scholar] [CrossRef]
- Sohail, M.F.; Rehman, M.; Sarwar, H.S.; Naveed, S.; Salman, O.; Bukhari, N.I.; Shahnaz, G. Advancements in the oral delivery of Docetaxel: Challenges, current state-of-the-art and future trends. Int. J. Nanomed. 2018, 13, 3145. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Shin, H.S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Cosco, D.; Mare, R.; Paolino, D.; Salvatici, M.C.; Cilurzo, F.; Fresta, M. Sclareol-loaded hyaluronan-coated PLGA nanoparticles: Physico-chemical properties and in vitro anticancer features. Int. J. Biol. Macromol. 2019, 132, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Bruno, F.; Castelli, G.; Puleio, R.; Bonacci, S.; Procopio, A.; Paolino, D. Meglumine Antimoniate-Loaded Aqueous-Core PLA Nanocapsules: Old Drug, New Formulation against Leishmania-Related Diseases. Macromol. Biosci. 2021, 2100046. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, M.; Reddy, A.; Yavvari, P.S.; Srivastava, A.; Bajaj, A. Interactions governing the entrapment of anticancer drugs by low-molecular-weight hydrogelator for drug delivery applications. RSC Adv. 2016, 6, 19751–19757. [Google Scholar] [CrossRef]
- Hoda, M.; Sufi, S.A.; Cavuturu, B.; Rajagopalan, R. Stabilizers influence drug–polymer interactions and physicochemical properties of disulfiram-loaded poly-lactide-co-glycolide nanoparticles. Future Sci. OA 2017, 4, 263. [Google Scholar] [CrossRef]
- Redhead, H.M.; Davis, S.S.; Illum, L. Drug delivery in poly (lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: In vitro characterisation and in vivo evaluation. J. Control. Rel. 2001, 70, 353–363. [Google Scholar] [CrossRef]
- He, H.; Zhang, J.; Xie, Y.; Lu, Y.; Qi, J.; Ahmad, E.; Wu, W. Bioimaging of intravenous polymeric micelles based on discrimination of integral particles using an environment-responsive probe. Mol. Pharm. 2016, 13, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef]
- Trindade, I.C.; Pound-Lana, G.; Pereira, D.G.S.; de Oliveira, L.A.M.; Andrade, M.S.; Vilela, J.M.C.; Mosqueira, V.C.F. Mechanisms of interaction of biodegradable polyester nanocapsules with non-phagocytic cells. Eur. J. Pharm. Sci. 2018, 124, 89104. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Pound-Lana, G.; Capelari-Oliveira, P.; Pontífice, T.G.; Silva, S.E.D.; Machado, M.G.C.; Mosqueira, V.C.F. Release, transfer and partition of fluorescent dyes from polymeric nanocarriers to serum proteins monitored by asymmetric flow field-flow fractionation. J. Chromatogr. A 2021, 1641, 461959. [Google Scholar] [CrossRef]
- Wang, X.Y.; Koller, R.; Wirth, M.; Gabor, F. Lectin-grafted PLGA microcarriers loaded with fluorescent model drugs: Characteristics, release profiles, and cytoadhesion studies. Sci. Pharm. 2014, 82, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, A.; Voci, S.; Paolino, D.; Fresta, M.; Cosco, D. Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules 2020, 25, 3174. [Google Scholar] [CrossRef]
