Therapeutic Potential of Natural Products as Innovative and New Frontiers for Combating Parasitic Diseases
Abstract
1. Introduction
2. Methods
3. Historical Context
4. Biological Targets for Antiparasitic Drug Discovery
4.1. Polyamine Biosynthesis
4.2. Folate Pathway
4.3. Sterol Metabolism
4.4. Other Metabolic Pathways
4.5. Cytoskeletal Pathway
4.6. Disruption of DNA Replication
5. Key Antiparasitic Natural Products
5.1. Plant-Derived Natural Products
5.2. Microbial Natural Products
5.3. Marine-Derived Natural Products
6. Advances in Natural Product Research as Potential Antiparasitic Agents
6.1. Advances Made in Isolation, Purification, Elucidation and Biological Assay of Natural Products
6.2. Computational Studies
Applications of Computational Techniques in Natural Product Research
- Virtual Screening
- Molecular Docking and Molecular Dynamics Simulations
- Repurposing
7. Innovative Approaches to NP-Based Drug Discovery
7.1. Combination Therapies
| Parasitic Infection | Combination Therapy | Geological Regions Mostly Affected by Drug Resistance | References |
|---|---|---|---|
| Malaria | a 1, b 2, c 3 | Southeast Asia | [189,190,191] |
| Babesiosis | d 4 | United States | [193] |
| Toxoplasmosis | e 5, f 6 | Brazil | [194] |
| Leishmaniasis | g 7, h 8, i 9 | North Bihar in India | [195,196,202] |
| Human African Trypanosomiasis | j 10, k 11, l 12 | Africa | [203,204,205,206] |
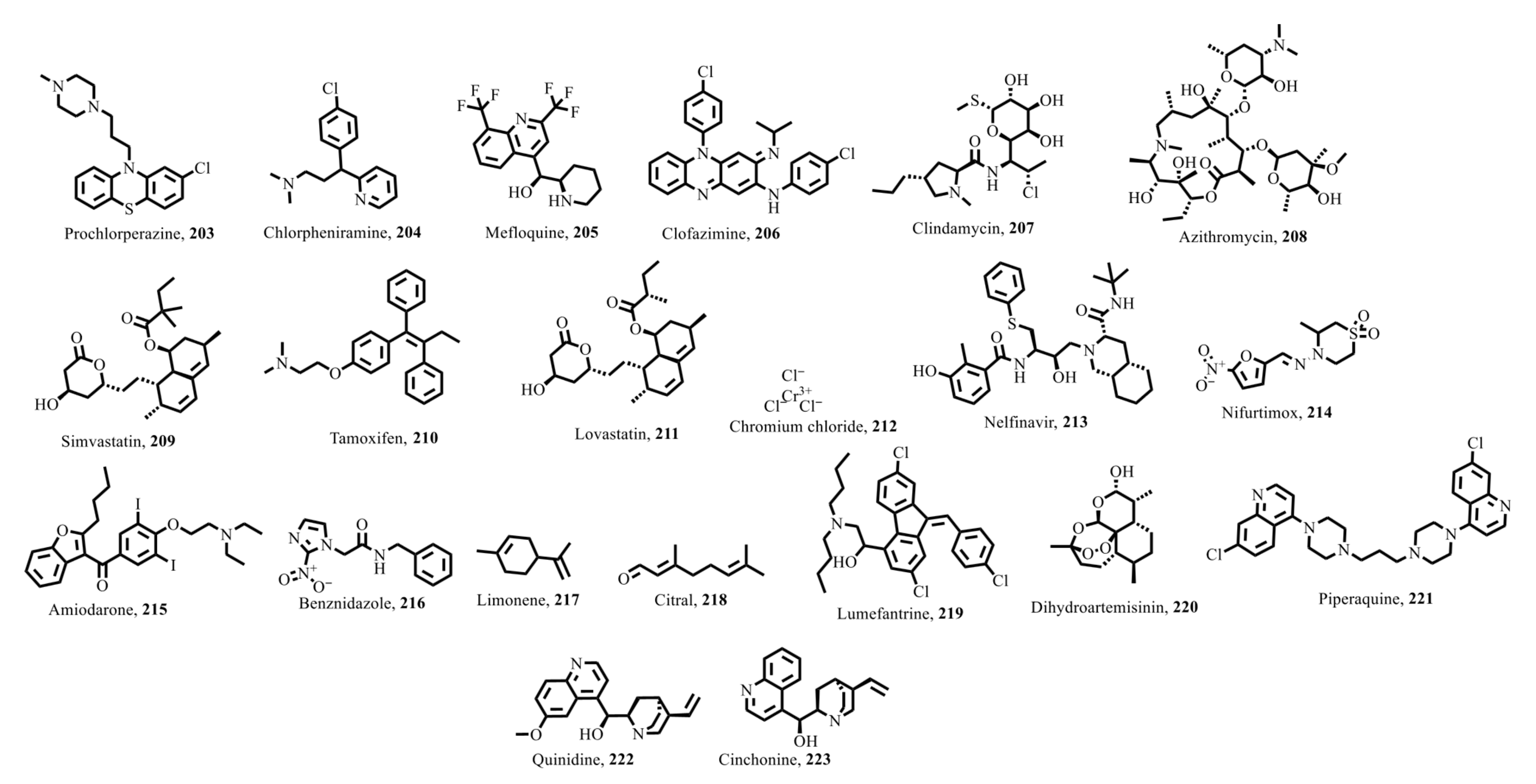
7.1.1. Combination Therapy for Malaria
7.1.2. Combination Therapy for Babesiosis
7.1.3. Toxoplasmosis
7.1.4. Leishmaniasis
7.1.5. Human African Trypanosomiasis
7.1.6. Other Parasitic Infections
7.1.7. Pharmacokinetic and Pharmacodynamic of Combinational Therapies
7.2. Nanotechnology: Enhancing the Delivery and Efficacy of Natural Products
7.3. Genetic Engineering
8. Clinical Trials in NP Research
8.1. Artemisinin Derivatives
8.2. Curcumin for Trichomonosis
8.3. Curcuma Longa + Camellia Sinensis in Livestock
8.4. Safety Profiles and Adverse Effects of Natural Products
9. Challenges and Future Directions in NP Research
9.1. Polypharmacology
9.2. Potential Innovations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs | Natural products |
| DALYs | Disability-adjusted life years |
| STH | Soil-transmitted helminthiases |
| HAT | Human African Trypanosomiasis |
| ACTs | Artemisinin-based combination therapies |
| MDA | Mass drug administration |
| LdSMT | L. donovani sterol methyltransferase |
| SMT | Sterol methyltransferase |
| SBVS | Structure-based virtual screening |
| HTS | High-throughput screening |
| LBVS | Ligand-based virtual screening |
| MM-PBSA | Molecular Mechanics Poisson–Boltzmann Surface Area |
| QSAR | Quantitative Structure–Activity Relationship |
References
- Kaminsky, R.; Mäser, P. Global impact of parasitic infections and the importance of parasite control. Front. Parasitol. 2025, 4, 1546195. [Google Scholar] [CrossRef]
- Victor, I. The Global Burden of Parasitic Diseases: Prevalence, Mortality and Economic Costs. Bio Med. 2024, 16, 760. [Google Scholar]
- WHO. World Malaria Report 2022. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 5 April 2025).
- WHO. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 7 July 2025).
- Keiser, J. Present drugs and future perspectives in treating soil-transmitted helminthiasis. Front. Trop. Dis. 2023, 4, 1282725. [Google Scholar] [CrossRef]
- Lapat, J.J.; Opee, J.; Apio, M.C.; Akello, S.; Ojul, C.L.; Onekalit, R.; Francis, O.J.; Lalweny, D.; Latigo, K.J.; Lebu, S. A One Health approach toward the control and elimination of soil-transmitted helminthic infections in endemic areas. IJID One Health 2024, 2, 100021. [Google Scholar] [CrossRef]
- Ponzo, E.; Midiri, A.; Manno, A.; Pastorello, M.; Biondo, C.; Mancuso, G. Insights into the epidemiology, pathogenesis, and differential diagnosis of schistosomiasis. Eur. J. Microbiol. Immunol. 2024, 14, 86–96. [Google Scholar] [CrossRef] [PubMed]
- WHO. Schistosomiasis (Bilharzia). Available online: https://www.who.int/health-topics/schistosomiasis#tab=tab_1 (accessed on 14 May 2025).
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 14 May 2025).
- WHO. Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 7 July 2025).
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African Trypanosomiasis (Sleeping Sickness)—Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr. Trop. Med. Rep. 2023, 10, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Utzinger, J.; Muhi, S.; Becker, S.L.; Keiser, J.; Khieu, V.; Gray, D.J. Strongyloidiasis. Nat. Rev. Dis. Primers 2024, 10, 6. [Google Scholar] [CrossRef]
- Yang, R.; Xu, M.; Zhang, L.; Liao, Y.; Liu, Y.; Deng, X.; Wang, L. Human Strongyloides stercoralis infection. J. Microbiol. Immunol. Infect. 2025, 58, 164–179. [Google Scholar] [CrossRef]
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A. The global prevalence of Strongyloides stercoralis infection. Pathogens 2020, 9, 468. [Google Scholar] [CrossRef]
- Pisarski, K. The global burden of disease of zoonotic parasitic diseases: Top 5 contenders for priority consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef]
- Mfeka, M.S.; Martinez-Oyanedel, J.; Chen, W.; Achilonu, I.; Syed, K.; Khoza, T. Comparative analyses and structural insights of new class glutathione transferases in Cryptosporidium species. Sci. Rep. 2020, 10, 20370. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.-N.; Fèvre, E.M.; Sripa, B. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177. [Google Scholar] [CrossRef] [PubMed]
- Moser, W.; Schindler, C.; Keiser, J. Drug combinations against soil-transmitted helminth infections. Adv. Parasitol. 2019, 103, 91–115. [Google Scholar] [PubMed]
- Gebreyesus, T.D.; Makonnen, E.; Tadele, T.; Mekete, K.; Gashaw, H.; Gerba, H.; Aklillu, E. Reduced efficacy of single-dose albendazole against Ascaris lumbricoides, and Trichuris trichiura, and high reinfection rate after cure among school children in southern Ethiopia: A prospective cohort study. Infect. Dis. Poverty 2024, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Tewelde, E.; Tadesse, S. Drug Discovery and Development for Soil-Transmitted Helminthiasis: Current Anthelmentics and Compounds in the Pipeline. In Roundworms-A Survey From Past to Present; IntechOpen: London, UK, 2022. [Google Scholar]
- Villamizar-Monsalve, M.A.; López-Abán, J.; Vicente, B.; Peláez, R.; Muro, A. Current drug strategies for the treatment and control of schistosomiasis. Expert Opin. Pharmacother. 2024, 25, 409–420. [Google Scholar] [CrossRef]
- Alwan, S.N.; Taylor, A.B.; Rhodes, J.; Tidwell, M.; McHardy, S.F.; LoVerde, P.T. Oxamniquine derivatives overcome Praziquantel treatment limitations for Schistosomiasis. PLoS Pathog. 2023, 19, e1011018. [Google Scholar] [CrossRef]
- Assefa, A.; Fola, A.A.; Tasew, G. Emergence of Plasmodium falciparum strains with artemisinin partial resistance in East Africa and the Horn of Africa: Is there a need to panic? Malar. J. 2024, 23, 34. [Google Scholar] [CrossRef]
- Swetanshu, P.S.; Yadav, S.; Nde, A.L.; Kumar, V.J. Drugs for the control of parasitic diseases: Current status and case studies. In Parasitic Infections: Immune Responses and Therapeutics; Wiley: Hoboken, NJ, USA, 2023; pp. 205–226. [Google Scholar]
- Garza-Tovar, T.F.; Sacriste-Hernández, M.I.; Juárez-Durán, E.R.; Arenas, R. An overview of the treatment of cutaneous leishmaniasis. Fac. Rev. 2020, 9, 28. [Google Scholar] [CrossRef]
- Buonfrate, D.; Rodari, P.; Barda, B.; Page, W.; Einsiedel, L.; Watts, M.R. Current pharmacotherapeutic strategies for Strongyloidiasis and the complications in its treatment. Expert Opin. Pharmacother. 2022, 23, 1617–1628. [Google Scholar] [CrossRef]
- Sparks, H.; Nair, G.; Castellanos-Gonzalez, A.; White, A.C., Jr. Treatment of Cryptosporidium: What we know, gaps, and the way forward. Curr. Trop. Med. Rep. 2015, 2, 181–187. [Google Scholar] [CrossRef]
- Prosty, C.; Hanula, R.; Levin, Y.; Bogoch, I.I.; McDonald, E.G.; Lee, T.C. Revisiting the evidence base for modern-day practice of the treatment of toxoplasmic encephalitis: A systematic review and meta-analysis. Clin. Infect. Dis. 2023, 76, e1302–e1319. [Google Scholar] [CrossRef]
- Haapanen, S.; Parkkila, S. Management of Entamoeba histolytica infection: Treatment strategies and possible new drug targets. In Antiprotozoal Drug Development and Delivery; Springer: Cham, Switzerland, 2021; pp. 259–269. [Google Scholar]
- Fokou, P.V.T.; Tali, M.B.T.; Mbouna, C.D.J.; Yamthe, L.R.T.; Sharifi-Rad, J.; Calina, D.; Radha; Kumar, M.; Tchouankeu, J.C.; Boyom, F.F. Natural products as transmission-blocking agents against malaria: A comprehensive review of bioactive compounds and their therapeutic potential. Malar. J. 2025, 24, 164. [Google Scholar] [CrossRef] [PubMed]
- Mao, E.Y.; Page, S.W.; Sleebs, B.E.; Gancheva, M.R.; Wilson, D.W. A review of natural products as a source of next-generation drugs against apicomplexan parasites. NPJ Antimicrob. Resist. 2025, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Dai, L.; Cao, X.; Ma, Y.; Gulnaz, I.; Miao, X.; Li, X.; Yang, X. Natural products in antiparasitic drug discovery: Advances, opportunities and challenges. Nat. Prod. Rep. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.M.; Nahar, L.; Zhang, K.Y.; Sarker, S.D. Antiparasitic natural products. In Annual Reports in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 55, pp. 115–151. [Google Scholar]
- Paloque, L.; Triastuti, A.; Bourdy, G.; Haddad, M. Natural products as antiparasitic agents. In Natural Antimicrobial Agents; Springer: Cham, Switzerland, 2018; pp. 215–245. [Google Scholar]
- Jayawardene, K.D.; Palombo, E.A.; Boag, P.R. Natural products are a promising source for anthelmintic drug discovery. Biomolecules 2021, 11, 1457. [Google Scholar] [CrossRef]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural products as a source for treating neglected parasitic diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Armson, A.; Lymbery, A.J.; Zahedi, A.; Ash, A. Medicinal plants as a source of antiparasitics: An overview of experimental studies. Pathog. Glob. Health 2023, 117, 535–553. [Google Scholar] [CrossRef]
- Ali, H.S.; Mishra, S. Natural Products as Antiparasitic, Antifungal, and Antibacterial Agents. In Drugs from Nature: Targets, Assay Systems and Leads; Springer: Singapore, 2024; pp. 367–409. [Google Scholar]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Zhang, Q.; Cui, X.; Zhu, L. Promising antiparasitic natural and synthetic products from marine invertebrates and microorganisms. Mar. Drugs 2023, 21, 84. [Google Scholar] [CrossRef]
- Mostafa, O.; Al-Shehri, M.; Moustafa, M. Promising antiparasitic agents from marine sponges. Saudi J. Biol. Sci. 2022, 29, 217–227. [Google Scholar] [CrossRef]
- McHale, D. The cinchona tree. Biologist 1986, 33, 45–53. [Google Scholar]
- Ain, Q.T.; Saleem, N.; Munawar, N.; Nawaz, R.; Naseer, F.; Ahmed, S. Quest for malaria management using natural remedies. Front. Pharmacol. 2024, 15, 1359890. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef]
- Su, X.-Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef]
