Manganese Oxide Carbon-Based Nanocomposite in Energy Storage Applications
Abstract
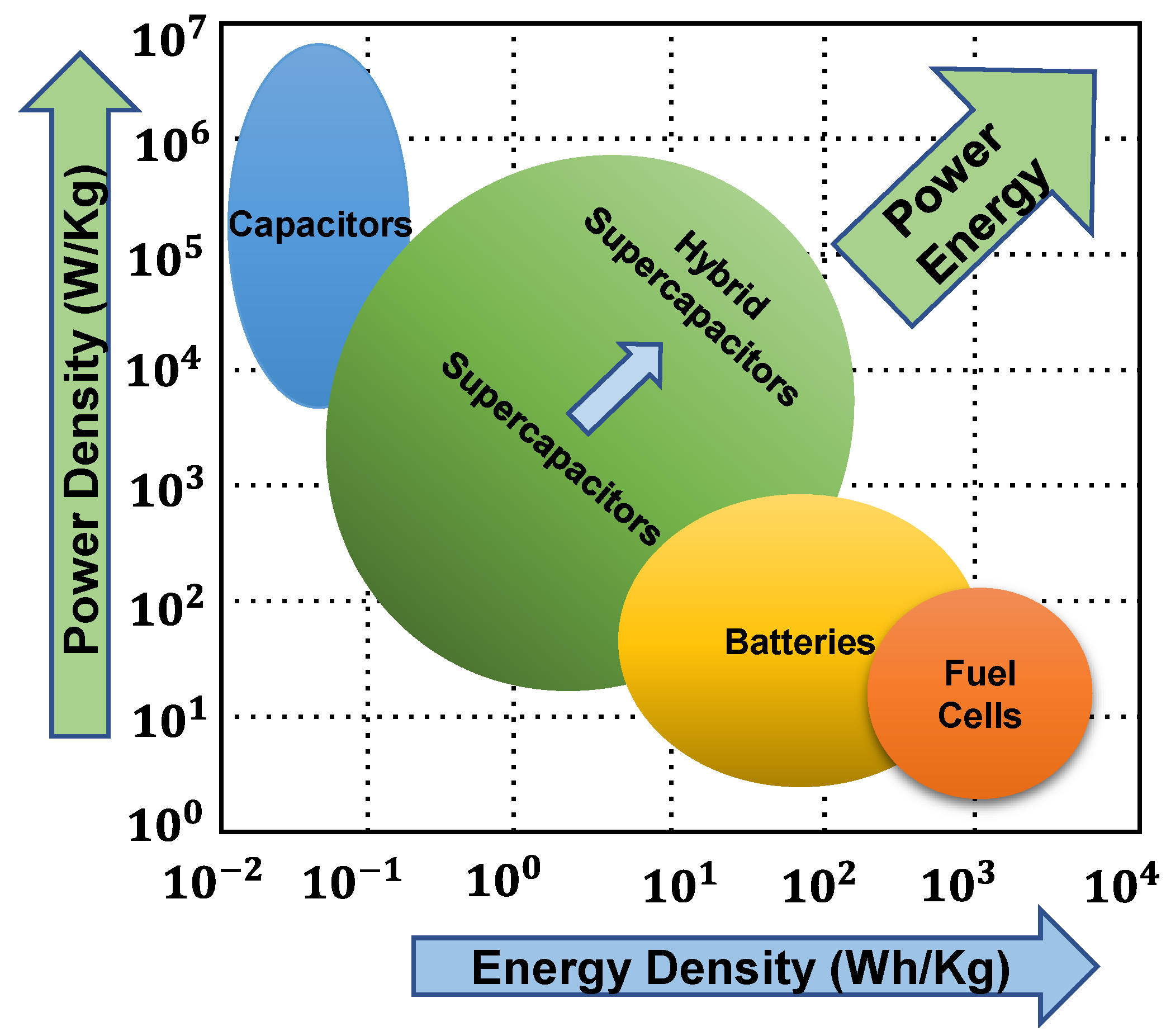
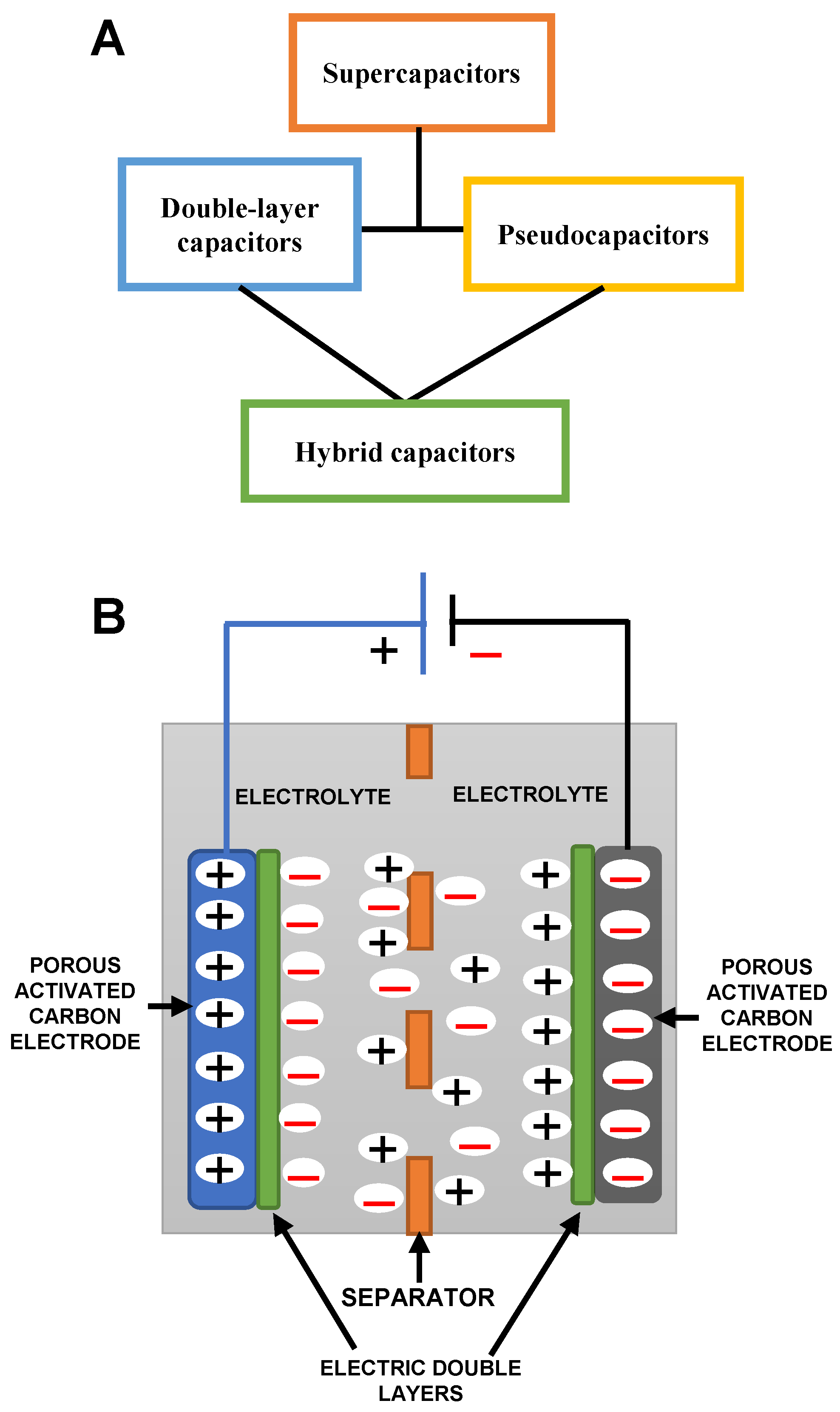
1. Introduction
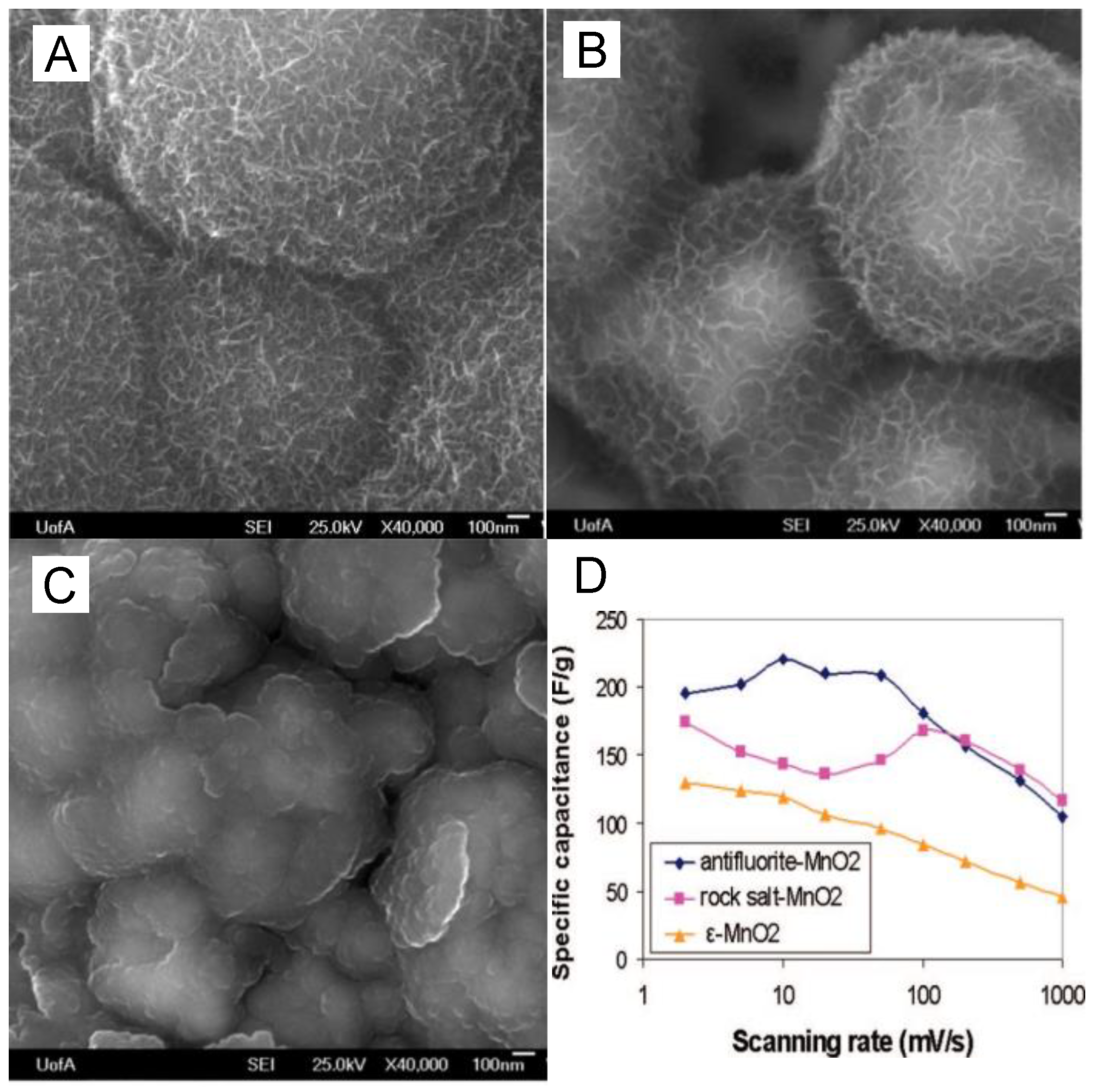
2. Manganese Oxide
3. Carbon-Based Material/MnOx Composite
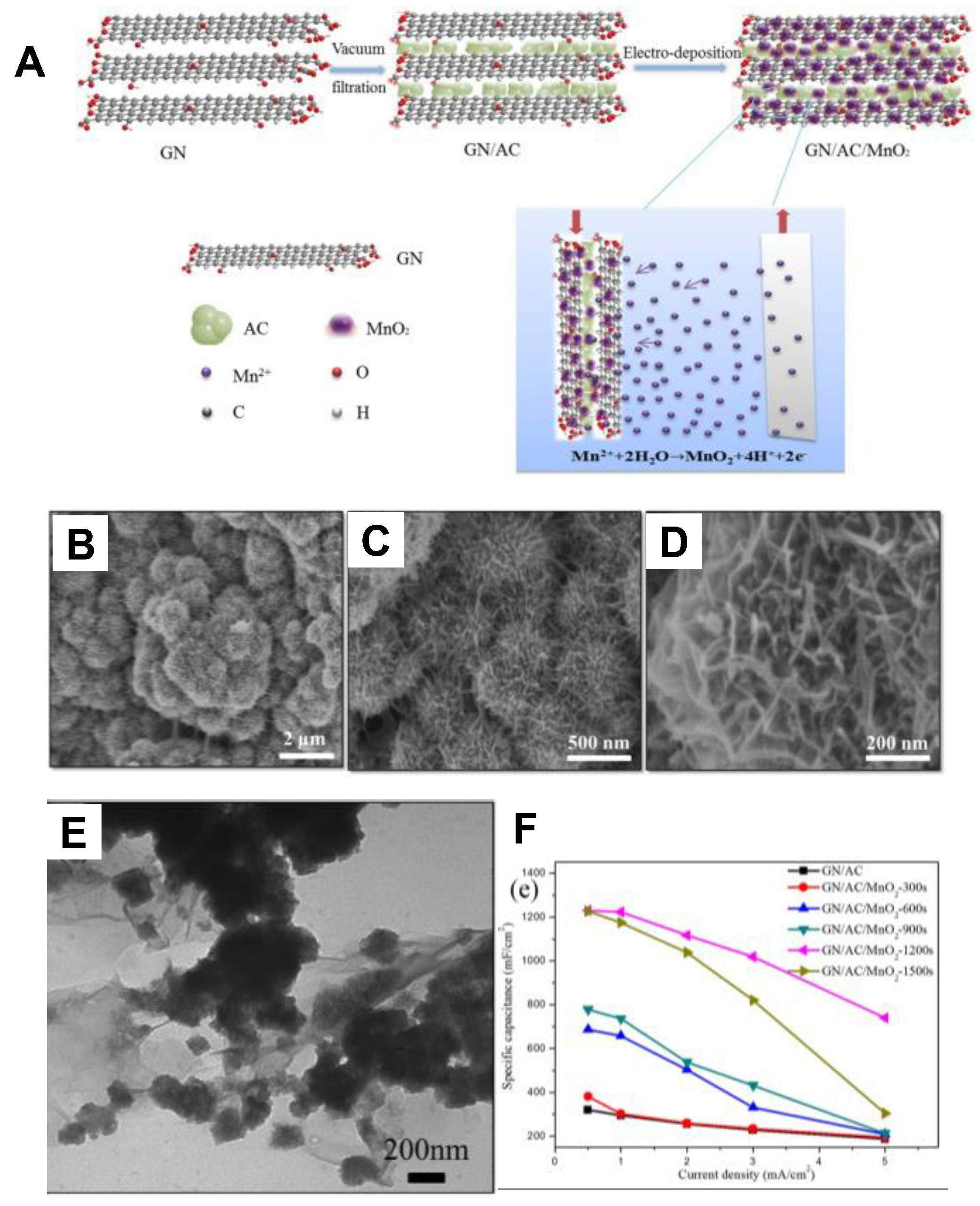
3.1. Activated Carbon/MnOx Composite
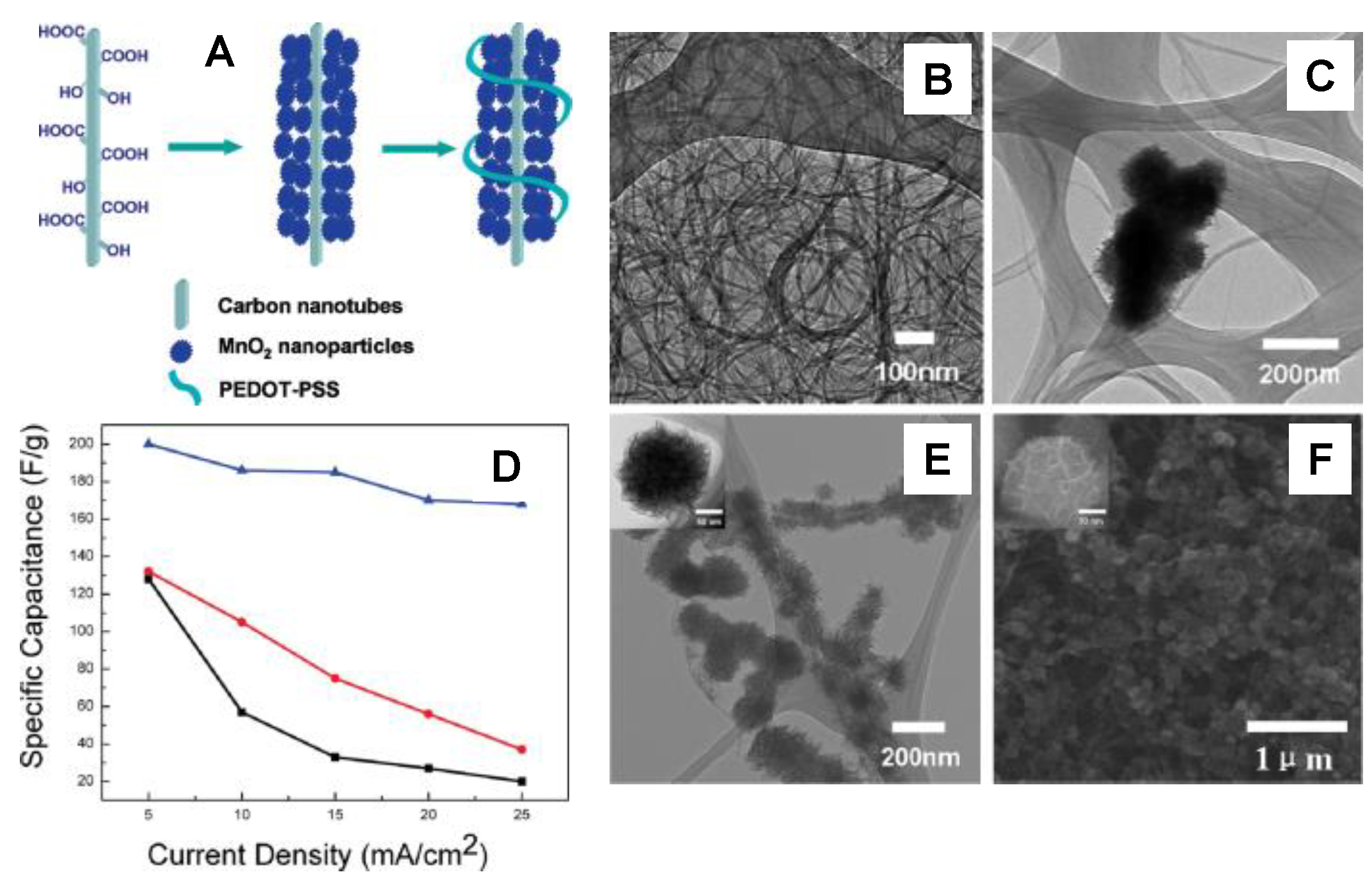
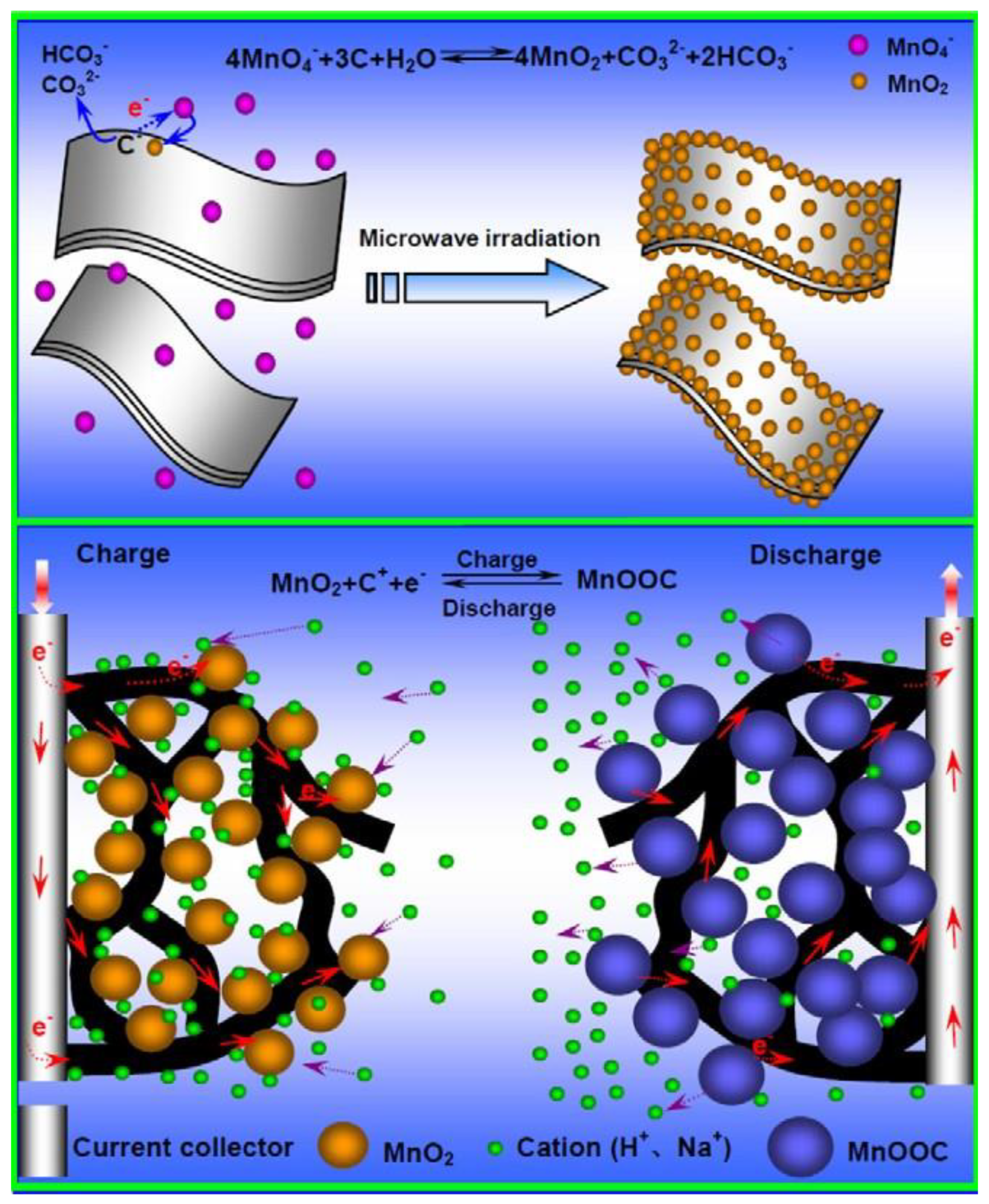
3.2. Carbon Nanotubes/MnOx Composite
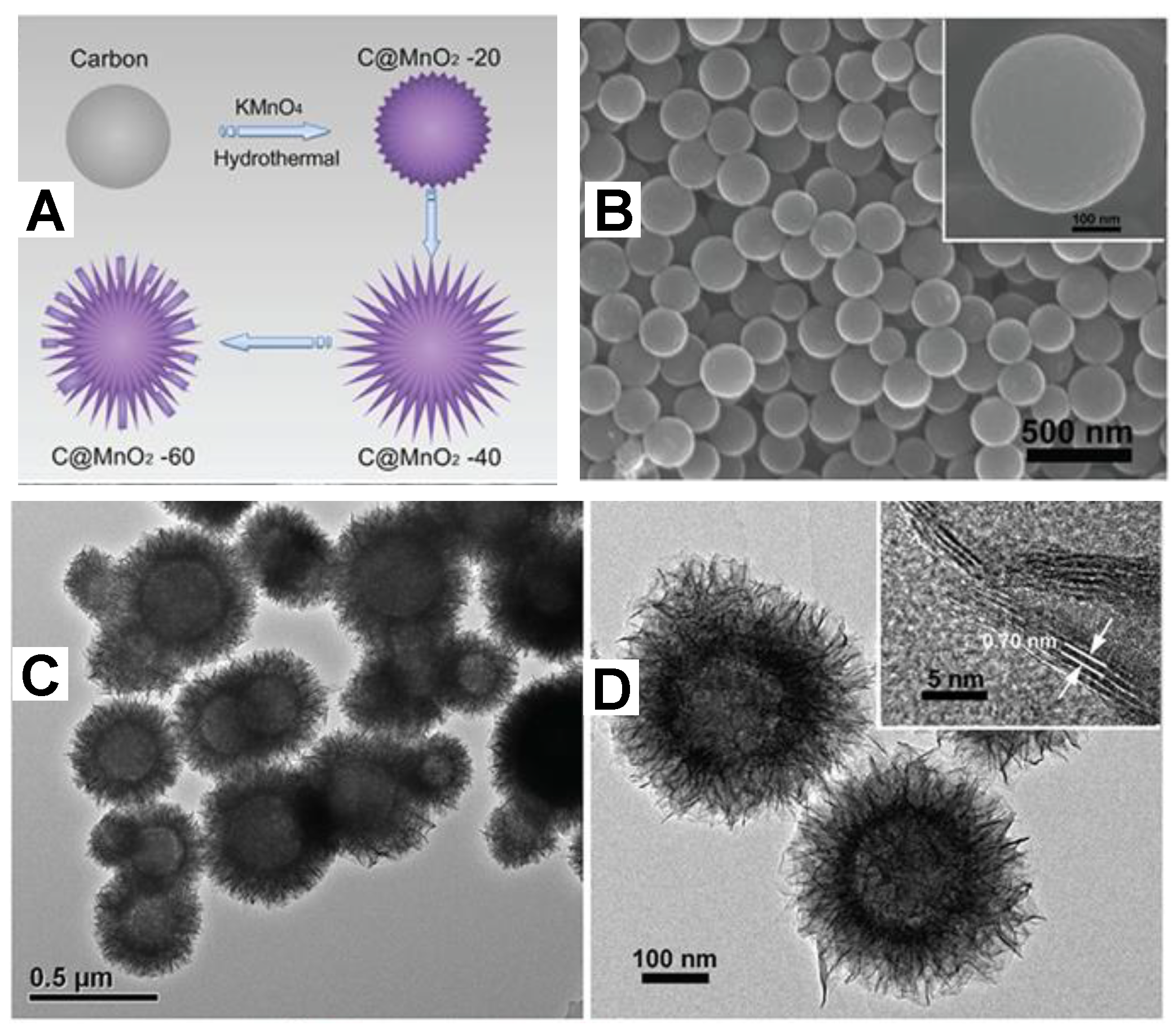
3.3. Carbon Spheres/MnOx Composite
3.4. Carbon Nanofibers/MnOx Composite
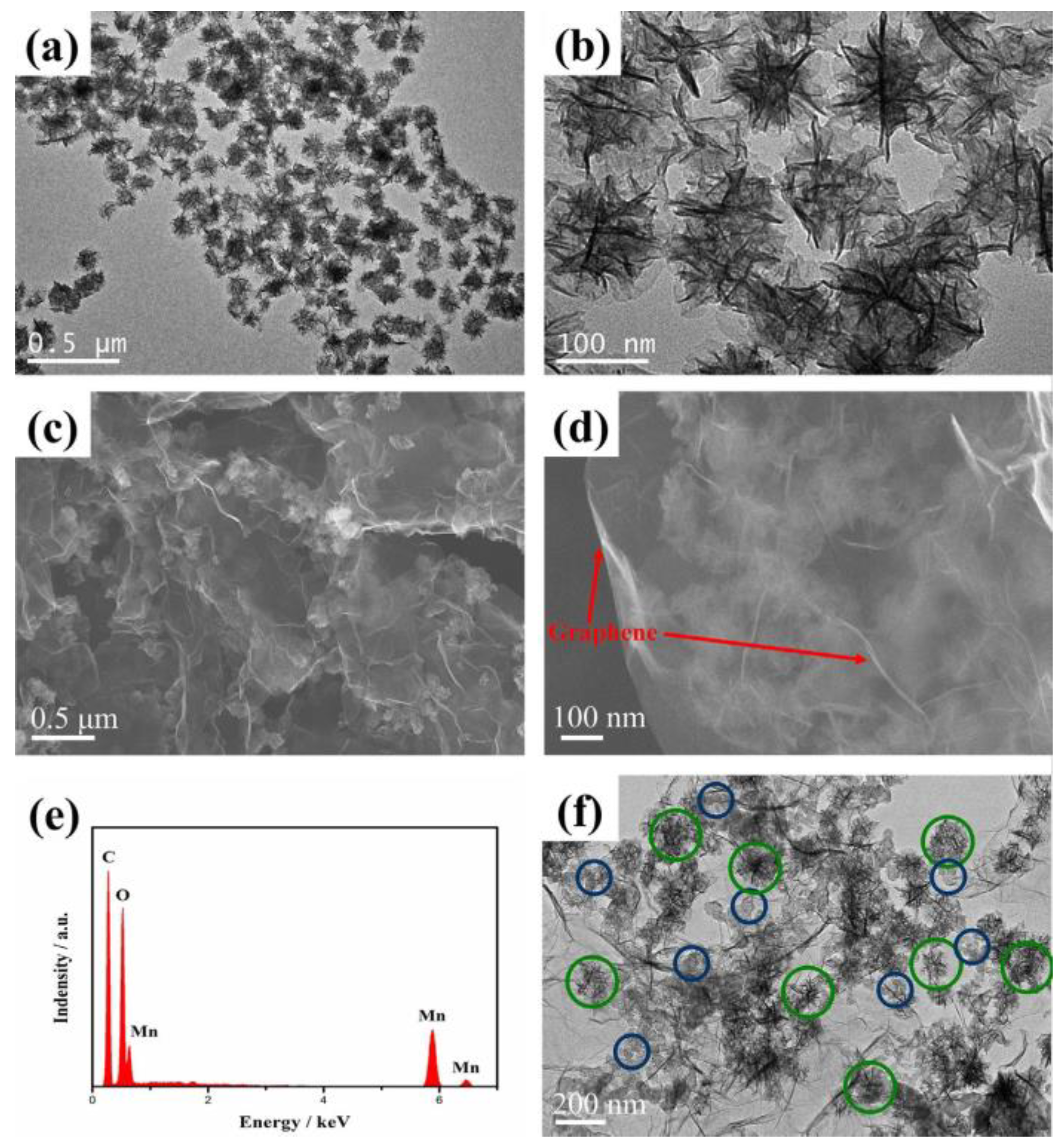
3.5. Graphene/MnOx Composite
3.6. Other Materials/MnOx Composite
4. Summary and Future Perspective
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, A.Y.; Saravanan, L.; Tseng, C.M.; Wang, F.K.; Huang, J.T. Effect of Composition Ratios on the Performance of Gra-phene/Carbon Nanotube/Manganese Oxide Composites toward Supercapacitor Applications. ACS Omega 2019, 5, 578–587. [Google Scholar] [CrossRef]
- Huang, T.; Zehai, Q.; Dewu, W.; Zhibiao, H. Bamboo-based activated carbon@ MnO2 nanocomposites for flexible high-performance supercapacitor electrode materials. Int. J. Electrochem. Sci. 2015, 10, 6312–6323. [Google Scholar]
- Xu, M.; Kong, L.; Zhou, W.; Li, H. Hydrothermal synthesis and pseudocapacitance properties of α-MnO2 hollow spheres and hollow urchins. J. Phys. Chem. C 2007, 111, 19141–19147. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef] [PubMed]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Ataherian, F.; Ghaemi, M.; Gholami, A. Capacitive behavior of nanostructured MnO2 prepared by sonochem-istry method. Electrochim. Acta 2007, 52, 2806–2814. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.; Chen, S.; Hammond, P.T.; Shao-Horn, Y. Carbon Nanotube/Manganese Oxide Ultrathin Film Electrodes for Electrochemical Capacitors. ACS Nano 2010, 4, 3889–3896. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nanosci. Technol. 2009, 320–329. [Google Scholar] [CrossRef]
- Lee, C.Y.; Tsai, H.M.; Chuang, H.J.; Li, S.Y.; Lin, P.; Tseng, T.Y. Characteristics and electrochemical performance of supercapacitors with manganese oxide-carbon nanotube nanocomposite electrodes. J. Electrochem. Soc. 2005, 152, A716. [Google Scholar] [CrossRef]
- Grover, S.; Shekhar, S.; Sharma, R.K.; Singh, G. Multiwalled carbon nanotube supported polypyrrole manganese oxide composite supercapacitor electrode: Role of manganese oxide dispersion in performance evolution. Electrochim. Acta 2014, 116, 137–145. [Google Scholar] [CrossRef]
- Dong, X.; Shen, W.; Gu, J.; Xiong, L.; Zhu, Y.; Li, A.H.; Shi, J. MnO2-Embedded-in-Mesoporous-Carbon-Wall Structure for Use as Electrochemical Capacitors. J. Phys. Chem. B 2006, 110, 6015–6019. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pinero, E.; Khomenko, V.; Frackowiak, E.; Beguin, F. Performance of manganese oxide/CNTs composites as elec-trode materials for electrochemical capacitors. J. Electrochem. Soc. 2004, 152, A229. [Google Scholar] [CrossRef]