- Ahsan, N.; Siddique, I.A.; Gupta, S.; Surolia, A. A routinely used protein staining dye acts as an inhibitor of wild type and mutant alpha-synuclein aggregation and modulator of neurotoxicity. Eur. J. Med. Chem. 2018, 143, 1174–1184. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Y.; Han, Y.; Henderson, S.C.; Majeska, R.J.; Weinbaum, S.; Schaer, M.B. In situ measuremen of solute transport in the bone lacunar-canalicular system. Proc. Natl. Acad. Sci. USA 2005, 102, 11911–11916. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein-vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2021, 118, 111538. [Google Scholar] [CrossRef]
- Voci, S.; Gagliardi, A.; Salvatici, M.C.; Fresta, M.; Cosco, D. Development of polyoxyethylene (2) oleyl ether-gliadin nanoparticles: Characterization and in vitro cytotoxicity. Eur. J. Pharm. Sci. 2021, 162, 105849. [Google Scholar] [CrossRef]
- Gagliardi, A.; Cosco, D.; Udongo, B.P.; Dini, L.; Viglietto, G.; Paolino, D. Design and Characterization of Glyceryl Monooleate-Nanostructures Containing Doxorubicin Hydrochloride. Pharmaceutics 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Giuliano, E.; Salvatici, M.C.; Celano, M.; Fresta, M.; Cosco, D. Phospholipid/zein hybrid nanoparticles as promising carriers for the protection and delivery of all-trans retinoic acid. Mater. Sci. Eng. 2021, 128, 112331. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Brij-stabilized zein nanoparticles as potential drug carriers. Colloids Surf. B 2021, 201, 111647. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ma, Y.; Dai, L.; Liao, W.; Zhang, L.; Liu, J.; Gao, Y. Fabrication, characterization, stability and re-dispersibility of curcumin-loaded gliadin-rhamnolipid composite nanoparticles using pH-driven method. Food Hydrocoll. 2021, 118, 106758. [Google Scholar] [CrossRef]
- Gulfam, M.; Kim, J.E.; Lee, J.M.; Ku, B.; Chung, B.H.; Chung, B.G. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir 2012, 28, 8216–8223. [Google Scholar] [CrossRef]
- Maity, M.; Dolui, S.; Maiti, N.C. Hydrogen bonding plays a significant role in the binding of coomassie brilliant blue-R to hemoglobin: FT-IR, fluorescence and molecular dynamics studies. Phys. Chem. Chem. Phys. 2015, 17, 31216–31227. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Vijayalakshmi, E.; Korrapati, P.S. Selective interactions of zein microspheres with different class of drugs: An in vitro and in silico analysis. AAPS PharmSciTech 2014, 15, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 1: Preparation and characterization. Food Hydrocoll. 2015, 45, 309–316. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.Q.; Chen, X.W.; Deng, Z.Y.; Yang, X.Q. Whole cereal protein-based Pickering emulsions prepared by zein-gliadin complex particles. J. Cereal Sci. 2019, 87, 46–51. [Google Scholar] [CrossRef]
