Abstract
Strongyloidiasis represents a public health problem in tropical and subtropical regions. The medicinal plants demonstrate the potential of plants as a source of molecules with helminthic activity. In this research, we assessed the potential of five extracts medicinally used in Mexico against Strongyloides venezuelensis third-stage infective larvae (L3). Plant methanol (MeOH) extracts of Argemone mexicana (chicalote), Jatropha dioica (Sangre de Drago), Lippia graveolens (oregano), Thymus vulgaris (tomillo), and Kalanchoe daigremontiana (aranto) were prepared by the maceration technique. The toxicity of the extracts was evaluated in human red blood cells by the hemolysis test and in monkey kidney epithelial cells (Vero cells) using the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In addition, we showed their antioxidant potential by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. The methanolic extracts of A. mexicana, J. dioica, L. graveolens, and T. vulgaris exhibited significant activity against L3 cultures at 72 and 96 h post-incubation. None of the extracts showed hemolytic effects on red cells or Vero cells. L. graveolens demonstrated the strongest antioxidant activity, with an EC50 of 19.80 µg/mL. Plant MeOH extracts used in this study showed a promising anthelmintic effect in vitro, making it a suitable candidate for future research in nematocidal therapies.
1. Introduction
Helminth-induced parasitosis remains a significant public health concern in developing countries, where these infections are closely associated with poverty and inadequate sanitation. Consequently, parasitic diseases are more prevalent in these regions [1]. Regarding strongyloidiasis, the World Health Organization (WHO) estimates that between 30 and 100 million people are infected globally [2]. Strongyloidiasis is a chronic infection caused by Strongyloides stercoralis that can lead to pulmonary symptoms, eosinophilia, and abdominal discomfort that have the feature of autoinfection. This disease is endemic in tropical and subtropical regions [3]. S. stercoralis is a soil-transmitted helminth (STH) that affects approximately 370 million people worldwide, predominantly in tropical and subtropical areas [4]. Disseminated and hyperinfection syndromes occur in immunocompromised individuals and involve widespread parasite massive dissemination, leading to high mortality rates [4,5]. Strongyloides venezuelensis is widely used as a laboratory model for the human parasite S. stercoralis [6]. Several studies involve the use of S. stercoralis as a model for in vitro and in vivo studies [7,8,9].
Moreover, S. stercoralis is a zoonotic nematode affecting canids and some primate species. Strongyloides papilosis has considerable importance in cattle, sheep, and goats around the world. Moreover, Strongyloides ransomi and Strongyloides westeri affect swine and horses, respectively. Finally, Strongyloides felis, Strongyloides tumefaciens, and Strongyloides planiceps affect cats and small wild carnivores [10].
Currently, no vaccines are available for strongyloidiasis; however, preventive measures such as proper sanitation, basic hygiene measures, and the use of appropriate footwear in endemic areas can help reduce the risk of infection [11]. The disease is primarily treated with ivermectin (IV) or albendazole (Alb) to reach total elimination in the patient to avoid autoinfection [12]. The recommended treatment involves oral ivermectin at 200 µg/kg/day for two consecutive days, with a second round of treatment after 14 days [13]. In immunocompromised patients, three treatment cycles are advised, each separated by a 14-day interval [14]. Since the introduction of IV, it has been considered the drug of choice first against veterinary nematode and arthropods and after against Onchocerca volvulus, Strongyloides stercolaris, and Sarcoptes scabiei [15]. However, IV-resistant nematode strains have been reported in grazing livestock, and there is concern about reduced susceptibility or potential resistance in human nematodes [16].
Plants and natural products with biological activity against various pathogens, including parasites [17] and nematodes [18], appear as a valuable source of bioactive metabolites with diverse therapeutic properties based on new treatments [19]. Plants contain a wide range of chemical constituents [20], many of which exhibit antifungal, antiparasitic, antiviral, and antibacterial activity [21,22]. In Mexico, medicinal plants have been used continuously from the pre-Hispanic era to the present. Among them, Argemone mexicana has demonstrated significant biological activity against Plasmodium falciparum and Plasmodium berghei [23,24]. Jatropha dioica is a plant widely distributed in the semi-desert regions of Mexico and the United States, traditionally used in folk medicine for pain and inflammation as well as to prevent hair loss and promote hair strengthening [25]. Additionally, this plant has been reported to exhibit nematocidal activity against Trichinella spiralis [18]. Lippia graveolens, Mexican oregano, is a rich source of flavonoids, sakuranetin, and cirsimaritin, useful in combating E. histolytica [26]. Thymus vulgaris (thyme), native to the Mediterranean basin, is widely utilized in Hispanic America as a culinary spice and is also appreciated for its anti-inflammatory and antimicrobial properties [27,28,29]. Moreover, thyme has demonstrated activity against Haemonchus contortus, T. spiralis, and Trypanosoma cruzi [30]. Kalanchoe daigremontiana, an introduced plant from Madagascar, has shown notable activity against Entamoeba histolytica and Trichomonas vaginalis [31].
In Mexico, the prevalence of intestinal parasitic infections (IPIs) remains high, especially in rural and marginalized communities, with national epidemiological surveys reporting frequent cases of Ascaris lumbricoides, Trichuris trichiura, and S. stercoralis among school-aged children [32,33]. These infections contribute significantly to the burden of disease in the country and reflect underlying socio-economic inequalities [32,34].
Therefore, this study aimed to evaluate the nematocidal activity of methanolic extracts from five medicinal plants traditionally used in Mexico against S. venezuelensis infective third-stage larvae (L3) as well as to assess their toxicity and antioxidant potential.
It is important to emphasize that the studies conducted are limited to the L3 larvae, which are the infective stage. This serves as a good model since it represents one of the stages that can be targeted in cases of autoinfection [35]. To determine the toxicity of the extracts, we evaluated their effects on two distinct cell lines: human erythrocytes and Vero cells [36].
2. Materials and Methods
2.1. Plant Material and Extraction
Between 2.5 and 3.5 kg of dry material was collected in Monterrey, Mexico (25°41′04″ N 100°19′05″ O at 540 m above sea level). An ethnobotanical and taxonomist expert, Prof. Marco Antonio Alvarado Vázquez from the Faculty of Biological Sciences, Universidad Autónoma de Nuevo León, carried out the collection and identification of the whole plant (Table 1). Specimens were identified and deposited in the herbarium of the Universidad Autónoma de Nuevo León. The botanical identifications were taxonomically validated using the WorldFlora Online (WFO) (http://www.worldfloraonline.org) (accessed on 15 February 2025) and the International Plant Names Index (IPNI) website (https://www.ipni.org) (accessed on 15 February 2025).