- Che, G.; Lakshmi, B.B.; Martin, C.R.; Fisher, E.R. Metal-Nanocluster-Filled Carbon Nanotubes: Catalytic Properties and Possible Applications in Electrochemical Energy Storage and Production. Langmuir 1999, 15, 750–758. [Google Scholar] [CrossRef]
- Das, R.K.; Liu, B.; Reynolds, J.R.; Rinzler, A.G. Engineered Macroporosity in Single-Wall Carbon Nanotube Films. Nano Lett. 2009, 9, 677–683. [Google Scholar] [CrossRef]
- Deng, X.; Bai, X.; Cai, Z.; Huang, M.; Chen, X.; Huang, B.; Chen, Y. Renewable carbon foam/δ-MnO2 composites with well-defined hierarchical microstructure as supercapacitor electrodes. J. Mater. Res. Technol. 2020, 9, 8544–8555. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Zhao, W.; Duan, C.; Ni, Y. Flexible N-doped reduced graphene oxide/carbon nanotube-MnO2 film as a multifunctional material for high-performance supercapacitors, catalysts and sensors. J. Mater. 2020, 6, 523–531. [Google Scholar]
- Wen, J.; Chen, X.; Huang, M.; Yang, W.; Deng, J. Core–shell-structured MnO2@carbon spheres and nitrogen-doped activated carbon for asymmetric supercapacitors with enhanced energy density. J. Chem. Sci. 2020, 132, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Cui, R.; Huang, H.; Liu, B.; Li, Y.; Sun, B. MnO2/Porous Carbon Nanotube/MnO2 Nanocomposites for High-Performance Supercapacitor. ACS Appl. Nano Mater. 2020, 3, 11152–11159. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Wang, J. Manganese-Oxide-Based Electrode Materials for Energy Storage Applications: How Close Are We to the Theoretical Capacitance? Adv. Mater. 2018, 30, e1802569. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Cao, R. Physical and electrochemical characterization of hydrous ruthenium oxide/ordered mesoporous carbon composites as supercapacitor. Microporous Mesoporous Mater. 2008, 111, 32–38. [Google Scholar] [CrossRef]
- Kim, H.; Popov, B.N. Characterization of hydrous ruthenium oxide/carbon nanocomposite supercapacitors prepared by a colloidal method. J. Power Sources 2002, 104, 52–61. [Google Scholar] [CrossRef]
- Ye, J.-S.; Cui, H.F.; Liu, X.; Lim, T.M.; Zhang, W.-D.; Sheu, F.-S. Preparation and Characterization of Aligned Carbon Nanotube-Ruthenium Oxide Nanocomposites for Supercapacitors. Small 2005, 1, 560–565. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, L.; Gao, B.; Su, L.; Zhang, X. Synthesis and utilization of RuO2·xH2O nanodots well dispersed on poly(sodium 4-styrene sulfonate) functionalized multi-walled carbon nanotubes for supercapacitors. J. Mater. Chem. 2008, 19, 246–252. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Zhang, Y.; Zhao, Y.; Yin, H.; Hua, Q.; Yuan, J.; Tang, J. A ternary composite RuO2@SWCNT/graphene for high performance electrochemical capacitors. Mater. Lett. 2020, 259, 126860. [Google Scholar] [CrossRef]