- Annang, F.; Pérez-Moreno, G.; García-Hernández, R.; Cordon-Obras, C.; Martín, J.; Tormo, J.; Rodríguez, L.; De Pedro, N.; Gómez-Pérez, V.; Valente, M. High-throughput screening platform for natural product–based drug discovery against 3 neglected tropical diseases: Human african trypanosomiasis, leishmaniasis, and chagas disease. J. Biomol. Screen. 2015, 20, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Deepika; Sharda, S.; Avasthi, A. Recent Advances in the Treatment of Parasitic Diseases: Current Status and Future. In Natural Product Based Drug Discovery Against Human Parasites; Springer: Singapore, 2023; pp. 249–286. [Google Scholar]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.D.A.; Biavatt, M.W.; Brun, R.; Da Costa, F.; de Castro, S.L.; Ferreira, V.F. The Potential of Secondary Metabolites from Plants as Drugs or Leads Against Protozoan Neglected Diseases–Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, N.; Futatsugi, N.; Fuji, H.; Suenaga, A.; Morimoto, G.; Yanai, R.; Ohno, Y.; Narumi, T.; Taiji, M. High-performance drug discovery: Computational screening by combining docking and molecular dynamics simulations. PLoS Comput. Biol. 2009, 5, e1000528. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Onguéné, P.A.; Mbah, J.A.; Lifongo, L.L.; Owono Owono, L.C.; Megnassan, E.; Sippl, W. Virtual screening of natural products as potential inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase (PfDHODH). J. Biomol. Struct. Dyn. 2013, 31, 1377–1392. [Google Scholar]
- Yoshino, R.; Yasuo, N.; Hagiwara, Y.; Ishida, T.; Inaoka, D.K.; Amano, Y.; Tateishi, Y.; Ohno, K.; Namatame, I.; Niimi, T. In silico, in vitro, X-ray crystallography, and integrated strategies for discovering spermidine synthase inhibitors for Chagas disease. Sci. Rep. 2017, 7, 6666. [Google Scholar] [CrossRef]
- Tasdemir, D.; Green, D.R.; Mangal, M.; Jaspars, M. Natural products as a source of novel drugs for treating protozoan parasitic diseases. Nat. Prod. Rep. 2021, 38, 2214–2235. [Google Scholar]
- Müller, J.; Hemphill, A. Drug target identification in protozoan parasites. Expert Opin. Drug Discov. 2016, 11, 815–824. [Google Scholar] [CrossRef]
- Tounta, V.; Liu, Y.; Cheyne, A.; Larrouy-Maumus, G. Metabolomics in infectious diseases and drug discovery. Mol. Omics 2021, 17, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Cowell, A.N.; Winzeler, E.A. Advances in omics-based methods to identify novel targets for malaria and other parasitic protozoan infections. Genome Med. 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A. Polyamines in protozoan pathogens. J. Biol. Chem. 2018, 293, 18746–18756. [Google Scholar] [CrossRef]
- Adinehbeigi, K.; Razi Jalali, M.H.; Shahriari, A.; Bahrami, S. In vitro antileishmanial activity of fisetin flavonoid via inhibition of glutathione biosynthesis and arginase activity in Leishmania infantum. Pathog. Glob. Health 2017, 111, 176–185. [Google Scholar] [CrossRef]
- Hazra, S.; Ghosh, S.; Sarma, M.D.; Sharma, S.; Das, M.; Saudagar, P.; Prajapati, V.K.; Dubey, V.K.; Sundar, S.; Hazra, B. Evaluation of a diospyrin derivative as antileishmanial agent and potential modulator of ornithine decarboxylase of Leishmania donovani. Exp. Parasitol. 2013, 135, 407–413. [Google Scholar] [CrossRef]
- Bertacine Dias, M.V.; Santos, J.C.; Libreros-Zuniga, G.A.; Ribeiro, J.A.; Chavez-Pacheco, S.M. Folate biosynthesis pathway: Mechanisms and insights into drug design for infectious diseases. Future Med. Chem. 2018, 10, 935–959. [Google Scholar] [CrossRef]
- Vickers, T.J.; Beverley, S.M. Folate metabolic pathways in Leishmania. Essays Biochem. 2011, 51, 63–80. [Google Scholar] [CrossRef]
- Camara, D.; Bisanz, C.; Barette, C.; Van Daele, J.; Human, E.; Barnard, B.; Van Der Straeten, D.; Stove, C.P.; Lambert, W.E.; Douce, R. Inhibition of p-aminobenzoate and folate syntheses in plants and apicomplexan parasites by natural product rubreserine. J. Biol. Chem. 2012, 287, 22367–22376. [Google Scholar] [CrossRef]
- Herrera-Acevedo, C.; de Menezes, R.P.B.; de Sousa, N.F.; Scotti, L.; Scotti, M.T.; Coy-Barrera, E. Kaurane-Type Diterpenoids as Potential Inhibitors of Dihydrofolate Reductase-Thymidylate Synthase in New World Leishmania Species. Antibiotics 2023, 12, 663. [Google Scholar] [CrossRef]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An overview on target-based drug design against kinetoplastid protozoan infections: Human African trypanosomiasis, Chagas disease and leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef] [PubMed]
- Dorsaz, S.; Snäkä, T.; Favre-Godal, Q.; Maudens, P.; Boulens, N.; Furrer, P.; Ebrahimi, S.N.; Hamburger, M.; Allémann, E.; Gindro, K. Identification and mode of action of a plant natural product targeting human fungal pathogens. Antimicrob. Agents Chemother. 2017, 61, e00829-17. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Kaur, H.; Palmo, T.; Nargotra, A.; Singh, K. Exploring natural product library as potential target against sterol C-24 methyltransferase protein of Leishmania donovani. Nat. Prod. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Kumari, D.; Kour, P.; Singh, C.P.; Choudhary, R.; Ali, S.M.; Bhayye, S.; Bharitkar, Y.P.; Singh, K. Anhydroparthenin as a dual-target inhibitor against Sterol C-24 methyltransferase and Sterol 14-α demethylase of Leishmania donovani: A comprehensive in vitro and in silico study. Int. J. Biol. Macromol. 2024, 269, 132034. [Google Scholar] [CrossRef]
- el Kouni, M.H. Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol. Ther. 2003, 99, 283–309. [Google Scholar] [CrossRef]
- Leroux, A.E.; Krauth-Siegel, R.L. Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol. Biochem. Parasitol. 2016, 206, 67–74. [Google Scholar] [CrossRef]
- Crichton, R. An overview of intermediary metabolism and bioenergetics. In Biological Inorganic Chemistry; Elsevier: Oxford, UK, 2012; pp. 91–115. [Google Scholar]
- Martinez-Peinado, N.; Lorente-Macías, Á.; García-Salguero, A.; Cortes-Serra, N.; Fenollar-Collado, Á.; Ros-Lucas, A.; Gascon, J.; Pinazo, M.-J.; Molina, I.J.; Unciti-Broceta, A. Novel purine chemotypes with activity against Plasmodium falciparum and Trypanosoma cruzi. Pharmaceuticals 2021, 14, 638. [Google Scholar] [CrossRef]
- Ponasik, J.; Strickland, C.; Faerman, C.; Savvides, S.; Karplus, P.; Ganem, B. Kukoamine A and other hydrophobic acylpolyamines: Potent and selective inhibitors of Crithidia fasciculata trypanothione reductase. Biochem. J. 1995, 311, 371–375. [Google Scholar] [CrossRef]
- Zuma, L.K.; Pooe, O.J.; Mabaso, N.H.; Alake, J.; Obakachi, V.A.; Yakobi, S.; Gasa, N.; Karpoormath, R.; Simelane, M. Assessing the efficacy of iso-mukaadial acetate and betulinic acid against selected Plasmodium falciparum glycolytic pathway proteins: In silico and in vitro studies. BMC Chem. 2025, 19, 16. [Google Scholar] [CrossRef]
- Hu, K.; Johnson, J.; Florens, L.; Fraunholz, M.; Suravajjala, S.; DiLullo, C.; Yates, J.; Roos, D.S.; Murray, J.M. Cytoskeletal components of an invasion machine—The apical complex of Toxoplasma gondii. PLoS Pathog. 2006, 2, e13. [Google Scholar] [CrossRef]
- Estes, R.; Vogel, N.; Mack, D.; McLeod, R. Paclitaxel arrests growth of intracellular Toxoplasma gondii. Antimicrob. Agents Chemother. 1998, 42, 2036–2040. [Google Scholar] [CrossRef]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Gigant, B.; Wang, C.; Ravelli, R.B.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Struck, N.S.; Xu, M.; Johnson, J.W.; Wang, W.; Pallant, D.; Cook, M.A.; Rambow, J.; Lemcke, S.; Gilberger, T.W. A non-reactive natural product precursor of the duocarmycin family has potent and selective antimalarial activity. Cell Chem. Biol. 2022, 29, 840–853.e6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chowdhury, S.R.; Jatte, K.K.; Chakrabarti, T.; Majumder, H.K.; Jha, T.; Mukhopadhyay, S. Anthocephaline, a new indole alkaloid and cadambine, a potent inhibitor of DNA topoisomerase IB of Leishmania donovani (LdTOP1LS), isolated from Anthocephalus cadamba. Nat. Prod. Commun. 2015, 10, 297–299. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Kumar, A.; Godinho, J.L.P.; Silva, S.T.D.M.; Zuma, A.A.; Saha, S.; Kumari, N.; Rodrigues, J.C.F.; Sundar, S.; Dujardin, J.-C. Voacamine alters Leishmania ultrastructure and kills parasite by poisoning unusual bi-subunit topoisomerase IB. Biochem. Pharmacol. 2017, 138, 19–30. [Google Scholar] [CrossRef]
- Mueller, D.; Davis, R.A.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial activity of azafluorenone alkaloids from the Australian tree Mitrephora diversifolia. J. Nat. Prod. 2009, 72, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Jacob, M.R.; Khan, S.I.; Zhao, J.; Tekwani, B.L.; Midiwo, J.O.; Walker, L.A.; Muhammad, I. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Nat. Prod. Commun. 2009, 4, 791–796. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Panseeta, P.; Kunchanawatta, S.; Distaporn, T.; Ruktasing, S.; Suksamrarn, A. Ceanothane-and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. Chem. Pharm. Bull. 2006, 54, 535–537. [Google Scholar] [CrossRef]
- Bala, K.; Melkani, I.; Singh, A.P.; Singh, A.P.; Kaur, J. Holarrhena antidysenterica in Inflammatory Bowel Disease: A potential. J. Drug Deliv. Ther. 2022, 12, 221–226. [Google Scholar] [CrossRef]
- Dua, V.K.; Verma, G.; Singh, B.; Rajan, A.; Bagai, U.; Agarwal, D.D.; Gupta, N.; Kumar, S.; Rastogi, A. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar. J. 2013, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Tamez, P.A.; Hoang, V.D.; Tan, G.T.; Hung, N.V.; Xuan, L.T.; Huong, L.M.; Cuong, N.M.; Thao, D.T.; Soejarto, D.D. Antimalarial Compounds from Rhaphidophora d ecursiva. J. Nat. Prod. 2001, 64, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sevik Kilicaslan, O.; Cretton, S.; Hausmann, E.; Quirós-Guerrero, L.; Karimou, S.; Kaiser, M.; Mäser, P.; Christen, P.; Cuendet, M. Antiprotozoal activity of natural products from Nigerien plants used in folk medicine. Front. Pharmacol. 2023, 14, 1190241. [Google Scholar] [CrossRef]
- Dofuor, A.K.; Djameh, G.I.; Amoa-Bosompem, M.; Kwain, S.; Osei, E.; Tetevi, G.M.; Ayertey, F.; Bolah, P.; Okine, L.K.; Kyeremeh, K. In vitro effects and mechanisms of action of Bidens pilosa in Trypanosoma brucei. J. Tradit. Complement. Med. 2022, 12, 260–268. [Google Scholar] [CrossRef]
- Manful, E.-E.; Dofuor, A.K.; Gwira, T.M. The role of tryptophan derivatives as anti-kinetoplastid agents. Heliyon 2024, 10, e23895. [Google Scholar] [CrossRef]
- Bairy, G.; Ozzin-Kholy Zolipou, C.O.; Nzoumbou-Boko, R. In vitro trypanocidal activity of extracts and compounds isolated from Vitellaria paradoxa. BMC Complement. Med. Ther. 2023, 23, 346. [Google Scholar] [CrossRef]
- de Souza Costa, D.; Leal, C.M.; Cajas, R.A.; Gazolla, M.C.; Silva, L.M.; de Carvalho, L.S.A.; Lemes, B.L.; de Moura, R.O.; de Almeida, J.; de Moraes, J. Antiparasitic properties of 4-nerolidylcatechol from Pothomorphe umbellata (L.) Miq.(Piperaceae) in vitro and in mice models with either prepatent or patent Schistosoma mansoni infections. J. Ethnopharmacol. 2023, 313, 116607. [Google Scholar] [CrossRef]
- Rocha, V.C.; Cajas, R.A.; Andrade-de-Siqueira, A.I.; Almeida, R.B.; Godoy-Silva, J.; Gonçalves, M.M.; Lago, J.H.G.; de Moraes, J. Evaluating the Antischistosomal Activity of Dehydrodieugenol B and Its Methyl Ether Isolated from Nectandra leucantha─ A Preclinical Study against Schistosoma mansoni Infection. ACS Omega 2023, 8, 40890–40897. [Google Scholar] [CrossRef]
- Pal, R.; Teli, G.; Akhtar, M.J.; Matada, G.S.P. The role of natural anti-parasitic guided development of synthetic drugs for leishmaniasis. Eur. J. Med. Chem. 2023, 258, 115609. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; de Carvalho, É.A.; Candido, A.C.B.; de Mendonça, R.P.; Fernanda da Silva, M.; Parreira, R.L.; Dias, F.G.; Ambrosio, S.R.; Arantes, A.T.; da Silva Filho, A.A. Licochalcone a exhibits leishmanicidal activity in vitro and in experimental model of Leishmania (Leishmania) infantum. Front. Vet. Sci. 2020, 7, 527. [Google Scholar] [CrossRef]