Table 1.
Plants used to extract bioactive molecules from the leaves of Argemone mexicana, Jatropha dioica, Lippia graveolens, Thymus vulgaris, and Kalanchoe daigremontiana.
Extraction was performed by subjecting 100 g of milled dry plant leaves to 500 mL of acetone-free absolute methanol (MeOH, Merck KGaA, Darmstadt, Germany, DE) in a 1 L ball-bottom flask for 48 h at 200 rpm on an orbital shaker at room temperature (24.0 ± 2.0 °C). Extracts were filtered and rotaevaporated (Yamato Scientific Co., Ltd., Harumi, Chuo-ku, Tokyo, Japan, JPN) followed by vacuum application for 96 h using a desiccator loaded with analytical silica gel absorbent beads with a moisture indicator (Merck) and stored at 4 °C in amber bottles until use. The extraction yield percentage (%) was determined as follows (1):
2.2. Qualitative Phytochemical Tests
The following phytochemical tests were performed to identify the functional groups of each extract based on the methodology reported by Rodríguez-Garza et al. (2023) [37]: KMnO4 (unsaturations), anthrone (carbohydrates), Lieberman–Buchard (sterols), Shinoda (flavonoids), NaOH (coumarins), Baljet (sesquiterpene lactones), saponins, sulfuric acid (quinones), ferric chloride (tannins), and Dragendorff (alkaloids).
2.3. Activity Against S. venezuelensis (L3)
The S. venezuelensis life cycle and the L3 larvae harvesting were described in previous research [18]. The S. venezuelensis life cycle was maintained through successive passages in 4-week-old male Wistar rats (150–200 g). Rats were subcutaneously injected with 12,000 infective third-stage larvae (L3) of S. venezuelensis in 500 μL of phosphate-buffered saline (PBS, pH~7.4). Fecal samples were collected from days 5 to 18 post-infection and cultured in 250 mL polyethylene containers with vermiculite and distilled water at 28 °C in a humid atmosphere (SANYO Electric Co. Ltd., Gunma, Japan) for 4 to 7 days. L3 larvae were harvested using the Baermann method and washed three times with distilled water, and their viability was assessed by light microscopy before experiments [18].
For the assays, 100–150 L3 per well were placed in a 96-well culture plate (Corning Incorporated, Corning, NY, USA) and incubated at 28 °C for 1 h for adaptation before treatment with methanolic extracts. The extracts and the positive control ivermectin (IV, Sigma-Aldrich®, Merck KGaA, Darmstadt, Germany, DE) were dissolved in 5% dimethyl sulfoxide (DMSO, Merck) and appropriately diluted. L3 cultures were treated with extract concentrations ranging from 1 to 500 µg/mL. All assays were conducted in a final volume of 200 µL per well at 28 °C in a humid atmosphere. Nematode mortality was defined as the inability to move within three minutes of observation under 40× magnification for at least 1 min, using visible light to stimulate movement at 0, 24, 48, 72, and 96 h post-treatment. The positive control (C+) consisted of 10 μM of IV, while the negative control (C−) included distilled water and a vehicle control of 0.4% DMSO. LC50 determination was performed at test concentrations of 1, 10, 100, 250, and 500 μg/mL at 0, 24, 48, 72, and 96 h.
2.4. Antioxidant Activity
The antioxidant potential of the plant extracts was tested by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay [38]. For this, we incubated 100 μL of 125 μM DPPH to 100 μL of each sample at different concentrations (31.25 to 500 µg/mL) for 30 min at 37 °C in darkness. Absorbance (Abs) at 517 nm was then measured with a spectrophotometer, Genesys 20 (Thermo-Fisher Scientific, Waltham, MA, USA), using 50 µM ascorbic acid as a positive control (C+) and MeOH as a blank. IC50 values were calculated as the concentration of the sample required to scavenge 50% of DPPH. The reduction % was calculated using Formula (2):
2.5. Red Cell Cytotoxicity
The activity of plant MeOH extracts was evaluated by the hemolysis test. The red cell cytotoxicity test was used as a model of cell toxicity, as previously reported by our research group [39]. The degree of hemolysis was determined by measuring Abs at 540 nm, whereas the IC50 values were calculated as the concentration of sample required to hemolyze 50% of human red blood cells. Sterile distilled water was used as a positive control for hemolysis because it causes erythrocyte lysis and total hemolysis in erythrocytes. The % of hemolysis was calculated with Formula (3):
2.6. Cytotoxic on Vero Cells
The African green monkey kidney epithelial cell line (Vero; ATCC CCL-81) was cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), 2 g/L sodium bicarbonate (NaHCO3), and a 1% penicillin–streptomycin solution (Gibco), referred to as complete DMEM medium. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 [40]. Vero cells were seeded at a density of 1 × 104 cells per well in flat-bottomed 96-well microplates (Corning Incorporated) containing complete DMEM medium. After 24 h of incubation at 37 °C with 5% CO2, cells were treated with methanolic extracts at concentrations ranging from 10 to 1000 µg/mL. Culture medium with or without 1% DMSO served as negative controls; the antineoplastic vincristine sulfate (Hospira, Warwickshire, UK) at 100 µg/mL was used as a positive control. Following 48 h of incubation, cell viability was assessed using the colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Affymetrix, Cleveland, OH, USA) reduction assay. MTT was added to each well at a final concentration of 0.5 mg/mL and incubated at 37 °C for an additional 3 h. The plates were then decanted, and formazan crystals were dissolved in 100 µL of DMSO. Optical density (OD) was measured at 570 nm [41] using a MULTISKAN GO microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Growth inhibition (%) was calculated using Formula (4):
2.7. Statistical Analysis
All assays were performed in triplicate and repeated at least three times. Results were expressed as means and standard error (SD) of the mean. Significant differences between groups were determined by a one-way ANOVA test. The post hoc Dunnett’s test was performed to determine statistical differences between treated and untreated controls. The post hoc Tukey’s honest significance test (HSD) was performed to determine any statistical differences between treatments. The Probit test was used to determine the mean cell lethal concentration (LC50), the immobilization of 50% of the L3 concentration (IC50), and the half-maximal effective concentration (EC50) of the radical scavenging activity. The analyses were performed with SPSS software Ver. 24 (IBM—SPSS, Armonk, NY, USA). All statistical analyses were considered significant at the p < 0.05 level.