- Chen, L.Y.; Hou, Y.; Kang, J.L.; Hirata, A.; Fujita, T.; Chen, M. Toward the Theoretical Capacitance of RuO2Reinforced by Highly Conductive Nanoporous Gold. Adv. Energy Mater. 2013, 3, 851–856. [Google Scholar] [CrossRef]
- Soudan, P.; Gaudet, J.; Guay, D.; Bélanger, D.; Schulz, R. Electrochemical Properties of Ruthenium-Based Nanocrystalline Materials as Electrodes for Supercapacitors. Chem. Mater. 2002, 14, 1210–1215. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Phase-controlled synthesis of MnO2 nanocrystals by anodic electrodeposition: Implica-tions for high-rate capability electrochemical supercapacitors. J. Phys. Chem. C 2008, 112, 15075–15083. [Google Scholar] [CrossRef]
- Munoz-Paez, A. Transition Metal Oxides: Geometric and Electronic Structures: Introducing Solid State Topics in Inorganic Chemistry Courses. J. Chem. Educ. 1994, 71, 381–388. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Hu, C.C.; Tsou, T.W. Ideal capacitive behavior of hydrous manganese oxide prepared by anodic deposition. Electrochem. Commun. 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L.L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, P.; Guo, B.; Cheng, Z.; Fan, H.; Yang, M.; Yang, X.; Li, J. Electrodeposition of manganese dioxide film on activated carbon paper and its application in supercapacitors with high rate capability. RSC Adv. 2014, 4, 64187–64192. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J. MnOx nanosheets for improved electrochemical performances through bilayer nano-architecting. J. Power Sources 2015, 286, 394–399. [Google Scholar] [CrossRef]
- Tang, X.; Liu, Z.-H.; Zhang, C.; Yang, Z.; Wang, Z. Synthesis and capacitive property of hierarchical hollow manganese oxide nanospheres with large specific surface area. J. Power Sources 2009, 193, 939–943. [Google Scholar] [CrossRef]
- Li, W.; Xu, K.; Li, B.; Sun, J.; Jiang, F.; Yu, Z.; Zou, R.; Chen, Z.; Hu, J. Cover Picture: MnO2 Nanoflower Arrays with High Rate Capability for Flexible Supercapacitors (ChemElectroChem 6/2014). ChemElectroChem 2014, 1, 960. [Google Scholar] [CrossRef]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.P.; Wang, Z.L. Low-Cost High-Performance Solid-State Asymmetric Supercapacitors Based on MnO2 Nanowires and Fe2O3 Nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef]
- Ma, S.-B.; Ahn, K.-Y.; Lee, E.-S.; Oh, K.-H.; Kim, K.-B. Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45, 375–382. [Google Scholar] [CrossRef]
- Kim, G.; Ryu, I.; Yim, S. Retarded saturation of the areal capacitance using 3D-aligned MnO2 thin film nanostructures as a supercapacitor electrode. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Xu, H.; Lu, L.; Zhao, H. One-step synthesis of mesoporous MnO2/carbon sphere composites for asymmetric elec-trochemical capacitors. J. Mater. Chem. A 2015, 3, 1127–1132. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2010, 40, 1697–1721. [Google Scholar] [CrossRef]