- Barroso, P.; Marco, J.; Korenaga, M.; Hashiguchi, Y. Antileishmanial activity of green tea (Camelia sinensis) catechins against Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis. In Studies on New and Old World Leishmaniasis and Their Transmission, with Particular Reference to Ecuador, Peru, Argentina and Pakistan; Research Report Series No. 8; Kyowa Printing & Co. Ltd.: Tokyo, Japan, 2007; pp. 104–110. [Google Scholar]
- Sosa, A.M.; Moya Alvarez, A.; Bracamonte, E.; Korenaga, M.; Marco, J.D.; Barroso, P.A. Efficacy of topical treatment with (−)-epigallocatechin gallate, a green tea catechin, in mice with cutaneous leishmaniasis. Molecules 2020, 25, 1741. [Google Scholar] [CrossRef] [PubMed]
- Inacio, J.D.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. In vitro and in vivo effects of (−)-epigallocatechin 3-O-gallate on Leishmania amazonensis. J. Nat. Prod. 2013, 76, 1993–1996. [Google Scholar] [CrossRef]
- Takahashi, H.T.; Novello, C.R.; Ueda-Nakamura, T.; Filho, B.P.D.; Palazzo de Mello, J.C.; Nakamura, C.V. Thiophene derivatives with antileishmanial activity isolated from aerial parts of Porophyllum ruderale (Jacq.) Cass. Molecules 2011, 16, 3469–3478. [Google Scholar] [CrossRef]
- Takahashi, H.T.; Britta, E.A.; Longhini, R.; Ueda-Nakamura, T.; de Mello, J.C.P.; Nakamura, C.V. Antileishmanial activity of 5-methyl-2, 2′: 5′, 2 ″-terthiophene isolated from Porophyllum ruderale is related to mitochondrial dysfunction in Leishmania amazonensis. Planta Medica 2013, 79, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Amang à Ngnoung, G.A.; Nganso Ditchou, Y.O.; Leutcha, P.B.; Dize, D.; Tatsimo, S.J.N.; Tchokouaha, L.R.Y.; Kowa, T.K.; Tembeni, B.; Mamoudou, H.; Poka, M. Antiplasmodial and antileishmanial activities of a new limonoid and other constituents from the stem bark of Khaya senegalensis. Molecules 2023, 28, 7227. [Google Scholar] [CrossRef]
- Muhammad, I.; Dunbar, D.C.; Khan, S.I.; Tekwani, B.L.; Bedir, E.; Takamatsu, S.; Ferreira, D.; Walker, L.A. Antiparasitic alkaloids from Psychotria klugii. J. Nat. Prod. 2003, 66, 962–967. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Kapingu, M.C.; Moshi, M.J.; Machumi, F.; Apers, S.; Cos, P.; Ferreira, D.; Marais, J.P.; Vanden Berghe, D.; Maes, L. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J. Nat. Prod. 2006, 69, 369–372. [Google Scholar] [CrossRef]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal Activity of the Constituents of Conyza filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef]
- Lima, H.G.; Gomes, D.C.; Santos, N.S.; Dias, Ê.R.; Botura, M.B.; Batatinha, M.J.M.; Branco, A. Prosopis juliflora Pods alkaloid-rich fraction: In vitro anthelmintic activity on goat gastrointestinal parasites and Its cytotoxicity on vero cells. Pharmacogn. Mag. 2017, 13, S684. [Google Scholar] [CrossRef]
- Brito, J.R.; Wilairatana, P.; Roquini, D.B.; Parra, B.C.; Gonçalves, M.M.; Souza, D.C.S.; Ferreira, E.A.; Salvadori, M.C.; Teixeira, F.S.; Lago, J.H.G. Neolignans isolated from Saururus cernuus L.(Saururaceae) exhibit efficacy against Schistosoma mansoni. Sci. Rep. 2022, 12, 19320. [Google Scholar] [CrossRef]
- Ayers, S.; Zink, D.L.; Mohn, K.; Powell, J.S.; Brown, C.M.; Murphy, T.; Brand, R.; Pretorius, S.; Stevenson, D.; Thompson, D. Scutiaquinones A and B, Perylenequinones from the Roots of Scutia myrtina with Anthelmintic Activity. J. Nat. Prod. 2007, 70, 425–427. [Google Scholar] [CrossRef]
- Otarigho, B.; Falade, M.O. Natural perylenequinone compounds as potent inhibitors of Schistosoma mansoni glutathione S-transferase. Life 2023, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, S.; Igoli, N.P.; Gray, A.I.; Oaikhena, E.E.; Alfayez, I.A.; de Koning, H.P.; Igoli, J.O. Antitrypanosomal and antileishmanial activity of compounds from some Nigerian plants. Exp. Parasitol. 2024, 266, 108844. [Google Scholar] [CrossRef] [PubMed]
- Toolabi, R.; Abai, M.R.; Sedaghat, M.M.; Vatandoost, H.; Shayeghi, M.; Tavakoli, S.; Aghdam, M.S. Larviciding activity of Acroptilon repens extract against Anopheles stephensi, Culex pipiens and Culex quinquefaciatus under laboratory conditions. Pharmacogn. J. 2018, 10, 453–456. [Google Scholar] [CrossRef]
- Firooziyan, S.; Osanloo, M.; Moosa-Kazemi, S.H.; Basseri, H.R.; Hajipirloo, H.M.; Sadaghianifar, A.; Amani, A.; Sedaghat, M.M. Preparation of a nanoemulsion of essential oil of Acroptilon repens plant and evaluation of its Larvicidal Activity Agianst Malaria Vector, Anopheles stephensi. J. Arthropod-Borne Dis. 2021, 15, 333. [Google Scholar] [CrossRef]
- Oaikhena, E.E.; Yahaya, U.A.; Abdulsalami, S.M.; Egbe, N.L.; Adeyemi, M.M.; Ungogo, M.A.; Ebiloma, G.U.; Zoiku, F.K.; Fordjour, P.A.; Elati, H.A. The activities of suaveolol and other compounds from Hyptis suaveolens and Momordica charantia against the aetiological agents of African trypanosomiasis, leishmaniasis and malaria. Exp. Parasitol. 2024, 263, 108807. [Google Scholar] [CrossRef]
- Muhammad, I.; Li, X.-C.; Jacob, M.R.; Tekwani, B.L.; Dunbar, D.C.; Ferreira, D. Antimicrobial and Antiparasitic (+)-trans-Hexahydrodibenzopyrans and Analogues from Machaerium m ultiflorum. J. Nat. Prod. 2003, 66, 804–809. [Google Scholar] [CrossRef]
- Hu, M.; Xu, M.; Chen, Y.; Ye, Z.; Zhu, S.; Cai, J.; Zhang, M.; Zhang, C.; Huang, R.; Ye, Q. Therapeutic potential of toosendanin: Novel applications of an old ascaris repellent as a drug candidate. Biomed. Pharmacother. 2023, 167, 115541. [Google Scholar] [CrossRef]
- Kwofie, K.D.; Tung, N.H.; Suzuki-Ohashi, M.; Amoa-Bosompem, M.; Adegle, R.; Sakyiamah, M.M.; Ayertey, F.; Owusu, K.B.-A.; Tuffour, I.; Atchoglo, P. Antitrypanosomal activities and mechanisms of action of novel tetracyclic iridoids from Morinda lucida Benth. Antimicrob. Agents Chemother. 2016, 60, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.M.; Murillo, J.; Garro, A.G.; Arbeláez, N.; Pineda, T.; Robledo, S.M.; Ravetti, S. Piperine, quercetin, and curcumin identified as promising natural products for topical treatment of cutaneous leishmaniasis. Parasitol. Res. 2024, 123, 185. [Google Scholar] [CrossRef]
- Dubois, O.; Allanic, C.; Charvet, C.; Guégnard, F.; Février, H.; Thery-Koné, I.; Cortet, J.; Koch, C.; Bouvier, F.; Fassier, T. Lupin (Lupinus spp.) seeds exert anthelmintic activity associated with their alkaloid content. Sci. Rep. 2019, 9, 9070. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, N.-Z.; Yao, Y.; Wang, T.; Hua, Q.; Zheng, X.; Cong, W.; Tan, F. Investigation of antiparasitic activity of two marine natural products, estradiol benzoate, and octyl gallate, on Toxoplasma gondii in vitro. Front. Pharmacol. 2022, 13, 841941. [Google Scholar] [CrossRef]
- Crump, A. Ivermectin: Enigmatic multifaceted ‘wonder’drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Papireddy, K.; Smilkstein, M.; Kelly, J.X.; Shweta; Salem, S.M.; Alhamadsheh, M.; Haynes, S.W.; Challis, G.L.; Reynolds, K.A. Antimalarial activity of natural and synthetic prodiginines. J. Med. Chem. 2011, 54, 5296–5306. [Google Scholar] [CrossRef]
- Umeda, K.; Iwasaki, A.; Taguchi, R.; Kurisawa, N.; Jeelani, G.; Nozaki, T.; Suenaga, K. Isolation and structure determination of akunolides, macrolide glycosides from a marine Okeania sp. cyanobacterium. J. Nat. Prod. 2023, 86, 2529–2538. [Google Scholar] [CrossRef]
- Umeda, K.; Kurisawa, N.; Jeelani, G.; Nozaki, T.; Suenaga, K.; Iwasaki, A. Isolation and structure determination of a new analog of polycavernosides from marine Okeania sp. cyanobacterium. Beilstein J. Org. Chem. 2024, 20, 645–652. [Google Scholar] [CrossRef]
- Osei, E.; Kwain, S.; Mawuli, G.T.; Anang, A.K.; Owusu, K.B.-A.; Camas, M.; Camas, A.S.; Ohashi, M.; Alexandru-Crivac, C.-N.; Deng, H.; et al. Paenidigyamycin A, Potent Antiparasitic Imidazole Alkaloid from the Ghanaian Paenibacillus sp. DE2SH. Mar. Drugs 2019, 17, 9. [Google Scholar] [CrossRef]
- Bernatchez, J.A.; Kil, Y.-S.; Barbosa da Silva, E.; Thomas, D.; McCall, L.-I.; Wendt, K.L.; Souza, J.M.; Ackermann, J.; McKerrow, J.H.; Cichewicz, R.H. Identification of Leucinostatins from Ophiocordyceps sp. as Antiparasitic Agents against Trypanosoma cruzi. ACS Omega 2022, 7, 7675–7682. [Google Scholar] [CrossRef]
- Brand, M.; Wang, L.; Agnello, S.; Gazzola, S.; Gall, F.M.; Raguž, L.; Kaiser, M.; Schmidt, R.S.; Ritschl, A.; Jelk, J. Antiprotozoal structure–activity relationships of synthetic leucinostatin derivatives and elucidation of their mode of action. Angew. Chem. Int. Ed. 2021, 60, 15613–15621. [Google Scholar] [CrossRef] [PubMed]
- Oluwabusola, E.T.; Tabudravu, J.N.; Al Maqbali, K.S.; Annang, F.; Pérez-Moreno, G.; Reyes, F.; Jaspars, M. Antiparasitic activity of bromotyrosine alkaloids and new analogues isolated from the Fijian marine sponge Aplysinella rhax. Chem. Biodivers. 2020, 17, e2000335. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.L.; Romanelli, M.M.; Borborema, S.E.; Johns, D.M.; Migotto, A.E.; Lago, J.H.G.; Tempone, A.G. Antitrypanosomal activity of isololiolide isolated from the marine hydroid Macrorhynchia philippina (Cnidaria, Hydrozoa). Bioorganic Chem. 2019, 89, 103002. [Google Scholar] [CrossRef]
- Cantillo-Ciau, Z.; Moo-Puc, R.; Quijano, L.; Freile-Pelegrín, Y. The tropical brown alga Lobophora variegata: A source of antiprotozoal compounds. Mar. Drugs 2010, 8, 1292–1304. [Google Scholar] [CrossRef]
- Kossuga, M.H.; Nascimento, A.M.; Reimão, J.Q.; Tempone, A.G.; Taniwaki, N.N.; Veloso, K.; Ferreira, A.G.; Cavalcanti, B.C.; Pessoa, C.; Moraes, M.O. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J. Nat. Prod. 2008, 71, 334–339. [Google Scholar] [CrossRef]
- Dong, S.-H.; Duan, Z.-K.; Bai, M.; Huang, X.-X.; Song, S.-J. Advanced technologies targeting isolation and characterization of natural products. TrAC Trends Anal. Chem. 2024, 175, 117711. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Bayram, E.; Lukić Bilela, L.; Cueto, M.; Díaz-Marrero, A.R.; Haznedaroglu, B.Z.; Jimenez, C.; Mandalakis, M.; Pereira, F.; Reyes, F. Advanced methods for natural products discovery: Bioactivity screening, dereplication, metabolomics profiling, genomic sequencing, databases and informatic tools, and structure elucidation. Mar. Drugs 2023, 21, 308. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Litaudon, M.; Touboul, D.; Queiroz, E.F. Innovative omics-based approaches for prioritisation and targeted isolation of natural products–new strategies for drug discovery. Nat. Prod. Rep. 2019, 36, 855–868. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Guillarme, D.; Wolfender, J.-L. Advanced high-resolution chromatographic strategies for efficient isolation of natural products from complex biological matrices: From metabolite profiling to pure chemical entities. Phytochem. Rev. 2024, 23, 1415–1442. [Google Scholar] [CrossRef]
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Matter, H. Integrating virtual screening in lead discovery. Curr. Opin. Chem. Biol. 2004, 8, 349–358. [Google Scholar] [CrossRef]
- Gasteiger, J. Chemoinformatics: Achievements and challenges, a personal view. Molecules 2016, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Chan, J.D. High-content approaches to anthelmintic drug screening. Trends Parasitol. 2021, 37, 780–789. [Google Scholar] [CrossRef]
- Marhöfer, R.J.; Noack, S.; Selzer, P.M. Antiparasitics discovery: From genotype to phenotype to compounds. Trends Parasitol. 2025, 41, 431–440. [Google Scholar] [CrossRef]
- Moshawih, S.; Goh, H.P.; Kifli, N.; Idris, A.C.; Yassin, H.; Kotra, V.; Goh, K.W.; Liew, K.B.; Ming, L.C. Synergy between machine learning and natural products cheminformatics: Application to the lead discovery of anthraquinone derivatives. Chem. Biol. Drug Des. 2022, 100, 185–217. [Google Scholar] [CrossRef]
