Abstract
Traditional materials synthesis often involves energy-intensive processes with significant waste generation and limited control over material properties. This review examines synthetic biology as a sustainable alternative for designing advanced materials with enhanced precision and versatility. It explores microbial biomineralization, detailing how microorganisms influence the formation of mineral deposits and participate in key biogeochemical cycles. It highlights recent research advancements in using a wide variety of microorganisms for the synthesis of inorganic materials such as metal and metal oxide nanoparticles, quantum dots, magnetic nanoparticles, and thin films. The review also discusses the production and properties of various biopolymers. Important factors that can influence the size, morphology, and uniformity of these biomaterials are covered in detail. Emphasis is placed on advancements utilizing synthetic biology tools, such as protein engineering and genome editing, and recent research for creating smart and responsive materials. Considering the present limitations of synthetic biology, challenges related to scale-up, yield, and uniformity are discussed, and suggestions for future research are detailed.
1. Introduction
The ongoing quest for materials of improved performance, designed for particular functionality and applications, has been achieved traditionally through synthetic routes that are high in energy demand, extreme in chemical conditions, and detrimental in environmental impact [1,2,3,4,5]. From the elevated temperatures of ceramic processing to the multi-step, intricate chemical syntheses of polymers, traditional material synthesis is geared to generate large amounts of waste, greenhouse gas emissions, and copious use of solvents and catalysts [2,6,7]. Furthermore, the degree of control over the properties of materials, including nanoscale architecture and precise chemical composition, may be lost because of the intrinsic limitations of these top–down approaches. These challenges necessitate a revolutionary change toward sustainable and specific material synthesis approaches. Synthetic biology is a new discipline that takes advantage of the natural capacity of living systems to construct biological entities with specific functions [8]. This strategy has the potential to transform materials science through the facilitation of a green, bottom–up route to the production of new materials with higher versatility and precision [5]. Microorganisms are very efficient biofactories that have the capacity to synthesize an incredibly large number of organic and inorganic materials under mild conditions and from renewable feedstocks. The potential to apply biological processes in the fabrication of materials presents appealing avenues of inquiry culminating in the development of sustainable alternatives for conventional paradigms of manufacture and addressing acute concerns in the area of environmental sustainability and depletion of resources [2,9,10]. Theoretical underpinnings of biofabricated materials are often directly inspired by nature. Living systems have evolved sophisticated methodologies for the synthesis of intricate architectures with extremely high precision and efficiency. From the complex silica structure of diatoms to the durable nacre of mollusc shells, nature provides the template for developing materials with specific properties [11]. Through imitation of these biological processes, researchers are developing bio-inspired materials with performance characteristics that exceed their traditional synthetic counterparts [12,13]. The intersection of bio-inspired design and synthetic biology is therefore catalyzing an exponential growth in research interest in the area of biofabrication and elevating it to a forefront position in materials science research [14].
The “biofabrication paradigm” is a fundamental departure from traditional materials synthesis, initiating an era where living organisms, particularly microorganisms, are conceptualized as programmable factories [12]. This paradigm shift moves away from conventional, often environmentally expensive, chemical processes and towards biologically mediated manufacturing, taking advantage of the inherent efficiency and diversity of living systems [15,16,17]. It envisions a future in which microbes, with their genetic and metabolic pathways exactly controlled, produce all kinds of advanced materials, from complex nanomaterials to elaborate biopolymers, in a sustainable manner [18,19,20]. Inspired by the efficiencies of nature’s solutions and driven by the accuracy of synthetic biology, the biofabrication paradigm will transform materials science, creating a future where design and fabrication are inseparably intertwined with biological processes. Nature has created processes for the synthesis of materials with complex properties, as in the case of bone’s capacity for self-healing and spider silk’s combination of rigidity and lightness [21]. Researchers are working on the creation of materials that can mimic natural processes to exhibit unprecedented properties. Microorganisms are at the forefront of this effort since they can be utilized to create bio-inspired materials with desired properties. The growing emphasis on naturally derived materials highlights the promise of synthetic biology to help advance materials science.
This article seeks to present a review of recent developments in microbial-mediated synthesis of inorganic and organic materials—in particular, how recent advancements in synthetic biology tools that utilize genetic and metabolic engineering have led to robust methods for industrial use. We will explore the ways in which microorganisms create molecules through biomineralization and focus on the microbial synthesis of inorganic nanostructures and biopolymers. We will discuss pathways microbes undertake to fabricate materials and highlight how microbes can be engineered to synthesize materials with control over their composition. We will discuss the prospects of microbes being used to engineer enhanced properties, improved biocompatibility, and biodegradability. We will deliberate the challenges that currently hinder the widespread adoption of these technologies, including issues related to scale-up, yield, product uniformity, and environmental considerations, and postulate future directions for research and development, highlighting the potential of emerging synthetic biology tools and synergistic approaches to overcome existing limitations.
2. Biomineralization
2.1. Overview
Biomineralization is the process by which living organisms produce minerals. Microbial pathways contribute to the formation of mineral deposits on the earth, such as carbonates, sulfides, and oxides [22]. Microbes are the key drivers of biogeochemical cycles, influencing the transformation of elements such as carbon, nitrogen, sulfur, and metals. Microbial biomineralization plays a vital role in these cycles by affecting the solubility and mobility of various elements [23]. Microbial processes such as respiration, fermentation, and photosynthesis can alter the pH of their surroundings; for example, the hydrolysis of urea by ureolytic bacteria releases ammonia, thus increasing pH and promoting the precipitation of carbonate minerals [22,24,25,26]. Conversely, the production of organic acids during the degradation of organic matter can decrease the pH, leading to mineral dissolution [27,28]. Microbes can also catalyze redox reactions by changing the oxidation state of metals and other elements, thereby forcing the precipitation or dissolution of minerals. For example, the reduction in ferric iron (Fe3+) to ferrous iron (Fe2+) by iron-reducing bacteria can lead to the formation of Fe2+-based minerals like siderite and magnetite [29,30]. Magnetotactic bacteria (MTB), which are capable of synthesizing magnetic nanoparticles (Fe3O4 or Fe3S4) using specialized organelles called magnetosomes, are an especially fascinating example of highly stereotyped and robust biomineralization with a lot of potential for industrial applications [31]. Similarly, diatoms, a group of photosynthetic algae, construct elaborate cell walls composed of amorphous silica (SiO2) called frustules. These frustules display hierarchical porosity and nanoscale patterning—features that have inspired the development of biomimetic materials for filtration, optics, and catalysis [11]. Furthermore, the microbial production of CO2 or H2S gases can influence mineral formation; CO2 production can increase carbonate saturation, while H2S production by sulfate-reducing bacteria (SRB) can lead to metal sulfide precipitation [32,33]. This influences sulfur cycling in nature and the availability of metals in different environments.
2.2. Bioremediation
A well-studied application of biomineralization is bioremediation, i.e., the use of microbes to remove pollutants from contaminated environments [34,35]. The origins of human-caused heavy metal contamination stem from both industrial and domestic sources. The metal ion contaminants that disperse into the environment experience difficulty degrading, introducing potential issues for human health and physical systems. In an effort to combat the bioaccumulation of these contaminants, many technological advancements, such as induced chemical precipitation, physical adsorption, ion exchange, and membrane filtration, have been in production but experience difficulties with progression due to financial constraints. Therefore, there is a focus on the advancement of biomineralization technology towards the goal of providing a stable and sustainable sequestration of heavy metals. One method of bioremediation is through microbial-induced carbonate precipitation [22,35]. This process produces the carbonate anion, CO32−, through microorganisms such as bacteria, fungi, or enzymes, which then precipitates the heavy metal ions into free, stable carbonates that do not leave harmful impacts on the environment. The precipitation of heavy metals can also be completed through other methods such as urea hydrolysis, denitrification, or sulfate reduction. Urea hydrolysis stands as the most advantageous method due to its time and money efficiency. Within this process, microorganisms decompose urea into compounds, which increases the overall pH, lessening the residual impact the contaminant would originally have on its surroundings [35,36,37]. Urea hydrolysis is driven largely by urease-producing bacteria (UPB), which can be easily ordered through commercial collection centers, making it an overall more preferable method of remediation.
Another method is phosphate precipitation, which utilizes microbial activity during the dissolution of inorganic phosphate and organic phosphorus to create an enzyme-induced phosphate precipitation reaction [38,39,40]. The process enables the precipitation of free metal ions into stable heavy metal phosphates, mitigating their toxic effects. Among the most well-studied methods of bioremediation, phosphate-solubilizing bacteria (PSB) play a crucial role in making organic phosphorus and inorganic phosphate soluble through the usage of enzymes and organic acids, which speed up the process [35]. Moreover, scientists have discovered that the addition of an increased amount of organic acids and microorganisms (such as Bacillus, Ochrobactrum, Pseudomonas, Marcescens, and Rahnella) improves the efficiency of the reaction, as they demonstrate increased decomposition rates [34,35]. The method of phosphate-based remediation introduces many advantages over carbonate precipitation, such as its greater thermodynamic stability, tolerance to lower pH levels, and lack of emission of harmful gases [38,41,42,43]. However, it remains an unattainable method to practice, as the cost is out of range for sustainable, long-term use. The efficiency of microbial precipitation is largely influenced by multiple conditions concerning its environment and the bacterial species involved in the heavy-metal deterioration process. In regard to phosphate precipitation-based bioremediation, differing species of bacteria exhibit varying degrees of tolerance to heavy metal ions [35]. As for carbonate precipitation, certain fungal species inherit higher capabilities of urea hydrolysis [44]. The situational differences in heavy-metal removal, lack of funding towards research, provision of materials, and overall high costs inhibit large steps towards technological advancements.
While bioremediation represents an important application of harnessing microbe-driven mineral formation, the processes involved in biomineralization also underpin their ability to synthesize a wider range of inorganic materials. Building on the understanding of how microorganisms control mineral precipitation for bioremediation, researchers have explored their potential in the synthesis of novel inorganic materials. Figure 1 highlights two mechanisms of biomineralization-induced precipitation.
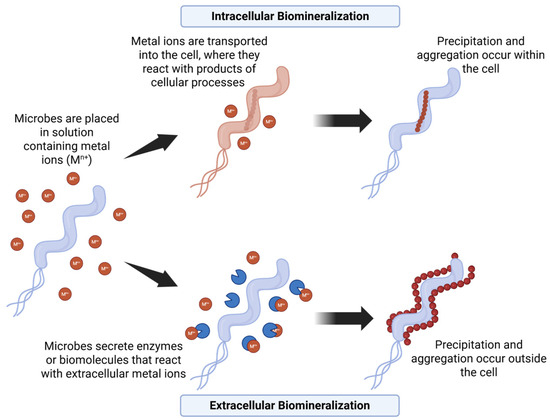
Figure 1.
