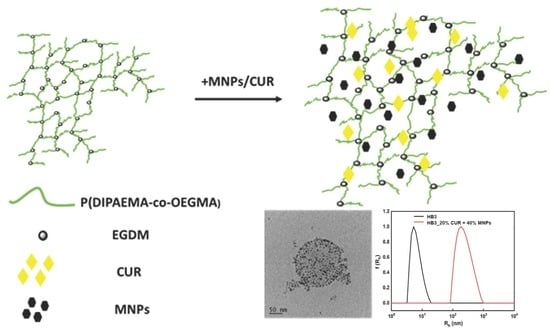
Amphiphilic P(OEGMA-co-DIPAEMA) Hyperbranched Copolymer/Magnetic Nanoparticle Hybrid Nanostructures by Co-Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hyperbranched P(OEGMA-co-DIPAEMA) Copolymer Synthesis
2.3. Synthesis of CoFe2O4 NPs
2.4. Preparation of P(OEGMA-co-DIPAEMA) Magnetic Nanoparticles Hybrid Nanostructures
2.5. Preparation of P(OEGMA-co-DIPAEMA) Magnetic Nanoparticle Hybrid Nanostructures Loaded with Curcumin
2.6. Methods
3. Results and Discussion
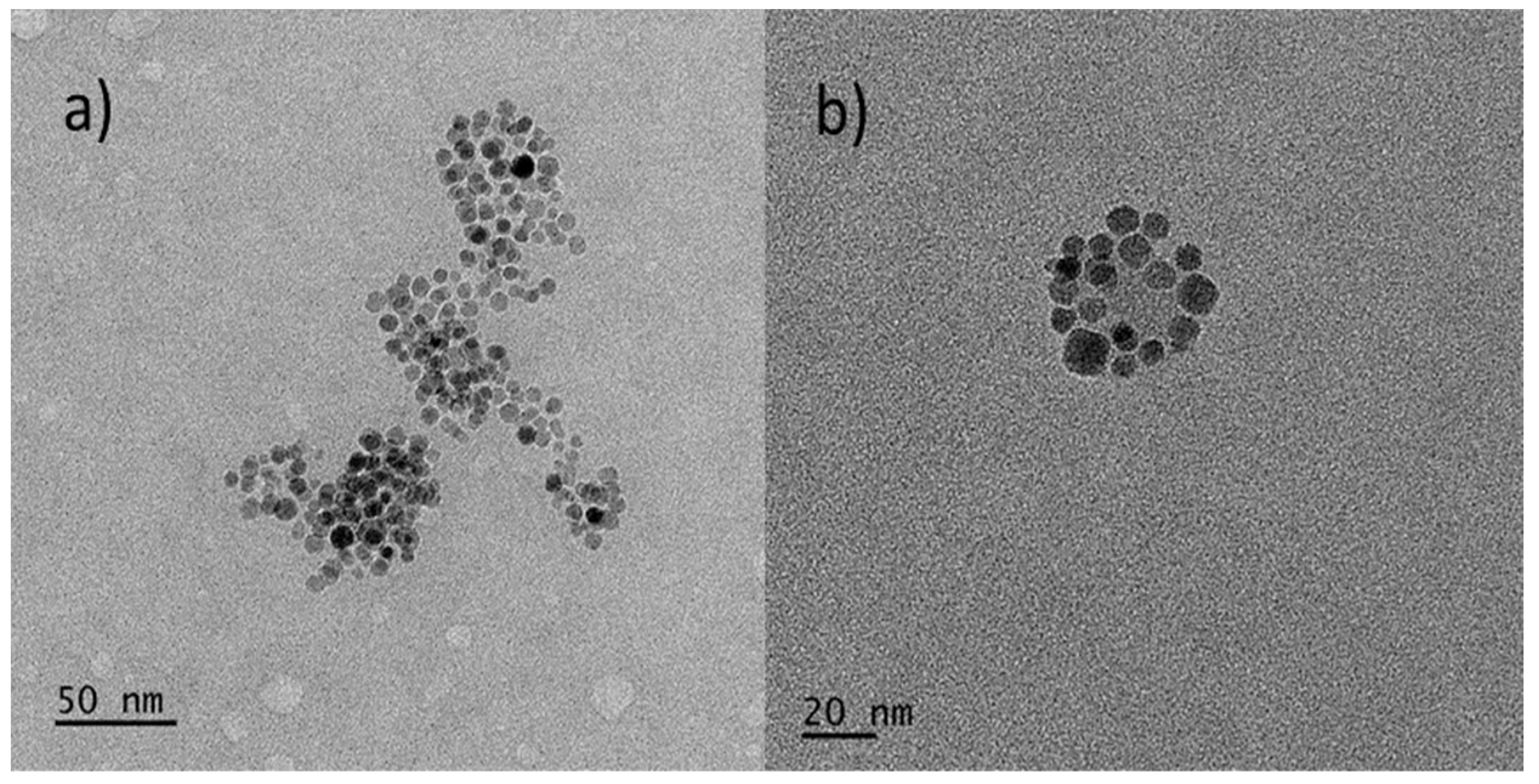
3.1. Characterization of CoFe2O4 NPs
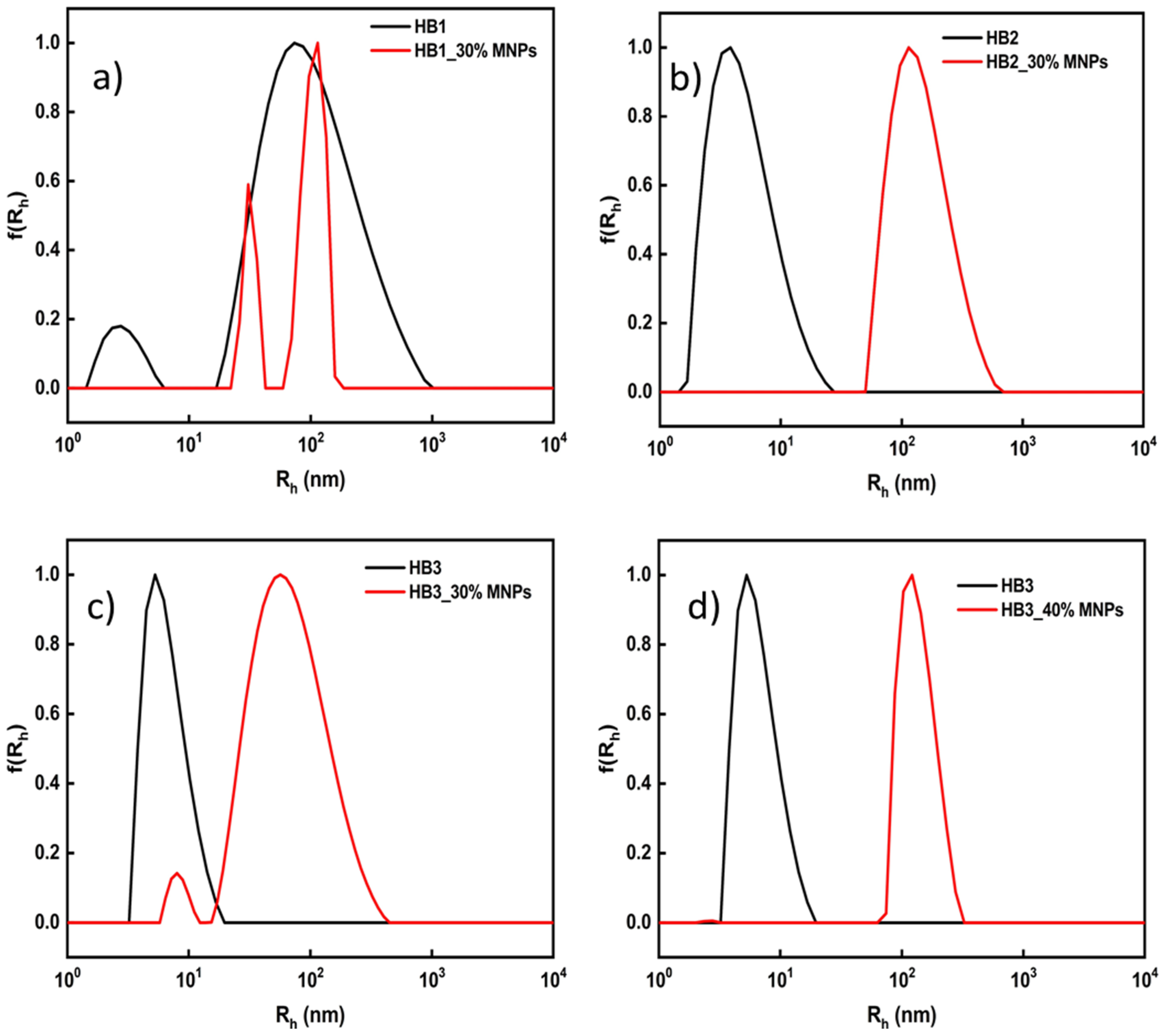
3.2. HB/MNP Hybrid Nanostructures
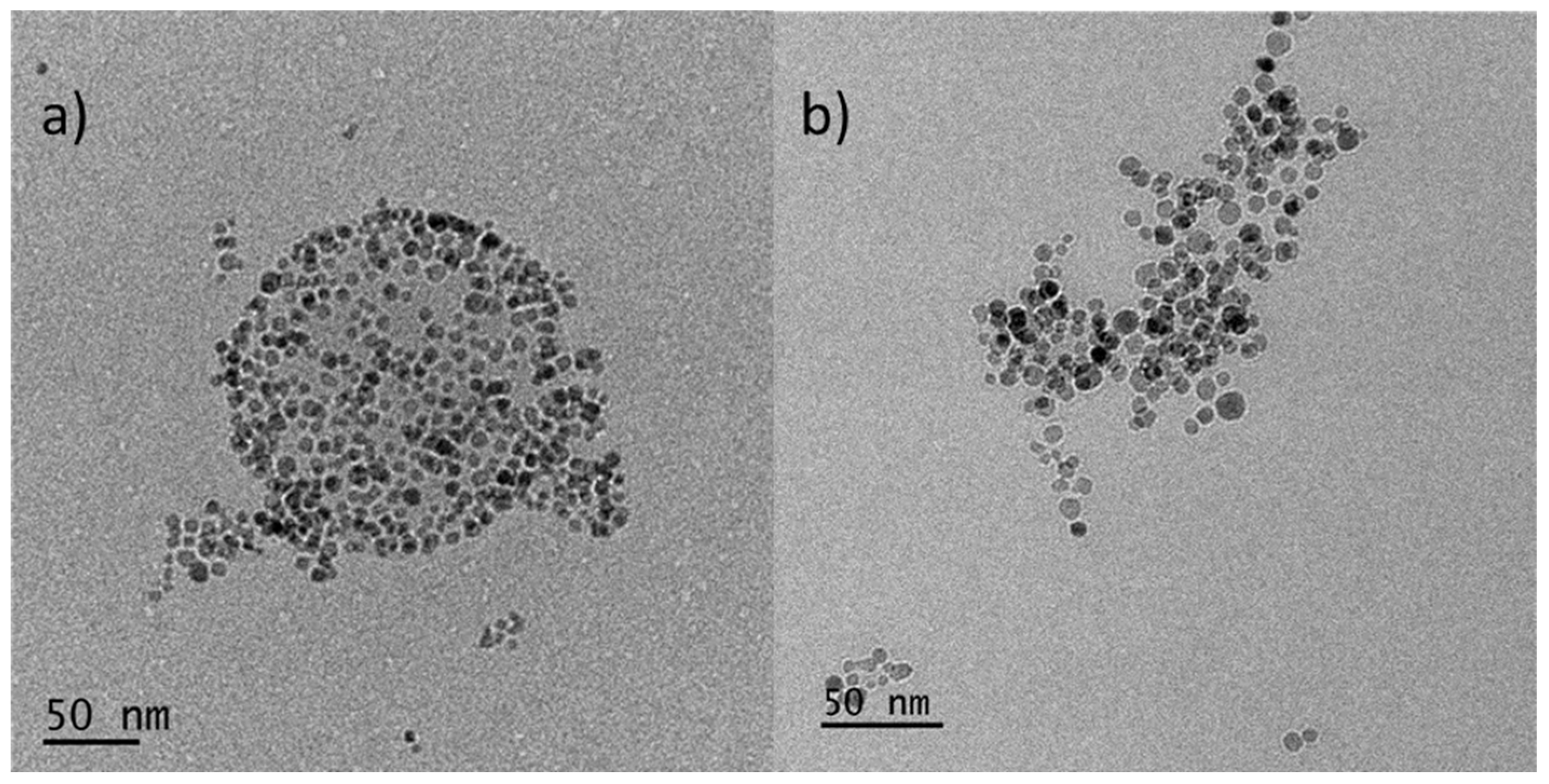
3.3. Co-Encapsulation of MNPs and CUR Simultaneously into P(OEGMA-co-DIPAEMA) Polymeric Aggregates
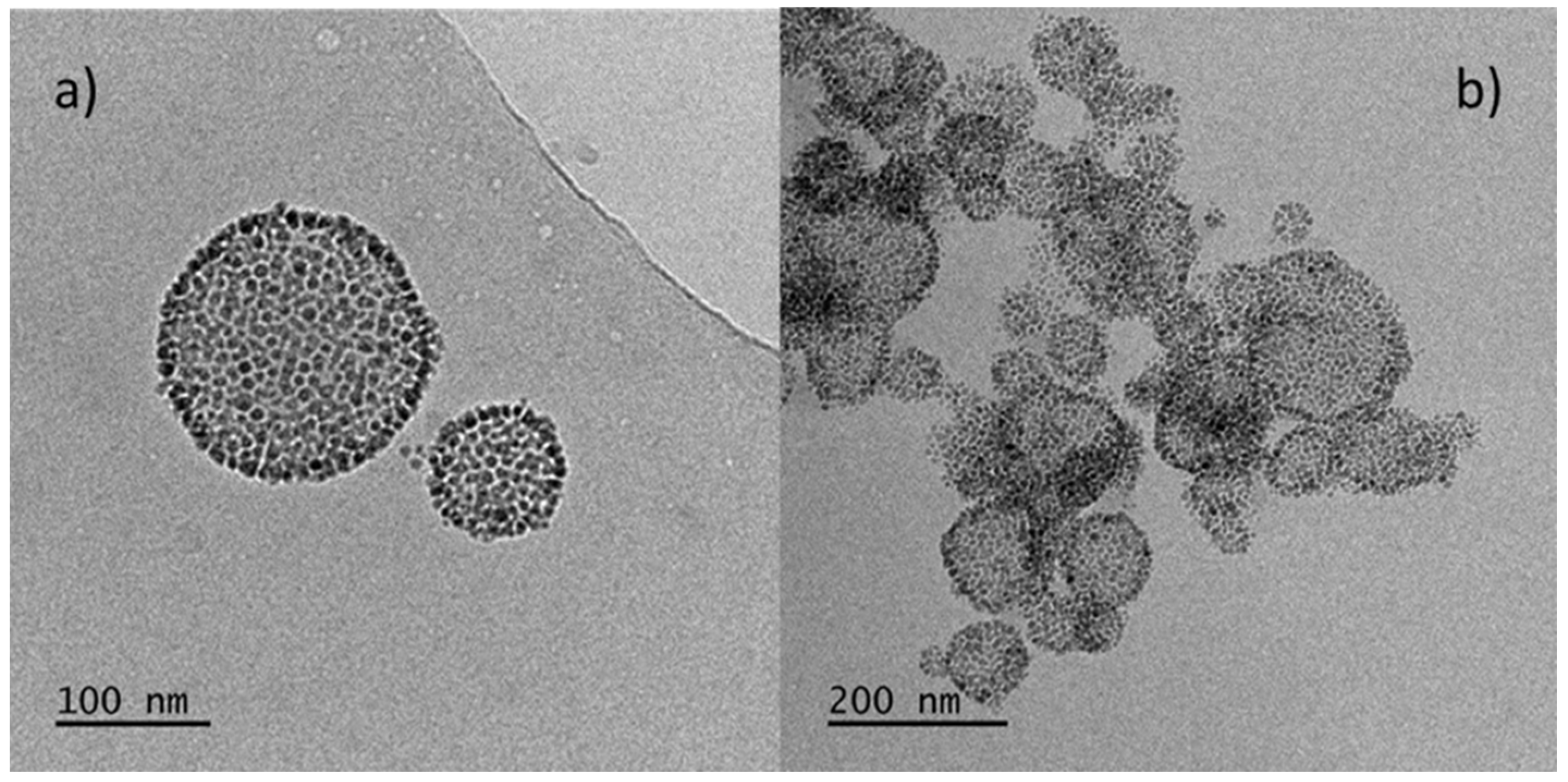
3.4. Cryo-TEM Imaging of the HB Based MNPs-Loaded and MNPS/CUR-Loaded Nanostructures
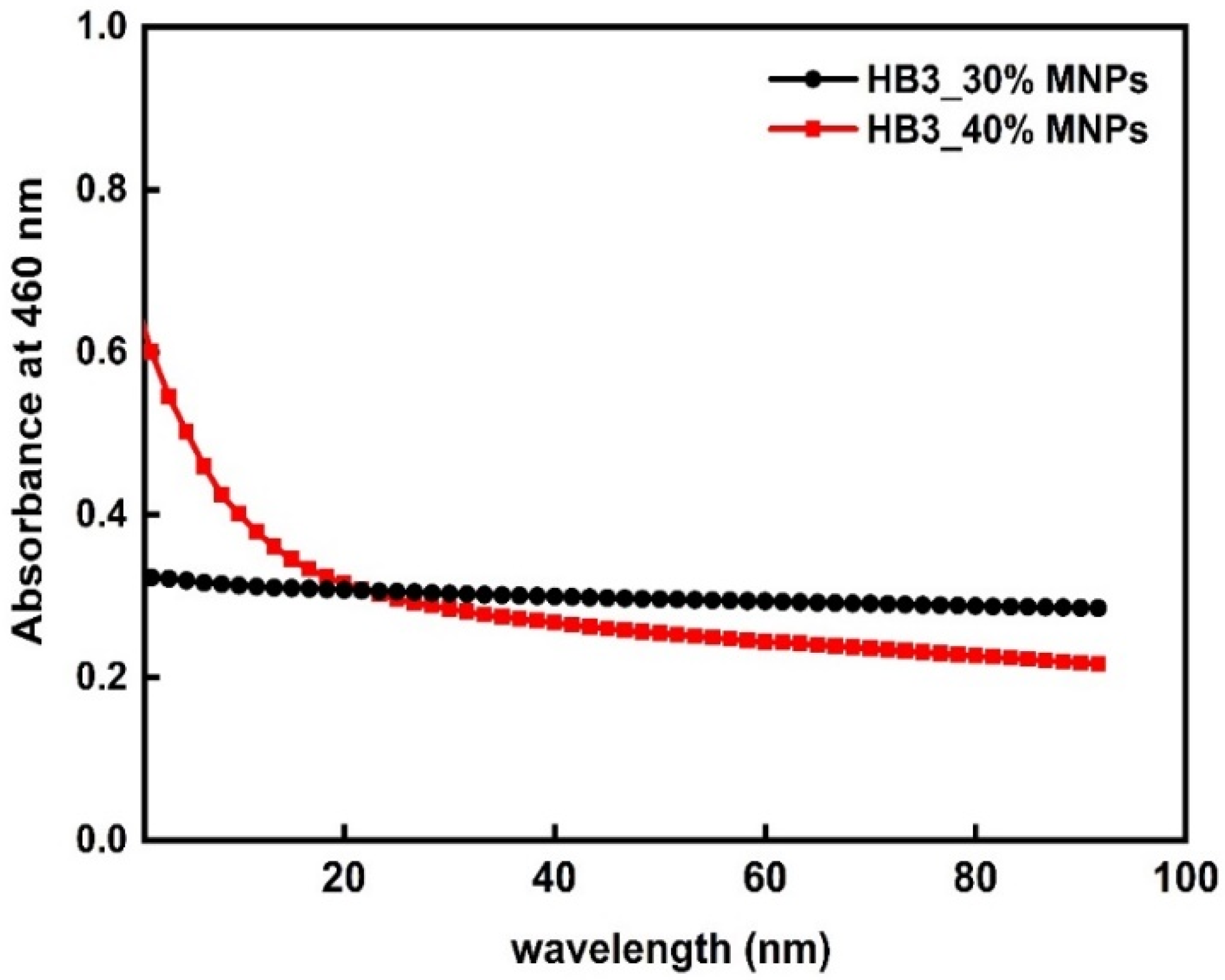
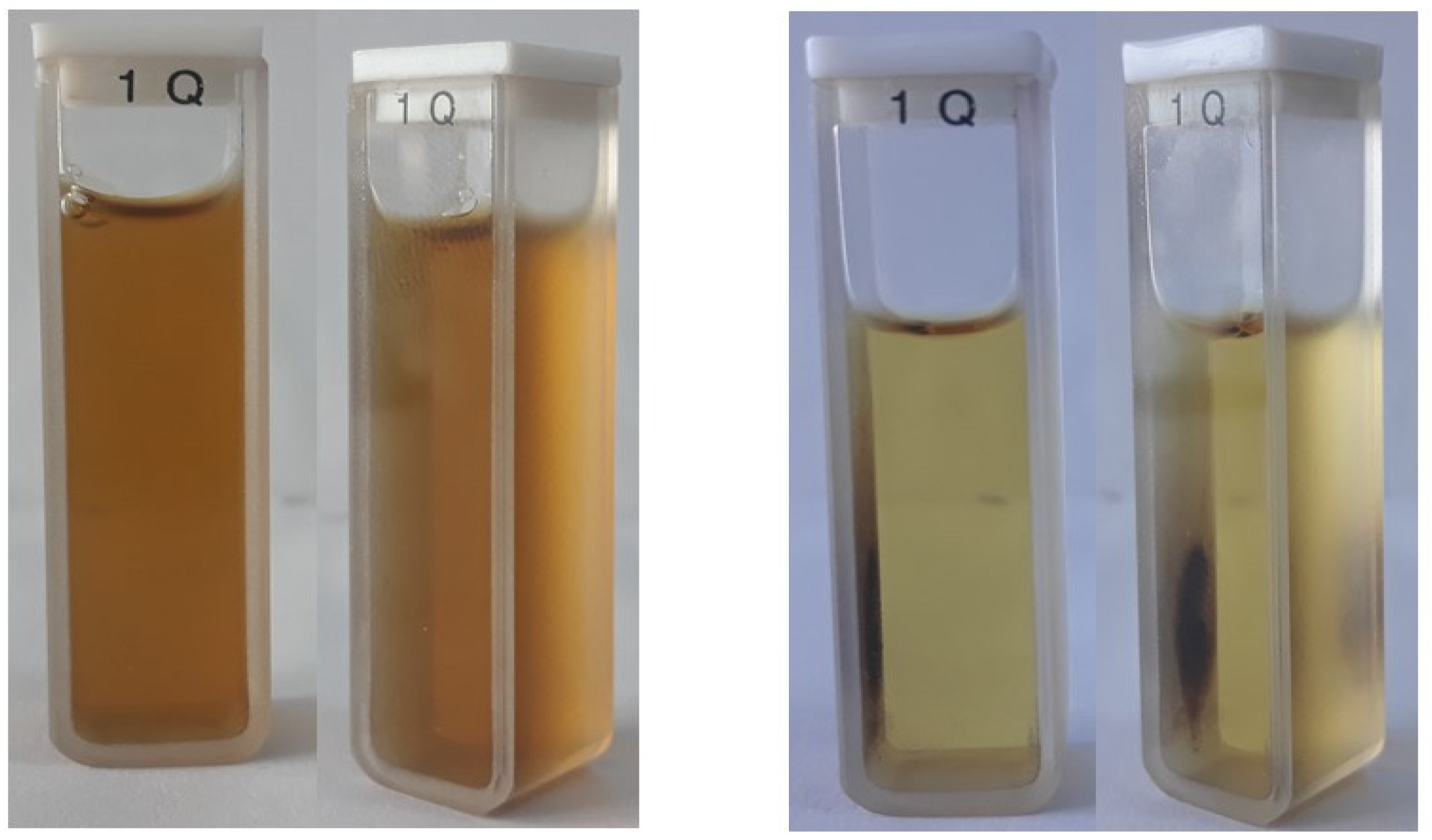
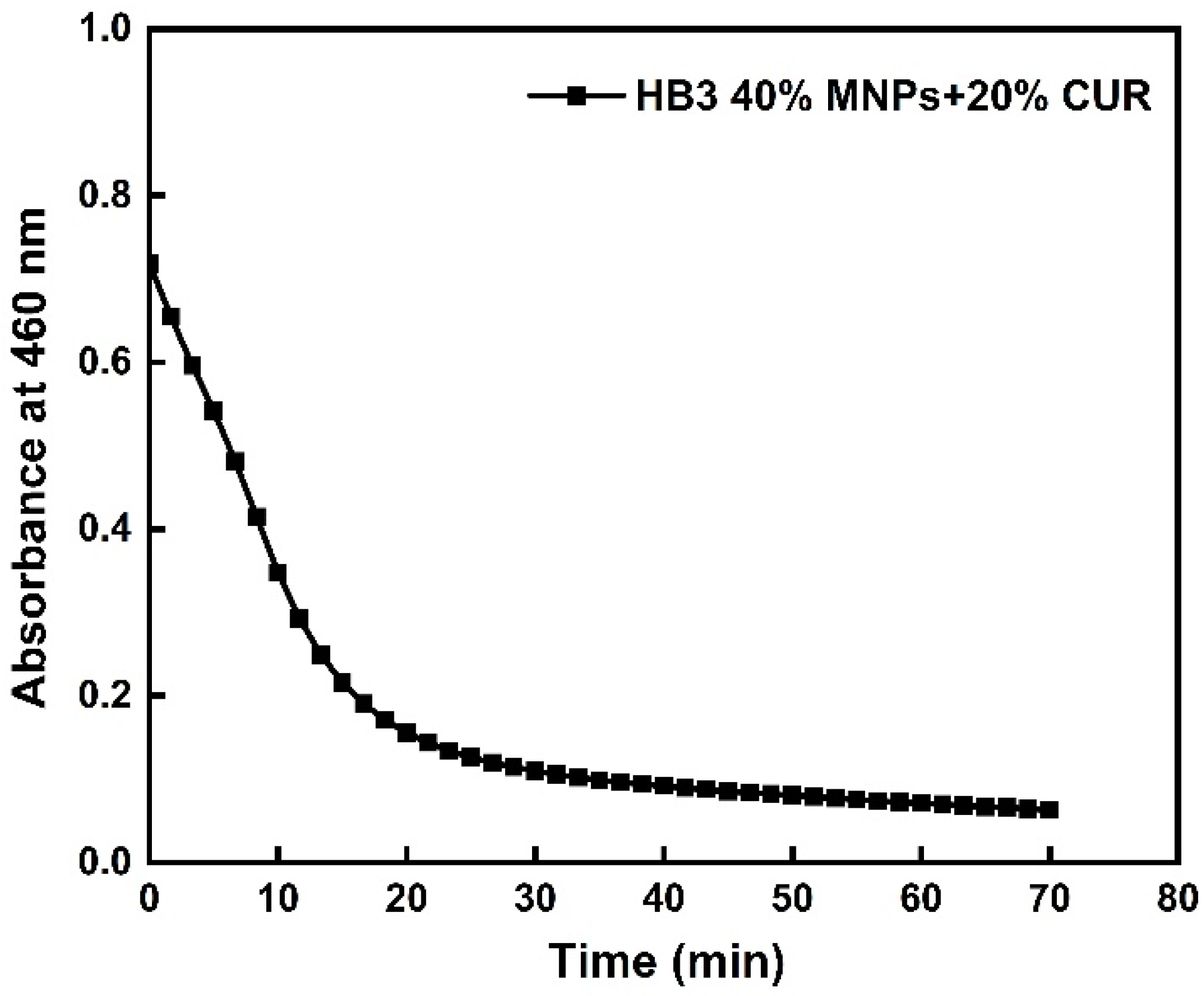
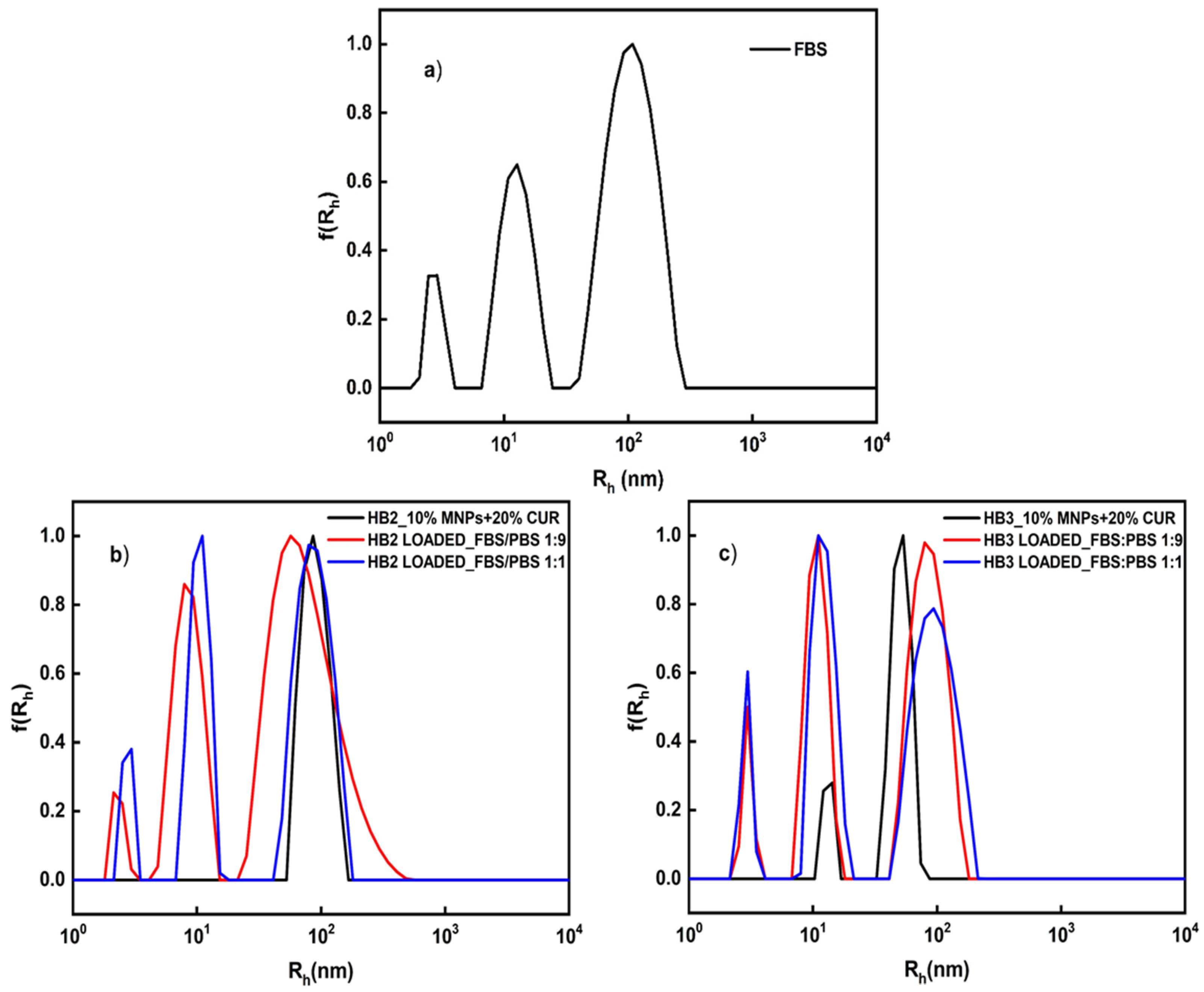
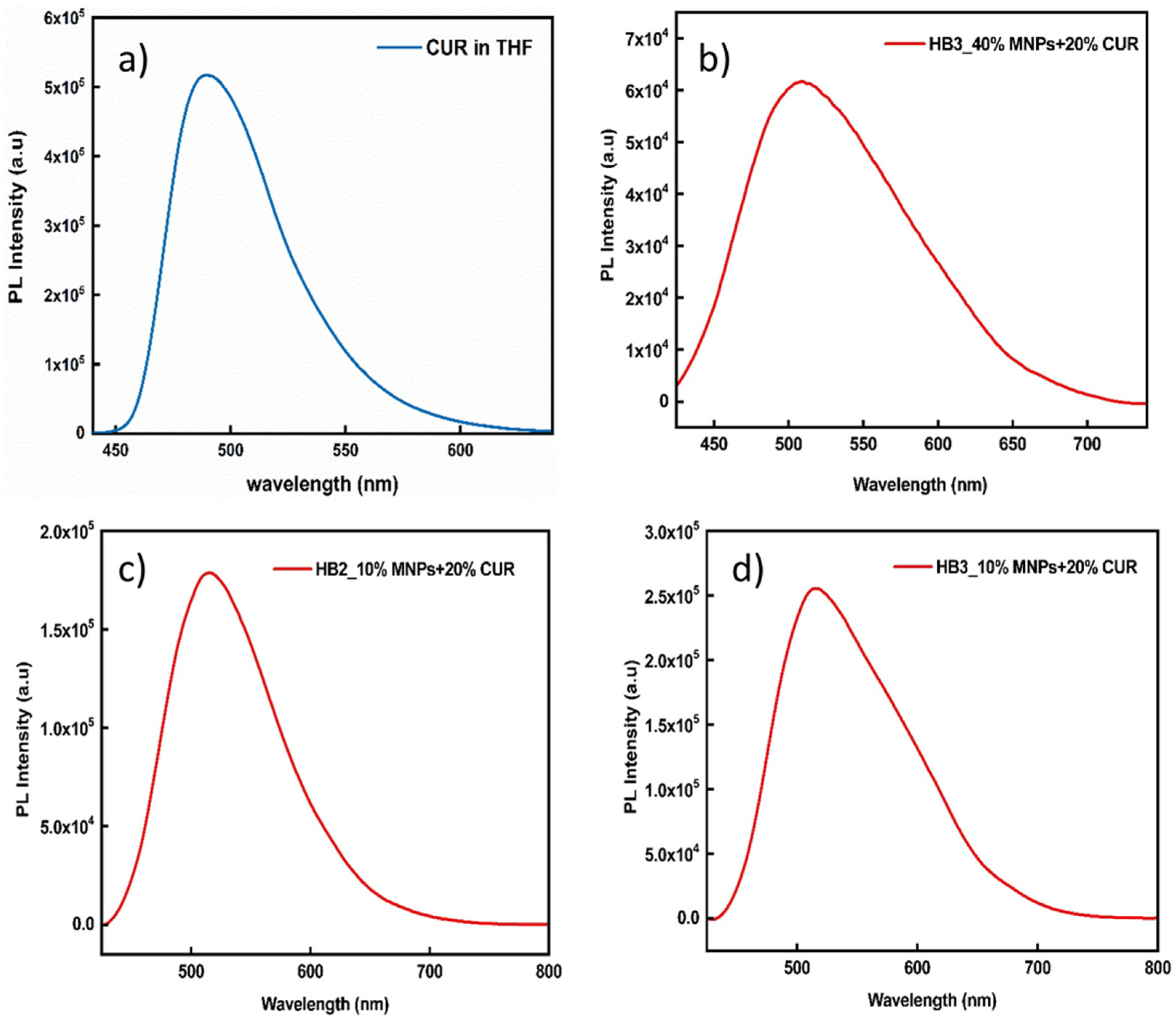
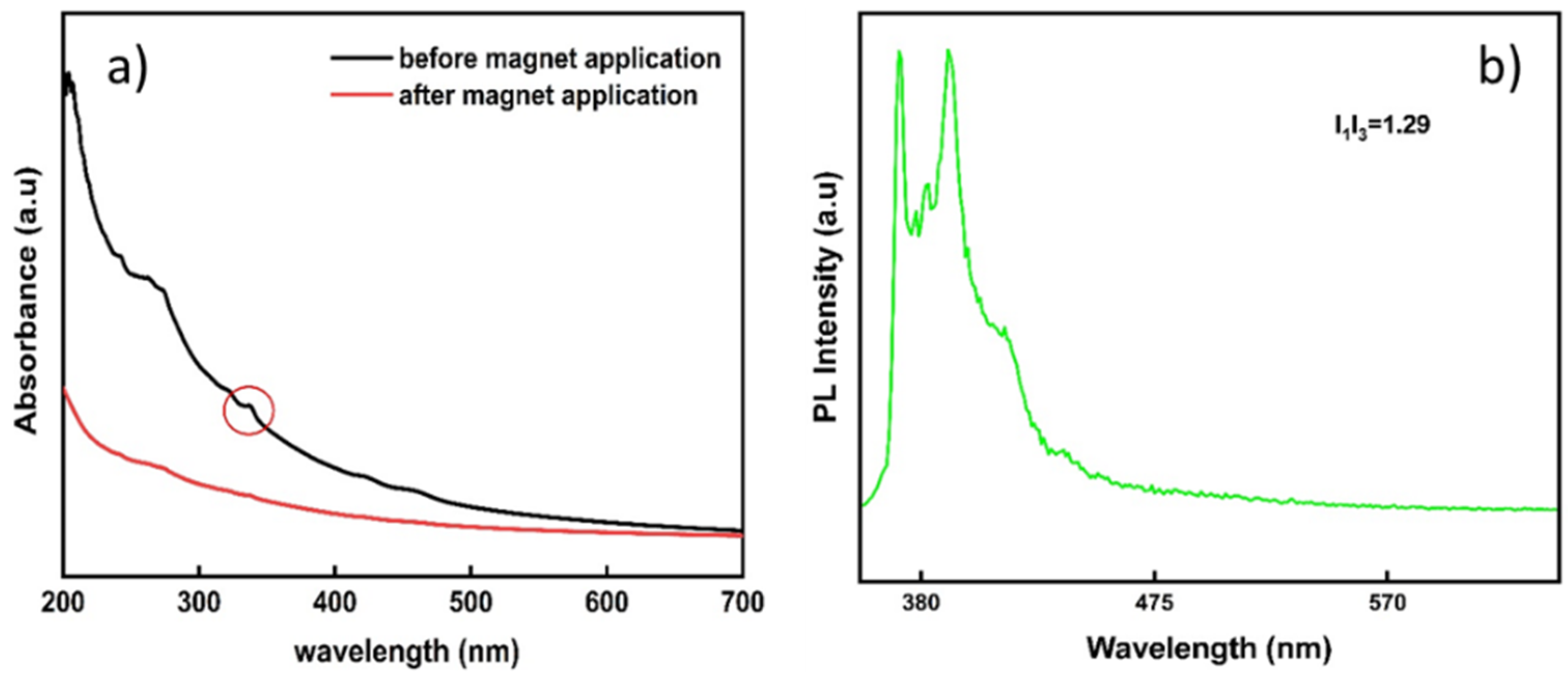
3.5. Stability of the Hybrid Nanostructures in Biological Media and Their Fluorescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Chroni, A.; Forys, A.; Trzebicka, B.; Alemayehu, A.; Tyrpekl, V.; Pispas, S. Poly [oligo (ethylene glycol) methacrylate]-b-poly [(vinyl benzyl trimethylammonium chloride)] Based Multifunctional Hybrid Nanostructures Encapsulating Magnetic Nanoparticles and DNA. Polymers 2020, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Indira, T.; Lakshmi, P. Magnetic nanoparticles—A review. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 1035–1042. [Google Scholar]