- Thewissen, B.G.; Celus, I.; Brijs, K.; Delcour, J.A. Foaming properties of wheat gliadin. J. Agric. Food. Chem. 2011, 59, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, K.; Zhong, Q. Casein/pectin nanocomplexes as potential oral delivery vehicles. Int. J. Pharm. 2015, 486, 59–68. [Google Scholar] [CrossRef]
- Klein, S.; Luchs, T.; Leng, A.; Distel, L.V.; Neuhuber, W.; Hirsch, A. Encapsulation of Hydrophobic Drugs in Shell-by-Shell Coated Nanoparticles for Radio—and Chemotherapy—An In Vitro Study. Bioengineering 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Majumder, D.; Roychoudhry, S.; Kundu, S.; Dey, S.K.; Saha, C. Hydrophobic quercetin encapsulated hemoglobin nanoparticles: Formulation and spectroscopic characterization. J. Biomol. Struct. Dyn. 2021, 1–10. [Google Scholar] [CrossRef]
- Das, S.; Bora, N.; Rohman, M.A.; Sharma, R.; Jha, A.N.; Roy, A.S. Molecular recognition of bio-active flavonoids quercetin and rutin by bovine hemoglobin: An overview of the binding mechanism, thermodynamics and structural aspects through multi-spectroscopic and molecular dynamics simulation studies. Phys. Chem. Chem. Phys. 2018, 20, 21668–21684. [Google Scholar] [CrossRef]
- Herrera, M.G.; Veuthey, T.V.; Dodero, V.I. Self-organization of gliadin in aqueous media under physiological digestive pHs. Colloids Surf. B Biointerfaces 2016, 141, 565–575. [Google Scholar] [CrossRef]
- Wouters, A.G.; Schaefer, S.; Joye, I.J.; Delcour, J.A. Relating the structural, air-water interfacial and foaming properties of wheat (Triticum aestivum L.) gliadin and maize (Zea mays L.) zein based nanoparticle suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 249–259. [Google Scholar] [CrossRef]
- Fang, R.; Jing, H.; Chai, Z.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Study of the physicochemical properties of the BSA: Flavonoid nanoparticle. Eur. Food Res. Technol. 2011, 233, 275–283. [Google Scholar] [CrossRef]
- Sengupta, P.; Sardar, P.S.; Roy, P.; Dasgupta, S.; Bose, A. Investigation on the interaction of Rutin with serum albumins: Insights from spectroscopic and molecular docking techniques. J. Photochem. Photobiol. B Biol. 2018, 183, 101–110. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S.; Kroll, J. Influence of a sugar moiety (rhamnosylglucoside) at 3-O position on the reactivity of quercetin with whey proteins. Int. J. Biol. Macromol. 2003, 32, 109–120. [Google Scholar] [CrossRef]
- Luo, Y. Perspectives on important considerations in designing nanoparticles for oral delivery applications in food. J. Agric. Food Inf. 2020, 2, 100031. [Google Scholar] [CrossRef]