- Nantasenamat, C.; Prachayasittikul, V. Maximizing computational tools for successful drug discovery. Expert Opin. Drug Discov. 2015, 10, 321–329. [Google Scholar] [CrossRef]
- Parihar, A.; Khan, R.; Kumar, A.; Kaushik, A.K.; Gohel, H. Computational Approaches for Novel Therapeutic and Diagnostic Designing to Mitigate SARS-CoV2 Infection: Revolutionary Strategies to Combat Pandemics; Elsevier Science: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorganic Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef]
- Masseroli, M.; Mons, B.; Bongcam-Rudloff, E.; Ceri, S.; Kel, A.; Rechenmann, F.; Lisacek, F.; Romano, P. Integrated Bio-Search: Challenges and trends for the integration, search and comprehensive processing of biological information. BMC Bioinform. 2014, 15 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Diao, Y.; Jiang, J.; Zhang, S.; Li, S.; Shan, L.; Huang, J.; Zhang, W.; Li, H. Discovery of natural products as novel and potent FXR antagonists by virtual screening. Front. Chem. 2018, 6, 140. [Google Scholar] [CrossRef]
- Alam, S.; Khan, F. Virtual screening, Docking, ADMET and System Pharmacology studies on Garcinia caged Xanthone derivatives for Anticancer activity. Sci. Rep. 2018, 8, 5524. [Google Scholar] [CrossRef]
- Chahal, V.; Kakkar, R. A combination strategy of structure-based virtual screening, MM-GBSA, cross docking, molecular dynamics and metadynamics simulations used to investigate natural compounds as potent and specific inhibitors of tumor linked human carbonic anhydrase IX. J. Biomol. Struct. Dyn. 2023, 41, 5465–5480. [Google Scholar] [CrossRef]
- Perez-Pineiro, R.; Burgos, A.; Jones, D.C.; Andrew, L.C.; Rodriguez, H.; Suarez, M.; Fairlamb, A.H.; Wishart, D.S. Development of a novel virtual screening cascade protocol to identify potential trypanothione reductase inhibitors. J. Med. Chem. 2009, 52, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Hert, J.; Willett, P.; Wilton, D.J.; Acklin, P.; Azzaoui, K.; Jacoby, E.; Schuffenhauer, A. New methods for ligand-based virtual screening: Use of data fusion and machine learning to enhance the effectiveness of similarity searching. J. Chem. Inf. Model. 2006, 46, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Villanueva, J.; Santos, R.; Hernández-Campos, A.; Giulianotti, M.A.; Castillo, R.; Medina-Franco, J.L. Towards a systematic characterization of the antiprotozoal activity landscape of benzimidazole derivatives. Bioorg. Med. Chem. 2010, 18, 7380–7391. [Google Scholar] [CrossRef] [PubMed]
- Guido, R.V.; Trossini, G.H.; Castilho, M.S.; Oliva, G.; Ferreira, E.I.; Andricopulo, A.D. Structure-activity relationships for a class of selective inhibitors of the major cysteine protease from Trypanosoma cruzi. J. Enzym. Inhib. Med. Chem. 2008, 23, 964–973. [Google Scholar] [CrossRef]
- Sakyi, P.O.; Broni, E.; Amewu, R.K.; Miller, W.A., III; Wilson, M.D.; Kwofie, S.K. Targeting Leishmania donovani sterol methyltransferase for leads using pharmacophore modeling and computational molecular mechanics studies. Inform. Med. Unlocked 2023, 37, 101162. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Borba, J.V.; Moreira-Filho, J.T.; Rimoldi, A.; Andrade, C.H.; Costa, F.T.M. QSAR-based virtual screening of natural products database for identification of potent antimalarial hits. Biomolecules 2021, 11, 459. [Google Scholar] [CrossRef]
- Guido, R.V.; Oliva, G.; Andricopulo, A.D. Structure-and ligand-based drug design approaches for neglected tropical diseases. Pure Appl. Chem. 2012, 84, 1857–1866. [Google Scholar] [CrossRef]
- Ferreira, L.L.; de Moraes, J.; Andricopulo, A.D. Approaches to advance drug discovery for neglected tropical diseases. Drug Discov. Today 2022, 27, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Muhseen, Z.T.; Hameed, A.R.; Al-Bhadly, O.; Ahmad, S.; Li, G. Natural products for treatment of Plasmodium falciparum malaria: An integrated computational approach. Comput. Biol. Med. 2021, 134, 104415. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.J.B.; Xavier, L.P.; Santos, A.V.; Pereira, H.D.M.; Santos, L.d.S.; Molfetta, F.A.d. Identification of potential inhibitors of Schistosoma mansoni purine nucleoside phosphorylase from neolignan compounds using molecular modelling approaches. J. Biomol. Struct. Dyn. 2022, 40, 8248–8260. [Google Scholar] [CrossRef]
- Barazorda-Ccahuana, H.L.; Goyzueta-Mamani, L.D.; Candia-Puma, M.A.; Freitas, C.S.d.; Vieria Tavares, G.d.S.; Lage, D.P.; Ferraz Coelho, E.A.; Chávez-Fumagalli, M.A. In silico-based screening for natural product’s structural analogs as new drugs candidate against leishmaniasis. bioRxiv 2022. bioRxiv:2022.07.22.501189. [Google Scholar] [CrossRef]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor–ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Vicente-Barrueco, A.; Román, Á.C.; Ruiz-Téllez, T.; Centeno, F. In silico research of new therapeutics rotenoids derivatives against Leishmania amazonensis infection. Biology 2022, 11, 133. [Google Scholar] [CrossRef]
- Herrmann, F.C.; Sivakumar, N.; Jose, J.; Costi, M.P.; Pozzi, C.; Schmidt, T.J. In silico identification and in vitro evaluation of natural inhibitors of Leishmania major pteridine reductase I. Molecules 2017, 22, 2166. [Google Scholar] [CrossRef]
- Kuhn, B.; Gerber, P.; Schulz-Gasch, T.; Stahl, M. Validation and use of the MM-PBSA approach for drug discovery. J. Med. Chem. 2005, 48, 4040–4048. [Google Scholar] [CrossRef]
- Asmare, M.M.; Yun, S.-I. E-pharmacophore and deep learning based high throughput virtual screening for identification of CDPK1 inhibitors of Cryptosporidium parvum. Comput. Biol. Chem. 2024, 112, 108172. [Google Scholar] [CrossRef]
- Rahman, F.; Tabrez, S.; Ali, R.; Akand, S.K.; Zahid, M.; Alaidarous, M.A.; Alsaweed, M.; Alshehri, B.M.; Banawas, S.; Bin Dukhyil, A.A. Virtual screening of natural compounds for potential inhibitors of Sterol C-24 methyltransferase of Leishmania donovani to overcome leishmaniasis. J. Cell. Biochem. 2021, 122, 1216–1228. [Google Scholar] [CrossRef]
- de Sá, É.R.; Souza, J.L.; Costa, R.K.; Barros, R.O.; de Lima, C.E.; Lima, F.d.C.; Ramos, R.M. Computational investigation of the alkaloids of Pilocarpus microphyllus species as phytopharmaceuticals for the inhibition of sterol 14α-demethylase protease of Trypanosoma cruzi. J. Biomol. Struct. Dyn. 2023, 41, 2555–2573. [Google Scholar] [CrossRef] [PubMed]
- Omolabi, K.F.; Agoni, C.; Olotu, F.A.; Soliman, M.E. ‘Finding the needle in the haystack’-will natural products fit for purpose in the treatment of cryptosporidiosis?–A theoretical perspective. Mol. Simul. 2021, 47, 636–649. [Google Scholar] [CrossRef]
- Adams, L.; Issahaku, A.R.; Agoni, C.; Afiadenyo, M.; Kusi, K.A.; Moane, S.; Obiri-Yeboah, D.; McKeon-Bennett, M. In silico identification of potential PvFKBP35 inhibitors from Entadrophragma angolense Limonoids extracts as antimalarial agents. Inform. Med. Unlocked 2023, 41, 101319. [Google Scholar] [CrossRef]
- Broni, E.; Kwofie, S.K.; Asiedu, S.O.; Miller, W.A., III; Wilson, M.D. A molecular modeling approach to identify potential antileishmanial compounds against the cell division cycle (Cdc)-2-related kinase 12 (crk12) receptor of Leishmania donovani. Biomolecules 2021, 11, 458. [Google Scholar] [CrossRef]
- Lobato-Tapia, C.A.; Moreno-Hernández, Y.; Olivo-Vidal, Z.E. In silico studies of four compounds of cecropia obtusifolia against malaria parasite. Molecules 2023, 28, 6912. [Google Scholar] [CrossRef] [PubMed]
- Crentsil, J.A.; Yamthe, L.R.T.; Anibea, B.Z.; Broni, E.; Kwofie, S.K.; Tetteh, J.K.A.; Osei-Safo, D. Leishmanicidal potential of hardwickiic acid isolated from Croton sylvaticus. Front. Pharmacol. 2020, 11, 753. [Google Scholar] [CrossRef]
- Adomako, A.K.; Gasu, E.N.; Mensah, J.O.; Borquaye, L.S. Antileishmanial natural products as potential inhibitors of the Leishmania pteridine reductase: Insights from molecular docking and molecular dynamics simulations. In Silico Pharmacology 2024, 12, 70. [Google Scholar] [CrossRef]
- Gallinger, T.L.; Aboagye, S.Y.; Obermann, W.; Weiss, M.; Grünweller, A.; Unverzagt, C.; Williams, D.L.; Schlitzer, M.; Haeberlein, S. First in silico screening of insect molecules for identification of novel anti-parasitic compounds. Pharmaceuticals 2022, 15, 119. [Google Scholar] [CrossRef]
- Al-Tannak, N.F.; Anyam, J.V.; Santali, E.Y.; Gray, A.I.; Ibeji, C.U.; Igoli, J.O. Anti-parasitic activity and computational studies on a novel labdane diterpene from the roots of Vachellia nilotica. Open Chem. 2024, 22, 20240005. [Google Scholar] [CrossRef]
- Chama, M.A.; Egyir, B.; Owusu, K.B.-A.; Armah, J.A.; Afiadenyo, M.; Kwofie, S.K. Evaluation of the Antitrypanosomal Activity of the Crude Extracts of Uvaria Ovata: In vitro and In silico Approach. Biomed. Biotechnol. Res. J. (BBRJ) 2024, 8, 172–180. [Google Scholar] [CrossRef]
- CDC. Choosing a Drug to Prevent Malaria. Available online: https://www.cdc.gov/malaria/hcp/drug-malaria/ (accessed on 7 July 2025).
- Pessanha de Carvalho, L.; Kreidenweiss, A.; Held, J. Drug Repurposing: A Review of Old and New Antibiotics for the Treatment of Malaria: Identifying Antibiotics with a Fast Onset of Antiplasmodial Action. Molecules 2021, 26, 2304. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.M.; Nyein, P.P.; Kyi, M.M.; Hanson, J. Bacterial coinfection in adults with severe malaria. Clin. Infect. Dis. 2021, 72, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Rai, A.K. Retinoic acid shows direct parasiticidal activity by targeting ergosterol pathway in Leishmania donovani: A potential therapeutic advancement. J. Biomol. Struct. Dyn. 2023, 41, 14473–14483. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Arya, H.; Saha, S.; Kumar, D.; Bhatt, T.K. Drug repurposing based novel anti-leishmanial drug screening using in-silico and in-vitro approaches. J. Biomol. Struct. Dyn. 2022, 40, 10812–10820. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.; Ojha, S. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Nketia, P.B.; Gasu, E.N.; Mensah, J.O.; Borquaye, L.S. In silico identification of α-bisabolol and letestuianin C as potential inhibitors of Trypanosoma brucei trypanothione reductase. J. Biomol. Struct. Dyn. 2024, 42, 8660–8672. [Google Scholar] [CrossRef]
- Berhanu, T.; Tewelde, E.; Yeshak, M.Y.; Bisrat, D.; Asres, K. Anthelmintic Potential and In Silico Studies of Ricinoleic Acid from the Seed Oil of Ricinus communis L. Int. J. Mol. Sci. 2025, 26, 1636. [Google Scholar] [CrossRef]
- Gahukar, R.; Mital, S. Castor oil. In Green Pesticides Handbook; CRC Press: Boca Raton, FL, USA, 2017; pp. 333–364. [Google Scholar]
- Haraguchi, A.; Harris, B.; Feasby, N.; Thekkiniath, J. Prevalence of Anti-parasitic Drug Resistance in Various Areas of the World. Indian J. Vet. Public Health 2024, 10, 1. [Google Scholar] [CrossRef]
- Ansbro, M.R.; Itkin, Z.; Chen, L.; Zahoranszky-Kohalmi, G.; Amaratunga, C.; Miotto, O.; Peryea, T.; Hobbs, C.V.; Suon, S.; Sá, J.M. Modulation of triple artemisinin-based combination therapy pharmacodynamics by Plasmodium falciparum genotype. ACS Pharmacol. Transl. Sci. 2020, 3, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- CDC. Malaria’s Impact Worldwide. Available online: https://www.cdc.gov/malaria/php/impact/index.html (accessed on 7 July 2024).
- Greenwood, B.M.; Fidock, D.A.; Kyle, D.E.; Kappe, S.H.; Alonso, P.L.; Collins, F.H.; Duffy, P.E. Malaria: Progress, perils, and prospects for eradication. J. Clin. Investig. 2008, 118, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 7 July 2025).