Mechanism of biomineralization using microbial-induced precipitation.
3. Synthesis of Advanced Materials
Biomineralization has long inspired materials scientists. By using synthetic biology to design materials, researchers are moving beyond traditional top–down approaches toward bottom–up, self-organizing systems with new functionalities. By accessing the metabolic potential of microorganisms, scientists have engineered biological systems that synthesize materials under less harsh conditions, using renewable biomass feedstocks and reducing toxic waste [1,3,45,46]. Another significant advantage of microbial-mediated synthesis is its versatility. Microorganisms are able to readily adjust to changing environments and can metabolize substrates with broad diversity, thereby enabling the production of a wide range of products [47].
3.1. Inorganic Nanostructures
Microorganisms can be genetically designed to synthesize inorganic nanostructures with defined size and shape, eliminating the necessity for toxic chemical reductants and high-temperature ovens. Naturally occurring microbial metabolic processes like photosynthesis and respiration can alter the chemical environment and lead to mineral or metal precipitation.
3.1.1. Metal Nanoparticles
Metal nanoparticles, especially those involving noble metals, are ubiquitous in catalytic converters [45]. Some metal nanoparticles are also utilized in drug delivery and therapeutics, making biocompatibility an essential requirement [48]. Due to their inherent ability to reduce metal ions, microorganisms have the ability to synthesize a range of metal nanoparticles [49]. By controlling the biological systems and reaction conditions, the size, shape, and composition of the nanoparticles can be precisely tuned [49,50].
The reduction in metal ions by microorganisms can occur both intracellularly and extracellularly (Figure 1). In intracellular synthesis, metal ions are transported into the microbial cell, where they are reduced and assembled into nanoparticles (NPs). This process involves specific enzymes (e.g., reductases) that act as bioreductants and stabilizing agents [3,51]. NPs are typically formed within the cell cytoplasm or periplasmic space. Bacteria like Shewanella oneidensis can transport gold (Au3+) and silver (Ag+) ions into the cell where they are reduced by intracellular reductases to their metallic form (Au0 and Ag0), leading to the accumulation of metal NPs [1,52]. It also utilizes nitrate reductase on its cell surface and in the periplasmic space to catalyze the reduction of gold and silver ions [53,54].
Extracellular synthesis occurs outside the cell, where metal ions are reduced by enzymes or biomolecules secreted by the microbe. Extracellular synthesis simplifies the purification process for obtaining the final product when compared to intracellular synthesis. Shewanella oneidensis employs extracellular electron shuttles, such as riboflavin, to transfer electrons to gold and silver ions in the surrounding environment [55,56]. Pseudomonas aeruginosa secretes enzymes such as NADH-dependent reductases extracellularly [49,52]. The bacterium also releases organic molecules, such as polysaccharides and proteins, which prevent aggregation of the resulting nanoparticles [54]. Similarly, certain fungi secrete a high concentration of enzymes and proteins from their mycelia, which act as primary reducing agents [57,58]. The proteins and polysaccharides in the fungal filtrate can act as capping agents and stabilize the synthesized NPs.
3.1.2. Metal Oxide Nanostructures
Metal oxide materials have diverse applications in fields such as catalysis, electronics, and medicine [46]. The mechanisms involved in microbial synthesis of metal oxide NPs often overlap with those in the synthesis of metal NPs. However, a key difference is that the metal ions are not reduced to their zero-valent state and instead form complexes with oxygen. Bio-precipitation is the most common mechanism for metal oxide formation within the cell [23,32,59]. Enzymes such as phosphatases or oxidases that are produced can alter the local chemical environment, leading to the precipitation of metal oxides. Similarly, metabolic activities can alter the pH of their surroundings, leading to the precipitation of metal ions as oxides or hydroxides. One example is the precipitation of zinc oxide (ZnO) NPs under alkaline conditions using fungal biomass (Aspergillus niger) [60,61]. Phytochemicals present in Azadirachta indica (neem) leaves were also used to synthesize metal oxide nanoparticles [8,62].
Microbial cell surfaces can also bind to metal ions through various functional groups (e.g., carboxyl, phosphate, hydroxyl). This creates a nucleation site for nanoparticle formation. Algae like Spirulina platensis have been successfully used as templates for the formation of titanium dioxide (TiO2) NPs through binding of titanium ions to the algal cell wall and its subsequent oxidation [54,63]. Here, the microbes do not actively mediate synthesis via metabolic processes but serve as structural scaffolds for abiotic mineral formation. This highlights the distinction between true microbial biosynthesis, where the organism plays an active biochemical role, and templated synthesis, where the organism’s surface facilitates material deposition. Some bacteria produce siderophores, which chelate with iron ions and facilitate their transport into the cell [64]. In certain cases, the iron ions are further processed to form iron oxide NPs, such as goethite (α-FeOOH) or hematite (α-Fe2O3) [23,65]. Microbial enzymes, such as reductases, can also support the synthesis of iron oxide (Fe2O3) nanoparticles [66].
3.1.3. Magnetic Nanoparticles
Microbial synthesis of magnetic nanoparticles, especially magnetite (Fe3O4), has been researched for producing biocompatible nanomaterials with targeted applications in drug delivery, magnetic hyperthermia therapy, and MRI contrast agents [29,67]. Magnetotactic bacteria (MTB) can naturally synthesize magnetic nanoparticles within specialized organelles called magnetosomes that contain magnetite or greigite (Fe3S4) crystals. The magnetosomes can control the size, shape, and crystal structure of the magnetic nanoparticles with precision [68]. Magnetospirillum magnetotacticum and Magnetospirillum gryphiswaldense are well-studied MTBs that produce uniform magnetite crystals within magnetosomes [69,70]. The process of magnetosome formation involves several steps: uptake of iron, formation of a membrane vesicle, regulation of iron transport and redox state, nucleation and growth of the magnetic crystal, and arrangement of magnetosomes in chains within the cell [68,71,72]. Specific pH conditions, oxygen availability, and the presence of other minerals have an influence on the transformation process [29]. The ability to control the properties of synthesized magnetic nanoparticles via genetics has allowed MTBs to play an outsized role in engineering chimeric magnetic nanoparticles necessary for novel research applications. Magnetogenetics is an emerging technology in biomedical research, allowing for noninvasive manipulation of cell signaling pathways via magnetic fields; an enduring challenge is developing a viable fusion of proteins and magnetic probes tailored to individual research needs. A recent study described a robust method for generating engineered ferritin fused to green fluorescent protein (GFP) as an efficient and biostable in vivo technique to manipulate cellular processes remotely [73].
Some bacteria and fungi can also produce magnetic nanoparticles extracellularly. These microorganisms secrete organic molecules that can reduce Fe3+ to Fe2+, leading to the formation of magnetite nanoparticles. Elblbesy et al. explored the extracellular production of nanoparticles by microbes, specifically using Magnetospirillum strain AMB-1 to produce magnetic nanoparticles [67]. The experiment showed that this nitrate-reducing Fe (II) oxidizing bacteria produced paramagnetic magnetite nanoparticles of ~50 nm size that possessed a low coercivity. Interestingly, even minute changes to incubation conditions under which biosynthesis was conducted caused small variations in the magnetic characteristics of the produced nanoparticles [67].
Shewanella oneidensis does not possess magnetosomes, but it can perform an extracellular reduction of iron ions [30]. It forms magnetite nanoparticles during the process of anaerobic respiration, where it uses iron oxides as electron acceptors. Sulfate-reducing bacteria are capable of producing magnetite nanoparticles during the process of sulfate reduction [74]. The mechanism involves the production of sulfide, which can react with iron ions to form iron sulfides. These iron sulfide minerals undergo transformation to form magnetite through processes like dissolution, recrystallization, and oxidation–reduction [74].
3.1.4. Semiconductor Nanocrystals
Quantum dots (QDs) are semiconductor nanocrystals ranging in size from 2 to 10 nanometers. Their unique optical and electronic properties arise from quantum confinement effects due to their small size, making them suitable for photocatalysis, optoelectronics, and bioimaging. Both intracellular and extracellular processes have been explored for the microbial synthesis of semiconductor nanocrystals [52,75,76,77,78,79].
Sulfate-reducing bacteria can react with metal ions to create nanocrystals of cadmium sulfide and zinc sulfide [4,80]. Through an intracellular process, they take up metal ions (Cd2+, Zn2+) into their cells. Sulfide ions (S2−), which are generated through sulfate reduction, then react with metal ions within the cell. This leads to the formation of CdS or ZnS QDs within the microbial cell. As expected, the size and properties of the QDs are influenced by the metal ion concentration, sulfide ion availability, temperature, pH, and enzymatic activity [78]. Escherichia coli has been engineered to synthesize CdS QDs intracellularly by introducing genes encoding gamma-glutamylcysteine synthetase (gamma-GCS) and glutathione synthetase (GS), which are involved in sulfate reduction [81]. Mi et al. reported the production of sulfide ions within the cells, leading to efficient CdS QD formation [77]. E. coli was also reported to synthesize CdTe quantum dots through an extracellular mechanism [76]. The fungus Fusarium oxysporum produces proteins and enzymes when exposed to a zinc ion-containing solution [82,83]. These secreted biomolecules convert Zn2+ to ZnS. The Gram-negative bacterium Pseudomonas putida can perform both intracellular and extracellular synthesis methods to make CdS QDs [75,84].
It is important to recognize that quantum dots are environmentally harmful due to the toxicity of their constituent materials [85]. Quantum dots can degrade and release heavy metals (e.g., Cd, Zn, Te) when improperly disposed of into soil and water [86]. This makes techniques like microbial bioremediation increasingly relevant; however, some studies have shown that quantum dots can be toxic to bacteria and algae. Nonetheless, microbial synthesis is a promising avenue for producing less toxic QDs. For example, a study investigating the effects of biogenic CdSQDs generated using F. oxysporum on bacterial viability and lettuce germination found that concentrations had to be at their highest to display antibacterial activity, while biogenic CdSQDs did not inhibit seed germination [87].
3.2. Thin Films Through Microbial Deposition
Microbial deposition leverages the ability of microorganisms to precisely control the nucleation and growth of inorganic materials [55]. It relies purely on biomineralization to precipitate the desired inorganic materials. Unlike traditional chemical vapor deposition or sputtering techniques, microbial deposition occurs under mild conditions, often requiring only room temperatures and neutral pH. For example, Bacillus subtilis precipitates CaCO3 through urea hydrolysis [25]; this process can be employed to create thin films of calcium carbonate for concrete reinforcement [88,89,90].