- Gutiérrez-Valenzuela, C.A.; Esquivel, R.; Guerrero-Germán, P.; Zavala-Rivera, P.; Rodríguez-Figueroa, J.C.; Guzmán-Z, R.; Lucero-Acuña, A. Evaluation of a combined emulsion process to encapsulate methylene blue into PLGA nanoparticles. RSC Adv. 2018, 8, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Barbero, N.; Barni, E.; Barolo, C.; Quagliotto, P.; Viscardi, G.; Napione, L.; Bussolino, F. A study of the interaction between fluorescein sodium salt and bovine serum albumin by steady-state fluorescence. Dyes Pigm. 2009, 80, 307–313. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Jofré, M.; Miranda, V.; Chandía, P.; González, R.; Hess, S.; Nishide, H. π-Stacking of rhodamine B onto water-soluble polymers containing aromatic groups. Polymer 2006, 47, 6496–6500. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Y.; Cai, K.; He, H.; Li, Y.; Lan, M.; Yin, L. High drug loading and sub-quantitative loading efficiency of polymeric micelles driven by donor–receptor coordination interactions. J. Am. Chem. Soc. 2018, 140, 1235–1238. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Waly, M.I.; Moghadam, M.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Nanostructured food proteins as efficient systems for the encapsulation of bioactive compounds. Food Sci. Hum. Wellness 2020, 9, 199–213. [Google Scholar] [CrossRef]
- Arpicco, S.; Battaglia, L.; Brusa, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Ceruti, M. Recent studies on the delivery of hydrophilic drugs in nanoparticulate systems. J. Drug. Deliv. Sci. Technol. 2016, 32, 298–312. [Google Scholar] [CrossRef]
- Sharma, K.; Deevenapalli, M.; Singh, D.; Chourasia, M.K.; Bathula, S.R. Preparation and characterization of paclitaxel-loaded gliadin nanoparticles. J. Biomater. Tissue Eng. 2014, 4, 399–404. [Google Scholar] [CrossRef]
- Sonekar, S.; Mishra, M.K.; Patel, A.K.; Nair, S.K.; Singh, C.S.; Singh, A.K. Formulation and evaluation of folic acid conjugated gliadin nanoparticles of curcumin for targeting colon cancer cells. J. Appl. Pharm. Sci. 2016, 6, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 2: Stability and functionality. Food Hydrocoll. 2015, 49, 127–134. [Google Scholar] [CrossRef]
- Abdelmoneem, M.A.; Mahmoud, M.; Zaky, A.; Helmy, M.W.; Sallam, M.; Fang, J.Y.; Elzoghby, A.O. Decorating protein nanospheres with lactoferrin enhances oral COX-2 inhibitor/herbal therapy of hepatocellular carcinoma. Nanomedicine 2018, 13, 2377–2395. [Google Scholar] [CrossRef]
- He, J.R.; Zhu, J.J.; Yin, S.W.; Yang, X.Q. Bioaccessibility and intracellular antioxidant activity of phloretin embodied by gliadin/sodium carboxymethyl cellulose nanoparticles. Food Hydrocoll. 2022, 122, 107076. [Google Scholar] [CrossRef]
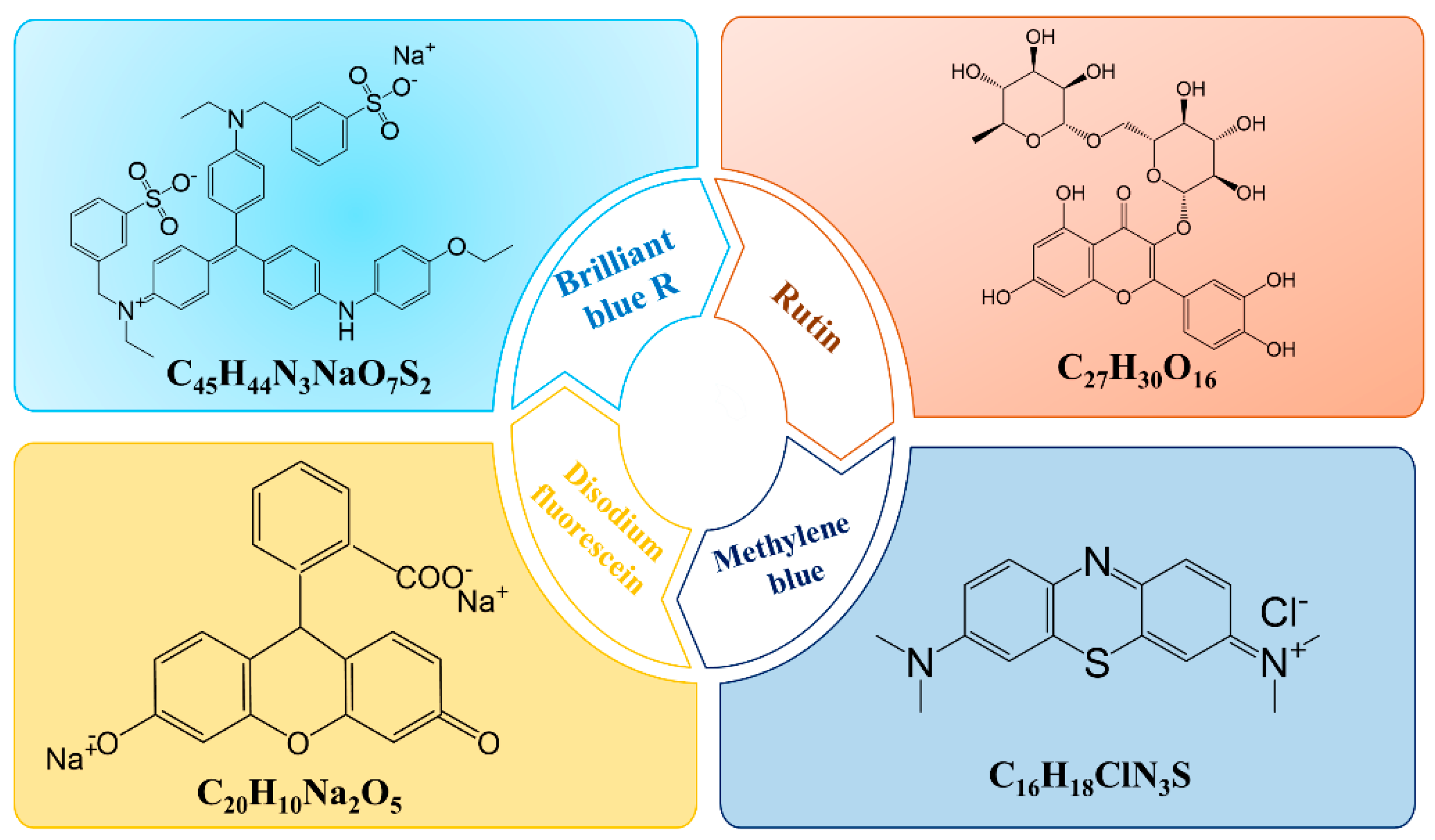

| Model Drug | LogP | Molecular Weight (g/mol) | Water Solubility (g/L) | Source |