- Wormser, G.P.; Prasad, A.; Neuhaus, E.; Joshi, S.; Nowakowski, J.; Nelson, J.; Mittleman, A.; Aguero-Rosenfeld, M.; Topal, J.; Krause, P.J. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin. Infect. Dis. 2010, 50, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Saha, B.; Pai, K.; Sundar, S.; Bhattacharyya, M.; Bodhale, N.P. The drug resistance mechanisms in Leishmania donovani are independent of immunosuppression. Cytokine 2021, 145, 155300. [Google Scholar] [CrossRef]
- Steinmetz, K.L.; Spack, E.G. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 2009, 9, S2. [Google Scholar] [CrossRef]
- Kumar, R.D. The Phases of Clinical Trials: From Preclinical Studies to Post-market Surveillance. Glob. J. Med. Clin. Case Rep. 2025, 11, 055–065. [Google Scholar]
- Soufizadeh, P.; Mansouri, V.; Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: Trends and insights. Lab. Anim. Res. 2024, 40, 17. [Google Scholar] [CrossRef]
- Wang, H.; Ciccocioppo, R.; Terai, S.; Shoeibi, S.; Carnevale, G.; De Marchi, G.; Tsuchiya, A.; Ishii, S.; Tonouchi, T.; Furuyama, K.; et al. Targeted animal models for preclinical assessment of cellular and gene therapies in pancreatic and liver diseases: Regulatory and practical insights. Cytotherapy 2025, 27, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Koszalka, P.; Subbarao, K.; Baz, M. Preclinical and clinical developments for combination treatment of influenza. PLoS Pathog. 2022, 18, e1010481. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, E.; Mejías, F.; Carrillo, E.; Sánchez, C.; Moreno, J. Potentiation of the leishmanicidal activity of nelfinavir in combination with miltefosine or amphotericin B. Int. J. Antimicrob. Agents 2018, 52, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, development, inventions and patent review of fexinidazole: The first all-oral therapy for human African trypanosomiasis. Pharmaceuticals 2022, 15, 128. [Google Scholar] [CrossRef]
- Veas, R.; Rojas-Pirela, M.; Castillo, C.; Olea-Azar, C.; Moncada, M.; Ulloa, P.; Rojas, V.; Kemmerling, U. Microalgae extracts: Potential anti-Trypanosoma cruzi agents? Biomed. Pharmacother. 2020, 127, 110178. [Google Scholar] [CrossRef]
- Barbosa Juliana Magalhães, C.; Pedra Rezende, Y.; de Melo Tatiana, G.; de Oliveira, G.; Cascabulho Cynthia, M.; Pereira Evelyn Nunes Goulart da, S.; Daliry, A.; Salem Kelly, S. Experimental Combination Therapy with Amiodarone and Low-Dose Benznidazole in a Mouse Model of Trypanosoma cruzi Acute Infection. Microbiol. Spectr. 2022, 10, e01852-21. [Google Scholar] [CrossRef]
- Gurgel do Vale, T.; Couto Furtado, E.; Santos, J.G.; Viana, G.S.B. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) N.E. Brown. Phytomedicine 2002, 9, 709–714. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef]
- Druilhe, P.; Brandicourt, O.; Chongsuphajaisiddhi, T.; Berthe, J. Activity of a combination of three cinchona bark alkaloids against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 1988, 32, 250–254. [Google Scholar] [CrossRef]
- Sakuma, M.; Setoguchi, A.; Endo, Y. Possible emergence of drug-resistant variants of Babesia gibsoni in clinical cases treated with atovaquone and azithromycin. J. Vet. Intern. Med. 2009, 23, 493–498. [Google Scholar] [CrossRef]
- Ji, S.; Liu, M.; Galon, E.M.; Rizk, M.A.; Li, J.; Li, Y.; Zafar, I.; Igarashi, I.; Xuan, X. In vitro screening of novel anti-Babesia gibsoni drugs from natural products. Parasitol. Int. 2021, 85, 102437. [Google Scholar] [CrossRef]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors 2021, 14, 263. [Google Scholar] [CrossRef]
- Antczak, M.; Dzitko, K.; Długońska, H. Human toxoplasmosis–Searching for novel chemotherapeutics. Biomed. Pharmacother. 2016, 82, 677–684. [Google Scholar] [CrossRef]
- Mady, R.F.; El-Hadidy, W.; Elachy, S. Effect of Nigella sativa oil on experimental toxoplasmosis. Parasitol. Res. 2016, 115, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Veronica, J.; Gundampati, R.K.; Sundar, S.; Maurya, R. Exploring the inhibitory activity of Withaferin-A against Pteridine reductase-1 of L. donovani. J. Enzym. Inhib. Med. Chem. 2016, 31, 1029–1037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borges, B.S.; Bueno, G.d.P.; Tomiotto-Pellissier, F.; Figueiredo, F.B.; Soares Medeiros, L.C. In vitro anti-Leishmania activity of triclabendazole and its synergic effect with amphotericin B. Front. Cell. Infect. Microbiol. 2023, 12, 1044665. [Google Scholar] [CrossRef]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Bueding, E.; Fisher, J. Factors affecting the inhibition of phosphofructokinase activity of Schistosoma mansoni by trivalent organic antimonials. Biochem. Pharmacol. 1966, 15, 1197–1211. [Google Scholar] [CrossRef]
- Czigle, S.; Nagy, M.; Mladěnka, P.; Tóth, J.; OEMONOM. Pharmacokinetic and pharmacodynamic herb-drug interactions—Part I. Herbal medicines of the central nervous system. PeerJ 2023, 11, e16149. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef]
- Oyanna, V.O.; Clarke, J.D. Mechanisms of intestinal pharmacokinetic natural product-drug interactions. Drug Metab. Rev. 2024, 56, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.T.; Hedges, L.; Sampson, K.E.; Lai, Y.; Mahabir, S.; Balogh, L.; Locuson, C.W. Pharmacokinetic Interaction of the Antiparasitic Agents Ivermectin and Spinosad in Dogs. Drug Metab. Dispos. 2011, 39, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Jiang, L.; Meng, Z.; Xu, J. Computational Drug Repurposing Based on a Recommendation System and Drug–Drug Functional Pathway Similarity. Molecules 2022, 27, 1404. [Google Scholar] [CrossRef]
- Handayani, H.; Savitri, A.D.; Wijaya, A.F.S.; Satriawan, H.; Jakhmola, V.; Rebezov, M.; Zainul, R. Anti-Inflammatory Effects of the Herbal Combination Sambiloto-Ginger-Turmeric (SIJAKUN). J. Med. Chem. Sci. 2024, 7, 637–648. [Google Scholar]
- King, C.H. Parasites and poverty: The case of schistosomiasis. Acta Trop. 2010, 113, 95–104. [Google Scholar] [CrossRef]
- Hao, L.; Haicha Pratama, I.; Eliza, Y.; Lubis, P. Parasitic diseases: Emerging challenges in urban environments and implications for public health interventions. J. Parasit. Dis. Diagn. Ther. 2023, 8, 151. [Google Scholar]
- Sanjai, C.; Gaonkar, S.L.; Hakkimane, S.S. Harnessing Nature’s Toolbox: Naturally Derived Bioactive Compounds in Nanotechnology Enhanced Formulations. ACS Omega 2024, 9, 43302–43318. [Google Scholar] [CrossRef]
- Nemati, S.; Mottaghi, M.; Karami, P.; Mirjalali, H. Development of solid lipid nanoparticles-loaded drugs in parasitic diseases. Discov. Nano 2024, 19, 7. [Google Scholar] [CrossRef]
- Singh, P.; Niveria, K.; Yadav, M.; Verma, A.K. Nanotechnology: Its Usages in Drug Delivery for the Treatment of Human Parasitic Diseases. In Natural Product Based Drug Discovery Against Human Parasites: Opportunities and Challenges; Singh, A., Rathi, B., Verma, A.K., Singh, I.K., Eds.; Springer Nature: Singapore, 2023; pp. 157–171. [Google Scholar]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in Nanoparticles in Targeted Drug Delivery-A Review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Saleem, K.; Khursheed, Z.; Hano, C.; Anjum, I.; Anjum, S. Applications of nanomaterials in leishmaniasis: A focus on recent advances and challenges. Nanomaterials 2019, 9, 1749. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, H.; Pang, S.; Ya, M.; Qiu, F.; Qin, P.; Zeng, C.; Lu, Y. Liposomes as multifunctional nano-carriers for medicinal natural products. Front. Chem. 2022, 10, 963004. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Aguiar, M.M.; Ferreira, L.A.; Ramos, G.S.; Santos, T.T.; Borges, G.S.; Vallejos, V.M.; De Morais, H.L. Liposomal amphotericin B for treatment of leishmaniasis: From the identification of critical physicochemical attributes to the design of effective topical and oral formulations. Pharmaceutics 2022, 15, 99. [Google Scholar] [CrossRef]
- Memvanga, P.B.; Nkanga, C.I. Liposomes for malaria management: The evolution from 1980 to 2020. Malar. J. 2021, 20, 327. [Google Scholar] [CrossRef]
- Gomes, D.C.; Medeiros, T.S.; Alves Pereira, E.L.; da Silva, J.F.O.; de Freitas Oliveira, J.W.; Fernandes-Pedrosa, M.d.F.; de Sousa da Silva, M.; da Silva-Júnior, A.A. From Benznidazole to new drugs: Nanotechnology contribution in Chagas disease. Int. J. Mol. Sci 2023, 24, 13778. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Iles, B.; Rodrigues, V.C.; Lima, A.L.; Machado, V.P.; Reatgui, W.S.; Bento da Silva, P.; Radichi, M.A.; Silva, T.C.; Teixeira, F.S. Praziquantel Nanoparticle Formulation for the Treatment of Schistosomiasis. ACS Appl. Nano Mater. 2025, 8, 3985–3997. [Google Scholar] [CrossRef]
- Tripathy, S.; Mahapatra, S.K.; Chattopadhyay, S.; Das, S.; Dash, S.K.; Majumder, S.; Pramanik, P.; Roy, S. A novel chitosan based antimalarial drug delivery against Plasmodium berghei infection. Acta Trop. 2013, 128, 494–503. [Google Scholar] [CrossRef]
- Hagras, N.A.-e.; Mogahed, N.M.F.H.; Sheta, E.; Darwish, A.A.-e.; El-Hawary, M.A.; Hamed, M.T.; Elwakil, B.H. The powerful synergistic effect of spiramycin/propolis loaded chitosan/alginate nanoparticles on acute murine toxoplasmosis. PLoS Neglected Trop. Dis. 2022, 16, e0010268. [Google Scholar] [CrossRef]
- Jain, R.; Sukla, S.; Panday, A. Kinetic modeling and release behavior of PLGA-loaded nanoparticle of anti-malarial drug using dialysis membrane. Nanomed. Nanotechnol. J. 2019, 2, 123. [Google Scholar]
- Oyeyemi, O.; Morenkeji, O.; Afolayan, F.; Dauda, K.; Busari, Z.; Meena, J.; Panda, A. Curcumin-artesunate based polymeric nanoparticle; antiplasmodial and toxicological evaluation in murine model. Front. Pharmacol. 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [PubMed]
- Susanti, D.; Haris, M.S.; Taher, M.; Khotib, J. Natural products-based metallic nanoparticles as antimicrobial agents. Front. Pharmacol. 2022, 13, 895616. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Kumar, G.S.; Roy, H.; Maddiboyina, B.; Leporatti, S.; Bohara, R.A. From nature to nanomedicine: Bioengineered metallic nanoparticles bridge the gap for medical applications. Discov. Nano 2024, 19, 85. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Investigating the toxicological effects of nanomaterials in food packaging associated with human health and the environment. J. Hazard. Mater. Lett. 2024, 5, 100125. [Google Scholar] [CrossRef]
- Arpitha, B.; Parthasarathy, P. Effect of nano-alumina and graphene oxide on the behavior of geopolymer composites: A state of the art of review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Green synthesis of metal oxide nanoparticles, and their various applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- Sunkari, S.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Alle, M.; Guttena, V. Microwave-irradiated green synthesis of gold nanoparticles for catalytic and anti-bacterial activity. J. Anal. Sci. Technol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Abd El Wahab, W.M.; El-Badry, A.A.; Mahmoud, S.S.; El-Badry, Y.A.; El-Badry, M.A.; Hamdy, D.A. Ginger (Zingiber Officinale)-derived nanoparticles in Schistosoma mansoni infected mice: Hepatoprotective and enhancer of etiological treatment. PLoS Neglected Trop. Dis. 2021, 15, e0009423. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Ullah, S.; Shah, S.I.; Nasir, F.; Shah, A.; Iqbal, Z.; Tahir, K.; Khan, U.A.; Yuan, Q. Synthesis of phytochemicals-stabilized gold nanoparticles and their biological activities against bacteria and Leishmania. Microb. Pathog. 2017, 110, 304–312. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Islam, N.U.; Ayaz, M.; Saravanan, M.; Ali, M.; Ahmad, I.; Shahid, M.; Shinwari, Z.K. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Yadav, P.; Verma, A. In silico study of Trianthema portulacastrum embedded iron oxide nanoparticles on glycogen synthase kinase-3β: A possible contributor to its enhanced in vivo wound healing potential. Front. Pharmacol. 2021, 12, 664075. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Ameliorative effect of biofabricated ZnO nanoparticles of Trianthema portulacastrum Linn. on dermal wounds via removal of oxidative stress and inflammation. RSC Adv. 2018, 8, 21621–21635. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.H.; Muzammil, S.; Hayat, S.; Khurshid, M.; Sayyid, A.H. Fabrication of chitosan and Trianthema portulacastrum mediated copper oxide nanoparticles: Antimicrobial potential against MDR bacteria and biological efficacy for antioxidant, antidiabetic and photocatalytic activities. Int. J. Biol. Macromol. 2023, 242, 124954. [Google Scholar] [CrossRef]
- Sabir, S.; Thani, A.S.B.; Abbas, Q. Nanotechnology in cancer treatment: Revolutionizing strategies against drug resistance. Front. Bioeng. Biotechnol. 2025, 13, 1548588. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Park, D.; Swayambhu, G.; Pfeifer, B.A. Heterologous biosynthesis as a platform for producing new generation natural products. Curr. Opin. Biotechnol. 2020, 66, 123–130. [Google Scholar] [CrossRef]
- Ben-Amar, A.; Mliki, A. Timely gene detection assay and reliable screening of genetically engineered plants using an improved direct PCR-based technology. Transgenic Res. 2021, 30, 263–274. [Google Scholar] [CrossRef]
- Tong, Y.; Whitford, C.M.; Blin, K.; Jørgensen, T.S.; Weber, T.; Lee, S.Y. CRISPR–Cas9, CRISPRi and CRISPR-BEST-mediated genetic manipulation in streptomycetes. Nat. Protoc. 2020, 15, 2470–2502. [Google Scholar] [CrossRef]
- American Society of Hospital Pharmacists. American Hospital Formulary Service Drug Information; Authority of the Board of Directors of the American Society of Hospital Pharmacists: Bethesda, MD, USA, 1984. [Google Scholar]
- Krysenko, S. Current Approaches for Genetic Manipulation of Streptomyces spp.—Key Bacteria for Biotechnology and Environment. BioTech 2025, 14, 3. [Google Scholar] [CrossRef]
- Maeder, C.I.; Maier, P.; Knop, M. 4 A Guided Tour to PCR-based Genomic Manipulations of S. cerevisiae (PCR-targeting). Methods Microbiol. 2007, 36, 55–78. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Tagboto, S.; Townson, S. Antiparasitic properties of medicinal plants and other naturally occurring products. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 2001; Volume 50, pp. 199–295. [Google Scholar]
- Saeed, M.E.M.; Krishna, S.; Greten, H.J.; Kremsner, P.G.; Efferth, T. Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res. 2016, 110, 216–226. [Google Scholar] [CrossRef]
- Perez del Villar, L.; Burguillo, F.J.; Lopez-Aban, J.; Muro, A. Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS ONE 2012, 7, e45867. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.S.; Long, X.; Su, X.; Lu, F. Artemisinin and its derivatives in treating helminthic infections beyond schistosomiasis. Pharmacol. Res. 2018, 133, 77–100. [Google Scholar] [CrossRef]
- Boulanger, D.; Dieng, Y.; Cisse, B.; Remoue, F.; Capuano, F.; Dieme, J.-L.; Ndiaye, T.; Sokhna, C.; Trape, J.-F.; Greenwood, B. Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 113–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, D.; Vercruysse, J.; Kongs, A.; Verle, P.; Dompnier, J.; Faye, P. Efficacy of artesunate and praziquantel in Schistosomahaematobium infected schoolchildren. Acta Trop. 2002, 82, 61–66. [Google Scholar] [CrossRef] [PubMed]
- N’goran, E.Z.K.; Utzinger, J.; Gnaka, H.N.; Yapi, A.; N’guessan, N.A.; Kigbafori, S.D.; Lengeler, C.; Chollet, J.; Shuhua, X.; Tanner, M. Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am. J. Trop. Med. Hyg. 2003, 68, 24–32. [Google Scholar] [CrossRef]
- Yi, Z.; Lu, M.; Feng, D.; Wang, Z.; Xiang, C.; Gou, Z. Clinical observation on prevention of schistosomiasis by oral artesunate in people exposed to epidemic water for a short time. Zhongguo Xuexichongbing Fangzhi Zazhi 2000, 2, 100–101. [Google Scholar]
- Li, Y.-S.; Chen, H.-G.; He, H.-B.; Hou, X.-Y.; Ellis, M.; McManus, D.P. A double-blind field trial on the effects of artemether on Schistosoma japonicum infection in a highly endemic focus in southern China. Acta Trop. 2005, 96, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xiao, S.; Wu, W.; Zhang, S.; Xie, H.; Xu, X.; Hu, X.; Cui, Q.; Chen, M.; Zheng, J. Preventive effect of artemether on schistosome infection. Chin. Med. J. 1998, 111, 123–127. [Google Scholar] [PubMed]
- Xiao, S.; Shi, Z.; Zhuo, S.; Wang, C.; Zhang, Z.; Chu, B.; Zhen, J.; Chen, M. Field studies on the preventive effect of oral artemether against schistosomal infection. Chin. Med. J. 1996, 109, 272–275. [Google Scholar] [PubMed]
- Borrmann, S.; Szlezák, N.; Faucher, J.-F.; Matsiegui, P.-B.; Neubauer, R.; Binder, R.K.; Lell, B.; Kremsner, P.G. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: A double-blind, randomized, placebo-controlled study. J. Infect. Dis. 2001, 184, 1363–1366. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, S.; Liu, Y.; Li, S.; Wu, L.; Gao, Z.; Tao, B.; Cheng, Y. Field observation on the prophylaxis of artesuante with 15 days interval against infection of Schistosoma japonicum. Chin. J. Zoonoses 1999, 15, 41–42. [Google Scholar]
- Lu, G.; Lin, G.; Sun, M.; Jiang, J.; Cui, J.; Wu, Q. Optimization of oral artesunate to prevent schistosoma schistosoma infection. J. Pathog. Biol. 2000, 3, 57–59. [Google Scholar]
- Hou, X.; Li, Y.; Luo, X.; Li, Y.; Yu, X.; Fu, X.; Zhou, Z.; Shi, M.; Liu, Z.; Wang, Y.; et al. Clinical study on acute schistosomiasis japonica treatment with artemether and praziquantel. Chin. J. Schisto. Control 2006, 18, 99–102. [Google Scholar]
- Utzinger, J.; N’Goran, E.K.; N’Dri, A.; Lengeler, C.; Shuhua, X.; Tanner, M. Oral artemether for prevention of Schistosoma mansoni infection: Randomised controlled trial. Lancet 2000, 355, 1320–1325. [Google Scholar] [CrossRef]
- Hua, H.-Y.; Liang, Y.-S.; Zhang, Y.; Wei, J.-F.; Guo, H.-X. The sensitivity of artesunate against Schistosoma japonicum decreased after 10 years of use in China. Parasitol. Res. 2010, 107, 873–878. [Google Scholar] [CrossRef]
- Liu, R.; Dong, H.-F.; Jiang, M.-S. The sensitivity of artesunate against Schistosoma japonicum decreased after 10 years of use in China? Parasitol. Res. 2012, 110, 1563–1564. [Google Scholar] [CrossRef]
- Hien, T.T.; Truong, N.T.; Minh, N.H.; Dat, H.D.; Dung, N.T.; Hue, N.T.; Dung, T.K.; Tuan, P.Q.; Campbell, J.I.; Farrar, J.J. A randomized controlled pilot study of artesunate versus triclabendazole for human fascioliasis in central Vietnam. Am. J. Trop. Med. Hyg. 2008, 78, 388–392. [Google Scholar] [CrossRef]
- Keiser, J.; Sayed, H.; El-Ghanam, M.; Sabry, H.; Anani, S.; El-Wakeel, A.; Hatz, C.; Utzinger, J.; El-Din, S.S.; El-Maadawy, W. Efficacy and safety of artemether in the treatment of chronic fascioliasis in Egypt: Exploratory phase-2 trials. PLoS Neglected Trop. Dis. 2011, 5, e1285. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Y.; Liu, G.; Xu, M. New clinical application prospects of artemisinin and its derivatives: A scoping review. Infect. Dis. Poverty 2023, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- WHO. Trichomoniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/trichomoniasis (accessed on 24 June 2025).