- Krasia-Christoforou, T.; Georgiou, T.K. Polymeric theranostics: Using polymer-based systems for simultaneous imaging and therapy. J. Mater. Chem. B 2013, 1, 3002–3025. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, M.; Saxena, S.; Tankhiwale, R.; Bajpai, M.; Mohan, Y.Á.; Bajpai, S. Magnetic nanoparticles for drug delivery applications. J. Nanosci. Nanotechnol. 2008, 8, 3247–3271. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, A.; Sergides, A.; Bakandritsos, A.; Pispas, S. PLMA-b-POEGMA amphiphilic block copolymers as nanocarriers for the encapsulation of magnetic nanoparticles and indomethacin. Polymers 2018, 10, 14. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Kafetzi, M.; Pispas, S.; Bao, X.; Yao, P. Amphiphilic QP (DMAEMA-co-LMA)-b-POEGMA Random-Block Terpolymers as Nanocarriers for Insulin. Biomedicines 2020, 8, 392. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.; Yuan, Y.; Chen, Y.; Cheng, F.; Jiang, S.-C. Amphiphilic hyperbranched copolymers bearing a hyperbranched core and a dendritic shell as novel stabilizers rendering gold nanoparticles with an unprecedentedly long lifetime in the catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 21173–21182. [Google Scholar] [CrossRef]