- Chang, H.-W.; Jeng-Lung, C.; Chen, J.-L.; Chen, C.-L.; Lee, J.-F.; Chen, J.-M.; Tsai, Y.-C.; Chang, C.-M.; Yeh, P.-H.; Chou, W.-C.; et al. Nanoflaky MnO2/functionalized carbon nanotubes for supercapacitors: An in situ X-ray absorption spectroscopic investigation. Nanoscale 2015, 7, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.H.P.; Nascimento, M.L.F.; de Oliveira, H.P. Carbon nanotube@MnO2@ polypyrrole composites: Chemical syn-thesis, characterization and application in supercapacitors. Mater. Res. 2016, 19, 1080–1087. [Google Scholar] [CrossRef]
- Bai, Z.; Li, H.; Li, M.; Li, C.; Wang, X.; Qu, C.; Yang, B. Flexible carbon nanotubes-MnO2/reduced graphene oxide-polyvinylidene fluoride films for supercapacitor electrodes. Int. J. Hydrogen Energy 2015, 40, 16306–16315. [Google Scholar] [CrossRef]
- Xiong, T.; Lee, W.S.V.; Huang, X.; Xue, J.M. Mn3O4/reduced graphene oxide based supercapacitor with ultra-long cycling performance. J. Mater. Chem. A 2017, 5, 12762–12768. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Bacsa, R.; Laurent, C.; Peigney, A.; Bacsa, W.; Vaugien, T.; Rousset, A. High specific surface area carbon nanotubes from catalytic chemical vapor deposition process. Chem. Phys. Lett. 2000, 323, 566–571. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, Y.; Chen, B.Z. A synthesis method of MnO2/activated carbon composite for electrochemical supercapacitors. J. Electrochem. Soc. 2015, 162, A1654. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Jo, J.; Jang, H.; Kim, I.; Kang, D.; Kim, K.Y. Activated carbon/manganese dioxide hybrid electrodes for high performance thin film supercapacitors. Appl. Phys. Lett. 2014, 104, 243901. [Google Scholar] [CrossRef]
- Xu, L.; Jia, M.; Li, Y.; Jin, X.; Zhang, F. High-performance MnO2-deposited graphene/activated carbon film electrodes for flexible solid-state supercapacitor. Sci. Rep. 2017, 7, 12857. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Tan, G.; Qiu, Y.; Guo, B.; Cheng, F.; Fan, H. High performance electrochemical capacitors based on MnO2/activated-carbon-paper. J. Mater. Chem. C 2015, 3, 6166–6171. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Moon, Y.S.; Kim, D.; Lee, G.; Hong, S.Y.; Kim, K.K.; Park, S.M.; Ha, J.S. Fabrication of flexible micro-supercapacitor array with patterned graphene foam/MWNT-COOH/MnOx electrodes and its application. Carbon 2015, 81, 29–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Wang, S. Hierarchically structured MnO2/graphene/carbon fiber and porous graphene hydrogel wrapped copper wire for fiber-based flexible all-solid-state asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 11215–11223. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Zhu, J.; Kharistal, D.J.; Zhao, W.; Lalia, B.S.; Hng, H.H.; Yan, Q. High-Power and High-Energy-Density Flexible Pseudocapacitor Electrodes Made from Porous CuO Nanobelts and Single-Walled Carbon Nanotubes. ACS Nano 2011, 5, 2013–2019. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, Y.; Hobson, T.; Liu, J. Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett. 2010, 10, 2727–2733. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, S.; Chen, M.; Zhang, Y.; Zheng, L.; Li, Q. Oxygen Evolution Assisted Fabrication of Highly Loaded Carbon Nanotube/MnO2 Hybrid Films for High-Performance Flexible Pseudosupercapacitors. Small 2016, 12, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.; Shin, D.; Shin, J.; Hwang, H.; Seo, B.; Lee, J.; Choi, W. DC-field-driven combustion waves for one-step fabrication of reduced manganese oxide/multi-walled carbon nanotube hybrid nanostructures as high-performance supercapacitor elec-trodes. J. Mater. Chem. A 2017, 5, 24707–24719. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Wang, L.; Sheng, P.; Peng, H. Fiber-based MnO2/carbon nanotube/polyimide asymmetric supercapacitor. Carbon 2017, 125, 595–604. [Google Scholar] [CrossRef]
- Wang, L.; Huang, M.; Chen, S.; Kang, L.; He, X.; Lei, Z.; Shi, F.; Xu, H.; Liu, Z.H. δ-MnO2 nanofiber/single-walled carbon nanotube hybrid film for all-solid-state flexible supercapacitors with high performance. J. Mater. Chem. A 2017, 5, 19107–19115. [Google Scholar] [CrossRef]
- Shi, P.; Li, L.; Hua, L.; Qian, Q.; Wang, P.; Zhou, J.; Sun, G.; Huang, W. Design of Amorphous Manganese Oxide@Multiwalled Carbon Nanotube Fiber for Robust Solid-State Supercapacitor. ACS Nano 2017, 11, 444–452. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Shi, E.; Shen, Q.; Shang, Y.; Wu, S.; Wei, J.; Wang, K.; Zhu, H.; Yuan, Q.; et al. Core-double-shell, carbon nanotube@ polypyrrole@ MnO2 sponge as freestanding, compressible supercapacitor electrode. ACS Appl. Mater. Interfaces 2014, 6, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Higgins, T.M.; McAteer, D.; Coelho, J.C.M.; Sanchez, B.M.; Gholamvand, Z.; Moriarty, G.; McEvoy, N.; Berner, N.C.; Duesberg, G.S.; Nicolosi, V.; et al. Effect of Percolation on the Capacitance of Supercapacitor Electrodes Prepared from Composites of Manganese Dioxide Nanoplatelets and Carbon Nanotubes. ACS Nano 2014, 8, 9567–9579. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, H.; Chen, M.; Liu, N.; Li, Q. Graphene-Patched CNT/MnO2 Nanocomposite Papers for the Electrode of High-Performance Flexible Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 3408–3416. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Lu, S.; Varanasi, C.V.; Liu, J. Flexible asymmetric supercapacitors with high energy and high power density in aqueous electrolytes. Nanoscale 2013, 5, 1067–1073. [Google Scholar] [CrossRef]