- Nagaraja, P. Antibiotic resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J. Med. Microbiol. 2008, 26, 155–157. [Google Scholar]
- Wachter, B.; Syrowatka, M.; Obwaller, A.; Walochnik, J. In vitro efficacy of curcumin on Trichomonas vaginalis. Wien Klin Wochenschr 2014, 126 (Suppl. 1), S32–S36. [Google Scholar] [CrossRef]
- Mohamadi, S.; Behboodi Moghadam, Z.; Godarzi, S.; Rezaei, E. A clinical trial of curcumin effect in comparison to metronidazole on the treatment of bacterial vaginosis. Sci. Rep. 2025, 15, 7479. [Google Scholar] [CrossRef]
- Knoll, S.; Dessì, G.; Tamponi, C.; Meloni, L.; Cavallo, L.; Mehmood, N.; Jacquiet, P.; Scala, A.; Cappai, M.G.; Varcasia, A. Practical guide for microscopic identification of infectious gastrointestinal nematode larvae in sheep from Sardinia, Italy, backed by molecular analysis. Parasites Vectors 2021, 14, 505. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic resistance and its mechanism: A review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Wangchuk, P.; Pearson, M.S.; Giacomin, P.R.; Becker, L.; Sotillo, J.; Pickering, D.; Smout, M.J.; Loukas, A. Compounds derived from the Bhutanese daisy, Ajania nubigena, demonstrate dual anthelmintic activity against Schistosoma mansoni and Trichuris muris. PLoS Neglected Trop. Dis. 2016, 10, e0004908. [Google Scholar] [CrossRef]
- Hidayatik, N.; Harini, S.L.; Triwidiawati, N.; Putri, S.I.; Proboningrat, A.; Kristianingtyas, L.; Khairullah, A.R.; Suwanti, L.T.; Hestianah, E.P.; Kuncorojakti, S.; et al. Ovicidal activity and cytotoxicity of ethanolic extract of turmeric (Curcuma longa) and green tea (Camellia sinensis) to treat digestive parasite of sheep. Open Vet. J. 2024, 14, 1467–1475. [Google Scholar] [CrossRef]
- Heydari, M.; Rauf, A.; Thiruvengadam, M.; Chen, X.; Hashempur, M.H. Editorial: Clinical safety of natural products, an evidence-based approach. Front. Pharmacol. 2022, 13, 960556. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Aspinall, S.; Beynon, A.; Ash, A.; Lymbery, A. Traditional medicinal plants in the treatment of gastrointestinal parasites in humans: A systematic review and meta-analysis of clinical and experimental evidence. Phytother. Res. 2023, 37, 3675–3687. [Google Scholar] [CrossRef]
- Soumya, S.J.; Arya, K.R.; Abhinand, C.S.; Nadh, A.G.; Rani, J.R.; Oommen, O.V.; Sudhakaran, P.R. Emerging Paradigms in Natural Products-Based Drug Discovery. In Biodiversity and Business: Bio Prospecting and Benefit Sharing; Krishna Panicker, L., Nelliyat, P., Oommen, O.V., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 231–246. [Google Scholar]