- Luzon, M.; Boyer, C.; Peinado, C.; Corrales, T.; Whittaker, M.; Tao, L.; Davis, T.P. Water-soluble, thermoresponsive, hyperbranched copolymers based on PEG-methacrylates: Synthesis, characterization, and LCST behavior. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2783–2792. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, J.; Su, Y.; Zhu, B.; Huang, W.; Zhou, Y.; Zhu, X.; Yan, D. Bioreducible unimolecular micelles based on amphiphilic multiarm hyperbranched copolymers for triggered drug release. Sci. China Chem. 2010, 53, 2497–2508. [Google Scholar] [CrossRef]
- Wang, D.; Chen, H.; Su, Y.; Qiu, F.; Zhu, L.; Huan, X.; Zhu, B.; Yan, D.; Guo, F.; Zhu, X. Supramolecular amphiphilic multiarm hyperbranched copolymer: Synthesis, self-assembly and drug delivery applications. Polym. Chem. 2013, 4, 85–94. [Google Scholar] [CrossRef]
- Irfan, M.; Seiler, M. Encapsulation using hyperbranched polymers: From research and technologies to emerging applications. Ind. Eng. Chem. Res. 2010, 49, 1169–1196. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Yu, B.; Wang, J.; Wang, C.; Zeng, W.; Hu, Y. Economical and environment-friendly synthesis of a novel hyperbranched poly (aminomethylphosphine oxide-amine) as co-curing agent for simultaneous improvement of fire safety, glass transition temperature and toughness of epoxy resins. Chem. Eng. J. 2017, 322, 618–631. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.-Z.; Cheng, S.-X.; Zhuo, R.-X.; Gu, Z.-W. Functionalized amphiphilic hyperbranched polymers for targeted drug delivery. Biomacromolecules 2008, 9, 2578–2585. [Google Scholar] [CrossRef]
