Abstract
Proteins are structurally and functionally diverse biomacromolecules that serve a variety of essential activities to ensure complex biological homeostasis. The desire to elucidate and enhance these biological functions has been at the forefront of research for many decades. However, generating active proteins via recombinant expression or through chemical total synthesis each has limitations in terms of yield and functionality. Nature has provided a solution to this problem through evolving protein ligases that catalyze the formation of amide bonds between peptides/proteins, which can be exploited by protein engineers to develop robust functional proteins. Here, we summarize the biochemical mechanisms and applications of multiple cysteine-based protein ligases, especially focusing on how they have been utilized for protein therapeutics and engineering, as well as how they inspired chemists to develop efficient methodologies for protein synthesis (e.g., native chemical ligation).
1. Introduction
Proteins are responsible for a wide range of biological functions in cells, acting as enzymes, transporters, signaling molecules, etc. There is a structure–function relationship that exists within proteins, meaning specific amino acids or post-translational modifications (PTMs) can alter the behavior of proteins and exert various biological impacts [1]. Therefore, decades of research have gone into elucidating their structures and functions and utilizing this information to develop more robust biomolecules and introduce them as therapeutic options. Protein/peptide-based therapeutics is currently a multi-billion-dollar industry that has produced life-saving treatments for diverse diseases like diabetes and cancer; however, the production of these proteins often requires synthetic techniques that have inherent limitations [2,3].
The introduction of recombinant protein expression in E. coli, starting in 1982, has been revolutionary in the production of proteins with desirable qualities, which soon became the major method for producing recombinant proteins in large quantities [3,4]. The alteration of amino acid sequences to modify protein structures has led to the synthesis of recombinant proteins that have increased pharmacokinetic properties, stability, activity, etc. [5]. However, a major limitation of bacterial expression is the unavailability of PTMs found in more complex eukaryotic systems which are vital for fully functional proteins. Many proteins require PTMs for proper folding, so, oftentimes, recombinant bacterial expression leads to low yields or dysfunctional eukaryotic proteins. The development of protein expression approaches using insect or mammalian cells can overcome this drawback and successfully introduce diverse PTMs onto the proteins of interest. Even though the PTMs are available, it is still very difficult to have precise control over site-specific modifications, which can be critical to elucidate specific PTMs on biological activity [6,7,8]. Therefore, the addition of site-specific PTMs can require the use of chemical protein/peptide synthesis.
Chemical synthesis via solid-phase peptide synthesis (SPPS) has allowed for the generation of peptides carrying site-specific PTMs, which represents an advantage over recombinant expression. Although SPPS has been optimized for faster production and simpler purification of peptides, the generation of large peptides/proteins presents a major challenge for this technique. Every amino acid coupling causes the overall yield to take a hit, so SPPS is currently limited to linear peptides containing ~40–50 amino acids [8,9]. To combat the size limitation of SPPS and PTM control issues found in recombinant expression, researchers have turned to the use of ligation reactions. Both chemical and enzymatic, these reactions allow for the fusing of peptides/proteins to incorporate exogenous functional groups or improve their structure–function relationship, without causing any side effects (e.g., denaturing the protein structures). Moreover, these ligation approaches enable the preparation of proteins/peptides that contain non-canonical structures (e.g., D- and β-amino acids) and unnatural post-translational modifications.
In this review, we will discuss nature’s gift to the field of protein synthesis and engineering through the availability of protein ligases that catalyze these ligation reactions both in vitro and in vivo. Although isolated from different hosts and require different recognition sequences, the protein ligases outlined here all proceed via a catalytic cysteine residue. The resulting thioester generated between the enzyme and peptide/protein substrate can be intercepted by an incoming primary amine of another peptide/protein donor to complete the ligation reaction. These cysteine-based protein ligases have been extensively optimized and proven to be powerful tools to produce therapeutic options and facilitate the incorporation of unnatural amino acids into proteins. In a similar catalytic manner, nature has also provided the cysteine-based mechanism of intein splicing, which potentially serves as the inspiration for chemical ligation methods, thus demonstrating the breadth of opportunities enzymatic protein ligation has given to the research world.
2. Sortase A
2.1. Mechanism and Engineering
Sortase A (SrtA) is a transpeptidase enzyme that is originally found in the cell wall of Staphylococcus aureus and naturally functions to anchor proteins to the cell wall [10,11,12,13]. This enzyme was discovered in 1999 by the Schneewind lab, which allowed for extensive study into its mechanism of action as a protein ligase [14,15]. Sortase A recognizes the sequence LPXTG and its catalytic cysteine residue acts a nucleophile to attack the carbonyl group of the amide bond between threonine (T) and glycine (G) [10,11]. This forms a thioacyl intermediate between sortase A and threonine and releases a C-terminal glycine. The enzyme then catalyzes the ligation between a primary amine donor on an N-terminal glycine to the LPXT-SrtA complex. Due to its nucleophilicity, the amine group attacks the carbonyl group of the thioacyl intermediate to release sortase A and ligates the two peptides through the formation of a new amide bond (Figure 1A). Seeing as though the primary amine donor must be on a glycine residue which is identical to the product released in the first step of catalysis, this ligation reaction is reversible. In addition, wild-type sortase A is Ca2+-dependent and has low catalytic efficiency, which requires a high enzyme to substrate concentration and thus limits its applicability for enzymatic protein ligation.
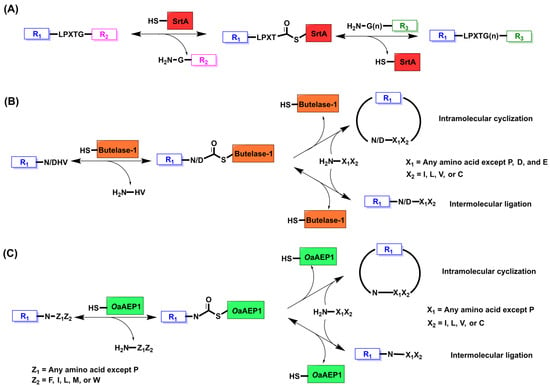
Figure 1.
Enzymatic peptide/protein ligation to produce native peptide/protein backbones. (A) Sortase A recognizes an LPXTG sequence and attacks the carbonyl group between T and G via its catalytic cysteine. This produces a thioester intermediate that can be intercepted by a primary amine on G to undergo an aminolysis reaction. R1 and R2 are peptide or protein sequences. (B) Butelase-1 recognizes an N/DHV sequence and attacks via its catalytic cysteine to produce a thioester with N/D. Butelase-1 can catalyze either a reversible intermolecular ligation or irreversible intramolecular cyclization with a primary amine. R1 is a peptide or protein sequence. X1 can be any amino acid except P, D, and E. X2 can be amino acids I, L, V, or C. (C) OaAEP1 recognizes an NZ1Z2 sequence, where Z1 is any amino acid except for P and Z2 can be amino acids F, I, L, M, or W. OaAEP1 can catalyze either intermolecular ligation or intramolecular cyclization with a primary amine via its catalytic cysteine residue. X1 can be any amino acid except P. X2 can be amino acids I, L, V, or C.
Since its initial application in enzymatic protein ligation, sortase A has been engineered to enhance its efficiency. Sortase A isolated from S. aureus has several setbacks, including Ca2+ dependency and low catalytic efficiency, which required optimization for ideal use [16,17]. To improve its catalytic efficiency, the 94R/D160N/D165A/K190E/K196T pentamutant of native sortase A, also known as eSrt, was developed by Chen et al. in 2015 [16,18]. Compared to the wild-type SrtA, eSrt has a 120-fold increased catalytic efficiency for LPETG coupling with a polyglycine peptide donor [18]. The catalytic efficiency of eSrt then allows for the usage of this enzyme with limited amounts of glycine substrate, which was a previous limitation for the wild-type enzyme [18]. Further improvements have been made to eSrt to eliminate the Ca2+ dependency that remained with this mutant. Hirakawa et al. identified two mutations, E105K and E108A, in the Ca2+ binding site that allowed sortase to have enzymatic function in the absence of Ca2+ [19]. However, these double mutations alone were not sufficient enough to promote ligation as they decreased the enzyme’s catalytic turnover rate [19]. This led to the successful development of the P94R/E105K/E108A/D160N/D165A/K190E/K196T heptamutant, which exhibits increased catalytic efficiency and Ca2+ independence compared to the wild type [19,20].