Some bacteria and fungi can actively transport metal ions into their cells, leading to intracellular precipitation. Pseudomonas sp. can accumulate uranium within their cells through active transport mechanisms where uranium ions are taken up and then precipitated as uranium phosphate [91]. Aspergillus niger and Paecilomyces javanicus also produce uranium phosphates on fungal mycelia in combination with extracellular processes [92]. These insights can be used to create thin biofilms of uranium compounds [93]. Another method is through passive absorption, where the microbial cell surfaces act as a template for nucleation and growth of minerals, owing to the presence of certain functional groups (e.g., carboxyl, phosphate) that bind to ions. A well-known example is diatoms, a type of algae that possess silica cell walls (frustules) with highly ordered nanoporous structures [11]. Research has shown that coating diatom frustules with other materials can fabricate thin films with tailored optical properties [94]. We have summarized the inorganic materials that can be synthesized microbially in Figure 2.
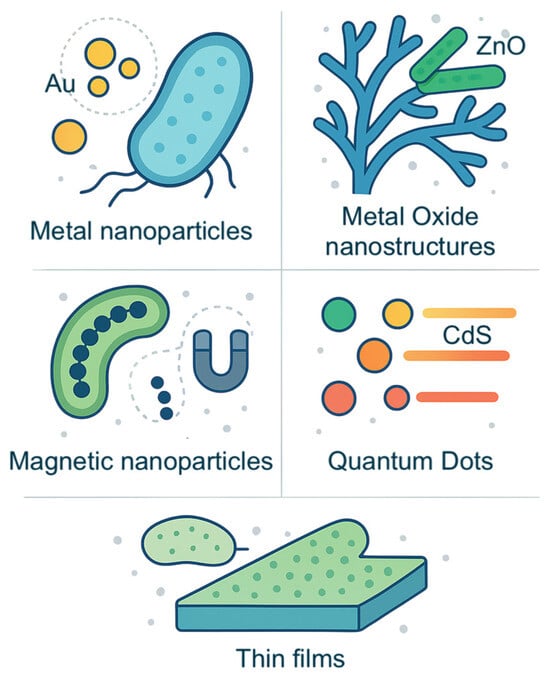
Figure 2.
Infographic representing the inorganic nanostructures described in Section 3.
3.3. Biopolymers
Microorganisms offer sustainable alternatives to traditional petroleum-based polymers. These microbial biopolymers are biodegradable, biocompatible, and synthesized from renewable resources. The production of microbial biopolymers involves (i) microbial selection and strain development, (ii) fermentation, i.e., nutrient consumption under controlled conditions, and (iii) extraction of biopolymer from the microbial cells [2,20].
Polysaccharides are made up of sugar monomers, which makes them an ideal avenue for biosynthesis. Some bacteria, such as Komagataeibacter xylinus, can synthesize bacterial cellulose with high purity and tensile strength [5,95,96,97]. K. xylinus converts glucose from its surroundings into UDP-glucose, which is then polymerized into glucan chains by cellulose synthase, an enzyme complex located at the bacterial cell membrane. The cellulose nanofibers produced have applications in wound healing, tissue engineering, and high-strength materials [2,20,98,99]. Similarly, xanthan gum, which is widely used in the food and cosmetic industry, can be produced by the bacterium Xanthomonas campestris from glucose starting materials [100].
Bacteria from the genera Azotobacter and Pseudomonas are capable of producing alginates, which are extensively used in wound dressings and drug delivery [5]. The enzymes alginate synthases and epimerases play a key role in determining the final structure and properties of the alginate product. An interesting example to demonstrate the versatility of microbial biopolymers is the production of polyhydroxyalkanoates (PHA) by Cupriavidus necator [101,102]. The type of PHA produced can be influenced by the carbon source provided to the bacteria—using glucose leads to polyhydroxybutyrate (PHB) production, while adding valerate leads to the production of polyhydroxybutyrate-co-hydroxyvalerate (PHBV) copolymer [103].
Bacteria are also capable of producing monomers for specific polymers, such as the synthesis of lactic acid by the Lactobacillus species [104]. These bacteria ferment carbohydrates like glucose or starch to produce lactic acid, which is then polymerized into polylactic acid. Similarly, succinic acid can be produced by bacteria like Actinobacillus succinogenes [105,106]. These bacteria can ferment renewable feedstocks containing glucose, glycerol, and agricultural byproducts to produce succinic acid [104,107,108].
Genetic alterations in microbes can also facilitate the synthesis of biopolymers with designed properties, including enhanced mechanical properties and biodegradability [96]. Researchers can genetically design bacteria to synthesize cellulose from alternative carbon sources, including waste streams [97]. Yeast can be made to secrete lipids with specific kinds of fatty acids, thereby offering a renewable feedstock for biopolymers [109]. Recently, researchers engineered bacteria and yeast to produce spider silk-like proteins [21]. These protein fibers have a high strength and elasticity, with potential to be used in textiles, biomedicine, and composite materials. Table 1 summarizes.

Table 1.
Summary of advanced materials discussed in Section 3.
4. Factors Affecting Material Properties
By manipulating biological and experimental parameters, we can tailor the properties of the resulting materials for specific applications. There are several factors that influence the morphology and properties of the resulting material (Figure 3), which are discussed in detail below.
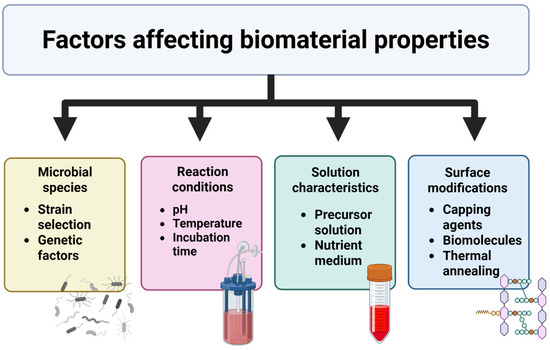
Figure 3.
General factors affecting the properties of biosynthesized materials.
4.1. Microbial Species/Strain Selection
Different organisms possess distinct enzyme machinery and metabolic processes, which directly influence the formation of the product. For example, researchers have found that careful selection of the strains of Fusarium oxysporum is required to produce monodisperse, spherical silver nanoparticles [15,48,79]. Other fungal species, or even different strains of Fusarium, could produce different shapes or a wider size distribution [3,45,49]. Also, S. xiamenensis was found to produce a higher yield of gold nanoparticles when compared to S. oneidensis [53].
Magnetotactic bacteria (MTB) clearly demonstrate how the choice of strain can affect the desired properties of the material produced. Genetic factors within MTB control iron uptake and crystal growth [71], leading to monodisperse Fe3O4 NPs. The size of magnetosomes (typically 35–120 nm) as well as their shape (cuboidal, elongated, and bullet-shaped) is genetically determined [29,68]. Specific proteins within the magnetosome guide crystal growth along preferred crystallographic planes, governing the final shape of the inorganic nanoparticles formed [29,68]. In contrast, sulfate-reducing bacteria (SRB) produce irregularly shaped Fe3O4 NPs extracellularly during anaerobic respiration [74].
4.2. Precursor and/or Nutrient Characteristics
As established with solution-based synthesis of inorganic nanoparticles, lower concentrations of metal salts lead to smaller nanoparticles, while higher concentrations of metal salts lead to larger nanoparticles due to agglomeration [62,110]. Carefully controlling the ratio of microbes and cations (e.g., Zn2+) in the growth medium will produce nanoparticles with a narrower size distribution [110,111]. In the synthesis of gold nanoparticles using Shewanella oneidensis, increasing the concentration of gold chloride (the metal precursor) in the growth medium generally led to an increase in the average size of the resulting gold nanoparticles [53,112]. Similarly, the size of palladium nanoparticles was controlled by fine-tuning the Pd/cell dry weight [65,112]. Additionally, the presence of other organic matter and salts in the growth medium can play a role in the nanoproduct [113]. Depending on the chemical composition of the product, the presence of specific ligands or additives can guide its morphology.
Biopolymers are also affected by the nutrient medium. In the synthesis of bacterial cellulose (BC) nanofibers, using glucose as the sugar source leads to a high yield of product [96]. In some cases, it has been shown that using glycerol can also lead to high BC yields [96,97]. Use of peptone in the medium improved BC yields from mannitol and sucrose [114]. The addition of polysaccharide pullulan can affect the bundling behavior of BC and improve the mechanical strength of BC [115]. In the biosynthesis of xanthan gum by Xanthomonas campestris, the choice of carbon source and composition of the nutrient medium controls the yield and molecular weight of the biopolymer [100,116,117].
4.3. Reaction pH, Temperature, and Time
Reaction pH, temperature, and time influence microbial enzyme activity and the kinetics of nanoparticle nucleation and growth [118]. Optimal ranges for synthesis vary depending on the microorganism and the target material. Therefore, these factors exhibit considerable variability across published research [15]. All factors are interactive, and therefore, they have to be carefully considered together in order to determine the best suitable conditions for a particular microorganism [119].
An initial increase in temperature generally increases the rate of synthesis; however, very high temperatures can denature microbial enzymes and lead to poor-quality products. In general, temperatures of 25–37 °C are ideal for the formation of uniform nanoparticles [46]. Higher temperatures can cause nanoparticle aggregation or loss of capping agent. Similarly, longer incubation times can lead to a higher yield; however, the product may be polydisperse with a larger size distribution due to continued growth or aggregation [111]. For biopolymers, longer fermentation times can lead to increased polymer chain elongation and higher molecular weight [116,120,121], but very long times may lead to degradation.
Synthesis of Ag nanoparticles by Bacillus sp. is more efficient at slightly alkaline pH, resulting in smaller, spherical nanoparticles [59,122,123,124]. This is because acidic pH could lead to delayed nucleation and agglomeration [125]. However, a pH that is too high may cause undesirable precipitation of hydroxides [126]. On the other hand, a slightly acidic pH promotes Fe3O4 precipitation by P. aeruginosa [72]. The formation of xanthan gum was increased at a pH of 7.0–7.5; however, further increase in the pH decreased the production [127]. For the production of bacterial cellulose, the ideal conditions are a lower pH of 3–7 and a temperature range of 25–30 °C [95].
4.4. Biomolecules and Capping Agents
Proteins and polysaccharides secreted by extracellular processes can act as capping and stabilizing agents, influencing product morphology. For example, specific proteins can act as templates, directing the growth of Ag NPs into different shapes like spherical and triangular [128,129]. Altering the reaction pH can affect the charge of these biomolecules, influencing their interaction with Ag+ ions and the final particle shape. In the biosynthesis of Au nanorods, some proteins bind to gold ions in a way that directs their assembly into rod-shaped structures rather than spheres [130]. Post-synthesis modifications such as thermal annealing or capping with additional ligands can be employed to fine-tune nanostructure properties.