- Amade, R.; Jover, E.; Caglar, B.; Mutlu, T.; Bertran, E. Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application. J. Power Sources 2011, 196, 5779–5783. [Google Scholar] [CrossRef]
- Qian, J.; Jin, H.; Chen, B.; Lin, M.; Lu, W.; Tang, W.M.; Xiong, W.; Chan, L.W.H.; Lau, S.P.; Yuan, J. Aqueous Manganese Dioxide Ink for Paper-Based Capacitive Energy Storage Devices. Angew. Chem. Int. Ed. 2015, 54, 6800–6803. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Li, X.; He, X. Ultrathin MnO2 nanosheets grown on hollow carbon spheres with enhanced capacitive performance. Phys. Lett. A 2020, 384, 126539. [Google Scholar] [CrossRef]
- Zhang, C.; Hatzell, K.B.; Boota, M.; Dyatkin, B.; Beidaghi, M.; Long, D.; Qiao, W.; Kumbur, E.C.; Gogotsi, Y. Highly porous carbon spheres for electrochemical capacitors and capacitive flowable suspension electrodes. Carbon 2014, 77, 155–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, Y.; Jiang, P. Carbon@MnO2 core–shell nanospheres for flexible high-performance supercapacitor electrode materials. J. Power Sources 2014, 259, 219–226. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Tang, C.-H.; Gong, H.; Li, X.; Wang, J. Hollow spheres of nanocarbon and their manganese dioxide hybrids derived from soft template for supercapacitor application. J. Power Sources 2013, 240, 713–720. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, M.; Chen, H.; Feng, L.; Wang, Z.; Yan, X.; Guan, S. Synthesis of honeycomb MnO2 nanospheres/carbon na-noparticles/graphene composites as electrode materials for supercapacitors. Appl. Surf. Sci. 2015, 357, 1024–1030. [Google Scholar] [CrossRef]
- Ranjusha, R.; Ramakrishna, S.; Nair, A.S.; Anjali, P.; Vineeth, S.; Sonia, T.S.; Sivakumar, N.; Subramanian, K.R.V.; Nair, S.V.; Balakrishnan, A. Fabrication and performance evaluation of button cell supercapacitors based on MnO2 nanowire/carbon nanobead electrodes. RSC Adv. 2013, 3, 17492–17499. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, X.H.; Xiao, X.; Zhai, T.; Dai, J.; Zhang, F.; Hu, B.; Wang, X.; Gong, L.; Chen, J.; et al. Flexible solid-state supercapacitors based on carbon nanoparticles/MnO2 nanorods hybrid structure. ACS Nano 2012, 6, 656–661. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.H.; Sim, H.J.; Lee, J.A.; Choi, A.Y.; Kim, Y.T.; Lepro, X.; Spinks, G.M.; Baughman, R.H.; Kim, S.J. Stretchable, Weavable Coiled Carbon Nanotube/MnO2/Polymer Fiber Solid-State Supercapacitors. Sci. Rep. 2015, 5, 9387. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199. [Google Scholar] [CrossRef]