Sortase A has a fairly tight specificity as it requires the recognition sequence of LPXTG; therefore, studies have engineered the enzyme to reprogram and broaden its specificity. The F40 sortase mutant was developed via phage display and contains mutations at the T164 and V168-Q172 residues [21,22]. Several amino acids with small side chains, specifically alanine, are recognized by the F40 sortase, thus enhancing its promiscuity [21]. The sequence APXTG showed preferential coupling over LPXTG, which is significant as this sequence is endogenously found in histone H3 [21,22]. Unfortunately, the F40 sortase remains Ca2+-dependent and exhibits slow kinetics, resulting in limitations to its application for enzymatic protein ligation [21,22,23]. Other studies have focused on reprogramming the eSrt mutant to maintain its catalytic efficiency while broadening its substrate specificity to recognize both LAXTG and LPXSG substrates [13,24].
2.2. Applications
2.2.1. Histone Modification In Vitro and In Cellulo
Sortase A has demonstrated, both in vitro and in cellulo, the capability of modifying the N-terminal tails of histone H3 [22,23]. The natural amino acid sequence of histone H3 tail includes the short sequence APATGG, which allows for the use of engineered F40 sortase [21,23]. To introduce scarless H3 tails in vitro, the F40 sortase was further mutated to cW11 sortase, which possesses the additional mutations at D160, K190, and K196 [22]. The feature that makes cW11 sortase most distinct is that its backbone is cyclized, as this significantly enhances the stability of the enzyme [22,25]. cW11 sortase is able to modify H3 in several ways, one of those being the ability to ligate N-terminal tails and install PTMs onto H3 tailless nucleosomes [22]. The expression of tailless H3 and addition of difficult PTMs, such as ubiquitination, by cW11 sortase accelerate assays examining nucleosome enzymatic and binding activities [22,26,27]. In addition to introducing full tails, cW11 sortase can also produce asymmetrically ligated H3 tails, allowing one to study the effects of site-specific PTMs in various combinations [22]. Ligation is only part of the enzymatic function of cW11 sortase, as it can also cleave H3 tails at the recognition sequence [22]. This allows for the isolation and characterization of H3 tails after treatment with various “reader” proteins and “writer” enzymes to quantify their effects on histone modifications [22]. Collectively, the applicability of cW11 sortase to modify histone H3 allows for the introduction, modification, and isolation of H3′s modified N-terminal tails.
Sortase A has also been used for the semisynthesis of histones H2B and H4 in vitro to investigate the mechanism of histone deacetylation by sirtuins and histone deacetylases (HDACs) [28,29]. The N-terminal tail on histone H2B contains the sequence HPDTG, which exhibits similarities to the recognition sequence LPXTG. To accommodate these sequence changes, Wang et al. developed W4 sortase by the site-specific mutagenesis of F40 sortase [28]. The full-length semisynthesis of H2B with site-specific N-terminal acetylation was catalyzed by W4 sortase, and these versions of histone H2B were incorporated into the recombinant nucleosome core particles [28]. Histone H4 also contains a sequence similar to LPXTG, i.e., LARRGG, so Xiao et al. hypothesized that semisynthetic H4 could be produced by sortase-mediated ligation (SML) with eSrt(2A-9) [29]. Although the substitution of alanine (A) at the P2 position was tolerated by eSrt(2A-9), the variant was incapable of tolerating arginine (R) at the P4 position, so the endogenous H4 sequence was modified to LARCGG since cysteine can be converted to an arginine analog [29]. The semisynthesis of histone H2B and H4 by sortase variants allowed both studies to probe the selectivity and kinetics of HDACs and sirtuins for specific acetylated residues [28,29].
The modification of histone H3 has been further expanded from in vitro to in cellulo through the use of SrtA for sortase-mediated metathesis (SMM) (Figure 2) [23]. Truncated histone H3 was synthetically produced with an LPATGG recognition sequence for 6M-Srt to cleave between T and G, and thus produce an α-amine glycine substrate [23]. To expand the substrate scope, a peptide also carrying the LPATGG sequence was introduced so that it could also be recognized by sortase A and provide the LPAT sequence needed for ligation [23]. This allows for the addition of a cell-penetrating peptide (CPP) for in cellulo delivery and α-amine glycine on histone H3, which is lacking the endogenous tail [23]. The use of SMM allows for the delivery of histone H3 tails with site-specific modifications such as PTMs to study their impact on epigenetics in live cells [23].
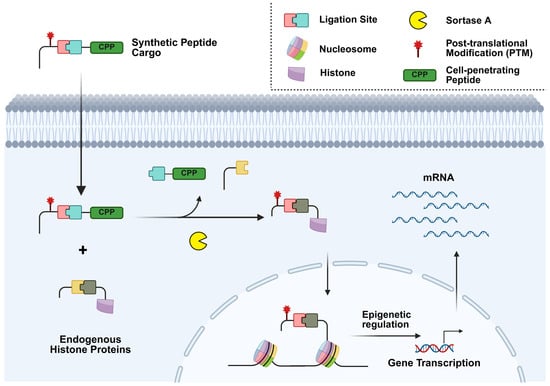
Figure 2.
Sortase-mediated metathesis (SMM) of histone N-terminal tails. The histone H3 N-terminal tail has been engineered with an LPATGG recognition motif for sortase A to deliver site specific post-translational modifications (PTMs) or small molecules. Control over the delivery of these modifications allows for the study of their biological implications and therapeutic applications.
2.2.2. Protein Engineering Through “Sortagging”
Protein ligation using sortase A creates proteins with terminal modifications; however, the combined use of sortase A and click chemistry allows for N-to-N and C-to-C fused peptides/proteins [30,31]. Click chemistry is a highly chemoselective reaction between two functional groups, such as azide and alkyne, which can be utilized for bio-orthogonal transformations [32,33]. Ploegh et al. in 2011 demonstrated the combination of SrtA-mediated ligation with click chemistry, coined as “sortagging”, using ubiquitin as the model protein [31]. Specifically, peptides with the recognition sequence LPETGG were synthesized with either an azide or cyclooctyne click handle [31]. N-terminal glycine on ubiquitin acted as the substrate for the SML reaction for each peptide; as a result, the incubation of both peptides produced a click reaction that formed a ubiquitin dimer [31]. Given that the click handles were present on the N-terminus of each peptide, the resulting ubiquitin dimer was an N-to-N fused protein [31]. This was extended to the production of C-to-C fused anti-GFP VHH antibody dimers, which were converted with a ~90% success rate and maintained their functionality [31]. This demonstrates the ability of “sortagging” to produce protein dimers that retain the identity of either the N- or C-terminus that is critical for function.
To improve the efficacy of cancer immunotherapy treatments, “sortagging” has been employed for the utilization of cytotoxic T lymphocytes (CTLs) that employ apoptosis to eliminate targeted cells [34,35]. He et al. set out to track CTL interaction with tumors from 4T1, a breast cancer cell line, by first utilizing click chemistry to attach the sortase A recognition sequence to each partner [34]. A thiol–maleimide click reaction was applied to attach sortase A to the CTL and a SPAAC reaction utilizing azide and dibenzocyclooctyne (DBCO) handles affixed an N-terminal polyglycine substrate to the tumor cell [34]. The introduction of a 5-Tamra-LPETG peptide provided the substrate for SML between sortase A on a CTL and the α-amine glycine donor from the tumor cell [34]. The resulting product of this ligation reaction is a fluorescently labeled tumor cell, thus allowing the tracking of CTL and tumor cell interactions in vivo [34]. To further validate this model, the authors were able to incubate this system with antitumor therapeutics, pexidartinib and cisplatin, to track their interaction with the tumor microenvironment (TME) [34]. This represents a more robust approach toward monitoring the TME, which will help in optimizing clinical immunotherapy treatments with CTLs.