5. Synergistic Approaches
The field of synthetic biology is naturally interdisciplinary, and its utility in material science has increased by its convergence with other technologies. The integration of synthetic biology with existing tools enables researchers to develop a robust approach to design and fabricate novel materials with increased complexity and functionality.
5.1. Protein Engineering and Genome Editing
One interesting approach is the combination of protein engineering with biomineralization. This entails creating proteins with specific shapes and chemical functionalities, acting as templates for mineral deposition [131,132,133]. These protein scaffolds can bind specific ions, thereby controlling the nucleation and growth of minerals with desired size, shape, and crystalline phases. For example, genetically engineered proteins, such as silk proteins or peptides, can be used to template the formation of calcium carbonate, silica, or metal nanoparticles [18,134,135,136,137,138,139]. The resulting materials exhibited enhanced mechanical strength, optical properties, and catalytic activity. Studies have shown that silver nanoparticles prepared using genetically engineered bacteria demonstrated precise control over nanoparticle size and distribution [140].
Similarly, using genome editing such as CRISPR-Cas9, researchers can modify microorganisms to enhance the production of specific building blocks for polymers. This involves deleting competing pathways, introducing new genes, or altering regulatory elements to fine-tune gene expression. An example is the engineering of bacteria to overproduce monomers like lactic acid (for polylactic acid production) or isoprene (for rubber synthesis) [19,104,141,142]. Additionally, new advancements in Cas9-based genetic engineering have allowed for the development of synthetic gene circuits that (1) greatly reduce the leakiness of a system (i.e., the basal expression of a gene in the absence of an inducer) and (2) achieve high maximum expression of a target product. CASwitch is one such system that combines low leakiness with high maximum expression, resulting in high fold induction (i.e., the ratio between maximum expression and leakiness) by combining two established network motifs: the Coherent Feed-Forward Loop (CFFL) and Mutual Inhibition (MI) [143].
5.2. Smart and Responsive Living Materials
Living materials offer unique advantages such as the ability to respond dynamically to their environment [144]. Engineered living tissues with tailored mechanical and biological properties could be used to repair or replace damaged organs and tissues. For example, researchers are exploring the use of 3D-printed living constructs containing cells and extracellular matrix components to create functional bone, cartilage, or skin [13,145,146]. Self-healing hydrogels can use biosynthesis to repair damaged tissues [147,148]. Bacteria can be programmed to synthesize and release therapeutic proteins at tumor sites [149,150,151]. While the term ‘living materials’ encompasses a plethora of technologies that combine living cells with nonliving scaffolds, these are distinct from engineered cells (otherwise known as artificial cells), which are nonliving and designed to mimic biological processes [152].
In the field of engineering, an interesting example is bacteria-containing composite materials that are programmed to secrete a mineralizing agent in response to damage. For example, concrete can be engineered to incorporate bacteria that secrete calcium carbonate to repair cracks and restore structural integrity [25,89,90]. Living cells can be engineered to sense specific environmental cues, such as changes in pH, temperature, or the presence of pollutants, and then trigger a visual response or release a signaling molecule [147,153,154]. Microorganisms can be engineered to detect specific pollutants in water and emit a fluorescent signal [154,155,156,157].
5.3. Resource Management and Recycling
Environmental chemistry is another area of synergy with synthetic biology. Microorganisms can be engineered to produce biofuels from renewable feedstocks, reducing our reliance on fossil fuels. For example, bacteria can produce high-energy-density biofuels from agricultural waste [10,158,159], while yeast can be programmed to synthesize biodegradable polymers for packaging applications [109,160,161]. Living filters composed of microorganisms can be used to remove heavy metals or organic pollutants from contaminated water [1,16,162]. Synthetic biology can play a key role toward a circular economy by facilitating the recycling of materials [109,163]. For example, enzymes can be engineered to break down complex polymers into their constituent monomers, which can then be used to synthesize new materials [164,165]. Microorganisms can be engineered to convert waste streams, such as agricultural waste or CO2 gas, into bioplastics or biofuels, closing the loop and reducing our reliance on non-renewable resources [1,22,109].
6. Challenges and Future Directions
Microbial synthesis has the potential to be a greener alternative to material synthesis by avoiding the use of toxic chemicals, generating less waste, and operating at mild conditions [14,16,33,54]. Microorganisms could be genetically engineered to produce substances with specific characteristics, such as enhanced strength, unique optical properties, or catalytic function, through the controlled synthesis mechanisms in the nanoscale. The process of microbial production supports the production of complex hierarchical structures through the self-assembly process, mimicking the biological formation processes for numerous materials. A variety of inorganic materials, ranging from metal nanoparticles, metal oxides, and sulfides to carbonates and phosphates, can be synthesized with the assistance of microorganisms [1,67]. However, the discipline also possesses numerous challenges that must be resolved in order to attain its full potential (Figure 4).
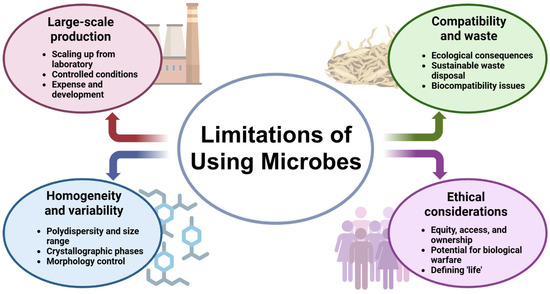
Figure 4.
Flowchart depicting the limitations of using microbes for materials synthesis.
6.1. Large-Scale Production
A key challenge is the transition from laboratory experiments to large-scale production [22]. While microorganisms are capable of producing inorganic materials quite well under controlled laboratory conditions, scaling up this process is fraught with numerous issues. Large bioreactors must have extremely controlled temperature, pH, nutrient availability, and oxygen conditions to promote constant microbial activity and material synthesis. Such conditions could be hard to achieve uniformly in a large volume. Process scale-up could increase the expense of bioreactor construction and operation, media preparation, and product purification. Moreover, growth conditions for microbes and synthesis of materials can be very different at the laboratory and production levels. A lot of process development is typically required to attain large scales and high yields and rates of production [14,54]. This can be achieved by creating novel bioreactors in which we can maintain control over important factors at large scales, for example, continuous-flow bioreactors, microfluidic bioreactors, or three-dimensional perfusion systems. In addition to technical scale-up challenges, regulatory frameworks and cost structures remain barriers to commercial adoption of microbial materials. Biomanufacturing of new nanomaterials must comply with biosafety and environmental guidelines, which vary across regions.
6.2. Polydispersity and Uniformity
Microbial growth and deposition rates can be slow compared to conventional techniques. Another problem is to regulate the properties and homogeneity of the produced materials. Microbe-produced materials, particularly nanoparticles, may be of varying sizes and shapes, which can alter their properties and performance [3,16,54]. It is desirable to have nanomaterials with a smaller size range for most applications in semiconductors and biology. Furthermore, regulating the crystal structure and shape of inorganic materials is extremely important. Microbial synthesis may sometimes produce amorphous or poorly crystalline material of non-uniform shape; batch and run consistency may be hard to obtain because of the inherent variability of biological systems [14,166]. Achieving uniform thin films over large areas can be challenging. Therefore, precise control over microbial metabolic pathways through genetic engineering techniques, optimization of precursor production, and designing microorganisms can aid uniform material synthesis [16,54]. Developing in situ methods of analyzing the synthesis process helps in controlling material properties and making them homogeneous.
6.3. Compatibility and Waste Production
For medical uses, the materials produced by the microorganisms must not cause adverse reactions in the host organism [14]. Certain microorganisms or their products can be harmful to human beings or other organisms. Non-toxic microorganisms must be chosen, and methods must be developed to remove any toxic byproducts [8,147]. Both in vivo and in vitro tests for biocompatibility must be conducted to ensure that the synthesized materials are safe and effective [5,98,99]. Finally, the synthesis of large amounts of material using microbes will generate enormous amounts of waste and biomass [1]. Disposal of these wastes must be conducted in a sustainable manner so as not to damage the environment. Even though microbial synthesis is generally greener than chemical synthesis, keen scrutiny of the degree to which the end materials are compatible with living things and their possible impact on the environment must be conducted. The release of engineered living materials in the environment, whether intentional or accidental, may have unexpected effects on ecosystems, biodiversity, and human health [167,168]. Strong risk assessments and control strategies are therefore crucial, but it can be difficult to predict long-term effects. Further research in the form of life-cycle assessments must be conducted to ascertain the effects of microbial synthesis on the environment.
6.4. Ethical Considerations
The emergence of technologies that enable the creation of living or biologically created materials brings forth ethical and societal considerations that require scrutiny. At the forefront is the question of defining “life” itself and the moral status of these novel entities. As we engineer organisms to produce materials with tailored properties, we blur the lines between living and non-living, potentially challenging traditional ethical frameworks. The potential does arise for these techniques to be used in dual applications, such as the development of biological weapons, raising serious security concerns [17,168,169,170,171]. The ability to manipulate biological systems with increasing accuracy requires the establishment of strict regulatory structures and international cooperation to prevent abuse [47,169]. In addition, the social implications of large-scale adoption of live materials should be considered. There is a need to address issues of equity, access, and ownership of technologies to ensure that the benefits are widespread and existing inequalities are not increased [8,47,168]. Public commitment and transparent communication are important for promoting informed dialog in these new and emerging areas of research. It is necessary to move forward with a sense of responsibility and acknowledge that synthetic biology has the potential to reshape our world in profound ways, for better or for worse.
7. Conclusions
This review highlights the potential of synthetic biology for materials design, shifting from traditional, environmentally burdensome methods to sustainable, biologically driven approaches. In particular, this review describes a synthesis of natural mechanisms for material production and technological advancements that enhance endogenous mechanisms, either by scaling up production, reducing the toxicity of products, or developing new applications. By harnessing microbial processes, researchers can create advanced materials with precision and versatility, moving beyond conventional synthesis. Microorganisms can act as programmable biofactories to facilitate the production of inorganic nanostructures and biopolymers with tailored properties. For instance, we have described how magnetotactic bacteria (MTBs) have the ability to synthesize magnetic nanoparticles via both intracellular and extracellular mechanisms. This endogenous ability has further been enhanced by synergistic approaches in genetic engineering, culminating in applications in the field of magnetogenetics. The ability to manipulate microbial species, precursor concentrations, and environmental parameters allows for fine-tuning material characteristics for specific applications. However, challenges related to scale-up, yield, uniformity, and environmental impact remain. We have summarized these considerations and conclusions in Table 2. Future research should prioritize the development of robust biomanufacturing platforms, the expansion of genetic engineering tools, and the encouragement of synergistic approaches to create multifunctional biomaterials.