3. Results and Discussion
3.1. Phytochemical Analysis
The extraction yield determined using Formula 1 of the total dry mass of the MeOH extracts of the leaves from A. mexicana was 11.30%, for J. dioica it was 15.99%, for L. graveolens was 43.33%, for T. vulgaris was 11.36%, and for K. daigremontiana was 10.04% (Table 2). All extracts tested were positive for the unsaturation and sterol tests. For carbohydrate and flavonoid tests, the extracts of A. mexicana and J. dioica exhibited negative reactions, respectively. The extracts from A. mexicana, J. dioica, and L. graveolens were positive for coumarins and sesquiterpene lactones. Additionally, the extracts of L. graveolens, T. vulgaris, and K. daigremontiana showed positive results for saponins and tannins.

Table 2.
Phytochemical results and extraction yield of methanolic extracts of dry leaves of Argemone mexicana, Jatropha dioica, Lippia graveolens, Thymus vulgaris, and Kalanchoe daigremontiana.
3.2. Anti-Strongyloides Venezuelensis Activity
We observed the concentration–response effects of the different extracts evaluated against S. venezuelensis (Figure 1). It can be observed that the extracts of A. mexicana (Figure 1a) and L. graveolens (Figure 1c) demonstrated significant activity at 250 and 500 μg/mL. J. dioica (Figure 1b) showed significant activity at 72 h post-treatment at 250 and 500 μg/mL, while T. vulgaris (Figure 1d) showed activity up to 96 h at the same concentrations. K. daigremontiana (Figure 1e) did not demonstrate any significant effect against the L3 larvae at any of the evaluated concentrations.
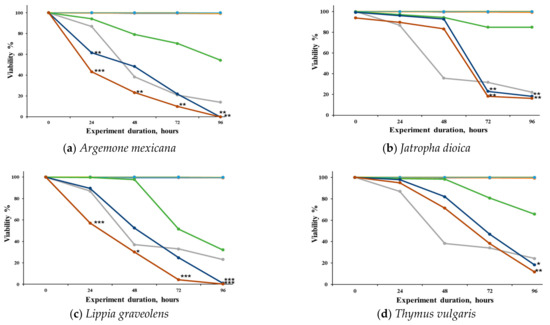
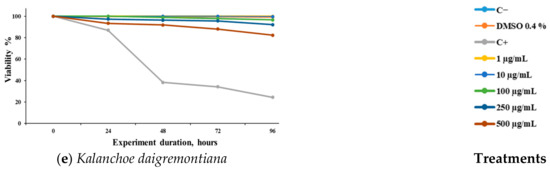
Figure 1.
Viability (%) of Strongyloides venezuelensis larvae (L3) exposed to methanolic extracts of five medicinal plants over time. The extracts were obtained from the leaves of (a) Argemone mexicana, (b) Jatropha dioica, (c) Lippia graveolens, (d) Thymus vulgaris, and (e) Kalanchoe daigremontiana. Larval viability was assessed at 0, 6, 12, 24, 48, and 96 h after treatment. A negative control (untreated larvae) and a positive control (C+, 10 μM IV) were included. Results are expressed as mean ± SD of three independent experiments, each performed in triplicate. Statistical significance was determined using ANOVA followed by Dunnett’s post hoc test (* p ˂ 0.05, ** p ˂ 0.01, and *** p ˂ 0.001).
The nematocidal activity of the extracts at different post-infection times is shown in Table 3. At 24 and 48 h, none of the treatments exhibited activity against L3 larvae. However, at 72 h, J. dioica and L. graveolens demonstrated activity with LC50 values below 200 µg/mL and 100 µg/mL, respectively. Finally, at 96 h, L. graveolens showed remarkable nematicidal activity with an LC50 < 100 µg/mL, while A. mexicana, J. dioica, and T. vulgaris exhibited LC50 values < 200 µg/mL. In contrast, K. daigremontiana was the extract with the lowest activity across all time points, with an LC50 > 500 µg/mL.

Table 3.
Nematocidal activities expressed in LC50 of methanolic extracts from dry leaves of Argemone mexicana, Jatropha dioica, Lippia graveolens, Thymus vulgaris, and Kalanchoe daigremontiana.
3.3. Hemolytic, Cytotoxic, and Antioxidant Test
The toxicity of the different methanolic extracts was evaluated on human erythrocytes and normal Vero cell cultures (Table 4). None of the extracts showed toxicity toward human erythrocytes. However, in Vero cells, J. dioica exhibited an IC50 greater than 1000 µg/mL, while K. daigremontiana, A. mexicana, and T. vulgaris presented IC50 values of 111.39, 202.99, and 404.52 µg/mL, respectively. Table 4 also displays the antioxidant activity of the extracts, assessed using the DPPH radical scavenging assay. The extract with the highest antioxidant capacity was L. graveolens (EC50 = 19.80 µg/mL), while those with the lowest antioxidant activities were A. mexicana (EC50 = 565.98 µg/mL) and K. daigremontiana (EC50 = 699.05 µg/mL).

Table 4.
Hemolytic, cytotoxic, and antioxidant activities of methanolic extracts of dry leaves of Argemone mexicana, Jatropha dioica, Lippia graveolens, Thymus vulgaris, and Kalanchoe daigremontiana.
4. Discussion
Strongiloides stercoralis is a medically significant parasitic nematode with a high annual incidence worldwide, particularly in tropical and subtropical regions [42,43]. Also, this nematode can develop chronic cryptic low-level infections in a process called autoinfection. Frequently, immunocompromised patients develop disseminated or hyperinfection syndromes with high mortality rates. This situation, linked to the limited availability of effective nematocidal drugs [44], underscores the need for novel treatment alternatives.