2.2.3. Bacterial Cell Wall Engineering
Owing to the native environment of sortase A, it has been utilized in modifying cell wall proteins in S. aureus [36]. Various cell surface proteins in S. aureus express the LPETG recognition sequence for sortase A, allowing for the engineering of these proteins to contain small molecules such as fluorophores, biotin, etc. [36]. Nelson et al. were the first to demonstrate surface cell engineering in bacteria by tracking fluorescent labeling and biotinylation of cell wall extracts via epifluorescence microscopy and MALDI-TOF MS, respectively [36]. These authors were also the first to introduce “sortagging” in S. aureus [36]. Through the utilization of an N-terminal azide group on an LPETG peptide and fluorophore-containing cyclooctyne probe, a [3 + 2] cycloaddition linked the fluorophore to the sortase A substrate. SML between the LPETG peptide and S. aureus cell wall proteins produced fluorescently labeled proteins that could be analyzed using epifluorescence microscopy [36]. This opens the horizon for the highly specific introduction of small molecules and engineering of bacterial proteins through “sortagging”.
Sortase A-mediated labeling in S. aureus has also been employed to attach photosensitizers to peptidoglycan [37]. An eosin photosensitizer was delivered on an LPETG peptide that binds to vancomycin, a glycopeptide antibiotic that binds to the D-Ala-D-Ala motif on the cell wall of S. aureus [37]. Sortase A then cleaves LPET with the photosensitizer to ligate it onto a peptidoglycan that can then be photoinduced to produce reactive oxygen species (ROS) and kill the bacterial cells [37]. SML then provides a potential mechanism to therapeutically target S. aureus as it is found in a variety of human afflictions including pneumonia and toxic shock syndrome [38,39].
3. Butelase
3.1. Mechanism and Engineering
Butelase-1 is an asparaginyl endopeptidase (AEP) isolated from the tropical plant Clitoria ternatea (C. ternatea) [40,41]. This ligase recognizes the motif Asn/Asp(Asx)-His-Val on a peptide and its catalytic cysteine residue acts as a nucleophile to produce an N-to-S acyl shift at the Asx carbonyl group [41,42,43], as a result forming a thioester intermediate between butelase-1 and Asx while releasing the His-Val dipeptide [42]. The thioester intermediate then undergoes an S-to-N acyl shift triggered by a nucleophilic N-terminal amine group to produce the ligated product [42]. This N-terminal amine group can either come from an incoming peptide in a reversible intermolecular reaction or N-terminus of the Asx peptide for an irreversible intramolecular reaction (Figure 1B) [42,44]. Therefore, one of the most impressive applications of butelase-1 is its ability to cyclize peptides with high efficiency as it has this native cyclase activity [40]. The first study to isolate and characterize butelase-1 demonstrated cyclization yields of over 95% for peptides with high catalytic efficiency; in addition, butelase-1 has demonstrated a 100-fold decrease in enzyme concentration needed for cyclization compared to sortase A [40,41,42].
Despite its successful applications as a peptide ligase and cyclase, the utilization of butelase-1 has been hindered by its difficult isolation from its native plant source and challenging recombinant expression in bacteria. Traditionally, butelase-1 has been isolated from the pods of C. ternatea and purified in a four-step process with a low yield of 5 mg kg−1 of the plant [45,46,47]. Examination of several parts of C. ternatea by Tam et al. revealed that butelase-1 extracted from shoots not only had higher ligation efficiency but was also able to be purified in a three-step process with a three-fold-greater yield than previously reported [45]. Extensive research has gone into improving the recombinant expression of butelase-1 in bacteria, mainly focusing on the promotion of disulfide bonds and improving its zymogen activation [45,48]. Advances in the recombinant expression of butelase-1 have found success through the employment of Rosetta-gami B (DE3) and SHuffle T7, two strains of E. coli that have mutated trxB and gor genes [46,48]. The mutation of these genes for reductase proteins may help facilitate the folding of butelase-1 as it naturally contains three disulfide bonds in its fully folded form [46,48]. AEPs found in plants are synthesized as zymogens which undergo irreversible autocleavage of their prodomains in acidic conditions to become active [49]. Several studies have worked to improve the activation of butelase-1 zymogen both in vitro and in vivo, which has led to the development of the engineered variant butelase AY [46,49,50]. Butelase AY contains mutations V237A and T238Y, which decrease the precipitation of the enzyme during its activation step compared to the WT and in turn improve its ligation efficiency and recombinant expression in E. coli RIL cells [50].
3.2. Applications
The Tam lab introduced the idea of unnatural amino acid incorporation using butelase-1 by demonstrating its ability to cyclize D-amino-acid-containing peptides [47,51]. As long as the Asx residue at the P1 position remained in the L form, butelase-1 was able to tolerate the majority of D-amino acids in the P1′′ and P2′′ positions [47]. The cyclization of bioactive peptides, such as D-θ-defensin, although distinctly slower than the L form, was successful and displayed antimicrobial activity against several bacterial strains [47]. The ability of butelase-1 to incorporate unnatural amino acids has been further explored with the engineered variant of the enzyme, butelase AY [50]. At the second position on the acceptor peptide, the unnatural amino acids methyl alanine (MetAla), 2-aminoisobutyric acid (Aib), and norvaline (NorVal) were all recognized [50]. However, NorVal had the best yield with 95% product conversion after 24 h [50]. Taken together, the utilization of butelase-1, both native and engineered, to develop peptides with unnatural amino acids opens the door for its potential use in bio-orthogonal systems and peptide therapeutics.
Expanding on the concept of butelase-1 employment for therapeutic purposes, this ligase has assisted in the synthesis of anticancer and antibacterial peptides [48,52]. Using a variant of butelase-1, engineered to improve its recombinant expression, Hu et al. were able to cyclize the peptide lunasin, which has shown anticancer activity in mouse and mammalian cells [48,53]. By redesigning the sequence of lunasin to contain the appropriate recognition amino acids for butelase-1, the peptide was cyclized with an over 90% yield [48]. Cyclized lunasin was then less susceptible to degradation by pepsin and trypsin, and better inhibited the proliferation of HepG2 cells compared to the linear peptide [48]. Butelase-1 mediated cyclization has also been employed to improve the stability of antimicrobial peptides for better target engagement [54]. Aside from cyclization, butelase-1 has been utilized as a ligase to develop peptide dendrimers, branched macromolecules, to act against antibiotic-resistant bacterial strains [52]. A thiodepsipeptide, where the thioester group was inserted between amino acids N and V, was utilized to increase the effectiveness of the ligation reaction between this peptide and a lysyl dendrimeric scaffold [52]. The synthesis of di-, tetra-, and octameric branched dendrimers was successful, and they displayed antimicrobial activities against several drug-resistant bacterial strains [52]. Butelase-1 ultimately possesses the potential to not only improve known peptide therapeutics but also allow for the development of peptide therapeutics for unmet clinical needs.