Table 2.
Comparison of traditional and microbial methods of materials synthesis.
Author Contributions
Conceptualization, R.R. and F.M.; writing—original draft preparation, R.R., G.B., and S.F.; writing—review and editing, R.R. and F.M.; visualization, R.R. and F.M.; supervision, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank our department for their continued support and our undergraduate students for their enthusiasm. R.R. gives gratitude to Pradeep Kumar Ragu Chanthar for his support. Authors used Grammarly software 14.1237.0 for editing/proofreading, BioRender 2025 for figures, and utilized the Paperpile extension as a reference management tool.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Annamalai, J.; Ummalyma, S.B.; Pandey, A.; Bhaskar, T. Recent Trends in Microbial Nanoparticle Synthesis and Potential Application in Environmental Technology: A Comprehensive Review. Environ. Sci. Pollut. Res. Int. 2021, 28, 49362–49382. [Google Scholar] [PubMed]
- Getahun, M.J.; Kassie, B.B.; Alemu, T.S. Recent Advances in Biopolymer Synthesis, Properties, & Commercial Applications: A Review. Process Biochem. 2024, 145, 261–287. [Google Scholar]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of Nanoparticles Using Microbes—A Review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar]
- Raouf Hosseini, M.; Nasiri Sarvi, M. Recent Achievements in the Microbial Synthesis of Semiconductor Metal Sulfide Nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 293–301. [Google Scholar]
- Hernández-Arriaga, A.M.; Campano, C.; Rivero-Buceta, V.; Prieto, M.A. When Microbial Biotechnology Meets Material Engineering. Microb. Biotechnol. 2022, 15, 149–163. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Arole, D.V.; Munde, S.V. Fabrication of Nanomaterials by Top-down and Bottom-up Approaches—An Overview. J. Mater. Sci 2014, 1, 89–93. [Google Scholar]
- Jain, A.; Bhatia, P.; Chugh, A. Microbial Synthetic Biology for Human Therapeutics. Syst. Synth. Biol. 2012, 6, 9–22. [Google Scholar]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st Century Manufacturing Paradigm. Biofabrication 2009, 1, 022001. [Google Scholar]
- Chen, H.-G.; Zhang, Y.-H.P. New Biorefineries and Sustainable Agriculture: Increased Food, Biofuels, and Ecosystem Security. Renew. Sustain. Energy Rev. 2015, 47, 117–132. [Google Scholar] [CrossRef]
- Reid, A.; Buchanan, F.; Julius, M.; Walsh, P.J. A Review on Diatom Biosilicification and Their Adaptive Ability to Uptake Other Metals into Their Frustules for Potential Application in Bone Repair. J. Mater. Chem. B Mater. Biol. Med. 2021, 9, 6728–6737. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Bashir, R. Biomimicry, Biofabrication, and Biohybrid Systems: The Emergence and Evolution of Biological Design. Adv. Healthc. Mater. 2017, 6, 1700496. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D Printing of Bioinspired Biomaterials for Tissue Regeneration. Adv. Healthc. Mater. 2020, 9, e2000208. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green Approach for Nanoparticle Biosynthesis by Fungi: Current Trends and Applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Yang, Y.; Waterhouse, G.I.N.; Chen, Y.; Sun-Waterhouse, D.; Li, D. Microbial-Enabled Green Biosynthesis of Nanomaterials: Current Status and Future Prospects. Biotechnol. Adv. 2022, 55, 107914. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Pereira, H.S.; Senra, R.L.; Ribon, A.d.O.B.; Mendes, T.A.d.O. Understanding the Emerging Potential of Synthetic Biology for Food Science: Achievements, Applications and Safety Considerations. Food Chem. Adv. 2023, 3, 100476. [Google Scholar] [CrossRef]
- Tamerler, C.; Sarikaya, M. Molecular Biomimetics: Nanotechnology and Bionanotechnology Using Genetically Engineered Peptides. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 1705–1726. [Google Scholar] [CrossRef]
- Isar, J.; Jain, D.; Joshi, H.; Dhoot, S.; Rangaswamy, V. MICROBIAL Isoprene Production: An Overview. World J. Microbiol. Biotechnol. 2022, 38, 122. [Google Scholar] [CrossRef]
- Kreyenschulte, D.; Krull, R.; Margaritis, A. Recent Advances in Microbial Biopolymer Production and Purification. Crit. Rev. Biotechnol. 2014, 34, 1–15. [Google Scholar] [CrossRef]
- Bittencourt, D.M.d.C.; Oliveira, P.; Michalczechen-Lacerda, V.A.; Rosinha, G.M.S.; Jones, J.A.; Rech, E.L. Bioengineering of Spider Silks for the Production of Biomedical Materials. Front. Bioeng. Biotechnol. 2022, 10, 958486. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of Calcium Carbonates and Their Engineered Applications: A Review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Férard, C. Significance, Mechanisms and Environmental Implications of Microbial Biomineralization. Comptes Rendus Geosci. 2010, 343, 160–167. [Google Scholar] [CrossRef]
- Keykha, H.A.; Zangani, A.; Romiani, H.M.; Asadi, A.; Kawasaki, S.; Radmanesh, N. Characterizing Microbial and CO2-Induced Carbonate Minerals: Implications for Soil Stabilization in Sandy Environments. Minerals 2023, 13, 976. [Google Scholar] [CrossRef]
- Elgendy, I.M.; Elkaliny, N.E.; Saleh, H.M.; Darwish, G.O.; Almostafa, M.M.; Metwally, K.; Yahya, G.; Mahmoud, Y.A.-G. Bacteria-Powered Self-Healing Concrete: Breakthroughs, Challenges, and Future Prospects. J. Ind. Microbiol. Biotechnol. 2024, 52, kuae051. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281. [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D. Silicate, Phosphate and Carbonate Mineral Dissolution Behaviour in the Presence of Organic Acids: A Review. Miner. Eng. 2017, 100, 115–123. [Google Scholar] [CrossRef]
- Tan, K.H. Degradation of Soil Minerals by Organic Acids. In SSSA Special Publications; Soil Science Society of America: Madison, WI, USA, 2015; pp. 1–27. ISBN 9780891189121. [Google Scholar]
- Moisescu, C.; Ardelean, I.I.; Benning, L.G. The Effect and Role of Environmental Conditions on Magnetosome Synthesis. Front. Microbiol. 2014, 5, 49. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Jimenez-Lopez, C.; Neal, A.L.; Rull-Perez, F.; Rodriguez-Navarro, A.; Fernandez-Vivas, A.; Iañez-Pareja, E. Magnetite Biomineralization Induced by Shewanella Oneidensis. Geochim. Cosmochim. Acta 2010, 74, 967–979. [Google Scholar] [CrossRef]
- Wan, J.; Ji, R.; Liu, J.; Ma, K.; Pan, Y.; Lin, W. Biomineralization in Magnetotactic Bacteria: From Diversity to Molecular Discovery-Based Applications. Cell Rep. 2024, 43, 114995. [Google Scholar] [CrossRef]
- Hoffmann, T.D.; Reeksting, B.J.; Gebhard, S. Bacteria-Induced Mineral Precipitation: A Mechanistic Review. Microbiology 2021, 167, 001049. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wang, C.-Y.; Ma, Y.-X.; Shen, M.-J.; Li, J.; Jiao, K.; Tay, F.R.; Niu, L.-N. Microbe-Mediated Extracellular and Intracellular Mineralization: Environmental, Industrial, and Biotechnological Applications. Adv. Mater. 2020, 32, e1907833. [Google Scholar] [CrossRef] [PubMed]
- Lai Huat, L.; Swee Pin, Y.; Yao, H.; Huangpu, J.; Shao Feng, S.; Swee Sen, T. Environmental Bioremediation: Microremediation, Mycoremediation, and Phytoremediation. Environ. Claims J. 2024, 36, 186–205. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Cui, M.; Zheng, J.; Chen, Z.; Pei, J.; Zhang, J. Mechanisms and Influencing Factors of Biomineralization Based Heavy Metal Remediation: A Review. Biogeotechnics 2023, 1, 100039. [Google Scholar] [CrossRef]
- Cui, M.-J.; Lai, H.-J.; Wu, S.-F.; Chu, J. Comparison of Soil Improvement Methods Using Crude Soybean Enzyme, Bacterial Enzyme or Bacteria Induced Carbonate Precipitation. Géotechnique 2022, 74, 18–26. [Google Scholar] [CrossRef]
- Qabany, A.A.L.; Soga, K. Effect of Chemical Treatment Used in MICP on Engineering Properties of Cemented Soils. In Bio- and Chemo-Mechanical Processes in Geotechnical Engineering; ICE Publishing: London, UK, 2014; pp. 107–115. ISBN 9780727760531. [Google Scholar]
- Zhou, Y.; Zhao, X.; Jiang, Y.; Ding, C.; Liu, J.; Zhu, C. Synergistic Remediation of Lead Pollution by Biochar Combined with Phosphate Solubilizing Bacteria. Sci. Total Environ. 2023, 861, 160649. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, J. Phosphate Microbial Mineralization Consolidation of Waste Incineration Fly Ash and Removal of Lead Ions. Ecotoxicol. Environ. Saf. 2020, 191, 110224. [Google Scholar] [CrossRef]
- Coelho, E.; Reis, T.A.; Cotrim, M.; Mullan, T.K.; Renshaw, J.; Rizzutto, M.; Corrêa, B. Talaromyces Amestolkiae Uses Organic Phosphate Sources for the Treatment of Uranium-Contaminated Water. Biometals 2022, 35, 335–348. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, Y.; Xu, H.; Yao, Y. Lead Removal by Phosphate Solubilizing Bacteria Isolated from Soil through Biomineralization. Chemosphere 2019, 224, 272–279. [Google Scholar] [CrossRef]