Plants synthesize a diversity of secondary metabolites with a wide range of biological activities, including significant antiparasitic properties [45]. The anthelmintic potential of plant extracts is primarily attributed to the presence of bioactive compounds such as alkaloids, flavonoids, coumarins, and terpenoids, among others [45,46]. These metabolites target helminths through various mechanisms that vary across different stages of the parasite’s life cycle. They can damage the cuticle, disrupt motility, or interfere with feeding, ultimately impairing the growth and reproductive capacity of the helminths [47]. For example, the alkaloid berberine has demonstrated the ability to intercalate into microtubules [48], disrupting chloride channels and subsequently affecting helminth motility [49]. However, caution is warranted, as improper use of berberine may result in toxicity [50].
The present study screened five methanolic extracts from Mexican plants against S. venezuelensis L3. We observed that L. graveolens, commonly known as Mexican oregano, showed the best results against L3 larvae, presenting a mean immobilization rate of 100 µg/mL at 72 and 96 h. These results combine low toxicity in contact with erythrocytes, Vero cells, and high antioxidant activity. This aligns with previous studies demonstrating the antiparasitic effects against T. cruzi cultures [51], H. contortus [52,53], and E. histolytica. Specifically, significant amoebicidal activity has been reported for its extracts, subfractions, the monoterpene carvacrol [54] and the isolated flavonoids pinocembrin, sakuranetin, cirsimaritin, and naringenin [55].
Previous studies conducted by Boyko, O. and Brygadyrenko, V. in 2023 [46] indicate the potential of several natural organic groups in vitro against S. papillosus and H. contortus, highlighting the potential of plant compounds to decrease their lethality [46]. In another study conducted by the same authors in 2023, they indicated the nematocidal potential of some essential oils, specifically eugenol, isoeugenol, thymol, and carvacrol, against S. papillosus and H. contortus. Therefore, the plants evaluated could represent a source of nematocidal compounds [56].
The antioxidant capacity of the extracts was evaluated using the DPPH radical scavenging assay, a widely employed method for determining the antiradical effects of various biological components, including plant extracts. In this study, L. graveolens demonstrated the strongest DPPH radical scavenging activity, as shown in Table 4. This effect may be attributed to its secondary metabolites (Table 2), such as flavonoids, polyphenols, monoterpenes, sesquiterpenes, and tannins [55,57,58], which are commonly found in extracts of the Lippia genus. Our findings align with those reported by Soto-Domínguez et al. in 2012 [59], where the aqueous extract of L. graveolens exhibited antioxidant activity without causing toxicity in an in vitro model using Artemia salina and an in vivo murine model. Histological and histochemical studies of liver and kidney tissues in these models revealed no adverse effects [59]. Additionally, the primary secondary metabolites of Mexican oregano, as previously mentioned, have been reported to possess antioxidant, anti-inflammatory, antiglycemic, and nutritional potential [26]. These findings underscore the beneficial effects of L. graveolens, as highlighted in the present study.
In a study evaluating the methanolic extract of J. dioica against Trichinella spiralis and S. venezuelensis, the extract exhibited an IC50 of 134.80 µg/mL against T. spiralis and an IC50 of 382.40 µg/mL at 72 h against S. venezuelensis [18]. The J. dioica MeOH extract demonstrated greater activity in the present investigation, with an LC50 < 200 µg/mL at 72 and 96 h post-treatment (Table 3). Aranda-López, in 2021, reported the effect against Taenia crassiceps of the naphthoquinone 4a, one component of J. dioica [60]; this demonstrated the antiparasitic potential of J. dioica. Jatropha dioica, commonly known as “Sangre de Drago” in Mexico, has also demonstrated cytotoxic, antimicrobial, and antifungal activities [61,62].
Previous research demonstrated the antiparasitic effects of the plants evaluated in the present study. A. mexicana has shown activity against E. histolytica [63] and T. vaginalis [64] as well as nematocidal effects against Meloidogyne incognita and M. juvanica [65] and activity against Schistosoma mansoni [66]. These biological effects have been attributed to isoquinoline alkaloids, such as berberine [66,67].
The MeOH extract of T. vulgaris demonstrated significant activity only after 96 h of culture at a 200 µg/mL concentration also with low toxicity to erythrocytes and Vero cells and high antioxidant activity. This activity is lower than that demonstrated against H. contortus and T. spiralis [30], probably due to its principal components such as carvacrol and thymol [26,68] that likely have less nematocidal activity.
In addition, K. daigremontiana has demonstrated activity against E. histolytica and T. vaginalis [31], with the flavonoid quercetin being the main compound with antiparasitic activity [69]. In this study, no significant effect was observed when evaluating it against S. venezuelensis L3 cultures.
Regarding the cytotoxic effect of the extracts against Vero cells, these have been previously evaluated in several investigations where it has been determined that some of these extracts show activity against various cells of tumor lineage without affecting healthy cells such as Vero and erythrocytes [70]. These cellular models are widely utilized by various authors to determine the toxicological or safety profiles of natural products, including plant extracts [41,71]. None of the extracts evaluated exhibited toxic effects on these cell types; this is based on several investigations where concentrations higher than 100 µg/mL were considered as non-toxic [72,73]. In the present investigation, all the determinations presented ranges higher than these. In addition, several plant-based studies on the use of plants against parasites have used Vero cells as a model to determine toxicity as well as to determine the selectivity indexes of such treatments [18,72].
This study provides a preliminary evaluation of the nematocidal, antioxidant, and cytotoxic activities of selected plant extracts; however, it has several limitations. First, the nematocidal activity was only assessed in vitro using S. venezuelensis third-stage infective larvae (L3), and further studies are needed to confirm these effects in vivo.
We adapted to the characteristics of our experimental model of S. venezuelensis and to our objective, which was to characterize plant extracts in terms of efficacy against the infective phase and toxicity to mammalian cells and to be able to propose new studies. We did not perform studies on L1 or L2 because the intention was to look for active principles on the host, and L1 and L2 are found in the soil. Also, we can cultivate the L3 phase for a week, conserving motility up to 85% for a week. In our experiments, we conserved the untreated controls for a week, but our results were recorded at 24, 48, 72 and 96 h following the general use to report information in three days after the treatment.
Second, while we evaluated cytotoxicity using hemolysis and the MTT assay in Vero cells, additional toxicological tests in different cell lines or animal models are required to fully assess the safety profile of these extracts. Third, it is necessary to identify the phytochemical composition of the extracts. Future studies incorporating a detailed chemical characterization and in vivo validation will be essential to further explore the potential of these plant extracts for nematocidal therapy.