4. OaAEP1
4.1. Mechanism and Engineering
Similar to butelase-1, OaAEP1 is a cysteine-based AEP isolated from Oldenlandia affinis which recognizes an Asn residue at the ligation site and proceeds through the same mechanism for protein ligation (Figure 1C) [54,55,56]. However, OaAEP1 has distinct differences from butelase-1 in that it has decreased catalytic efficiency but has better recombinant expression in bacteria [51,57]. When determining the crystal structure of OaAEP1, Tam et al. discovered a C247A mutation that increased the activity of the enzyme by 160-fold [51,58,59]. C247 is positioned near the substrate pocket, so substitution for a smaller side chain with alanine increases the acceptability of the incoming nucleophilic amine [57]. Also, the hydrophobic nature of alanine was hypothesized to reduce the capability of water to hydrolyze the thioester intermediate before the ligation reaction [57]. Early recombinant expression of OaAEP1 demonstrated a specificity for the C-terminal NGL motif and N-terminal GL nucleophile for intramolecular cyclization [55]. This poses a problem as the ligation reaction then becomes reversible as the released peptide can act as a nucleophile for a subsequent ligation reaction. The C247A mutation helps alleviate some of this reversibility problem and increases the promiscuity of OaAEP1 by demonstrating the ability to accept a GV nucleophile, and it produces an NGV product that has poor ligation efficiency [59,60]. However, when using OaAEP1 variants with a tolerated substrate, it is oftentimes necessary to introduce metal cations to quench the reversible byproduct [61,62]. This OaAEP1 variant is further being engineered to broaden the acceptance for various substrates and nucleophiles. One study has developed OaAEP1-C247A-aa55-351, which can recognize NAL and accept RL nucleophiles with high catalytic efficiency [62]. This variant also resolves other issues such as purification, low pH requirements for activation, etc., that exist with OaAEP1 C247A [62].
4.2. Applications
Due to the ability of OaAEP1 to act as a cyclase, it has been sought out for its ability to perform head-to-tail cyclization. Oftentimes, this enzyme has been used for in vitro ligations; however, one study was able to recombinantly express a truncated version of OaAEP1-C247A in E. coli to perform in vivo cyclization [63]. Specifically, OaAEP1-C247A was co-expressed with its substrate murine dihydrofolate reductase (mDHFR), which contained prodomains, to reveal an N-terminal GL nucleophile and C-terminal NGL recognition sequence [63]. Cyclized mDHFR was purified and obtained in a similar yield to the linear mDHFR from E. coli while displaying increased thermal stability and no loss of enzymatic function [63]. Aside from producing macrocycles itself, the ligation capabilities of OaAEP1-C247A have been used to facilitate intramolecular cyclization for the creation of phage-displayed peptide libraries [64]. An NGL substrate containing a chloroacetyl group on its N-terminus was introduced to OaAEP1-C247A, which catalyzed its ligation with a phage displaying a GL-containing peptide [64]. This peptide also contains a cysteine which undergoes an SN2 reaction with the chloroacetyl group for intramolecular cyclization [64]. Using the transcription factor TEAD4 as a proof of concept, the authors were able to perform several rounds of selection and enriched for several cyclic peptides, one of which had a KD value 16-fold lower than its linearized counterpart [64]. These studies highlight the promise of OaAEP1-C247A in producing and assisting in the enrichment of macrocycles for highly specific targets.
OaAEP1 has also contributed to the development of noncanonical amino acid incorporation by accepting peptides containing unnatural or D-amino acids [65,66,67]. Lang et al. used genetic code expansion (GCE) to incorporate an azide glycylglycine moiety linked via an isopeptide bond to lysine—AzGGisoK—on an internal residue in ubiquitin, which then underwent a Staudinger reduction to become GGisoK [65]. A desthiobiotin-NGLH probe, dtb-NGLH, provided the substrate for the ligation reaction catalyzed by OaAEP1-C247A to label the ubiquitin molecule with ~95% yield in a 1 h incubation period [65]. The authors further explored the capabilities of OaAEP1-247A by dual-labeling ubiquitin, with its N-terminus acquiring an alkyne probe for proceeding click reactions, and catalyzing the ubiquitination and SUMOylation of POI with the GGisoK residue [65,66]. In this study, although the unnatural amino acid (UAA) was present, it was GG that served as the nucleophile. Rehm et al. expanded on the OaAEP1-catalyzed ligation with a UAA nucleophile by integrating an Ac-G[Dap]LGV peptide where the diaminopropionic acid (Dap) residue acted as the amine nucleophile for an NGL substrate [67]. High-yield product conversion was seen at a more neutral pH both with and without the Ni2+ quencher; furthermore, Ac-G[d-Dap]LGV and Ac-vGl[d-Dap]G also had high product yields, demonstrating the compatibility of this ligase with D-amino acids [67]. As Leu was important for ligation efficiency, the Dap-Leu motif was incorporated into a model protein, MCoTI-II, and had an 83% product conversion when ligated with an NGL-His6-carrying eGFP protein [67]. OaAEP1-catalyzed ligation of UAAs expands the capabilities of protein synthesis for site-specific side chain modifications and the incorporation of biochemical probes for techniques like bio-orthogonal reactions.
5. Transglutaminase
5.1. Mechanism and Engineering
Transglutaminase 2 (TGM2) is a member of the transglutaminase protein family, which are enzymes that facilitate transamidation reactions in the presence of Ca2+ [68,69]. This enzymatic reaction was first discovered in 1957 by Sarkar et al., who discovered that in the presence of Ca2+, amines were being incorporated into soluble proteins found in guinea pig livers [70]. Decades later, it has been elucidated that TGM2 is the enzyme responsible for this crosslinking, which occurs between the γ-carboxamide group of glutamine and a primary amine [68,71,72]. TGM2 has a conserved catalytic cysteine, i.e., C277, which exerts a nucleophilic attack on the γ-carboxamide group of glutamine to produce a thioester intermediate and release ammonia [68,69]. The thioester intermediate is then attacked by a primary amine, such as lysine, to release the enzyme and create an isopeptide bond between glutamine and lysine (Figure 3A) [68]. TGM2 is heavily reliant on the surrounding concentration of Ca2+, as mutagenesis studies have shown that a loss of any of its Ca2+ binding sites reduces or eliminates the transamidation reaction [69]. However, there is a microbial version of TGM isolated from Streptomyces mobaraense that is Ca2+-independent while still retaining the ability to generate isopeptide bonds [73].
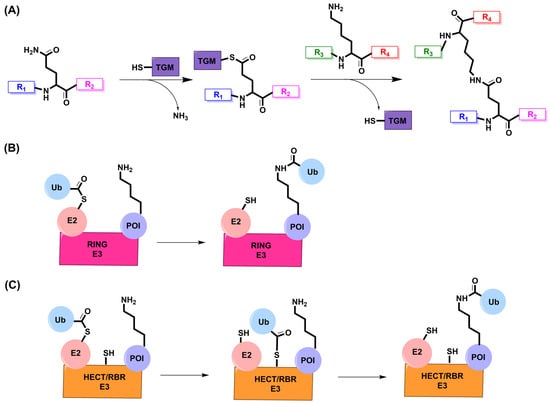
Figure 3.
Enzymatic peptide ligation through isopeptide bond formation. (A) A catalytic cysteine on TGM attacks the γ-carboxamide group of glutamine to produce a thioester intermediate and release ammonia. The thioester intermediate undergoes an aminolysis reaction with a primary amine on lysine to release the enzyme and create an isopeptide bond. R1–R4 are peptide or protein sequences. (B) One-step transfer of ubiquitin from E2 onto a protein substrate by RING E3 ligases. The thioester intermediate between E2 and ubiquitin undergoes an aminolysis reaction with lysine on the protein substrate. (C) Two-step transfer of ubiquitin from E2 onto a protein substrate by HECT and RBR E3 ligases. A catalytic cysteine on HECT/RBR attacks the thioester to transfer ubiquitin onto the C-terminus of the E3 ligase. The thioester intermediate between HECT/RBR and ubiquitin undergoes an aminolysis reaction with lysine on the protein substrate. POI: protein of interest.