- Deng, Y.; Saucier-Sawyer, J.K.; Hoimes, C.J.; Zhang, J.; Seo, Y.-E.; Andrejecsk, J.W.; Saltzman, W.M. The effect of hyperbranched polyglycerol coatings on drug delivery using degradable polymer nanoparticles. Biomaterials 2014, 35, 6595–6602. [Google Scholar] [CrossRef]
- Jafari, M.; Abolmaali, S.S.; Najafi, H.; Tamaddon, A.M. Hyperbranched polyglycerol nanostructures for anti-biofouling, multifunctional drug delivery, bioimaging and theranostic applications. Int. J. Pharm. 2020, 576, 118959. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, F.; Zhu, B.; Zhu, X. Hyperbranched polymers for bioimaging. RSC Adv. 2013, 3, 2071–2083. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly (ethylene imine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. J. Hazard. Mater. 2009, 170, 35–42. [Google Scholar] [CrossRef]
- Harinath, Y.; Reddy, D.H.K.; Sharma, L.S.; Seshaiah, K. Development of hyperbranched polymer encapsulated magnetic adsorbent (Fe3O4@SiO2–NH2-PAA) and its application for decontamination of heavy metal ions. J. Environ. Chem. Eng. 2017, 5, 4994–5001. [Google Scholar] [CrossRef]
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Bombelli, F.B.; Metrangolo, P.; Resnati, G. 19F magnetic resonance imaging (MRI): From design of materials to clinical applications. Chem. Rev. 2015, 115, 1106–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jin, Y.; Zhu, X.; Yan, D. Synthesis and applications of stimuli-responsive hyperbranched polymers. Prog. Polym. Sci. 2017, 64, 114–153. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, A.; Ansari, M.M.; Vyawahare, A.; Jayamurugan, G.; Khan, R. Hyperbranched polymer-functionalized magnetic nanoparticle-mediated hyperthermia and niclosamide bimodal therapy of colorectal cancer cells. ACS Biomater. Sci. Eng. 2019, 6, 1102–1111. [Google Scholar] [CrossRef]