5. Conclusions
This study demonstrated the high nematocidal activity of crude methanolic extracts from L. graveolens compared to the other evaluated extracts against S. venezuelensis in a time- and concentration-dependent manner, exhibiting low cytotoxic activity in red cells and high antioxidant activity.
Our findings highlight the potential of Lippia graveolens as a nematocidal agent, warranting further studies on its bioactive compounds and in vivo efficacy to support future therapeutic applications.
Author Contributions
Conceptualization, J.H.E.-L., A.F.B.-R., and J.L.-A.; methodology, A.M., B.V.-S., and J.L.-A.; investigation, J.H.E.-L., A.C.-M., and U.C.-V.; formal analyses, J.H.E.-L., D.G.G.-H., and J.L.-A.; writing—original draft, J.H.E.-L., H.L.-G., and M.K.; writing—review and editing, J.H.E.-L., A.M., M.K., A.F.B.-R., and B.V.-S.; resources, A.M., B.V.-S., and J.L.-A.; supervision, A.M. and J.L.-A.; project administration, A.M. and B.V.-S.; funding acquisition, J.H.E.-L., H.L.-G., A.F.B.-R., and J.L.-A.; visualization, H.L.-G., B.V.-S., and A.C.-M.; software, A.C.-M. and A.F.B.-R.; data curation, J.H.E.-L., M.K., D.G.G.-H., and U.C.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIN/AEI/doi 10.13039/501100011033 co-funded by European Union grant PID2021-127471OB-I00 and financed to J.L.-A., J.H.E.-L. and H.L.-G. received a postdoctoral grant funded by the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONHACYT). M.K. was funded by the grant APVV-20-0058, “The potential of the essential oils from aromatic plants for medical use and food preservation”. M.K. was also supported by the grant VEGA 1/0059/24, “Chemical properties and biological activity (in vitro, in vivo and in situ) of plant volatile mixtures, their main components and inclusion systems”. A.C.-M. was funded by the grant CF-2023-I-1254 under the project “Nanotecnología en farmacia verde para tratamiento antineoplásico” under the program Ciencia Básica y de Frontera of the CONAHCYT.
Institutional Review Board Statement
This study with human erythrocytes was carried out under the approval of the ethics committee of the UANL, College of Medicine (Registration Number HI11002-2025), and under the consent of healthy donors, following the provisions of the Official Mexican Technical Standard NOM-253-SSA1-2012. All animal procedures adhered to the ethical guidelines and regulations of Spain RD 53/2013 and Di 2010/63/CE. The experiments were conducted at the accredited Animal Experimentation Facilities of the University of Salamanca (Reg. No.: PAE/SA/001). The study protocols received approval from the Ethics Committee of USAL CEI 1080.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated or analyzed during the present study are available on request from the corresponding author.
Acknowledgments
SNII-CONAHCYT of Mexico, and Slovak Research and Development Agency (APVV-20-0058) from Slovakia are thankfully acknowledged for their financial support in this research. The authors also thank the Fundación USAL—Banco Santander, Spain. Special thanks to the crew of the Herbarium of FCB-UANL for their support in the identification of the plants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Truscott, J.E.; Turner, H.C.; Farrell, S.H.; Anderson, R.M. Soil-Transmitted Helminths. In Advances in Parasitology; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 94, pp. 133–198. [Google Scholar]
- Estrongiloidiasis-Enfermedades Infecciosas-Manual MSD Versión Para Profesionales. Available online: https://www.msdmanuals.com/es/professional/enfermedades-infecciosas/nematodos-gusanos-redondos/estrongiloidiasis (accessed on 13 December 2024).
- Siddiqui, A.A.; Berk, S.L. Diagnosis of Strongyloides Stercoralis Infection. Clin. Infect. Dis. 2001, 33, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides Stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed]
- Montes, M.; Sawhney, C.; Barros, N. Strongyloides Stercoralis: There but Not Seen. Curr. Opin. Infect. Dis. 2010, 23, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Toma, H. Strongyloides Venezuelensis Infections in Mice. Int. J. Parasitol. 1990, 20, 57–62. [Google Scholar] [CrossRef]
- Moraes, D.; Levenhagen, M.A.; Costa-Cruz, J.M.; Costa Netto, A.P.D.; Rodrigues, R.M. In Vitro Efficacy of Latex and Purified Papain from Carica Papaya against Strongyloides Venezuelensis Eggs and Larvae. Rev. Inst. Med. Trop. 2017, 59, e7. [Google Scholar] [CrossRef]
- Carvalho, C.O.; Chagas, A.C.S.; Cotinguiba, F.; Furlan, M.; Brito, L.G.; Chaves, F.C.M.; Stephan, M.P.; Bizzo, H.R.; Amarante, A.F.T. The Anthelmintic Effect of Plant Extracts on Haemonchus Contortus and Strongyloides Venezuelensis. Vet. Parasitol. 2012, 183, 260–268. [Google Scholar] [CrossRef]
- Legarda-Ceballos, A.L.; López-Abán, J.; del Olmo, E.; Escarcena, R.; Bustos, L.A.; Rojas-Caraballo, J.; Vicente, B.; Fernández-Soto, P.; San Feliciano, A.; Muro, A. In Vitro and in Vivo Evaluation of 2-Aminoalkanol and 1,2-Alkanediamine Derivatives against Strongyloides Venezuelensis. Parasit. Vectors 2016, 9, 364. [Google Scholar] [CrossRef]