- Zeng, G.; Qiao, S.; Wang, X.; Sheng, M.; Wei, M.; Chen, Q.; Xu, H.; Xu, F. Immobilization of Cadmium by Burkholderia Sp. QY14 through Modified Microbially Induced Phosphate Precipitation. J. Hazard. Mater. 2021, 412, 125156. [Google Scholar] [CrossRef]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, Adsorption and Rhizosphere Effect: The Mechanisms for Phosphate-Induced Pb Immobilization in Soils-A Review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Lin, H.; Dong, Y.; Li, B.; He, Y.; Liu, C.; Chen, X. A Novel Constructed Carbonate-Mineralized Functional Bacterial Consortium for High-Efficiency Cadmium Biomineralization. J. Hazard. Mater. 2021, 401, 123269. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Biological Synthesis of Metal Nanoparticles by Microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar]
- Vanlalveni, C.; Ralte, V.; Zohmingliana, H.; Das, S.; Anal, J.M.H.; Lallianrawna, S.; Rokhum, S.L. A Review of Microbes Mediated Biosynthesis of Silver Nanoparticles and Their Enhanced Antimicrobial Activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef]
- Sridhar, S. Microbial Genetics; Wisdom Press: New Delhi, India, 2013; ISBN 9789382006213. [Google Scholar]
- Quester, K.; Avalos-Borja, M.; Castro-Longoria, E. Biosynthesis and Microscopic Study of Metallic Nanoparticles. Micron 2013, 54–55, 1–27. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of Metal Nanoparticles from Fungi and Metal Salts: Scope and Application. Nanoscale Res. Lett. 2016, 11, 98. [Google Scholar]
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Optimization of Biological Synthesis of Silver Nanoparticles Using Fusarium Oxysporum. Iran. J. Pharm. Res. 2013, 12, 289–298. [Google Scholar]
- Durán, N.; Marcato, P.D.; Alves, O.L.; Souza, G.I.H.D.; Esposito, E. Mechanistic Aspects of Biosynthesis of Silver Nanoparticles by Several Fusarium Oxysporum Strains. J. Nanobiotechnology 2005, 3, 8. [Google Scholar] [CrossRef]
- Tauseef, A.; Hisam, F.; Hussain, T.; Caruso, A.; Hussain, K.; Châtel, A.; Chénais, B. Nanomicrobiology: Emerging Trends in Microbial Synthesis of Nanomaterials and Their Applications. J. Clust. Sci. 2023, 34, 639–664. [Google Scholar]
- Wu, J.-W.; Ng, I.-S. Biofabrication of Gold Nanoparticles by Shewanella Species. Bioresour. Bioprocess. 2017, 4, 50. [Google Scholar] [CrossRef]
- Grasso, G.; Zane, D.; Dragone, R. Microbial Nanotechnology: Challenges and Prospects for Green Biocatalytic Synthesis of Nanoscale Materials for Sensoristic and Biomedical Applications. Nanomaterials 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Tsukiyama, T.; Tachimi, T.; Saitoh, N.; Nomura, T.; Nagamine, S. Microbial Deposition of Gold Nanoparticles by the Metal-Reducing Bacterium Shewanella Algae. Electrochim. Acta 2007, 53, 186–192. [Google Scholar] [CrossRef]
- Kitching, M.; Ramani, M.; Marsili, E. Fungal Biosynthesis of Gold Nanoparticles: Mechanism and Scale up: Fungal Biosynthesis of AuNPs. Microb. Biotechnol. 2015, 8, 904–917. [Google Scholar] [CrossRef]
- Hammad, S.E.; El-Rouby, M.N.; Abdel-Aziz, M.M.; El-Sayyad, G.S.; Elshikh, H.H. Endophytic Fungi–assisted Biomass Synthesis of Gold, and Zinc Oxide Nanoparticles for Increasing Antibacterial, and Anticancer Activities. Biomass Convers. Biorefin. 2025, 15, 2285–2302. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Gowramma, B.; Keerthi, U.; Rafi, M.; Muralidhara Rao, D. Biogenic Silver Nanoparticles Production and Characterization from Native Stain of Corynebacterium Species and Its Antimicrobial Activity. 3 Biotech 2015, 5, 195–201. [Google Scholar] [CrossRef]
- Rilda, Y.; Rinaldi, R.; Syukri, S.; Armaini, A.; Refinel, R.; Agustien, A.; Pardi, H. Biosynthesis of Zinc Oxide (ZnO) Using the Biomass of Aspergillus Niger to Impart Cotton Fabric with Antimicrobial Properties. ChemistrySelect 2022, 7, e202103824. [Google Scholar] [CrossRef]
- Sharma, J.L.; Dhayal, V.; Sharma, R.K. White-Rot Fungus Mediated Green Synthesis of Zinc Oxide Nanoparticles and Their Impregnation on Cellulose to Develop Environmental Friendly Antimicrobial Fibers. 3 Biotech 2021, 11, 269. [Google Scholar] [CrossRef]
- Hajiali, S.; Daneshjou, S.; Daneshjoo, S.; Khajeh, K. Biosynthesis Optimization of Antibacterial-Magnetic Iron Oxide Nanoparticles from Bacillus Megaterium. Biol. Trace Elem. Res. 2025, 203, 467–484. [Google Scholar] [CrossRef]
- Vasanth, V.; Murugesh, K.A.; Susikaran, S. Synthesis of Titanium Dioxide Nanoparticles Using Spirulina Platensis Algae Extract. Pharma Innov. J. 2022, 11, 266–269. [Google Scholar]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial Siderophores and Their Potential Applications: A Review. Environ. Sci. Pollut. Res. Int. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- De Windt, W.; Boon, N.; Van den Bulcke, J.; Rubberecht, L.; Prata, F.; Mast, J.; Hennebel, T.; Verstraete, W. Biological Control of the Size and Reactivity of Catalytic Pd(0) Produced by Shewanella Oneidensis. Antonie Van Leeuwenhoek 2006, 90, 377–389. [Google Scholar] [CrossRef] [PubMed]
- ELtarahony, M.; Zaki, S.; Kheiralla, Z.; Abd-El-Haleem, D. Biogenic Synthesis of Iron Oxide Nanoparticles via Optimization of Nitrate Reductase Enzyme Using Statistical Experimental Design. J. Adv. Biotechnol 2016, 5, 667–684. [Google Scholar]
- Elblbesy, M.A.; Madbouly, A.; Hamdan, T. Bio-Synthesis of Magnetite Nanoparticles by Bacteria. Am. J. Nano Res. Appl. 2014, 2, 98. [Google Scholar]
- Hossain, S.; Bahreini, B.; Pasteur, E. Magnetotactic Bacteria and Magnetosomes–an Overview. Mater. Sci. Eng. 2024, 8, 83–100. [Google Scholar] [CrossRef]
- Noguchi, Y.; Fujiwara, T.; Yoshimatsu, K.; Fukumori, Y. Iron Reductase for Magnetite Synthesis in the Magnetotactic Bacterium Magnetospirillum Magnetotacticum. J. Bacteriol. 1999, 181, 2142–2147. [Google Scholar] [CrossRef]
- Fdez-Gubieda, M.L.; Muela, A.; Alonso, J.; García-Prieto, A.; Olivi, L.; Fernández-Pacheco, R.; Barandiarán, J.M. Magnetite Biomineralization in Magnetospirillum Gryphiswaldense: Time-Resolved Magnetic and Structural Studies. ACS Nano 2013, 7, 3297–3305. [Google Scholar] [CrossRef]
- Amor, M.; Ceballos, A.; Wan, J.; Simon, C.P.; Aron, A.T.; Chang, C.J.; Hellman, F.; Komeili, A. Magnetotactic Bacteria Accumulate a Large Pool of Iron Distinct from Their Magnetite Crystals. Appl. Environ. Microbiol. 2020, 86, e01278-20. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, S.; Khan, S.; Rentschler, S.; Laufer, S.; Deigner, H.-P. Biosynthesis of Iron Oxide Magnetic Nanoparticles Using Clinically Isolated Pseudomonas Aeruginosa. Sci. Rep. 2021, 11, 20503. [Google Scholar] [CrossRef]
- Liße, D.; Monzel, C.; Vicario, C.; Manzi, J.; Maurin, I.; Coppey, M.; Piehler, J.; Dahan, M. Engineered Ferritin for Magnetogenetic Manipulation of Proteins and Organelles inside Living Cells. Adv. Mater. 2017, 29, 1700189. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Xie, Q.; Wang, J.; Yue, Z.; Wei, L.; Yang, Y.; Li, L.; Chen, T. Reduction and Transformation of Nanomagnetite and Nanomaghemite by a Sulfate-Reducing Bacterium. Geochim. Cosmochim. Acta 2019, 256, 66–81. [Google Scholar] [CrossRef]
- Pan, J.; Qian, H.; Sun, Y.; Miao, Y.; Zhang, J.; Li, Y. Microbially Synthesized Nanomaterials: Advances and Applications in Biomedicine. Precis. Med. Eng. 2025, 2, 100019. [Google Scholar] [CrossRef]
- Bao, H.; Lu, Z.; Cui, X.; Qiao, Y.; Guo, J.; Anderson, J.M.; Li, C.M. Extracellular Microbial Synthesis of Biocompatible CdTe Quantum Dots. Acta Biomater. 2010, 6, 3534–3541. [Google Scholar] [CrossRef]
- Mi, C.; Wang, Y.; Zhang, J.; Huang, H.; Xu, L.; Wang, S.; Fang, X.; Fang, J.; Mao, C.; Xu, S. Biosynthesis and Characterization of CdS Quantum Dots in Genetically Engineered Escherichia Coli. J. Biotechnol. 2011, 153, 125–132. [Google Scholar] [CrossRef]
- Tusher, M.M.H. Microbial Synthesis of Cadmium Selenide Quantum Dots (CdSe QDs), Influencing Factors and Applications. Opt. Quantum Electron. 2023, 55, 332. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and Their Role in Phytopathogens Management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Su, Z.; Li, X.; Xi, Y.; Xie, T.; Liu, Y.; Liu, B.; Liu, H.; Xu, W.; Zhang, C. Microbe-Mediated Transformation of Metal Sulfides: Mechanisms and Environmental Significance. Sci. Total Environ. 2022, 825, 153767. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Tuan, H.-Y.; Tien, C.-W.; Lo, W.-H.; Liang, H.-C.; Hu, Y.-C. Augmented Biosynthesis of Cadmium Sulfide Nanoparticles by Genetically Engineered Escherichia Coli. Biotechnol. Prog. 2009, 25, 1260–1266. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Darezereshki, E.; Bakhtiari, F.; Fazaelipoor, M.H.; Hosseini, M.R. Characterization of Zinc Sulfide (ZnS) Nanoparticles Biosynthesized by Fusarium Oxysporum. Mater. Sci. Semicond. Process. 2013, 16, 374–378. [Google Scholar] [CrossRef]
- Senapati, S.; Syed, A.; Khan, S.; Pasricha, R.; Khan, M.; Kumar, R.; Ahmad, A. Extracellular Biosynthesis of Metal Sulfide Nanoparticles Using the Fungus Fusarium Oxysporum. Curr. Nanosci. 2014, 10, 588–595. [Google Scholar] [CrossRef]