- Ghosh, S.; Das, S.K.; Sinha, K.; Ghosh, B.; Sen, K.; Ghosh, N.; Sil, P.C. The Emerging Role of Natural Products in Cancer Treatment. Arch. Toxicol. 2024, 98, 2353–2391. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Manjunatha, U.H.; Mikolajczak, S.; Ashigbie, P.G.; Diagana, T.T. Drug discovery for parasitic diseases: Powered by technology, enabled by pharmacology, informed by clinical science. Trends Parasitol. 2023, 39, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ruenchit, P. Exploring bioactive molecules released during inter-and intraspecific competition: A paradigm for novel antiparasitic drug discovery and design for human use. Curr. Res. Parasitol. Vector-Borne Dis. 2025, 7, 100256. [Google Scholar] [CrossRef] [PubMed]
- Nduati, E.W.; Kamau, E.M. Multiple synergistic interactions between atovaquone and antifolates against Plasmodium falciparum in vitro: A rational basis for combination therapy. Acta Trop. 2006, 97, 357–363. [Google Scholar] [CrossRef]
- Baker, N.; de Koning, H.P.; Mäser, P.; Horn, D. Drug resistance in African trypanosomiasis: The melarsoprol and pentamidine story. Trends Parasitol. 2013, 29, 110–118. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, R.; Liu, Y.; Yu, M.; He, Z.; Xiao, J.; Li, K.; Liu, G.; Ning, Q.; Li, Y. Progress in antileishmanial drugs: Mechanisms, challenges, and prospects. PLoS Neglected Trop. Dis. 2025, 19, e0012735. [Google Scholar] [CrossRef]
- Méndez-Lucio, O.; Naveja, J.J.; Vite-Caritino, H.; Prieto-Martínez, F.D.; Medina-Franco, J.L. One drug for multiple targets: A computational perspective. J. Mex. Chem. Soc. 2016, 60, 168–181. [Google Scholar] [CrossRef]
- Kabir, A.; Muth, A. Polypharmacology: The science of multi-targeting molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Palve, V.; Liao, Y.; Rix, L.L.R.; Rix, U. Turning liabilities into opportunities: Off-target based drug repurposing in cancer. Semin. Cancer Biol. 2021, 68, 209–229. [Google Scholar] [CrossRef]
- Osafo, N.; Agyare, C.; Obiri, D.D.; Antwi, A.O. Mechanism of action of nonsteroidal anti-inflammatory drugs. In Nonsteroidal Anti-Inflammatory Drugs; IntechOpen: London, UK, 2017. [Google Scholar]