- Thamsborg, S.M.; Ketzis, J.; Horii, Y.; Matthews, J.B. Strongyloides Spp. Infections of Veterinary Importance. Parasitology 2017, 144, 274–284. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Zhou, X.; Li, J.; Yan, G.; James, A.A.; Chen, X. Review: Strongyloidiasis: An Emerging Infectious Disease in China. Am. J. Trop. Med. Hyg. 2013, 88, 420–425. [Google Scholar] [CrossRef]
- Henriquez-Camacho, C.; Gotuzzo, E.; Echevarria, J.; White, A.C.; Terashima, A.; Samalvides, F.; Pérez-Molina, J.A.; Plana, M.N. Ivermectin versus Albendazole or Thiabendazole for Strongyloides Stercoralis Infection. Cochrane Database Syst. Rev. 2016, 2016, CD007745. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Díaz-Menéndez, M.; Pérez-Ayala, A.; Ferrere, F.; Monje, B.; Norman, F.; López-Vélez, R. Tratamiento de las enfermedades causadas por parásitos. Enferm. Infecc. Microbiol. Clin. 2010, 28, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Corti, M. Strongyloides Stercoralis in Immunosuppressed Patients. Arch. Clin. Infect. Dis. 2016, 11, e27510. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, G. Anthelmintic Resistance in Equine Parasites—Detection, Potential Clinical Relevance and Implications for Control. Vet. Parasitol. 2012, 185, 2–8. [Google Scholar] [CrossRef]
- Parasitol, I.J.; Article, R. Anthelmintics Resistance; How to Overcome It? Iran. J. Parasitol. 2013, 8, 18–32. [Google Scholar]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Igarashi, I. Effects of Methanolic Extract from Turmeric (Curcuma longa) against the In Vitro Multiplication of Several Babesia Species and Theileria Equi. Parasitologia 2021, 1, 188–196. [Google Scholar] [CrossRef]
- Rodríguez-Garza, N.E.; Gomez-Flores, R.; Quintanilla-Licea, R.; Elizondo-Luévano, J.H.; Romo-Sáenz, C.I.; Marín, M.; Sánchez-Montejo, J.; Muro, A.; Peláez, R.; López-Abán, J. In Vitro Anthelmintic Effect of Mexican Plant Extracts and Partitions Against Trichinella Spiralis and Strongyloides Venezuelensis. Plants 2024, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Patel, P.; Patel, R. Effect of Different Extracts from Celosia Argentea on Calcium and Phosphate Inhibition in Vitro. Int. J. Pharm. Pharm. Sci. 2011, 3, 337–339. [Google Scholar]
- Chang, Y.C.; Chang, F.R.; Khalil, A.T.; Hsieh, P.W.; Wu, Y.C. Cytotoxic Benzophenanthridine and Benzylisoquinoline Alkaloids from Argemone mexicana. Z. Naturforschung-Sect. C J. Biosci. 2003, 58, 521–526. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, D. Berberine: A Pioneer Remedy for Various Ailments. Pharma Innov. 2018, 7, 194–200. [Google Scholar]
- Swayze, E.E.; Griffey, R.H.; Bennett, C.F. Nucleic Acids (Deoxyribonucleic Acid and Ribonucleic Acid). Compr. Med. Chem. II 2007, 2, 1037–1052. [Google Scholar] [CrossRef]
- Bhattacharjee, I.; Chatterjee, S.K.; Chandra, G. Isolation and Identification of Antibacterial Components in Seed Extracts of Argemone mexicana L. (Papaveraceae). Asian Pac. J. Trop. Med. 2010, 3, 547–551. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Garza-Vega, L.M.; Torres-Hernández, Á.D.; Quintanilla-Licea, R.; Chávez-Montes, A. Argemone mexicana (Papaveraceae) y Berberina-Tesoros Ocultos de La Medicina Herbal. Rev. De Cienc. Agroaliment. Y Biotecnología 2024, 1, 5–11. [Google Scholar] [CrossRef]
- Aguilar-Galaviz, L.; Cadena-Iñiguez, J.; Ortega-Amaro, M.A.; García-Flores, D.A.; Loera-Alvarado, G. Sangre de Drago (Jatropha Dioica Sessé) Un Recurso Vegetal Infrautilizado Del Semidesierto Mexicano. Agro-Divulgación 2024, 4, 73–76. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia Graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.d.; Costa, K.S.d.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G.d. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Miranda, F.; Pinoargote Véliz, S. Evaluación de Extracto Etanólico de Hojas de Tomillo Thymus Vulgaris Como Inhibidor de Virulencia En Vibrio Parahaemolyticus. Ciencia Unemi 2021, 14, 81–91. [Google Scholar] [CrossRef]
- Strothmann, A.L.; Berne, M.E.A.; Capella, G.d.A.; Moura, M.Q.d.; Terto, W.D.d.S.; Costa, C.M.d.; Pinheiro, N.B. Antiparasitic Treatment Using Herbs and Spices: A Review of the Literature of the Phytotherapy. Braz. J. Vet. Med. 2022, 44, e004722. [Google Scholar] [CrossRef]
- Chávez-Montes, A.; Bazaldúa Rodríguez, A.F.; Larqué-García, H.; Gutiérrez-Soto, G.; Elizondo-Luévano, J.H. Actividad Antiparasitaria In-Vitro Del Extracto Metanólico de Kalanchoe Daigremontiana (Crassulaceae) En Contra de Entamoeba Histolytica (Amoebida: Entamoebidae) y Trichomonas Vaginalis (Trichomonadida: Trichomonadidae). Sci. Agric. Vita 2024, 1, 1–9. [Google Scholar] [CrossRef]
- Zavala, G.A.; van Dulm, E.; Doak, C.M.; García, O.P.; Polman, K.; Campos-Ponce, M. Ascariasis, Amebiasis and Giardiasis in Mexican Children: Distribution and Geographical, Environmental and Socioeconomic Risk Factors. J. Parasit. Dis. 2020, 44, 829–836. [Google Scholar] [CrossRef]
- Vega, J.T.S.; López, R.H.; Galicia, A.E.M.; Fuentes, H.A.C.; Castor, A.C.T.; Aguilar, D.I.S.; Aya, D.A.P. Strongyloidiasis in Mexico: A Neglected Disease. EAS J. Parasitol. Infect. Dis. 2023, 5, 43–51. [Google Scholar] [CrossRef]
- Morales-Espinoza, E.M.; Sánchez-Pérez, H.J.; García-Gil, M.d.M.; Vargas-Morales, G.; Méndez-Sánchez, J.D.; Pérez-Ramírez, M. Intestinal Parasites in Children, in Highly Deprived Areas in the Border Region of Chiapas, Mexico. Salud Publica de Mex. 2003, 45, 379–388. [Google Scholar] [CrossRef]