- Meikle, S.; Piñeiro, Y.; López, M.B.; Rivas, J.; Santin, M. Surface functionalization superparamagnetic nanoparticles conjugated with thermoresponsive poly (epsilon-lysine) dendrons tethered with carboxybetaine for the mild hyperthermia-controlled delivery of VEGF. Acta Biomater. 2016, 40, 235–242. [Google Scholar] [CrossRef]
- Shi, Y.; Lei, G.; Li, Y.; Zhang, X.; Peng, R.; Hu, J.; Yuan, Z.; Liu, Y.; Shen, X.; Sun, N. In situ preparation of non-viral gene vectors with folate/magnetism dual targeting by hyperbranched polymers. Eur. Polym. J. 2020, 127, 109584. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Arun, A.; Malrautu, P.; Laha, A.; Ramakrishna, S. Gelatin Nanofibers in Drug Delivery Systems and Tissue Engineering. Eng. Sci. 2021, 16, 71–81. [Google Scholar]
- Hu, X.; Liu, G.; Li, Y.; Wang, X.; Liu, S. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J. Am. Chem. Soc. 2015, 137, 362–368. [Google Scholar] [CrossRef]
- Zhao, C.; Han, Q.; Qin, H.; Yan, H.; Qian, Z.; Ma, Z.; Zhang, X.; Li, X. Biocompatible hyperbranched polyester magnetic nanocarrier for stimuli-responsive drug release. J. Biomater. Sci. Polym. Ed. 2017, 28, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Shi, Y.; Wang, Y.; Zheng, Y.; Liu, H.; Hu, Q.; Wei, S.; Gu, H.; Guo, Z. Electrospun iron/cobalt alloy nanoparticles on carbon nanofibers towards exhaustive electrocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2021, 405, 126585. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. Multi-responsive poly (oligo (ethylene glycol) methyl methacrylate)-co-poly (2-(diisopropylamino) ethyl methacrylate) hyperbranched copolymers via reversible addition fragmentation chain transfer polymerization. Polym. Chem. 2021, 12, 6582–6593. [Google Scholar] [CrossRef]
- Repko, A.; Vejpravová, J.; Vacková, T.; Zákutná, D.; Nižňanský, D. Oleate-based hydrothermal preparation of CoFe2O4 nanoparticles, and their magnetic properties with respect to particle size and surface coating. J. Magn. Magn. Mater. 2015, 390, 142–151. [Google Scholar] [CrossRef]
- Gališinová, J.; Andriamainty, F.; Malík, I.; Čižmárik, J.; Karlovska, J.; Sichrovska, L. A Study of Local Anaesthetics. Part 202. Determination of the Critical Micellar Concentration of Carbisocainium Chloride in Water Using Spectral Methods and the Probe Pyrene. Eur. Pharm. J. 2013, 60, 1–6. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.; Ma, D.; Jin, W. Preparation of CoFe2O4 Nanocrystallites by Solvothermal Process and Its Catalytic Activity on the Thermal Decomposition of Ammonium Perchlorate. J. Nanomater. 2010, 2010, 842816. [Google Scholar] [CrossRef]
- Fang, S.; Tong, Z.; Nie, P.; Liu, G.; Zhang, X. Raspberry-like nanostructured silicon composite anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 18766–18773. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Selective localization of preformed nanoparticles in morphologically controllable block copolymer aggregates in solution. Acc. Chem. Res. 2012, 45, 1657–1666. [Google Scholar] [CrossRef]