Microbial TGM (mTGM) is highly commercially available due to its Ca2+ independence and low cost but exhibits a short half-life at high temperatures, approximately 2 min at 60 °C [74]. This is a major problem considering mTGM is widely used in the food industry for production of meat, cheese, etc., and these processes require several hours at temperatures of 60 °C and above [74,75]. Many early studies looked to random mutagenesis to identify residues important to the thermostability of mTGM; however, it was the utilization of saturation mutagenesis of these hotspots in the N-terminus that produced a triple mutant with improved thermostability [76,77]. S23V-Y24N-K294L mTGM exhibited a 12-fold-higher half-life at 60 °C compared to the wildtype, which has been further improved into FRAPD-TGm1 via addition of the mutations S2P and S199A to increase enzymatic activity by approximately 115% [76,77,78]. The Liu lab has spent several years creating even more robust versions of this mutant (FRAPD-TGm2, FRAPD-TGm2A, and FRAPD-TGm3), with the latter two having half-lives of over 120 min above 60 °C [74,79]. FRAPD-TGm2 contains E28T-A265P-A287P mutations, granting it a two-fold-higher activity over FRAPD-TGm1 [79]. Adding mutations S116A and S179L for increased flexibility generated FRAPD-TGm2A and resulted in an additional 1.84-fold-higher activity over FRAPD-TGm2 at 79.15 U/mg [74]. To increase the surface negative charge, FRAPD-TGm3 was generated from FRAPD-TGm2 with N96E-S144E-N163D-R183E-R208E-K325E mutations and demonstrated the highest specific activity at 83.7 U/mg [79].
Aside from thermostability, mTGM has also been engineered to increase its substrate tolerance as adjacent amino acids to glutamine have been shown to impact the transamidation reaction. It has been demonstrated that the presence of arginine is not well tolerated by the wild-type mTGM, which has made this a target for increased substrate specificity [80]. One study mutated FRAPD-TGm2 and determined that the mutation Y278E shifted the substrate preference of GGGGQR from 0.05 to 0.93 and increased its activity by 1.15-fold, signifying that it is possible for transamidation to occur in the presence of arginine [81]. However, this study took place under virtual mutagenesis and molecular docking and this mutation was not stable on the physical enzyme, which limits its application and requires further optimization [81].
5.2. Applications
Although most of the attention received for TGM focuses on its inhibition for the treatment of various diseases, this enzyme has several applications as a tool for protein ligation by forming isopeptide bonds [82]. TGM has been employed in the production of site-specific antibody–drug conjugates (ADCs) utilizing various glutamine or lysine residues on the antibody [83,84,85]. ADCs present a promising therapeutic option for cancer by combining the specificity of antibodies for tumor cells with the cytotoxicity of small-molecule drugs; however, traditional syntheses of ADCs produce heterogenous mixtures with little control over the site of conjugation [86]. Although there are many native glutamine and lysine residues in IgG antibodies, only very specific residues are capable of undergoing a transamidation reaction with TGM, which allows for site-specific ADCs (Figure 4) [83]. For example, among the ~60 glutamine residues found in IgG1, only Q295 in the Fc domain has the ability to be modified by mTGM [85,87]. However, a nearby N-glycan at residue N297 prevents mTGM from accessing Q295, which has been the focus of several studies to remove this glycan and make Q295 available for modification [82,88]. This was first accomplished by using an amidase, PNGase, to convert the N297 into D297, which did allow for transamidation at Q295 but with alteration to the structure and function of IgG1 [82,88]. Other work has utilized an endoglycosidase, EndoS2, to trim the glycan down to its core, which then allows for mTGM to modify Q295 without compromising the IgG1 antibody [83]. An alternative approach has also been to generate mutant mTGM that has acquired specificity for Q295 in the presence of N-glycated N297 [84]. Studies have also shown that despite the inability of TGM to modify ~80 native lysine residues on IgG1, there is a C-terminal lysine, K447, that can act as an acyl acceptor [85]. Normally, this lysine is cleaved by carboxypeptidase B when expressed in HEK 293 cells; however, the addition of amino acids following K447 blocks its cleavage and allows for TGM-mediated transamidation of this residue [85,89]. Taken together, the ability to use glutamine or lysine residues on IgG antibodies for site-specific TGM-mediated conjugation signifies an important route for ADC production. Referred to as “meat glue”, TGM also has a role in the food industry as it is capable of crosslinking meat pieces in combination with binding agents such as sodium caseinate and κ-carrageenan [90,91]. These crosslinks improve emulsion stability and meat gelation, which results in better meat quality and texture and reduces cooking loss [91,92]. To increase the nutritional value of meat products, TGM has been used to crosslink meat with plant-based products [93,94].
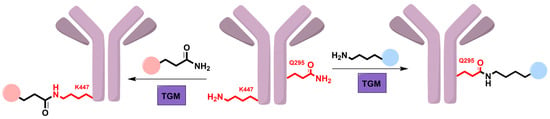
Figure 4.
The production of site-specific ADCs via an isopeptide bond formation catalyzed by TGM. Despite ~60 glutamine residues existing on IgG antibodies, studies have shown that only Q295 on the Fc domain can act as an acyl donor for a ligation reaction by TGM. Similarly, K447 located on the C-terminus of the Fc domain is the only lysine residue that can act as an acyl acceptor. Drugs bearing either a primary amine or glutamine residue can then be conjugated to these residues in a site-specific manner.
6. E3 Ligase
6.1. Mechanism
In order to regulate the expression of proteins, cells have developed refined machinery through the ubiquitin proteasome pathway (UPP). Proteins marked for degradation are modified with a small molecule, ubiquitin, through an isopeptide bond between the ε-amino group on lysine and the C-terminus of ubiquitin [95,96]. Ubiquitin is passed down through E1, E2, and E3 ligases; however, it is the role of E3s to recruit E2s and facilitate the transfer of ubiquitin onto the POI [97,98]. There are over 600 E3 ligases, which can be classified into three major families: RING (really interesting new gene), HECT (homologous to E6AP C-terminus), and RBR (RING-between-RING) [99,100]. RING E3s are the largest family and can either act as monomers or in complex with several adaptor proteins for the transfer of ubiquitin [99,100]. RING E3s act in a one-step transfer as they bind both the substrate and E2–ubiquitin complex at the same time; as a result, the thioester intermediate between E2 and ubiquitin can be attacked by lysine for an aminolysis reaction (Figure 3B) [101]. HECT E3s catalyze this reaction in a two-step process by first binding to the E2–ubiquitin complex on its N-terminus, which is attacked by the C-terminal catalytic cysteine on HECT to form an E3–ubiquitin complex via a transthiolation reaction [99,101]. This complex is then positioned closely to the substrate for the aminolysis reaction to transfer ubiquitin onto the protein [99]. RBR E3s are considered a hybrid between RING and HECT seeing as though their RING1 domain binds E2, similar to RING E3s, and their RING2 domain has a catalytic cysteine for the transthiolation reaction seen in HECT E3s (Figure 3C) [101,102]. Seeing as though E3s are responsible for binding the substrate for ubiquitination, these ligases are responsible for substrate specificity through the recognition of different degrons and post-translational modifications [103].