|---|---|---|---|---|
| Brilliant blue R (BB) | −0.17 | 825.97 | 10 | Pubchem/National Diagnostic |
| Disodium fluorescein (DF) | −0.67 | 376.30 | 500 | Pubchem/Merck |
| Methylene blue (MB) | 0.75 | 319.86 | 43.6 | Pubchem |
| Rutin | 0.15 | 610.50 | 0.125 | Pubchem/Drug Bank |
| Model Drug | Amount of Compound Initially Added (mg/mL) | Mean Sizes (nm) | Polydispersity Index (PdI) |
|---|---|---|---|
| Brilliant blue R (BB) | 0.050 | 154 ± 1 | 0.176 ± 0.010 |
| 0.100 | 190 ± 5 ** | 0.216 ± 0.030 * | |
| 0.200 | 161 ± 2 | 0.255 ± 0.040 ** | |
| 0.400 | 150 ± 6 | 0.284 ± 0.041 ** | |
| Disodium fluorescein (DF) | 0.050 | 152 ± 2 | 0.197 ± 0.008 |
| 0.100 | 162 ± 8 | 0.200 ± 0.021 | |
| 0.200 | 170 ± 4 * | 0.316 ± 0.024 ** | |
| 0.400 | 208 ± 3 ** | 0.335 ± 0.006 ** | |
| Methylene blue (MB) | 0.050 | 152 ± 1 | 0.223 ± 0.016 ** |
| 0.100 | 240 ± 3 ** | 0.208 ± 0.010 ** | |
| 0.200 | 858 ± 13 ** | 0.957 ± 0.074 ** | |
| 0.400 | >1000 ** | 0.900 ± 0.053 ** | |
| Rutin | 0.050 | 215 ± 14 ** | 0.423 ± 0.058 ** |
| 0.100 | 180 ± 13 | 0.417 ± 0.064 ** | |
| 0.200 | 237 ± 14 ** | 0.466 ± 0.080 ** | |
| 0.400 | 370 ± 73 ** | 0.491 ± 0.085 ** |
| Model Compound | Amount of Compound Initially Added (mg/mL) | LC (%) |
|---|---|---|
| Brilliant blue R (BB) | 0.050 | 1.79 ± 1.0 |
| 0.100 | 3.68 ± 0.18 | |
| 0.200 | 6.57 ± 0.33 | |
| 0.400 | 11.87 ± 1.0 | |
| Disodium fluorescein (DF) | 0.050 | 0.21 ± 0.0105 |
| 0.100 | 0.23 ± 0.0115 | |
| 0.200 | 0.37 ± 0.0185 | |
| 0.400 | 0.87 ± 0.004 | |
| Methylene blue (MB) | 0.050 | 0.46 ± 0.073 |
| 0.100 | 0.86 ± 0.043 | |
| 0.200 | 0.19 ± 0.010 | |
| 0.400 | 0.41 ± 0.021 | |
| Rutin | 0.050 | 0.37 ± 0.190 |
| 0.100 | 0.46 ± 0.023 | |
| 0.200 | 1.79 ± 1.0 | |
| 0.400 | 3.68 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voci, S.; Fresta, M.; Cosco, D. Influence of the Physico-Chemical Properties of Model Compounds on the Mean Sizes and Retention Rate of Gliadin Nanoparticles. Nanomanufacturing 2021, 1, 160-170. https://doi.org/10.3390/nanomanufacturing1030011
Voci S, Fresta M, Cosco D. Influence of the Physico-Chemical Properties of Model Compounds on the Mean Sizes and Retention Rate of Gliadin Nanoparticles. Nanomanufacturing. 2021; 1(3):160-170. https://doi.org/10.3390/nanomanufacturing1030011
Chicago/Turabian StyleVoci, Silvia, Massimo Fresta, and Donato Cosco. 2021. "Influence of the Physico-Chemical Properties of Model Compounds on the Mean Sizes and Retention Rate of Gliadin Nanoparticles" Nanomanufacturing 1, no. 3: 160-170. https://doi.org/10.3390/nanomanufacturing1030011
APA StyleVoci, S., Fresta, M., & Cosco, D. (2021). Influence of the Physico-Chemical Properties of Model Compounds on the Mean Sizes and Retention Rate of Gliadin Nanoparticles. Nanomanufacturing, 1(3), 160-170. https://doi.org/10.3390/nanomanufacturing1030011