- Kim, M.P.; Yi, G.-R. Nanostructured colloidal particles by confined self-assembly of block copolymers in evaporative droplets. Front. Mater. 2015, 2, 45. [Google Scholar] [CrossRef]
- Selianitis, D.; Pispas, S. P (MMA-co-HPMA)-b-POEGMA copolymers: Synthesis, micelle formation in aqueous media and drug encapsulation. Polym. Int. 2021, 70, 1508–1522. [Google Scholar] [CrossRef]
- Skandalis, A.; Selianitis, D.; Pispas, S. PnBA-b-PNIPAM-b-PDMAEA Thermo-Responsive Triblock Terpolymers and Their Quaternized Analogs as Gene and Drug Delivery Vectors. Polymers 2021, 13, 2361. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Teng, C.P.; Win, K.Y.; Chen, Y.; Zhang, X.; Yang, D.P.; Li, Z.; Ye, E. Polymeric encapsulation of turmeric extract for bioimaging and antimicrobial applications. Macromol. Rapid Commun. 2019, 40, 1800216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Ha, P.T.; Sao Nguyen, A.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025001. [Google Scholar] [CrossRef]


| Sample | Mw a (g/mol) (×104) | Mw b (g/mol) (×104) | Mw/Mn b | %wt PDIPAEMA c | %wt POEGMA c |
|---|---|---|---|---|---|
| HB1 | 33.3 | 1.2 | 1.21 | 10 | 90 |
| HB2 | 48.6 | 0.8 | 1.19 | 29 | 71 |
| HB3 | 35.1 | 1.1 | 1.24 | 54 | 46 |
| Sample | Hyperbranched Copolymer | HB/MNP Hybrid Nanostructures | |||
|---|---|---|---|---|---|
| Rh a (nm) | PDI a | MNPs % | Rh a (nm) | PDI a | |
| HB1 | 3/97 | 0.5 | 10 | 152 | 0.23 |
| 20 | 27/131 b | 0.41 | |||
| 30 | 32/105 b | 0.29 | |||
| HB2 | 4 | 0.5 | 10 | 165 | 0.27 |