- Gordon, C.; Kurscheid, J.; Jones, M.; Gray, D.; McManus, D. Soil-Transmitted Helminths in Tropical Australia and Asia. Trop. Med. Infect. Dis. 2017, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic Activity Screening of Some Indigenous Thai Plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Biological Activity and Lymphoma Cell Growth Inhibition by Selected Mexican Medicinal Plants. Life 2023, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of Antiradical Properties of Antioxidants Using DPPH Assay: A Critical Review and Results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Clark-Pérez, D.L.; Romo-Sáenz, C.I.; Ramírez-Villalobos, J.M.; Tamez-Guerra, P.; Caballero-Hernández, D.; Delgado-Miranda, A.L.; García, A.; Elizondo-Luevano, J.H.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro and In Vivo Antitumor Activity of Lophocereus marginatus (DC.) S. Arias & Terrazas Endophytic Aspergillus versicolor and Metarhizium anisopliae Extracts Against the Murine Lymphoma L5178Y-R. Microorganisms 2024, 12, 2310. [Google Scholar] [CrossRef]
- Yeap, S.K.; Alitheen, N.B.; Ali, A.M.; Omar, A.R.; Raha, A.R.; Suraini, A.A.; Muhajir, A.H. Effect of Rhaphidophora Korthalsii Methanol Extract on Human Peripheral Blood Mononuclear Cell (PBMC) Proliferation and Cytolytic Activity toward HepG2. J. Ethnopharmacol. 2007, 114, 406–411. [Google Scholar] [CrossRef]
- Berrington, D.; Lall, N. Anticancer Activity of Certain Herbs and Spices on the Cervical Epithelial Carcinoma (HeLa) Cell Line. Evid.-Based Complement. Altern. Med. 2012, 2012, 564927. [Google Scholar] [CrossRef]
- Troiano, G.; Nante, N. Human Trichinellosis in Italy: An Epidemiological Review since 1989. J. Prev. Med. Hyg. 2019, 60, E71–E75. [Google Scholar] [CrossRef]
- Mirzaei, L.; Ashrafi, K.; Atrkar Roushan, Z.; Mahmoudi, M.R.; Shenavar Masooleh, I.; Rahmati, B.; Saadat, F.; Mirjalali, H.; Sharifdini, M. Strongyloides Stercoralis and Other Intestinal Parasites in Patients Receiving Immunosuppressive Drugs in Northern Iran: A Closer Look at Risk Factors. Epidemiol. Health 2021, 43, e2021009. [Google Scholar] [CrossRef]
- Buonfrate, D.; Rodari, P.; Barda, B.; Page, W.; Einsiedel, L.; Watts, M.R. Current Pharmacotherapeutic Strategies for Strongyloidiasis and the Complications in Its Treatment. Expert. Opin. Pharmacother. 2022, 23, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, M.; Tava, A.; Mancini, S.; Tedesco, D.; Perrucci, S. In Vitro Anthelmintic Activity of Saponins from Medicago spp. Against Sheep Gastrointestinal Nematodes. Molecules 2020, 25, 242. [Google Scholar] [CrossRef] [PubMed]
- Boyko, O.; Brygadyrenko, V. Survival of Nematode Larvae Strongyloides Papillosus and Haemonchus Contortus under the Influence of Various Groups of Organic Compounds. Diversity 2023, 15, 254. [Google Scholar] [CrossRef]
- da Silva, G.D.; de Lima, H.G.; de Sousa, N.B.; de Jesus Genipapeiro, I.L.; Uzêda, R.S.; Branco, A.; Costa, S.L.; Batatinha, M.J.M.; Botura, M.B. In Vitro Anthelmintic Evaluation of Three Alkaloids against Gastrointestinal Nematodes of Goats. Vet. Parasitol. 2021, 296, 109505. [Google Scholar] [CrossRef] [PubMed]
- Raghav, D.; Ashraf, S.M.; Mohan, L.; Rathinasamy, K. Berberine Induces Toxicity in HeLa Cells through Perturbation of Microtubule Polymerization by Binding to Tubulin at a Unique Site. Biochemistry 2017, 56, 2594–2611. [Google Scholar] [CrossRef]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin—Old Drug, New Tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef]
- Molina-Garza, Z.J.; Bazaldúa-Rodríguez, A.F.; Quintanilla-Licea, R.; Galaviz-Silva, L. Anti-Trypanosoma Cruzi Activity of 10 Medicinal Plants Used in Northeast Mexico. Acta Trop. 2014, 136, 14–18. [Google Scholar] [CrossRef]
- López-Aroche, U.; Salinas-Sánchez, D.O.; Mendoza de Gives, P.; López-Arellano, M.E.; Liébano-Hernández, E.; Valladares-Cisneros, G.; Arias-Ataide, D.M.; Hernández-Velázquez, V. In Vitro Nematicidal Effects of Medicinal Plants from the Sierra de Huautla, Biosphere Reserve, Morelos, Mexico against Haemonchus Contortus Infective Larvae. J. Helminthol. 2008, 82, 25–31. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Delgado-Núñez, E.J.; Bahena-Vicencio, A.; Villa-Mancera, A.; Zamilpa, A.; González-Cortazar, M.; Rivero-Pérez, N.; Flores-Franco, G.; López-Arellano, M.E.; Mendoza de Gives, P. In Vitro Nematocidal Properties from Two Extracts: Lippia Graveolens Leaves and Delonix Regia Flowers Against Eggs and Infective Larvae of Haemonchus Contortus. J. Med. Food 2022, 25, 329–337. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Mata-Cárdenas, B.; Vargas-Villarreal, J.; Bazaldúa-Rodríguez, A.; Kavimngeles-Hernández, I.; Garza-González, J.; Hernández-García, M. Antiprotozoal Activity against Entamoeba Histolytica of Plants Used in Northeast Mexican Traditional Medicine. Bioactive Compounds from Lippia Graveolens and Ruta Chalepensis. Molecules 2014, 19, 21044–21065. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal Activity against Entamoeba Histolytica of Flavonoids Isolated from Lippia Graveolens Kunth. Molecules 2020, 25, 2464. [Google Scholar] [CrossRef] [PubMed]