- Trapali, M.I. Therapeutic Uses of Aspirin. In Pain Management-From Acute to Chronic and Beyond; IntechOpen: London, UK, 2023. [Google Scholar]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Tan, X.; Jiao, J.; Lai, S.; Zhang, H.; Kan, Q.; He, Z.; Sun, M.; Sun, J. Iron ion-coordinated carrier-free supramolecular co-nanoassemblies of dual DNA topoisomerase-targeting inhibitors for tumor suppression. Acta Biomater. 2022, 144, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Liu, Y.; An, S.; Jiang, X. Glutathione-Induced In Situ Michael Addition between Nanoparticles for Pyroptosis and Immunotherapy. Angew. Chem. Int. Ed. 2023, 62, e202301866. [Google Scholar] [CrossRef]
- Martin, E.J.; Jansen, J.M. Biased diversity for effective virtual screening. J. Chem. Inf. Model. 2020, 60, 4116–4119. [Google Scholar] [CrossRef]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-based virtual screening: Advances and applications in drug discovery. Front. Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef]
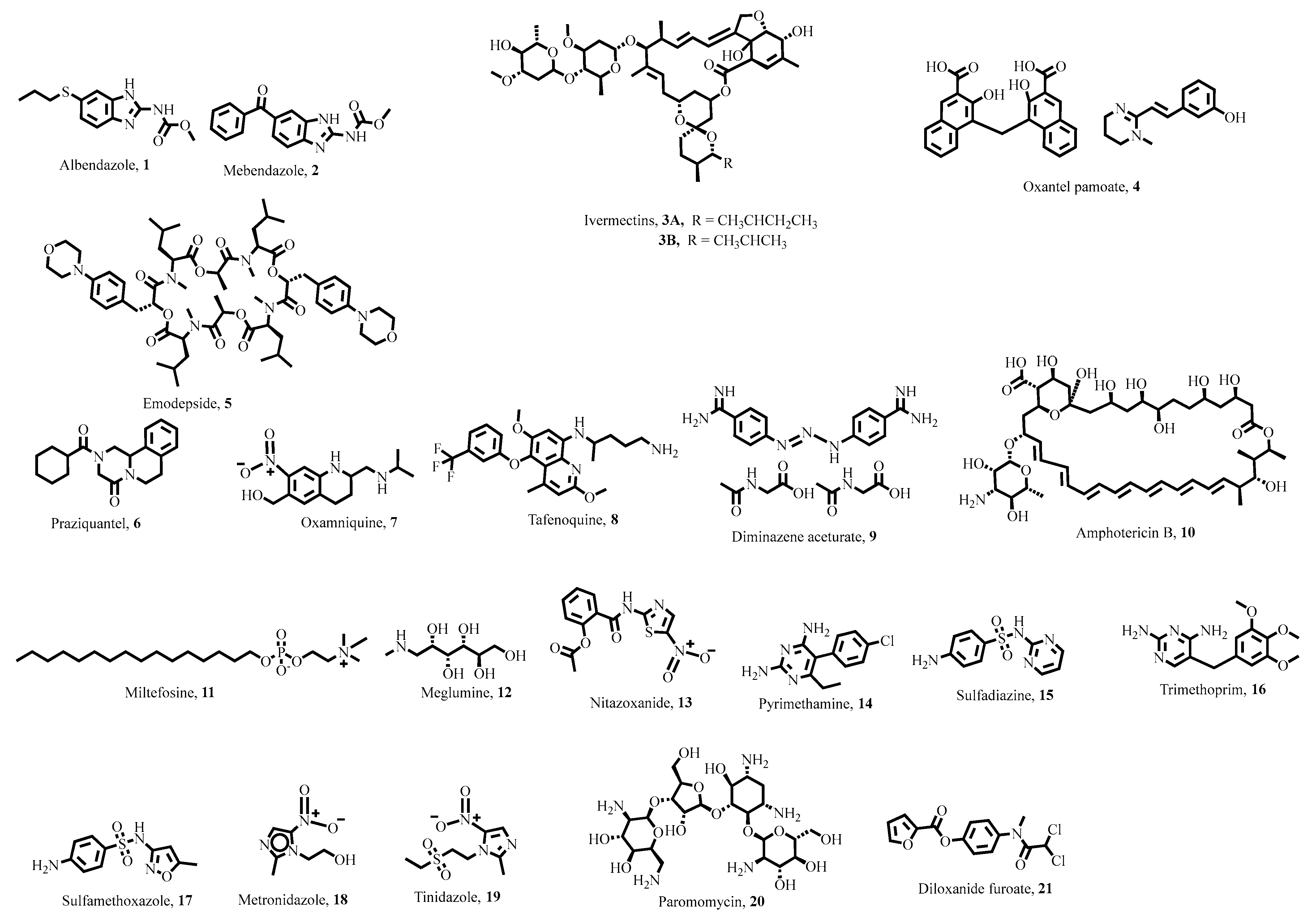
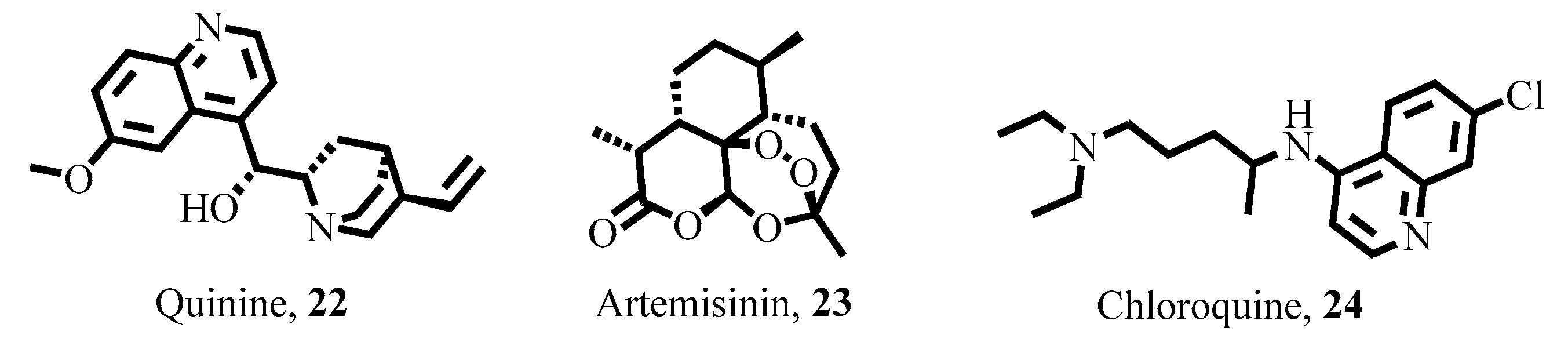
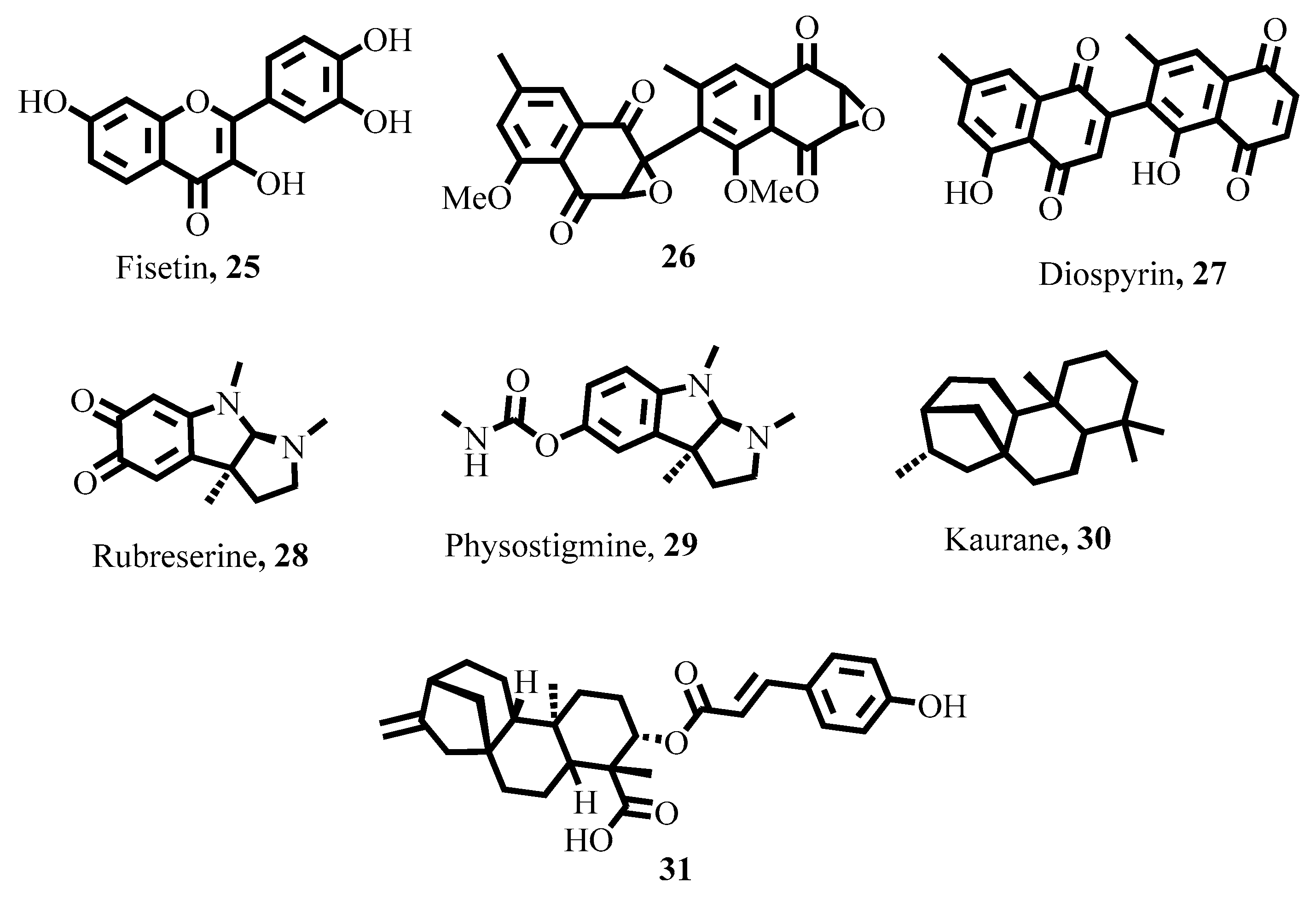


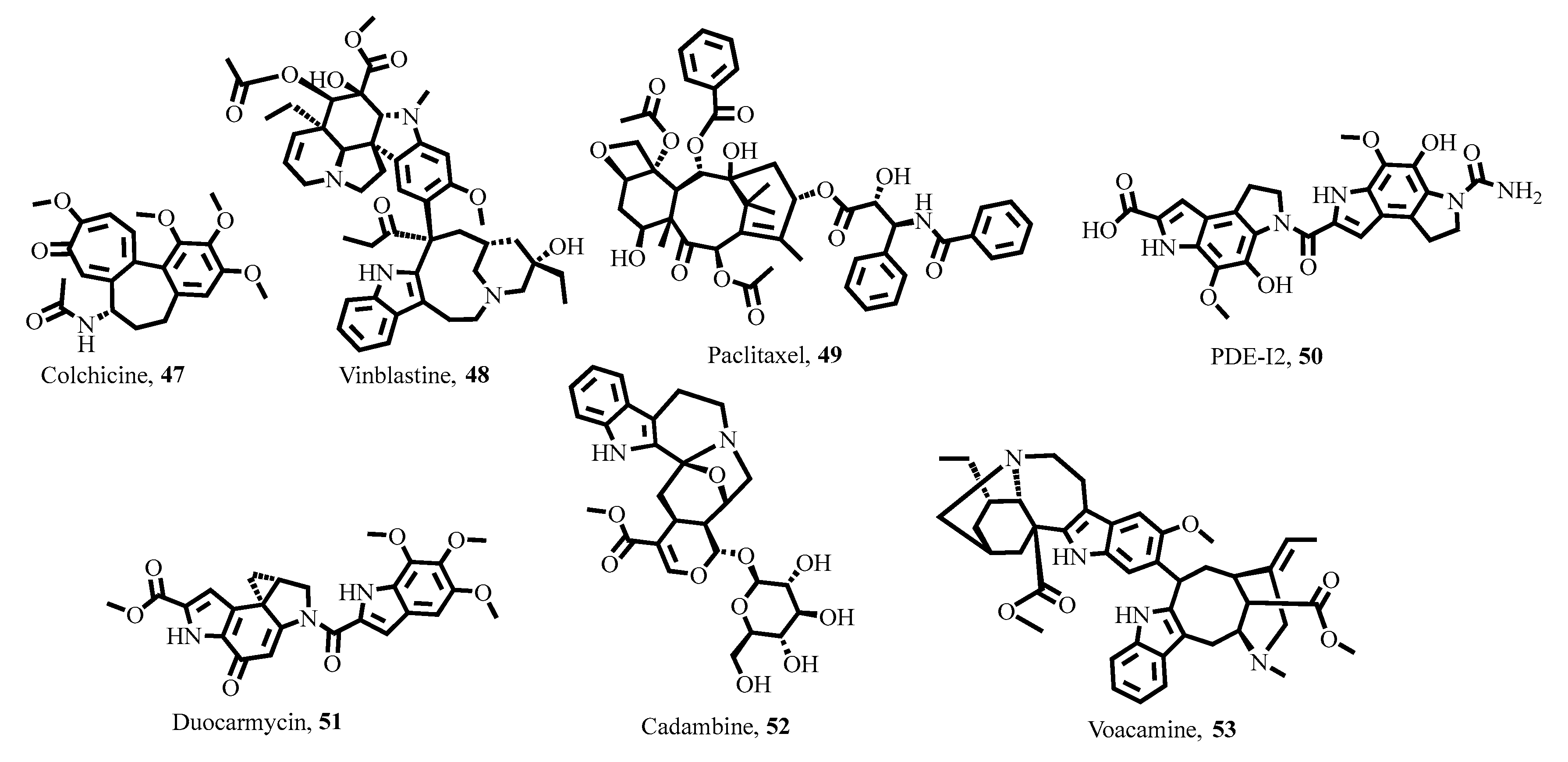
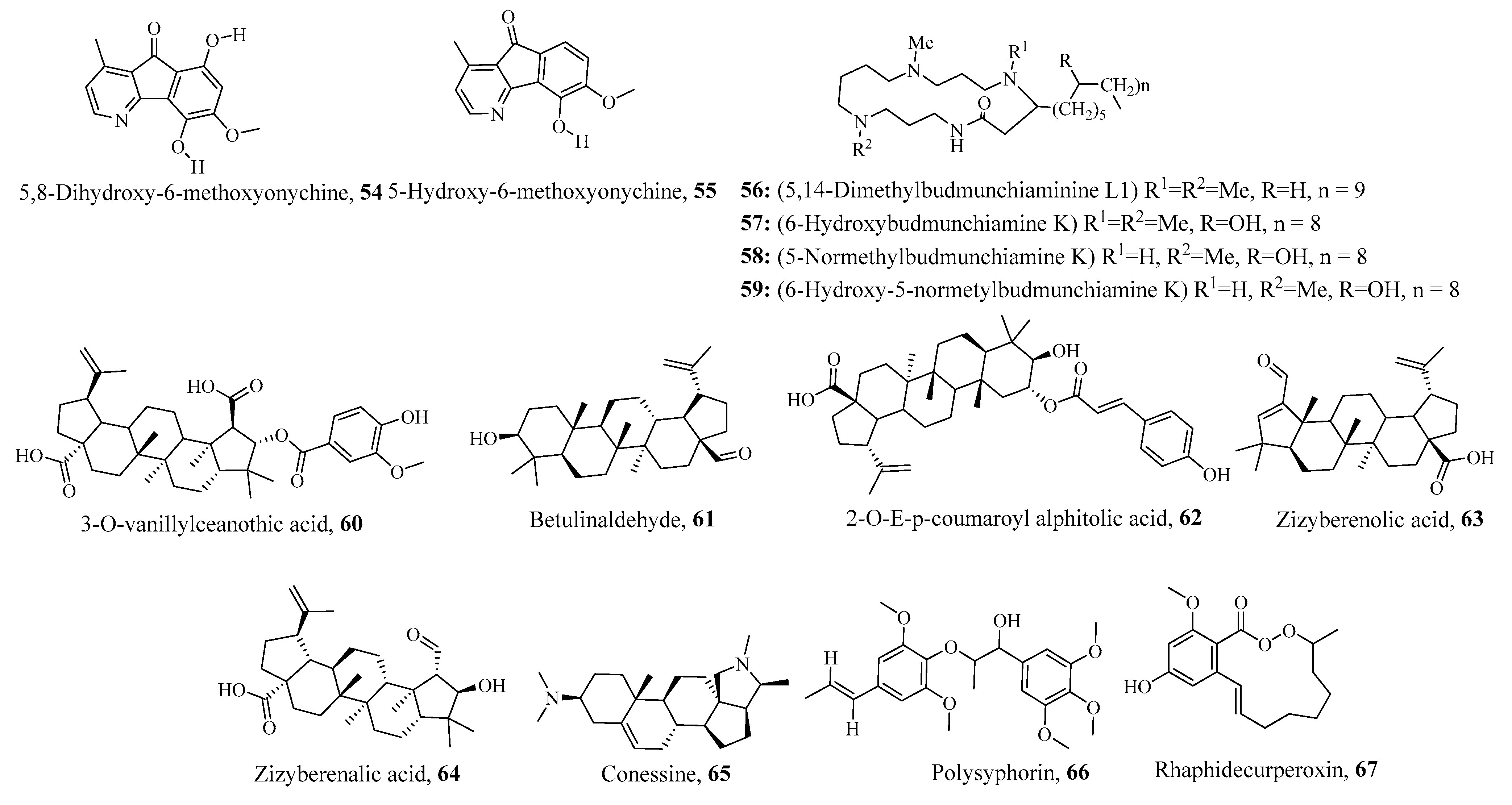
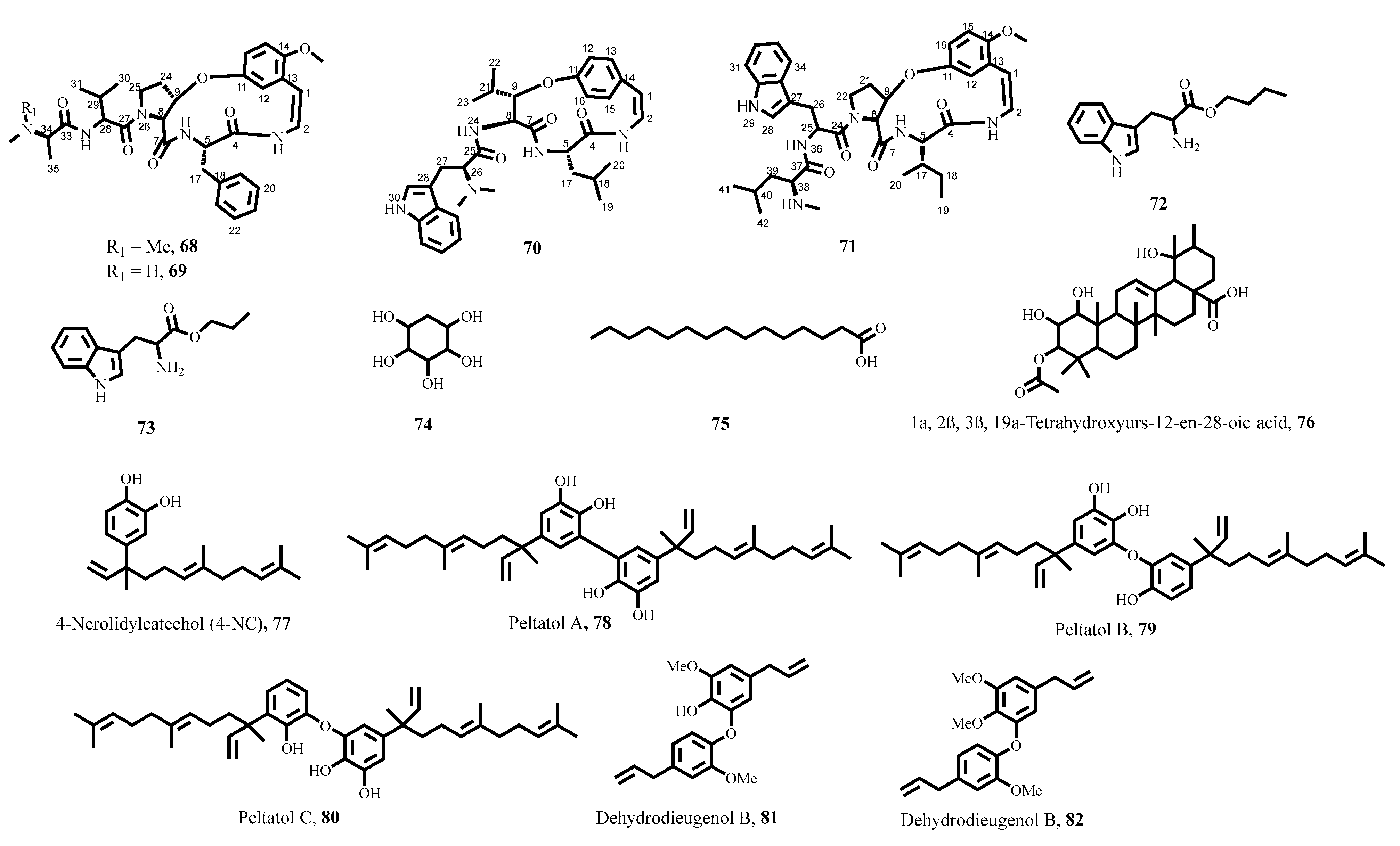


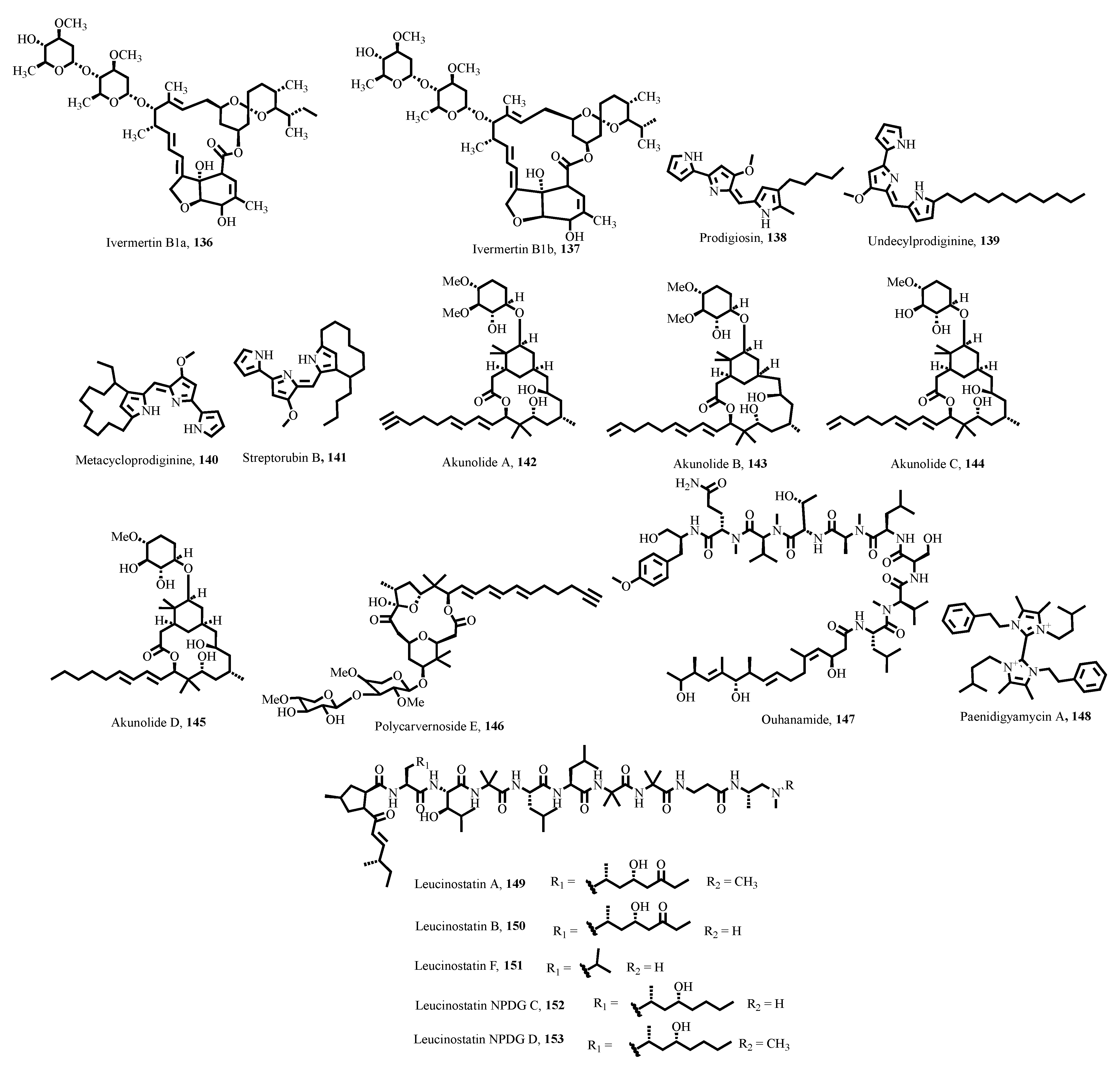
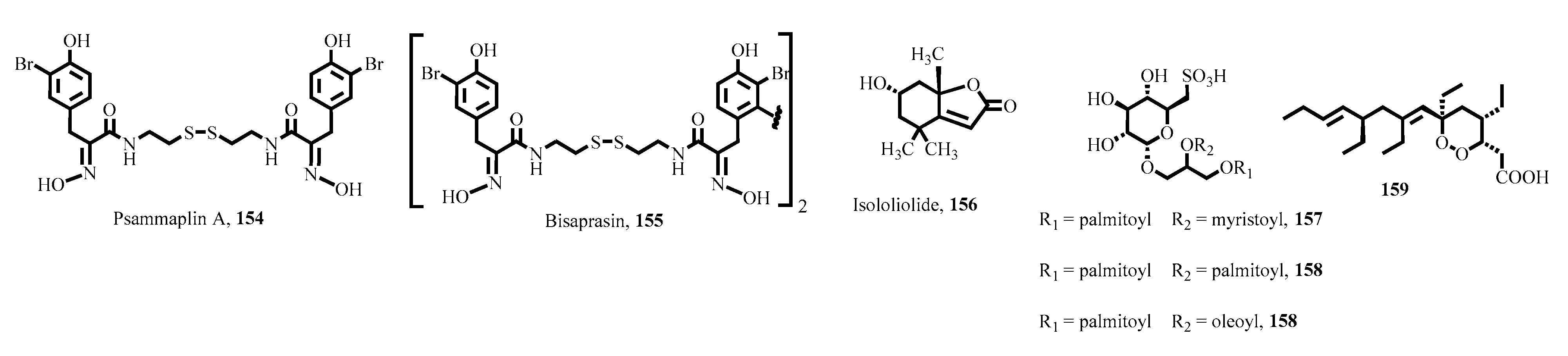

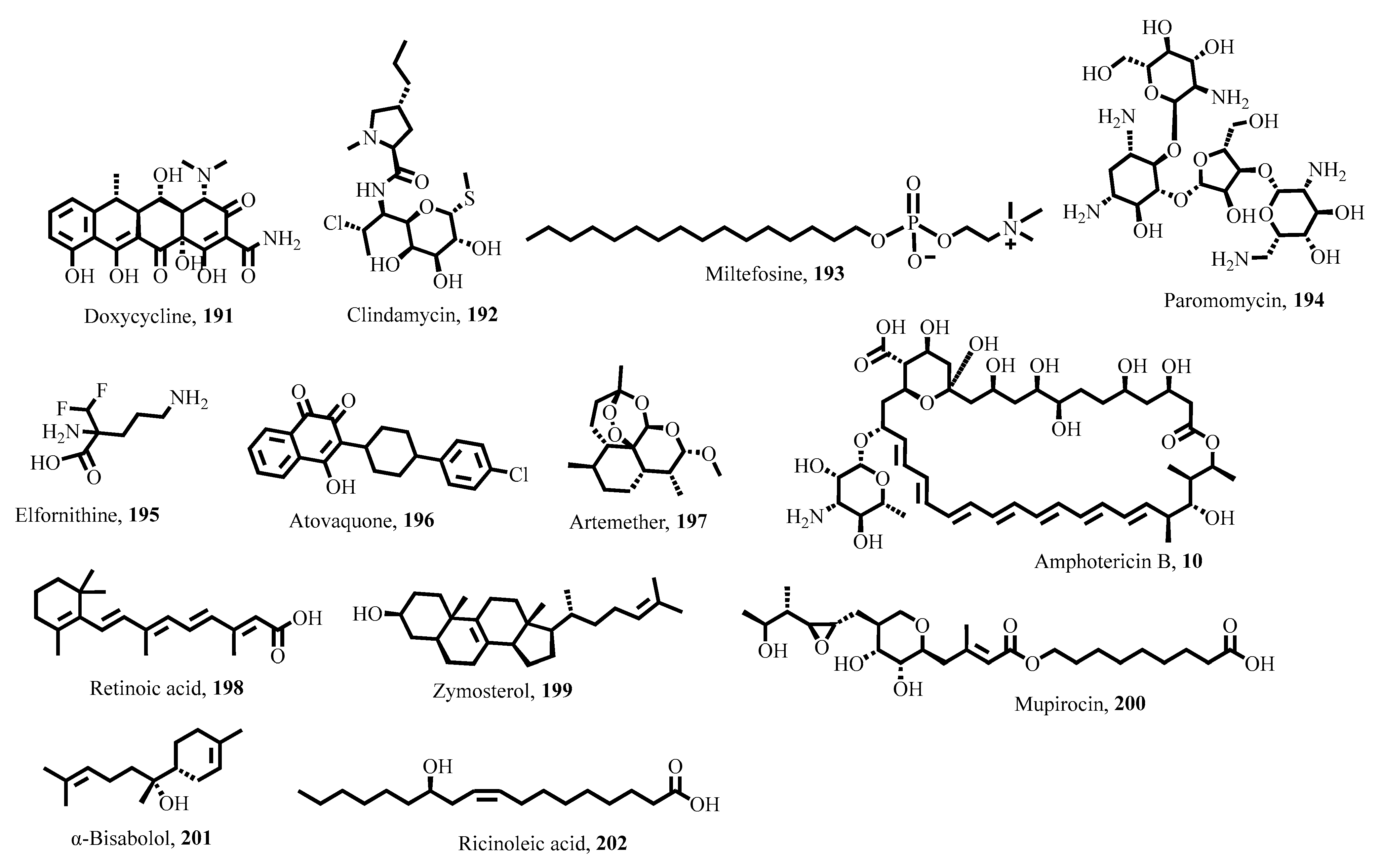
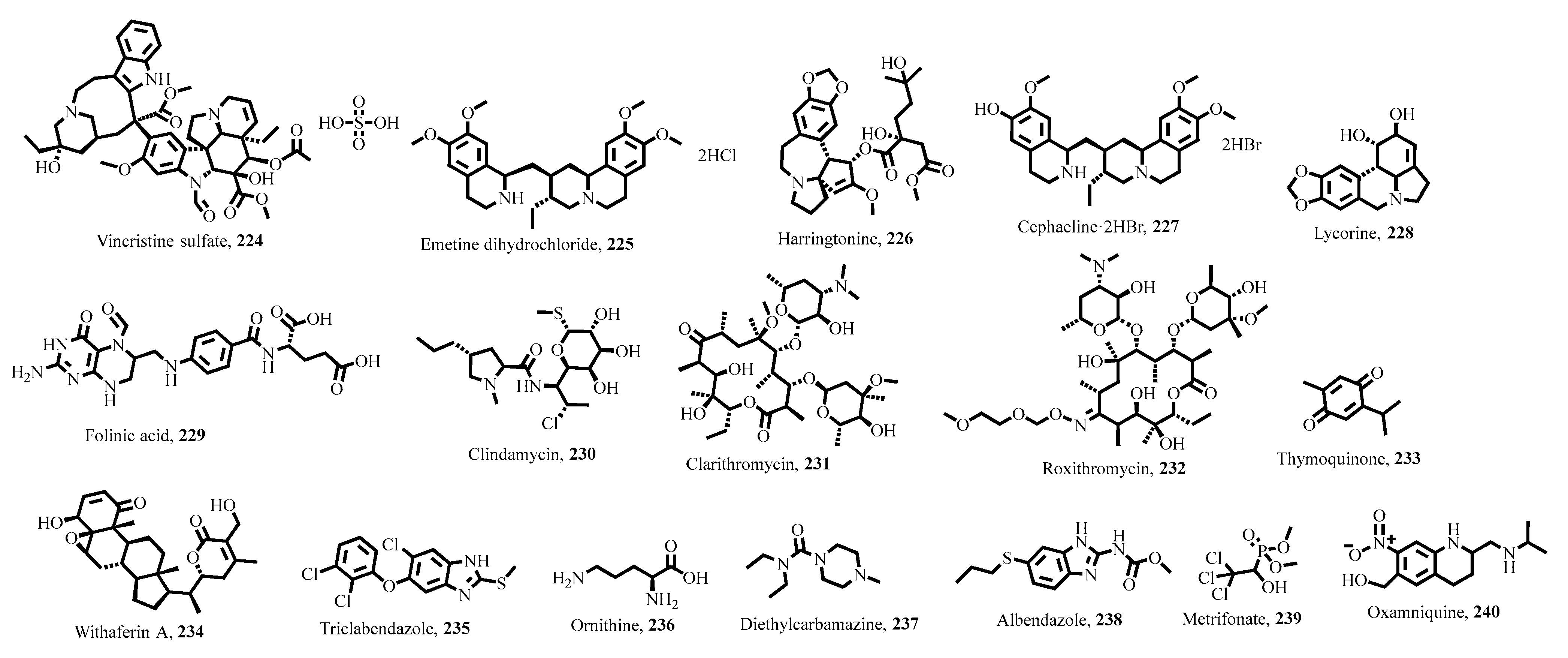
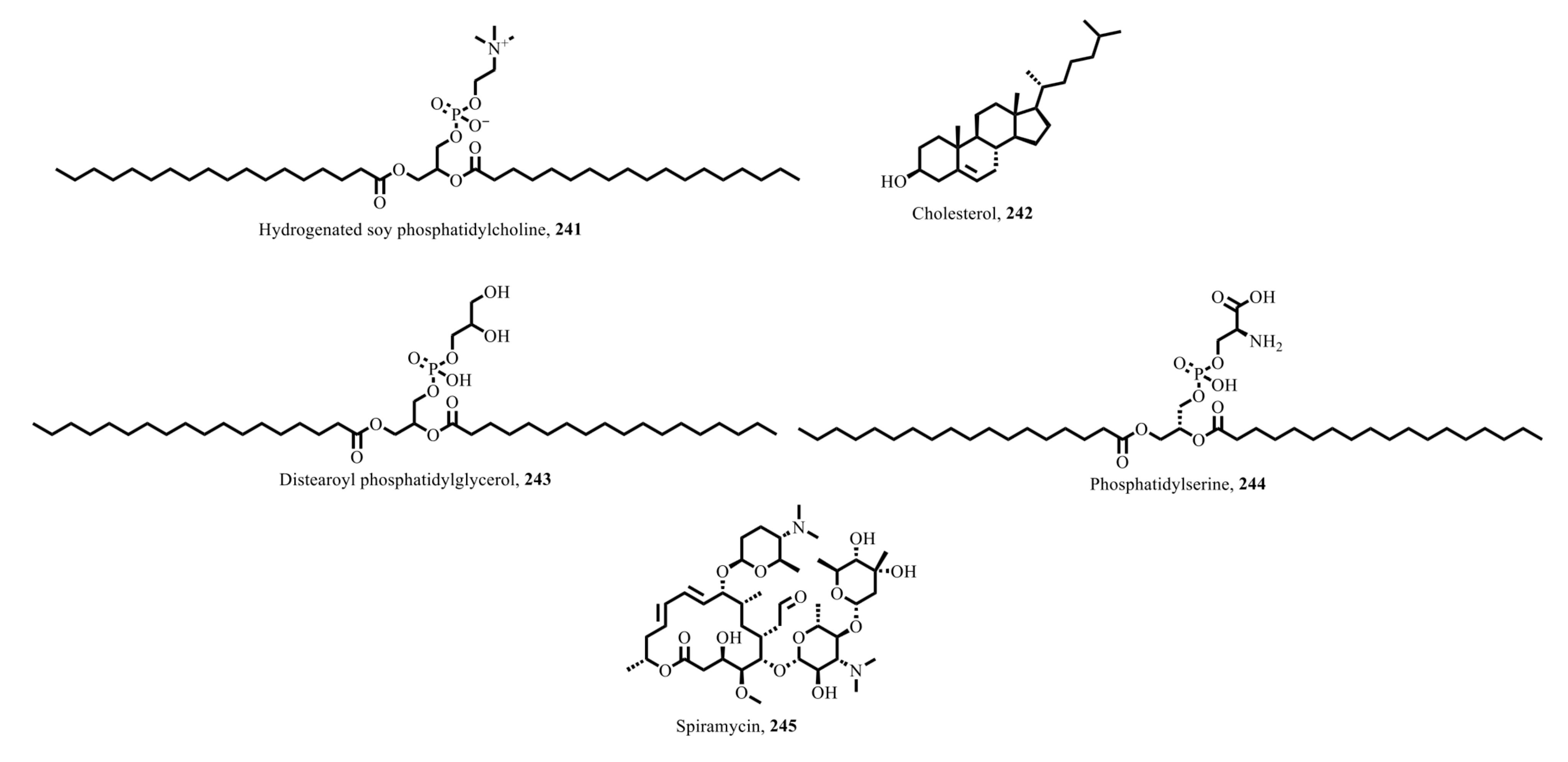
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakyi, P.O.; Twumasi, E.B.; Twumasi, M.A.; Akolgo, G.A.; Amewu, R.K.; Osei-Safo, D. Therapeutic Potential of Natural Products as Innovative and New Frontiers for Combating Parasitic Diseases. Parasitologia 2025, 5, 49. https://doi.org/10.3390/parasitologia5030049
Sakyi PO, Twumasi EB, Twumasi MA, Akolgo GA, Amewu RK, Osei-Safo D. Therapeutic Potential of Natural Products as Innovative and New Frontiers for Combating Parasitic Diseases. Parasitologia. 2025; 5(3):49. https://doi.org/10.3390/parasitologia5030049
Chicago/Turabian StyleSakyi, Patrick Opare, Emmanuella Bema Twumasi, Mary Ayeko Twumasi, Gideon Atinga Akolgo, Richard Kwamla Amewu, and Dorcas Osei-Safo. 2025. "Therapeutic Potential of Natural Products as Innovative and New Frontiers for Combating Parasitic Diseases" Parasitologia 5, no. 3: 49. https://doi.org/10.3390/parasitologia5030049
APA StyleSakyi, P. O., Twumasi, E. B., Twumasi, M. A., Akolgo, G. A., Amewu, R. K., & Osei-Safo, D. (2025). Therapeutic Potential of Natural Products as Innovative and New Frontiers for Combating Parasitic Diseases. Parasitologia, 5(3), 49. https://doi.org/10.3390/parasitologia5030049