- Bao, L.; Zang, J.; Li, X. Flexible Zn2SnO4/MnO2 core/shell nanocable− carbon microfiber hybrid composites for high-performance supercapacitor electrodes. Nano Lett. 2011, 11, 1215–1220. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Yang, H.; Sun, Y.; Hu, C.; Huang, Y. Flexible Asymmetric Micro-Supercapacitors Based on Bi2O3and MnO2 Nanoflowers: Larger Areal Mass Promises Higher Energy Density. Adv. Energy Mater. 2015, 5, 1401882. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, W.-Q.; Wang, J.-W.; Chen, B.-Z. Fabrication and electrochemical performance of nanoflake MnO2@carbon fiber coaxial nanocables for supercapacitors. J. Appl. Electrochem. 2016, 46, 241–249. [Google Scholar] [CrossRef]
- Dang, W.; Dong, C.; Zhang, Z.; Chen, G.; Wang, Y.; Guan, H. Self-grown MnO2 nanosheets on carbon fiber paper as high-performance supercapacitors electrodes. Electrochim. Acta 2016, 217, 16–23. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, L.; Xu, C.; Kang, F. High-performance supercapacitors based on graphene/MnO2/activated carbon fiber felt composite electrodes in different neutral electrolytes. RSC Adv. 2016, 6, 12525–12529. [Google Scholar] [CrossRef]
- Ramezani, M.; Fathi, M.; Mahboubi, F. Facile synthesis of ternary MnO2/graphene nanosheets/carbon nanotubes composites with high rate capability for supercapacitor applications. Electrochim. Acta 2015, 174, 345–355. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, L.; Deng, L.; Wang, J.; Kang, L.; Liu, Z.-H. Graphene–MnO2 nanocomposite for high-performance asymmetrical electrochemical capacitor. Mater. Res. Bull. 2014, 49, 577–583. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Lv, J.; Dang, Q.; Kang, L.; Lei, Z.; Yang, Z.; Hao, Z.; Liu, Z.-H. Graphene/MnO2 hybrid film with high capacitive performance. Electrochim. Acta 2015, 154, 300–307. [Google Scholar] [CrossRef]
- Mahmood, M.; Rasheed, A.; Ayman, I.; Rasheed, T.; Munir, S.; Ajmal, S.; Agboola, P.O.; Warsi, M.F.; Shahid, M. Synthesis of Ultrathin MnO2 Nanowire-Intercalated 2D-MXenes for High-Performance Hybrid Supercapacitors. Energy Fuels 2021, 35, 3469–3478. [Google Scholar] [CrossRef]
- Ma, Z.; Fan, L.; Jing, F.; Zhao, J.; Liu, Z.; Li, Q.; Li, J.; Fan, Y.; Dong, H.; Qin, X.; et al. MnO2 Nanowires@NiCo-LDH Nanosheet Core–Shell Heterostructure: A Slow Irreversible Transition of Hydrotalcite Phase for High-Performance Pseudocapacitance Electrode. ACS Appl. Energy Mater. 2021. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Chen, B.; Dai, G.; Situ, Y.; Huang, H. High-performance adjustable manganese oxides hybrid nanostructure for supercapacitors. Electrochim. Acta 2021, 381, 138213. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]

| Material | C/Fg−1 | PD (kW/kg) | ED (Wh/kg) | C (Retention Cycle) | Ref. |
|---|---|---|---|---|---|
| α-MnO2 | 167 | - | - | 89% (350) | [3] |
| Layered Structure MnO2 | 344 (5 mVs−1) | - | - | - | [6] |
| MnO2 nanospheres | 299 | - | - | 97.6% (1000) | [37] |
| MnO2 nanoflower array | 314 | - | - | - | [38] |
| Bilayer MnOx/Ni foam | 559.5 | - | - | - | [36] |
| α-MnOx/CNTs/Ni | 415 (5 mVs−1) | 79% (1000) | [11] | ||
| MnO2/GNS/CNT | 367 | - | - | 83% (3000) | [87] |
| MnO2/VACNTs | 642 (10 mVs−1) | - | - | - | [70] |