6.2. Applications
Besides the in vitro applications of E3 for protein engineering [17], one area of drug discovery that has been rapidly developing is the production of proteolysis targeting chimeras (PROTACs) that hijack the UPP to selectively degrade proteins of interest (POIs), which is a representative application example of E3-mediated isopeptide bond formation in vivo. PROTACs consist of a warhead that will bind to a POI, an E3 ligand, and a linker connecting the two pieces together [104,105]. Several PROTACs have been developed for cancer therapeutics; however, their efficacy remains limited by aspects such as off-target degradation, large molecular weight, and varying expression of E3 ligases in different tumor cells [105,106,107,108,109]. To overcome these limitations, attention has been turned to the combination of click chemistry with PROTACs to deliver small pieces of the PROTAC into cells with an in situ assembly [110,111]. Wang et al. developed a click-release PROTAC (crPROTAC) which used αvβ3 integrin, as it is highly expressed on cancer cells, to deliver a click handle for the bio-orthogonal reaction in tumor cells [112]. A tetrazide (Tz) group was attached to an αvβ3 integrin binding peptide, c (RGDyK), which, when inside the cell, reacted with a trans-cyclooctene (TCO) containing PROTAC [112]. The ARV-771 PROTAC was used to recruit the Von Hippel–Lindau (VHL) E3 ligase from the RING family to selectively degrade BRD4 in HeLa cells [112]. The authors found that not only did dosing this crPROTAC induce increased activity in cancer cell lines over normal cell lines, but this PROTAC also acted in a prodrug in that BRD4 was only degraded if both click handles were present to activate the PROTAC [112]. ARV-771 has also been employed in other studies developing tissue-specific delivery and click-mediated activation of PROTACs for cancer therapeutics [113]. Yu et al. employed nanoparticles (NPs) that exposed a DBCO click handle only in the tumor microenvironment, which underwent a click reaction with an azide-modified polymeric PROTAC (POLY-PROTAC) [113]. By undergoing this click reaction in cancer-specific tissues, the ARV-771 PROTAC demonstrated 3.9-fold-higher accumulation in an MDA-MB-231 tumor xenograft model, which correlated with higher activity and decreased tumor volume [113]. These PROTACs still have limitations in that they depend on an endogenous E3 ligase, VHL, which is susceptible to resistance and has varying expression in different tissues. ClickRNA-PROTACs overcome this limitation by exhibiting spatiotemporal control of degradation in selected tumor cells. Li et al. designed an IGF2 mRNA transcript that encodes for the E3 ligase SIAH1 fused to a SNAPTag which covalently attaches to a benzylguanine (BG) group containing DBCO [114]. To complete the PROTAC, DBCO undergoes a click reaction with an azide group attached to the POI. IGF2 mRNA is specific to adrenocortical carcinoma, so this clickRNA-PROTAC can act as a targeted therapy option [114]. Collectively, these studies show the potential for targeted cancer treatments by utilizing bio-orthogonal chemistry to overcome the limitations of traditional PROTACs for therapeutic applications.
Another route to improve the tissue specificity of PROTACs is to create antibody-based PROTACs (AbTACs) where the chosen antibody will bind to tumor-specific antigens [115,116]. Once bound to the antigen, the AbTAC is internalized and releases the PROTAC, which has been demonstrated using a BRD4 PROTAC fused with an antibody specific for HER2+ cell lines [116,117]. Another feature of AbTACs that addresses the limitations of traditional PROTACs is the ability to improve the pharmacokinetic parameters of high-affinity degraders that have low bioavailability [115,118]. One of the earliest AbTAC studies came from Dragovich et al., where they synthesized a BET PROTAC, GNE-987, that had picomolar potency in acute myeloid leukemia (AML) cell lines; however, when transitioning to in vivo animal studies, this PROTAC had poor pharmacokinetic properties for oral or intravenous administration [118]. The conjugation of GNE-987 with the CLL1-targeting antibody via a cleavable disulfide linkage allowed for BET-mediated degradation by GNE-987 to shrink tumor volumes in AML mouse models, thus demonstrating how antibody conjugation can enhance PROTAC development for clinical therapeutics [115,118]. James Well’s lab has also developed AbTACs to target membrane proteins and membrane-bound E3 ligases, such as RNF43, and bring them in close proximity to facilitate degradation [119,120].
7. Intein Splicing as the Inspiration for Native Chemical Ligation (NCL)
The existence of inteins was first discovered in 1990 by two independent groups, Kane et al. and Hirata et al., and for the past several decades, they have been heavily studied for their roles in protein ligation [121,122]. Inteins are believed to act as self-catalyzing enzymes which are able to undergo cis- or trans-splicing to fuse together their external protein sequences known as the N- and C-exteins [123,124]. Canonical intein splicing is a multistep process, starting with either cysteine or serine on the N-intein, which acts as a nucleophile and attacks the adjacent carbonyl group linked to the N-extein [125,126,127,128]. This produces a reversible N-to-S or N-to-O acyl shift to produce a linear intermediate. A trans(thio)esterification step, promoted by either cysteine or serine on the C-extein, links the N- and C-exteins together. To resolve the branched intermediate that is a product of this reaction, there is a rate-limiting irreversible cyclization of a conserved C-terminal asparagine. A succinimide is produced release the connected exteins from the intein. The succinimide formed on the intein undergoes a hydrolysis reaction to restore the intein. Finally, the exteins undergo an acyl shift, either S to N or O to N, to create the native peptide bond and complete the ligation reaction (Figure 5A).
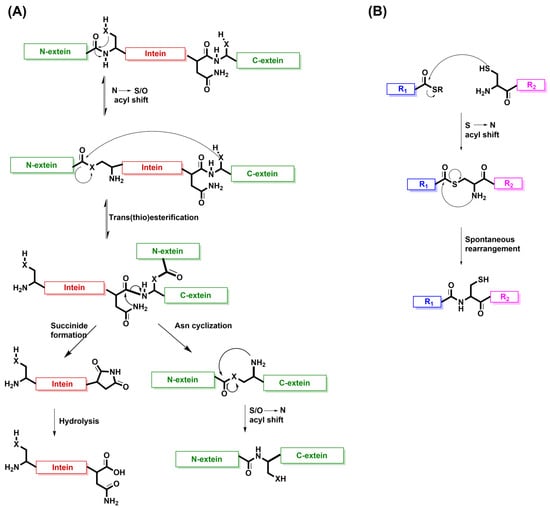
Figure 5.
The mechanism of intein splicing as the inspiration behind NCL. (A) The multistep mechanism of class 1 canonical intein splicing. Cysteine or serine on the N-intein attacks the adjacent carbonyl group linked to the N-extein. A reversible N-to-S or N-to-O acyl shift produces a linear intermediate that undergoes a trans(thio)esterification step promoted by either cysteine or serine on the C-extein. The irreversible cyclization of a conserved C-terminal asparagine releases intein from the fused N- and C-exteins. The resulting succinimide is hydrolyzed to restore the intein. The exteins undergo an acyl shift, either S to N or O to N, to create the native peptide bond and complete the ligation reaction. X represents either S or O. (B) The mechanism of NCL. A cysteine residue attacks the carbonyl group of a C-terminal thioester on a separate peptide. The thioester intermediate undergoes a spontaneous S-to-N acyl shift to form a native peptide bond and the ligated product. R1 and R2 are peptide or protein sequences.
When comparing the mechanism of intein splicing with that of native chemical ligation (NCL), there are many similarities, which suggest that nature inspired the design of this chemical ligation. The concept of NCL to produce a peptide with a native backbone structure was first introduced in the pioneering study published by the Kent lab in 1994 [129]. In this reaction, an activated cysteine residue attacks the carbonyl group of a C-terminal thioester belonging to another peptide. This produces a thioester intermediate between the peptides that undergoes a spontaneous S-to-N acyl shift to form a native peptide bond that results in the ligated product (Figure 5B). The mechanism of NCL looks very similar to the transthioesterification and subsequent S-to-N acyl shift that occur with intein splicing. Therefore, this demonstrates how nature has gifted the world of protein engineering with a mechanism that can be implemented into chemical peptide synthesis [130].
8. Summary and Outlook
Given the vast amount of roles proteins play in biological homeostasis, decades of research have gone into understanding their functions and harnessing them to improve the research world. However, the generation of these proteins by both recombinant expression and chemical synthesis has proven to be difficult. The limitations involved in recombinant expression in bacteria often involve an absence or lack of control over PTMs, which are important for the function and folding of proteins, as a result leading to low yields or dysfunctional proteins. Chemical synthesis via SPPS retains the ability to induce site-specific modifications; however, issues with yield only allow the production of ~40–50 amino-acid-containing linear peptide sequences. Notably, nature has given us a gift through the availability of protein ligases which can catalyze the fusion of peptides/proteins synthesized either recombinantly or chemically. In this review, we highlighted protein ligases that all contain a catalytic cysteine residue to facilitate the formation of both native peptide and isopeptide bonds between peptides/proteins.