- Oliva-Arancibia, B.; Órdenes-Aenishanslins, N.; Bruna, N.; Ibarra, P.S.; Zacconi, F.C.; Pérez-Donoso, J.M.; Poblete-Castro, I. Co-Synthesis of Medium-Chain-Length Polyhydroxyalkanoates and CdS Quantum Dots Nanoparticles in Pseudomonas Putida KT2440. J. Biotechnol. 2017, 264, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sobhanan, J.; Jones, P.; Kohara, R.; Sugino, S.; Vacha, M.; Subrahmanyam, C.; Takano, Y.; Lacy, F.; Biju, V. Toxicity of Nanomaterials due to Photochemical Degradation and the Release of Heavy Metal Ions. Nanoscale 2020, 12, 22049–22058. [Google Scholar] [CrossRef]
- Calvo-Olvera, A.; De Donato-Capote, M.; Pool, H.; Rojas-Avelizapa, N.G. In Vitro Toxicity Assessment of Fungal-Synthesized Cadmium Sulfide Quantum Dots Using Bacteria and Seed Germination Models. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2021, 56, 713–722. [Google Scholar] [CrossRef]
- Feng, J.; Chen, B.; Sun, W.; Wang, Y. Microbial Induced Calcium Carbonate Precipitation Study Using Bacillus Subtilis with Application to Self-Healing Concrete Preparation and Characterization. Constr. Build. Mater. 2021, 280, 122460. [Google Scholar] [CrossRef]
- Ahmad, S.S.E.; Elmahdy, M.A.R.; ELShami, A.A.; Yousry, E.-S.M. Bacterial Sustainable Concrete for Repair and Rehabilitation of Structural Cracks. J. Sustain. Cem.-Based Mater. 2024, 20, e03188. [Google Scholar] [CrossRef]
- Fan, Q.; Fan, L.; Quach, W.-M.; Zhang, R.; Duan, J.; Sand, W. Application of Microbial Mineralization Technology for Marine Concrete Crack Repair: A Review. J. Build. Eng. 2023, 69, 106299. [Google Scholar] [CrossRef]
- Renninger, N.; Knopp, R.; Nitsche, H.; Clark, D.S.; Keasling, J.D. Uranyl Precipitation by Pseudomonas Aeruginosa via Controlled Polyphosphate Metabolism. Appl. Environ. Microbiol. 2004, 70, 7404–7412. [Google Scholar] [CrossRef]
- Liang, X.; Hillier, S.; Pendlowski, H.; Gray, N.; Ceci, A.; Gadd, G.M. Uranium Phosphate Biomineralization by Fungi. Environ. Microbiol. 2015, 17, 2064–2075. [Google Scholar] [CrossRef]
- Manobala, T.; Shukla, S.K.; Rao, T.S.; Kumar, M.D. Uranium Sequestration by Biofilm-Forming Bacteria Isolated from Marine Sediment Collected from Southern Coastal Region of India. Int. Biodeterior. Biodegrad. 2019, 145, 104809. [Google Scholar] [CrossRef]
- Toster, J.; Harnagea, C.; Iyer, K.; Rosei, F.; Raston, C. Controlling Anatase Coating of Diatom Frustules by Varying the Binding Layer. CrystEngComm 2012, 14, 3446–3450. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for Cost-Effective and Enhanced Production of Bacterial Cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N.; et al. Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Rehm, B.H.; Moradali, M.F. (Eds.) Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; ISBN 9783527818310. [Google Scholar]
- Rosalam, S.; England, R. Review of Xanthan Gum Production from Unmodified Starches by Xanthomonas Comprestris sp. Enzym. Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Ronďošová, S.; Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertus, S. Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus Necator. Fermentation 2022, 8, 451. [Google Scholar] [CrossRef]
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.; Ramarao, B.V.; Misra, M. A Review on Polyhydroxyalkanoate (PHA) Production through the Use of Lignocellulosic Biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving Biological Production of poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Co-Polymer: A Critical Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Tian, X.; Chen, H.; Liu, H.; Chen, J. Recent Advances in Lactic Acid Production by Lactic Acid Bacteria. Appl. Biochem. Biotechnol. 2021, 193, 4151–4171. [Google Scholar] [CrossRef]
- Dessie, W.; Xin, F.; Zhang, W.; Jiang, Y.; Wu, H.; Ma, J.; Jiang, M. Opportunities, Challenges, and Future Perspectives of Succinic Acid Production by Actinobacillus Succinogenes. Appl. Microbiol. Biotechnol. 2018, 102, 9893–9910. [Google Scholar] [PubMed]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus Succinogenes: Advances on Succinic Acid Production and Prospects for Development of Integrated Biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Eiteman, M.A.; Ramalingam, S. Microbial Production of Lactic Acid. Biotechnol. Lett. 2015, 37, 955–972. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial Production of Lactic Acid: The Latest Development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Amara, N.I.; Chukwuemeka, E.S.; Obiajulu, N.O.; Chukwuma, O.J. Yeast-Driven Valorization of Agro-Industrial Wastewater: An Overview. Environ. Monit. Assess. 2023, 195, 1252. [Google Scholar] [CrossRef]
- Saravanan, M.; Gopinath, V.; Chaurasia, M.K.; Syed, A.; Ameen, F.; Purushothaman, N. Green Synthesis of Anisotropic Zinc Oxide Nanoparticles with Antibacterial and Cytofriendly Properties. Microb. Pathog. 2018, 115, 57–63. [Google Scholar] [CrossRef]
- Jacob, P.J.; Masarudin, M.J.; Hussein, M.Z.; Rahim, R.A. Optimization of Process Parameters Influencing the Sustainable Construction of Iron Oxide Nanoparticles by a Novel Tropical Wetlands Streptomyces spp. J. Clean. Prod. 2019, 232, 193–202. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kimber, R.L.; Singh, V.K.; Shende, S.; Behal, A.; Sushkova, S.; Mandzhieva, S.; Lloyd, J.R. Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella. Appl. Environ. Microbiol. 2021, 87, e01390-21. [Google Scholar] [CrossRef]
- Khort, A.; Brookman-Amissah, M.; Hedberg, J.; Chang, T.; Mei, N.; Lundberg, A.; Sturve, J.; Blomberg, E.; Odnevall, I. Influence of Natural Organic Matter on the Transformation of Metal and Metal Oxide Nanoparticles and Their Ecotoxic Potency In Vitro. NanoImpact 2022, 25, 100386. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of Bacterial Cellulose Using Different Carbon Sources and Culture Media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Hu, H.; Catchmark, J.M.; Demirci, A. Effects of Pullulan Additive and Co-Culture of Aureobasidium Pullulans on Bacterial Cellulose Produced by Komagataeibacter Hansenii. Bioprocess Biosyst. Eng. 2022, 45, 573–587. [Google Scholar]
- Chavan, S.; Baig, M. Relationship of Biomass and Xanthan Gum Production by Xanthomonas Campestris: Optimization of Parameters. Br. Biotechnol. J. 2016, 11, 1–8. [Google Scholar] [CrossRef]
- Trindade, R.A.; Munhoz, A.P.; Burkert, C.A.V. Impact of a Carbon Source and Stress Conditions on Some Properties of Xanthan Gum Produced by Xanthomonas Campestris Pv. Mangiferaeindicae. Biocatal. Agric. Biotechnol. 2018, 15, 167–172. [Google Scholar] [CrossRef]
- Zulfiqar, Z.; Khan, R.R.M.; Summer, M.; Saeed, Z.; Pervaiz, M.; Rasheed, S.; Shehzad, B.; Kabir, F.; Ishaq, S. Plant-Mediated Green Synthesis of Silver Nanoparticles: Synthesis, Characterization, Biological Applications, and Toxicological Considerations: A Review. Biocatal. Agric. Biotechnol. 2024, 57, 103121. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for Biogenic Microbial Synthesis of Silver Nanoparticles through Response Surface Methodology, Characterization, Their Antimicrobial, Antioxidant, and Catalytic Potential. Sci. Rep. 2021, 11, 770. [Google Scholar]
- Nejadmansouri, M.; Shad, E.; Razmjooei, M.; Safdarianghomsheh, R.; Delvigne, F.; Khalesi, M. Production of Xanthan Gum Using Immobilized Xanthomonas Campestris Cells: Effects of Support Type. Biochem. Eng. J. 2020, 157, 107554. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Zeng, Y.; Li, X.; Jiang, X.; Wang, Y.; Xie, T.; Zhang, Y. Biosynthesis of Xanthan Gum by Xanthomonas Campestris LRELP-1 Using Kitchen Waste as the Sole Substrate. Carbohydr. Polym. 2016, 151, 684–691. [Google Scholar] [CrossRef]
- Baker, S.; Mohan Kumar, K.; Santosh, P.; Rakshith, D.; Satish, S. Extracellular Synthesis of Silver Nanoparticles by Novel Pseudomonas Veronii AS41G Inhabiting Annona Squamosa L. and Their Bactericidal Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136 Pt C, 1434–1440. [Google Scholar] [CrossRef]
- Saravana Kumar, P.; Balachandran, C.; Duraipandiyan, V.; Ramasamy, D.; Ignacimuthu, S.; Al-Dhabi, N.A. Extracellular Biosynthesis of Silver Nanoparticle Using Streptomyces Sp. 09 PBT 005 and Its Antibacterial and Cytotoxic Properties. Appl. Nanosci. 2015, 5, 169–180. [Google Scholar] [CrossRef]
- Guo, E.; Chen, G.; Yu, D.; Qiu, Y.; Li, S.; Yu, Y. Optimization of Dry Anaerobic Co-Fermentation of Sludge and Corn Straw with Magnetite (Fe3O4). J. Environ. Chem. Eng. 2022, 10, 108618. [Google Scholar]
- Tyagi, S.; Tyagi, P.K.; Gola, D.; Chauhan, N.; Bharti, R.K. Extracellular Synthesis of Silver Nanoparticles Using Entomopathogenic Fungus: Characterization and Antibacterial Potential. SN Appl. Sci. 2019, 1, 1545. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Balakumaran, M.D.; Murugan, R.; Dhanapal, K.; Kalaichelvan, P.T. Phytogenic Synthesis of Silver Nanoparticles, Optimization and Evaluation of in Vitro Antifungal Activity against Human and Plant Pathogens. Microbiol. Res. 2016, 192, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Krishna Leela, J.; Sharma, G. Studies on Xanthan Production from Xanthomonas Campestris. Bioprocess Biosyst. Eng. 2000, 23, 687–689. [Google Scholar]