| 20 | 132 | 0.19 | |||
| 30 | 142 | 0.3 | |||
| HB3 | 11 | 0.39 | 10 | 27/120 b | 0.5 |
| 20 | 9/67 b | 0.47 | |||
| 30 | 12/67 b | 0.44 | |||
| 40 | 133 | 0.25 | |||
| Sample | % MNPs | % CUR | Rh a (nm) | PDI a |
|---|---|---|---|---|
| HB2 | 10 | 20 | 89 | 0.26 |
| HB3 | 10 | 20 | 13/51 | 0.36 |
| 40 | 20 | 237 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selianitis, D.; Forys, A.; Trzebicka, B.; Alemayehu, A.; Tyrpekl, V.; Pispas, S. Amphiphilic P(OEGMA-co-DIPAEMA) Hyperbranched Copolymer/Magnetic Nanoparticle Hybrid Nanostructures by Co-Assembly. Nanomanufacturing 2022, 2, 53-68. https://doi.org/10.3390/nanomanufacturing2010004
Selianitis D, Forys A, Trzebicka B, Alemayehu A, Tyrpekl V, Pispas S. Amphiphilic P(OEGMA-co-DIPAEMA) Hyperbranched Copolymer/Magnetic Nanoparticle Hybrid Nanostructures by Co-Assembly. Nanomanufacturing. 2022; 2(1):53-68. https://doi.org/10.3390/nanomanufacturing2010004
Chicago/Turabian StyleSelianitis, Dimitrios, Aleksander Forys, Barbara Trzebicka, Adam Alemayehu, Václav Tyrpekl, and Stergios Pispas. 2022. "Amphiphilic P(OEGMA-co-DIPAEMA) Hyperbranched Copolymer/Magnetic Nanoparticle Hybrid Nanostructures by Co-Assembly" Nanomanufacturing 2, no. 1: 53-68. https://doi.org/10.3390/nanomanufacturing2010004
APA StyleSelianitis, D., Forys, A., Trzebicka, B., Alemayehu, A., Tyrpekl, V., & Pispas, S. (2022). Amphiphilic P(OEGMA-co-DIPAEMA) Hyperbranched Copolymer/Magnetic Nanoparticle Hybrid Nanostructures by Co-Assembly. Nanomanufacturing, 2(1), 53-68. https://doi.org/10.3390/nanomanufacturing2010004