Although these protein ligases can all be isolated from natural sources, many of them have undergone extensive optimization. Studies focusing on sortase A and OaAEP1 have generated several different mutants to improve their catalytic efficiencies and substrate specificities, while butelase-1 and mTGM have been engineered to improve recombinant expression and thermal stability, respectively. Engineered variants of these ligases have expanded the toolbox for many chemical biology applications, diversifying the field of proteomics. For example, butelase-1 and OaAEP1 have been integral in unnatural amino acid incorporation and protein cyclization, while sortase A and E3 ligases can be combined with click chemistry to develop potent and selective cancer therapeutic options. Further expansion of this synthetic toolbox has come from the implementation of NCL for the chemical ligation of proteins, which has a natural counterpart through the mechanism of intein splicing. The employment of protein ligases is rapidly expanding, revolutionizing therapeutics and biological tools, indeed demonstrating that nature is the greatest gift of all.
Author Contributions
Y.R. drafted the manuscript and prepared the figures. H.L. assisted in editing the manuscript and preparing the figures. Q.Z. conceptualized the review, edited the manuscript, and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
The research in Zheng’s lab was financially supported by the NIH (R35 GM150676) and startup funds from Purdue University for Q.Z.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Listov, D.; Goverde, C.A.; Correia, B.E.; Fleishman, S.J. Opportunities and Challenges in Design and Optimization of Protein Function. Nat. Rev. Mol. Cell Biol. 2024, 25, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in Peptide-Based Drug Development: Delivery Platforms, Therapeutics and Vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.B.; Samanta, D. Engineering Protein-Based Therapeutics through Structural and Chemical Design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.S. Human Insulin from Recombinant DNA Technology. Science 1983, 219, 632–637. [Google Scholar] [CrossRef]
- Schellenberger, V.; Wang, C.; Geething, N.C.; Spink, B.J.; Campbell, A.; To, W.; Scholle, M.D.; Yin, Y.; Yao, Y.; Bogin, O.; et al. A Recombinant Polypeptide Extends the in Vivo Half-Life of Peptides and Proteins in a Tunable Manner. Nat. Biotechnol. 2009, 27, 1186–1190. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Kurotani, A.; Takagi, T.; Toyama, M.; Shirouzu, M.; Fukami, Y.; Yokoyama, S. Multiple Post-Translational Modifications Affect Heterologous Protein Synthesis. J. Biol. Chem. 2012, 287, 27106–27116. [Google Scholar] [CrossRef]
- Berrade, L.; Camarero, J.A. Expressed Protein Ligation: A Resourceful Tool to Study Protein Structure and Function. Cell. Mol. Life Sci. 2009, 66, 3909–3922. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Sayers, J.; Premdjee, B.; Payne, R.J. Rapid and Efficient Protein Synthesis through Expansion of the Native Chemical Ligation Concept. Nat. Rev. Chem. 2018, 2, 0122. [Google Scholar] [CrossRef]
- Bondalapati, S.; Jbara, M.; Brik, A. Expanding the Chemical Toolbox for the Synthesis of Large and Uniquely Modified Proteins. Nat. Chem. 2016, 8, 407–418. [Google Scholar] [CrossRef]
- Jacobitz, A.W.; Kattke, M.D.; Wereszczynski, J.; Clubb, R.T. Sortase Transpeptidases: Structural Biology and Catalytic Mechanism. Adv. Protein Chem. Struct. Biol. 2017, 109, 223–264. [Google Scholar] [CrossRef]
- Spirig, T.; Weiner, E.M.; Clubb, R.T. Sortase Enzymes in Gram-Positive Bacteria. Mol. Microbiol. 2011, 82, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Surface Proteins of Staphylococcus Aureus. Microbiol. Spectr. 2019, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Amacher, J.F.; Antos, J.M. Sortases: Structure, Mechanism, and Implications for Protein Engineering. Trends Biochem. Sci. 2024, 49, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, G.; Ton-That, H.; Schneewind, O. Staphylococcus Aureus Sortase, an Enzyme That Anchors Surface Proteins to the Cell Wall. Science 1999, 285, 760–763. [Google Scholar] [CrossRef]
- Bradshaw, W.J.; Davies, A.H.; Chambers, C.J.; Roberts, A.K.; Shone, C.C.; Acharya, K.R. Molecular Features of the Sortase Enzyme Family. FEBS J. 2015, 282, 2097–2114. [Google Scholar] [CrossRef]
- Antos, J.M.; Truttmann, M.C.; Ploegh, H.L. Recent Advances in Sortase-Catalyzed Ligation Methodology. Curr. Opin. Struct. Biol. 2016, 38, 111–118. [Google Scholar] [CrossRef]
- Pihl, R.; Zheng, Q.; David, Y. Nature-Inspired Protein Ligation and Its Applications. Nat. Rev. Chem. 2023, 7, 234–255. [Google Scholar] [CrossRef]
- Chen, I.; Dorr, B.M.; Liu, D.R. A General Strategy for the Evolution of Bond-Forming Enzymes Using Yeast Display. Proc. Natl. Acad. Sci. USA 2011, 108, 11399–11404. [Google Scholar] [CrossRef]
- Hirakawa, H.; Ishikawa, S.; Nagamune, T. Ca2+-Independent Sortase-A Exhibits High Selective Protein Ligation Activity in the Cytoplasm of Escherichia coli. Biotechnol. J. 2015, 10, 1487–1492. [Google Scholar] [CrossRef]
- Wuethrich, I.; Peeters, J.G.C.; Blom, A.E.M.; Theile, C.S.; Li, Z.; Spooner, E.; Ploegh, H.L.; Guimaraes, C.P. Site-Specific Chemoenzymatic Labeling of Aerolysin Enables the Identification of New Aerolysin Receptors. PLoS ONE 2014, 9, e109883. [Google Scholar] [CrossRef]
- Piotukh, K.; Geltinger, B.; Heinrich, N.; Gerth, F.; Beyermann, M.; Freund, C.; Schwarzer, D. Directed Evolution of Sortase A Mutants with Altered Substrate Selectivity Profiles. J. Am. Chem. Soc. 2011, 133, 17536–17539. [Google Scholar] [CrossRef] [PubMed]
- Whedon, S.D.; Lee, K.; Wang, Z.A.; Zahn, E.; Lu, C.; Yapa Abeywardana, M.; Fairall, L.; Nam, E.; DuBois-Coyne, S.; De Ioannes, P.; et al. Circular Engineered Sortase for Interrogating Histone H3 in Chromatin. J. Am. Chem. Soc. 2024, 146, 33914–33927. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, Y.; Liu, X.; Xiao, Y.; Wu, M. A General Method to Edit Histone H3 Modifications on Chromatin Via Sortase-Mediated Metathesis. Angew. Chem. Int. Ed. 2022, 61, e202209945. [Google Scholar] [CrossRef] [PubMed]
- Dorr, B.M.; Ham, H.O.; An, C.; Chaikof, E.L.; Liu, D.R. Reprogramming the Specificity of Sortase Enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 13343–13348. [Google Scholar] [CrossRef]
- Zhulenkovs, D.; Jaudzems, K.; Zajakina, A.; Leonchiks, A. Enzymatic Activity of Circular Sortase A under Denaturing Conditions: An Advanced Tool for Protein Ligation. Biochem. Eng. J. 2014, 82, 200–209. [Google Scholar] [CrossRef]