- Boyko, O.; Brygadyrenko, V. Survival of Nematode Larvae after Treatment with Eugenol, Isoeugenol, Thymol, and Carvacrol. Front. Biosci. (Elite Ed.) 2023, 15, 25. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Ramos-Payán, R.; Contreras-Angulo, L.A.; Gutiérrez-Grijalva, E.P.; León-Félix, J.; Villicaña, C.; Angulo-Escalante, M.A.; Heredia, J.B. Cytotoxic Activity of Polyphenol Extracts from Three Oregano Species: Hedeoma Patens, Lippia Graveolens and Lippia Palmeri, and Antiproliferative Potential of Lippia Graveolens against Two Types of Breast Cancer Cell Lines (MDA-MB-231 and MCF-7). Molecules 2022, 27, 5240. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.M.; Fonseca, F.S.A.; Silva, J.C.R.L.; Silva, A.M.; Silva, J.R.; Martins, E.R. Essential Oil Composition in Natural Population of Lippia Origanoides (Verbenaceae) during Dry and Rainy Seasons. Rev. Biol. Trop. 2019, 67, 278–285. [Google Scholar] [CrossRef]
- Soto-Domínguez, A.; García-Garza, R.; Ramírez-Casas, Y.; Morán-Martínez, J.; Serrano-Gallardo, L.B. El Extracto Acuoso de Orégano (Lippia Graveolens HBK) Del Norte de México Tiene Actividad Antioxidante Sin Mostrar Un Efecto Tóxico in Vitro e in Vivo. Int. J. Morphol. 2012, 30, 937–944. [Google Scholar] [CrossRef]
- Aranda-López, Y.; López-López, L.; Castro, K.E.N.; Ponce-Regalado, M.D.; Becerril-Villanueva, L.E.; Girón-Pérez, M.I.; Del Río-Araiza, V.H.; Morales-Montor, J. Cysticidal Effect of a Pure Naphthoquinone on Taenia Crassiceps Cysticerci. Parasitol. Res. 2021, 120, 3783–3794. [Google Scholar] [CrossRef]
- Silva-Belmares, Y.; Rivas-Morales, C.; Viveros-Valdez, E.; de la Cruz-Galicia, M.G.; Carranza-Rosales, P. Antimicrobial and Cytotoxic Activities from Jatropha Dioica Roots. Pak. J. Biol. Sci. 2014, 17, 748–750. [Google Scholar] [CrossRef]
- Fernández-Villascan, C.; Patiño-Herrera, R.; Patino, I.; Octavio Sánchez Vargas, L.; Salado-Leza, D.; Pérez, E. Invasive Candidiasis: A Promising Approach Using Jatropha Dioica Extracts and Nanotechnology. Chem. Biodivers. 2024, 22, e202402339. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Castro-Ríos, R.; Sánchez-García, E.; Hernández-García, M.E.; Vargas-Villarreal, J.; Rodríguez-Luis, O.E.; Chávez-Montes, A. In Vitro Study of Antiamoebic Activity of Methanol Extracts of Argemone mexicana on Trophozoites of Entamoeba Histolytica HM1-IMSS. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7453787. [Google Scholar] [CrossRef]
- Elizondo-Luevano, J.H.; Verde-Star, J.; González-Horta, A.; Castro-Ríos, R.; Hernández-García, M.E.; Chávez-Montes, A. In Vitro Effect of Methanolic Extract of Argemone mexicana against Trichomonas Vaginalis. Korean J. Parasitol. 2020, 58, 135–145. [Google Scholar] [CrossRef]
- Singh, L.; Gupta, S. Ethnopharmacological Aspects of Argemone mexicana Linn., a Significant Plant Species, in Traditional System of Medicine. Int. Arch. Appl. Sci. Technol. 2019, 10, 143–150. [Google Scholar]
- Elizondo-Luévano, J.H.; Castro-Ríos, R.; Vicente, B.; Fernández-Soto, P.; López-Aban, J.; Muro, A.; Chávez-Montes, A. In Vitro Antischistosomal Activity of the Argemone mexicana Methanolic Extract and Its Main Component Berberine. Iran. J. Parasitol. 2021, 16, 91–100. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Hernández-García, M.E.; Pérez-Narváez, O.A.; Castro-Ríos, R.; Chávez-Montes, A. Berberina, Curcumina y Quercetina Como Potenciales Agentes Con Capacidad Antiparasitaria. Rev. Biol. Trop. 2020, 68, 1241–1249. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Darwish, N.H.E.; Sudha, T.; Bahlouli, S.; Kellou, D.; Benelmouffok, A.B.; Chader, H.; Rajabi, M.; Benali, Y.; Mousa, S.A. In Vitro Antifungal and Topical Anti-Inflammatory Properties of Essential Oil from Wild-Growing Thymus Vulgaris (Lamiaceae) Used for Medicinal Purposes in Algeria: A New Source of Carvacrol. Sci. Pharm. 2020, 88, 33. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Pérez-Narváez, O.A.; Sánchez-García, E.; Castro-Ríos, R.; Hernández-García, M.E.; Chávez-Montes, A. In-Vitro Effect of Kalanchoe Daigremontiana and Its Main Component, Quercetin against Entamoeba Histolytica and Trichomonas Vaginalis. Iran. J. Parasitol. 2021, 16, 394–401. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Gomez-Flores, R.; Verde-Star, M.J.; Tamez-Guerra, P.; Romo-Sáenz, C.I.; Chávez-Montes, A.; Rodríguez-Garza, N.E.; Quintanilla-Licea, R. In Vitro Cytotoxic Activity of Methanol Extracts of Selected Medicinal Plants Traditionally Used in Mexico against Human Hepatocellular Carcinoma. Plants 2022, 11, 2862. [Google Scholar] [CrossRef]
- De La Cruz-Jiménez, L.; Hernández-Torres, M.A.; Monroy-García, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; Gonzalez-Villasana, V.; Viveros-Valdez, E. Biological Activities of Seven Medicinal Plants Used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Olounladé, P.A.; Azando, E.V.B.; Hounzangbé-Adoté, M.S.; Ha, T.B.T.; Leroy, E.; Moulis, C.; Fabre, N.; Magnaval, J.F.; Hoste, H.; Valentin, A. In Vitro Anthelmintic Activity of the Essential Oils of Zanthoxylum Zanthoxyloides and Newbouldia Laevis against Strongyloides Ratti. Parasitol. Res. 2012, 110, 1427–1433. [Google Scholar] [CrossRef]
- Aderogba, M.A.; McGaw, L.J.; Bagla, V.P.; Eloff, J.N.; Abegaz, B.M. In Vitro Antifungal Activity of the Acetone Extract and Two Isolated Compounds from the Weed, Pseudognaphalium Luteoalbum. S. Afr. J. Bot. 2014, 94, 74–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).