- Chakraborty, I.; Feliu, N.; Roy, S.; Dawson, K.; Parak, W.J. Protein-Mediated Shape Control of Silver Nanoparticles. Bioconjug. Chem. 2018, 29, 1261–1265. [Google Scholar]
- Chakraborty, I.; Parak, W.J. Protein-induced Shape Control of Noble Metal Nanoparticles. Adv. Mater. Interfaces 2019, 6, 1801407. [Google Scholar]
- Murphy, C.J.; Chang, H.-H.; Falagan-Lotsch, P.; Gole, M.T.; Hofmann, D.M.; Hoang, K.N.L.; McClain, S.M.; Meyer, S.M.; Turner, J.G.; Unnikrishnan, M.; et al. Virus-Sized Gold Nanorods: Plasmonic Particles for Biology. Acc. Chem. Res. 2019, 52, 2124–2135. [Google Scholar]
- Duman, E.; Şahin Kehribar, E.; Ahan, R.E.; Yuca, E.; Şeker, U.Ö.Ş. Biomineralization of Calcium Phosphate Crystals Controlled by Protein-Protein Interactions. ACS Biomater. Sci. Eng. 2019, 5, 4750–4763. [Google Scholar]
- Mohammadi, P.; Gandier, J.-A.; Nonappa; Wagermaier, W.; Miserez, A.; Penttilä, M. Bioinspired Functionally Graded Composite Assembled Using Cellulose Nanocrystals and Genetically Engineered Proteins with Controlled Biomineralization. Adv. Mater. 2021, 33, e2102658. [Google Scholar]
- Nie, Z.; Zhang, Y.; Tang, R.; Wang, X. Biomimetic Mineralization: An Emerging Organism Engineering Strategy for Biomedical Applications. J. Inorg. Biochem. 2022, 232, 111815. [Google Scholar]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and Peptide-Directed Syntheses of Inorganic Materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar]
- Tamerler, C.; Kacar, T.; Sahin, D.; Fong, H.; Sarikaya, M. Genetically Engineered Polypeptides for Inorganics: A Utility in Biological Materials Science and Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2007, 27, 558–564. [Google Scholar]
- Qin, K.; Zheng, Z.; Wang, J.; Pan, H.; Tang, R. Biomineralization Strategy: From Material Manufacturing to Biological Regulation. Giant 2024, 19, 100317. [Google Scholar] [CrossRef]
- Miserez, A.; Yu, J.; Mohammadi, P. Protein-Based Biological Materials: Molecular Design and Artificial Production. Chem. Rev. 2023, 123, 2049–2111. [Google Scholar]
- Schulz, A.; Wang, H.; Rijn, P.; Böker, A. Synthetic Inorganic Materials by Mimicking Biomineralization Processes Using Native and Non-Native Protein Functions. J. Mater. Chem. 2011, 21, 18903–18918. [Google Scholar]
- Wang, Y.; Guo, J.; Zhou, L.; Ye, C.; Omenetto, F.G.; Kaplan, D.L.; Ling, S. Design, Fabrication, and Function of Silk-Based Nanomaterials. Adv. Funct. Mater. 2018, 28, 1805305. [Google Scholar]
- Chandraker, S.K.; Kumar, R. Biogenic Biocompatible Silver Nanoparticles: A Promising Antibacterial Agent. Biotechnol. Genet. Eng. Rev. 2024, 40, 3113–3147. [Google Scholar] [CrossRef]
- Russo, P.; De Simone, N.; Capozzi, V.; Mohedano, M.L.; Ruiz-Masó, J.Á.; Del Solar, G.; López, P.; Spano, G. Selection of Riboflavin Overproducing Strains of Lactic Acid Bacteria and Riboflavin Direct Quantification by Fluorescence. Methods Mol. Biol. 2021, 2280, 3–14. [Google Scholar]
- Kant, G.; Pandey, A.; Kumari, S.; Bux, F.; Srivastava, S. Enhancing Bio-Isoprene Production in Escherichia Coli through a Combinatorial Optimization Approach. Process Biochem. 2024, 144, 210–219. [Google Scholar]
- De Carluccio, G.; Fusco, V.; di Bernardo, D. Engineering a Synthetic Gene Circuit for High-Performance Inducible Expression in Mammalian Systems. Nat. Commun. 2024, 15, 3311. [Google Scholar]
- Wang, Y.; Liu, Y.; Li, J.; Chen, Y.; Liu, S.; Zhong, C. Engineered Living Materials (ELMs) Design: From Function Allocation to Dynamic Behavior Modulation. Curr. Opin. Chem. Biol. 2022, 70, 102188. [Google Scholar]
- Sadeghianmaryan, A.; Ahmadian, N.; Wheatley, S.; Alizadeh Sardroud, H.; Nasrollah, S.A.S.; Naseri, E.; Ahmadi, A. Advancements in 3D-Printable Polysaccharides, Proteins, and Synthetic Polymers for Wound Dressing and Skin Scaffolding—A Review. Int. J. Biol. Macromol. 2024, 266, 131207. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.G.; Curtin, C.; O’Brien, F. Articulation Inspired by Nature: A Review of Biomimetic and Biologically Active 3D Printed Scaffolds for Cartilage Tissue Engineering. Biomater. Sci. 2022, 10, 2462–2483. [Google Scholar] [CrossRef] [PubMed]
- Heveran, C.M.; Williams, S.L.; Qiu, J.; Artier, J.; Hubler, M.H.; Cook, S.M.; Cameron, J.C.; Srubar, W.V., III. Biomineralization and Successive Regeneration of Engineered Living Building Materials. Matter 2020, 2, 481–494. [Google Scholar] [CrossRef]
- Bi, S.; He, C.; Liu, R.; Zhao, X.; Liu, J.; Gu, J.; Liu, W.; Yan, B. On-Demand Dissociable Antibacterial Self-Healing Hydrogel with Rapid Arginine-Related Biosynthesis Capacity to Promote Full-Thickness Bacteria-Infected Wound Healing. Ind. Eng. Chem. Res. 2023, 62, 7492–7503. [Google Scholar] [CrossRef]
- Yang, M.; Yang, F.; Chen, W.; Liu, S.; Qiu, L.; Chen, J. Bacteria-Mediated Cancer Therapies: Opportunities and Challenges. Biomater. Sci. 2021, 9, 5732–5744. [Google Scholar] [CrossRef]
- Fan, J.-X.; Niu, M.-T.; Qin, Y.-T.; Sun, Y.-X.; Zhang, X.-Z. Progress of Engineered Bacteria for Tumor Therapy. Adv. Drug Deliv. Rev. 2022, 185, 114296. [Google Scholar] [CrossRef]
- Lin, D.; Feng, X.; Mai, B.; Li, X.; Wang, F.; Liu, J.; Liu, X.; Zhang, K.; Wang, X. Bacterial-Based Cancer Therapy: An Emerging Toolbox for Targeted Drug/gene Delivery. Biomaterials 2021, 277, 121124. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Z.; Gao, Z.; Wan, M.; Zhou, M.; Mao, C.; Shen, J. Artificial Cells: Past, Present and Future. ACS Nano 2022, 16, 15705–15733. [Google Scholar] [CrossRef]
- Choi, S. Electrogenic Bacteria Promise New Opportunities for Powering, Sensing, and Synthesizing. Small 2022, 18, e2107902. [Google Scholar] [CrossRef]
- Shin, H.J. Genetically Engineered Microbial Biosensors for in Situ Monitoring of Environmental Pollution. Appl. Microbiol. Biotechnol. 2011, 89, 867–877. [Google Scholar] [CrossRef]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of Environmental Pollutants by Bioluminescent Bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Belkin, S. Microbial Whole-Cell Sensing Systems of Environmental Pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Belkin, S. Genetically Engineered Microorganisms for Pollution Monitoring. In NATO Science Series; Springer: Dordrecht, The Netherlands, 2007; pp. 147–160. ISBN 9781402047268. [Google Scholar]
- Parakh, P.D.; Nanda, S.; Kozinski, J.A. Eco-Friendly Transformation of Waste Biomass to Biofuels. Curr. Biochem. Eng. 2020, 6, 120–134. [Google Scholar] [CrossRef]
- Zeng, J.; Zeng, H.; Wang, Z. Review on Technology of Making Biofuel from Food Waste. Int. J. Energy Res. 2022, 46, 10301–10319. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Zhang, L.; Zeng, D.-W.; Liao, S.; Fan, Y.; Champreda, V.; Runguphan, W.; Zhao, X.-Q. Engineering Yeast Cell Factories to Produce Biodegradable Plastics and Their Monomers: Current Status and Prospects. Biotechnol. Adv. 2023, 68, 108222. [Google Scholar] [CrossRef]
- Kitamoto, H.K.; Shinozaki, Y.; Cao, X.-H.; Morita, T.; Konishi, M.; Tago, K.; Kajiwara, H.; Koitabashi, M.; Yoshida, S.; Watanabe, T.; et al. Phyllosphere Yeasts Rapidly Break down Biodegradable Plastics. AMB Express 2011, 1, 44. [Google Scholar] [CrossRef]
- Naidu, S.; Singh, I.K.; Singh, A. Microbial Synthesis of Magnetic Nanoparticles for Plant Science and Agriculture. Plant Nano Biol. 2023, 4, 100036. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Kumar, S.; Das, A.; Kumar, R.; Bains, A.; Sridhar, K.; Duhan, J.S.; Sharma, M. Recovery of Agricultural Waste Biomass: A Path for Circular Bioeconomy. Sci. Total Environ. 2023, 870, 161904. [Google Scholar] [CrossRef]
- Chen, C.-C.; Dai, L.; Ma, L.; Guo, R. Enzymatic Degradation of Plant Biomass and Synthetic Polymers. Nat. Rev. Chem. 2020, 4, 114–126. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- de Jesús Montaño López, J.; Duran, L.A.; Avalos, J. Physiological Limitations and Opportunities in Microbial Metabolic Engineering. Nat. Rev. Microbiol. 2021, 20, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J.; Parameswaran, B.; Kumar, S.; Khare, S.K. (Eds.) Biosynthetic Technology and Environmental Challenges, 1st ed.; Energy, Environment, and Sustainability; Springer: Singapore, 2017; ISBN 9789811074332. [Google Scholar]
- Ahmed, I.A. Ethical Issues of Microbial Products for Industrialization. In Interdisciplinary Biotechnological Advances; Springer Nature: Singapore, 2023; pp. 393–411. ISBN 9789819917365. [Google Scholar]
- Meena, S.S.; Mohanty, A. Ethical, Patent, and Regulatory Issues in Microbial Engineering. In Engineering of Microbial Biosynthetic Pathways; Springer: Singapore, 2020; pp. 133–142. ISBN 9789811526039. [Google Scholar]
- Casadevall, A. The Future of Biological Warfare: The Future of Biological Warfare. Microb. Biotechnol. 2012, 5, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Granato, E.T.; Meiller-Legrand, T.A.; Foster, K.R. The Evolution and Ecology of Bacterial Warfare. Curr. Biol. 2019, 29, R521–R537. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).