| MnO2/porous CNT/MnO2 | 341.5 (2 mVs−1) | 98% (6000) | [20] | ||
| Mixed nanocomposite (MWCNT/PPy: MnO2) | 365 | - | 44 | - | [12] |
| Co-axial (MWCNT/Ppy/MnO2) | 270 | - | 36 | - | [12] |
| MWCNT@MnO2@Ppy | 272.7 | - | - | - | [46] |
| LbL-MWNT/MnO2 | ** 246 (1000 mVs−1) | - | - | - | [8] |
| MnxOy/MWCNT hybrid | 757 | - | - | 100% (10,000) | [62] |
| CNTs-MnO2/rGO-PVDF | 276.3 | 4.83 | 49.1 | 83% (1000) | [47] |
| δ-MnO2/SWCNT | * 964 | a 8.15 × 10−4 | b 3.18 × 10−5 | - | [64] |
| CNT/MnO2 hybrid | 300 | a 2.5 × 10−3 | b 2.6 × 10−5 | - | [61] |
| MnO2 nanosheets/MWCNT | 1035 | 81 | 25.3 | 98.9% (10,000) | [71] |
| MnO2/CNT/CP | 200 | - | - | >99% (1000) | [60] |
| Graphene-CNT/MnO2 | 486.6 | - | 24.8 | - | [68] |
| MnO2/ACP | 485.4 | - | - | 85% (2000) | [35] |
| Bamboo-Based AC@MnO2 | 221.45 (5 mVs−1) | 89.29% (1000) | [2] | ||
| AC/MnO2 | 290 | - | - | - | [53] |
| MnO2/AC | 332.6 (2 mVs−1) | 99.99% (2000) | [51] | ||
| MnO2/ACP | 640.8 (10 mVs−1) | - | - | - | [55] |
| MnO2@CS | 150 | 9.627 | 8 | 74.4% (1000) | [19] |
| MnO2/hollow CS | 227.5 | - | - | 96% (5000) | [72] |
| Highly porous CS film | 168 | - | - | 95% (5000) | [73] |
| MnO2/CS | 307.6 | - | - | 96.6% (1000) | [42] |
| Carbon@MnO2 nanospheres | 252 (2 mVs−1) | - | bb 3.7 × 10−4 | 74% (2000) | [74] |
| MnO2 NW/C nanobead | 1200 | 32 | 96 | - | [77] |
| CNP/MnO2 nanorods | 800 (5 mVs−1) | 14 | 4.8 | 97.3% (10,000) | [79] |
| MnO2/CNF | 56.8 | 20.8 | 30.6 | 94% (5000) | [81] |
| Zn2SnO4/MnO2/CMF | 621.6 (2 mVs−1) | 32 | 36.8 | 98.8% (1000) | [82] |
| Nanoflake MnO2@CF | 511.8 (2 mVs−1) | - | - | - | [84] |
| MnO2/CFP | 713.7 | - | - | 86.8% (1200) | [85] |
| Graphene/MnO2 | 310 (2 mVs−1) | - | - | 88% (15,000) | [7] |
| MnO2/CNP/Graphene | 255 (2 mVs−1) | - | - | 83% (1000) | [76] |
| GR-MnO2 | 274 (10 mVs−1) | 0.225 | 23.9 | 96% (1000) | [88] |
| [RGO/MnO2]10 | 446 (5 mVs−1) | - | - | 96% (1000) | [89] |
| Mn3O4/rGO | ** 52.2 | aa 0.018 | bb 3.13 | 100% (10,000) | [48] |
| GN/AC/MnO2 | ** 1231 | aa 0.02 | bb 2.7 × 10−4 | 82.8% (10,000) | [54] |
| Graphene/MnO2/ACFF | * 1.516 | - | - | 100% (5000) | [86] |
| N-rGO/CNT-MnO2 film | 418 (50 mVs−1) | 12.526 | 45.72 | - | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wayu, M. Manganese Oxide Carbon-Based Nanocomposite in Energy Storage Applications. Solids 2021, 2, 232-248. https://doi.org/10.3390/solids2020015
Wayu M. Manganese Oxide Carbon-Based Nanocomposite in Energy Storage Applications. Solids. 2021; 2(2):232-248. https://doi.org/10.3390/solids2020015
Chicago/Turabian StyleWayu, Mulugeta. 2021. "Manganese Oxide Carbon-Based Nanocomposite in Energy Storage Applications" Solids 2, no. 2: 232-248. https://doi.org/10.3390/solids2020015
APA StyleWayu, M. (2021). Manganese Oxide Carbon-Based Nanocomposite in Energy Storage Applications. Solids, 2(2), 232-248. https://doi.org/10.3390/solids2020015