- Li, Z.; Tong, Z.; Gong, Q.; Ai, H.; Peng, S.; Chen, C.; Chu, G.-C.; Li, J.-B. The Expedient, CAET-Assisted Synthesis of Dual-Monoubiquitinated Histone H3 Enables Evaluation of Its Interaction with DNMT1. Chem. Sci. 2023, 14, 5681–5688. [Google Scholar] [CrossRef]
- Li, W.; Cao, P.; Xu, P.; Sun, F.; Wang, C.; Zhang, J.; Dong, S.; Wilson, J.R.; Xu, D.; Fan, H.; et al. Rapid Reconstitution of Ubiquitinated Nucleosome Using a Non-Denatured Histone Octamer Ubiquitylation Approach. Cell Biosci. 2024, 14, 81. [Google Scholar] [CrossRef]
- Wang, Z.A.; Whedon, S.D.; Wu, M.; Wang, S.; Brown, E.A.; Anmangandla, A.; Regan, L.; Lee, K.; Du, J.; Hong, J.Y.; et al. H2B Deacylation Selectivity: Exploring Chromatin’s Dark Matter with an Engineered Sortase. J. Am. Chem. Soc. 2022, 144, 3360–3364. [Google Scholar] [CrossRef]
- Xiao, Y.; Zou, K.; Yang, J.; Wu, M. Deciphering Histone H4 Lysine Acetylation and Methylation via Sortase-Mediated Semisynthesis. Cell Rep. Phys. Sci. 2023, 4, 101638. [Google Scholar] [CrossRef]
- Zou, Z.; Ji, Y.; Schwaneberg, U. Empowering Site-Specific Bioconjugations In Vitro and In Vivo: Advances in Sortase Engineering and Sortase-Mediated Ligation. Angew. Chem. Int. Ed. 2024, 63, e202310910. [Google Scholar] [CrossRef]
- Witte, M.D.; Cragnolini, J.J.; Dougan, S.K.; Yoder, N.C.; Popp, M.W.; Ploegh, H.L. Preparation of Unnatural N-to-N and C-to-C Protein Fusions. Proc. Natl. Acad. Sci. USA 2012, 109, 11993–11998. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Saxon, E.; Bertozzi, C.R. Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liang, C.; Yu, X.-H.; Ma, X.; Qu, Y.; Zhuang, W.-R.; Li, W.; Nie, W.; Ren, Y.; Lei, Y.; et al. Chemistry-Enabled Intercellular Enzymatic Labeling for Monitoring the Immune Effects of Cytotoxic T Lymphocytes In Vivo. Anal. Chem. 2024, 96, 13996–14003. [Google Scholar] [CrossRef]
- Ito, H.; Seishima, M. Regulation of the Induction and Function of Cytotoxic T Lymphocytes by Natural Killer T Cell. J. Biomed. Biotechnol. 2010, 2010, 641757. [Google Scholar] [CrossRef]
- Nelson, J.W.; Chamessian, A.G.; McEnaney, P.J.; Murelli, R.P.; Kazmierczak, B.I.; Spiegel, D.A. Correction to A Biosynthetic Strategy for Re-Engineering the Staphylococcus Aureus Cell Wall with Non-Native Small Molecules. ACS Chem. Biol. 2011, 6, 971. [Google Scholar] [CrossRef]
- Jiang, F.; Cai, C.; Wang, X.; Han, S. A Dual Biomarker-Targeting Probe Enables Signal-on Surface Labeling of Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2023, 93, 129428. [Google Scholar] [CrossRef]
- Self, W.H.; Wunderink, R.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Balk, R.A.; Fakhran, S.S.; Chappell, J.D.; Casimir, G.; Courtney, D.M.; et al. Staphylococcus aureus Community-Acquired Pneumonia: Prevalence, Clinical Characteristics, and Outcomes. Clin. Infect. Dis. 2016, 63, 300–309. [Google Scholar] [CrossRef]
- Silversides, J.A.; Lappin, E.; Ferguson, A.J. Staphylococcal Toxic Shock Syndrome: Mechanisms and Management. Curr. Infect. Dis. Rep. 2010, 12, 392–400. [Google Scholar] [CrossRef]
- Nguyen, G.K.T.; Kam, A.; Loo, S.; Jansson, A.E.; Pan, L.X.; Tam, J.P. Butelase 1: A Versatile Ligase for Peptide and Protein Macrocyclization. J. Am. Chem. Soc. 2015, 137, 15398–15401. [Google Scholar] [CrossRef]
- Nguyen, G.K.T.; Wang, S.; Qiu, Y.; Hemu, X.; Lian, Y.; Tam, J.P. Butelase 1 Is an Asx-Specific Ligase Enabling Peptide Macrocyclization and Synthesis. Nat. Chem. Biol. 2014, 10, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Hemu, X.; Zhang, X.; Bi, X.; Liu, C.-F.; Tam, J.P. Butelase 1-Mediated Ligation of Peptides and Proteins. In Enzyme-Mediated Ligation Methods; Nuijens, T., Schmidt, M., Eds.; Springer: New York, NY, USA, 2019; pp. 83–109. [Google Scholar] [CrossRef]
- Morgan, H.E.; Turnbull, W.B.; Webb, M.E. Challenges in the Use of Sortase and Other Peptide Ligases for Site-Specific Protein Modification. Chem. Soc. Rev. 2022, 51, 4121–4145. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.T.; Cao, Y.; Wang, W.; Liu, C.F.; Tam, J.P. Site-Specific N-Terminal Labeling of Peptides and Proteins Using Butelase 1 and Thiodepsipeptide. Angew. Chem. Int. Ed. 2015, 54, 15694–15698. [Google Scholar] [CrossRef] [PubMed]
- Hemu, X.; Zhang, X.; Nguyen, G.K.T.; To, J.; Serra, A.; Loo, S.; Sze, S.K.; Liu, C.-F.; Tam, J.P. Characterization and Application of Natural and Recombinant Butelase-1 to Improve Industrial Enzymes by End-to-End Circularization. RSC Adv. 2021, 11, 23105–23112. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, R.; Jia, F.; Huang, Y.; Huang, Z.; Hou, Y.; Hu, S.-Q. Enzymatic Properties of Recombinant Ligase Butelase-1 and Its Application in Cyclizing Food-Derived Angiotensin I-Converting Enzyme Inhibitory Peptides. J. Agric. Food Chem. 2021, 69, 5976–5985. [Google Scholar] [CrossRef]
- Nguyen, G.K.T.; Qiu, Y.; Cao, Y.; Hemu, X.; Liu, C.-F.; Tam, J.P. Butelase-Mediated Cyclization and Ligation of Peptides and Proteins. Nat. Protoc. 2016, 11, 1977–1988. [Google Scholar] [CrossRef]
- Zhao, J.; Song, W.; Huang, Z.; Yuan, X.; Huang, Y.; Hou, Y.; Liu, K.; Jin, P.; Hu, S.-Q. “Top-down” Overexpression Optimization of Butelase-1 in Escherichia coli and Its Application in Anti-Tumor Peptides. Int. J. Biol. Macromol. 2024, 276, 133933. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, G.; Huang, Y.; Hou, Y.; Hu, S.-Q. Study on Activation Mechanism and Cleavage Sites of Recombinant Butelase-1 Zymogen Derived from Clitoria ternatea. Biochimie 2022, 199, 12–22. [Google Scholar] [CrossRef]
- Singh, A.K.; Antonenko, A.; Kocyła, A.; Krężel, A. An Efficient and Easily Obtainable Butelase Variant for Chemoenzymatic Ligation and Modification of Peptides and Proteins. Microb. Cell Fact. 2024, 23, 325. [Google Scholar] [CrossRef]
- Cui, Y.; Han, D.; Bai, X.; Shi, W. Development and Applications of Enzymatic Peptide and Protein Ligation. J. Pept. Sci. 2025, 31, e3657. [Google Scholar] [CrossRef]
- Cao, Y.; Nguyen, G.K.T.; Chuah, S.; Tam, J.P.; Liu, C.-F. Butelase-Mediated Ligation as an Efficient Bioconjugation Method for the Synthesis of Peptide Dendrimers. Bioconjug. Chem. 2016, 27, 2592–2596. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.F.; Chen, N.; Macasieb, J.; de Lumen, B.O. Chemopreventive Property of a Soybean Peptide (Lunasin) That Binds to Deacetylated Histones and Inhibits Acetylation1. Cancer Res. 2001, 61, 7473–7478. [Google Scholar] [PubMed]
- Zhao, J.; Ge, G.; Huang, Y.; Hou, Y.; Hu, S.-Q. Butelase 1-Mediated Enzymatic Cyclization of Antimicrobial Peptides: Improvements on Stability and Bioactivity. J. Agric. Food Chem. 2022, 70, 15869–15878. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.S.; Durek, T.; Kaas, Q.; Poth, A.G.; Gilding, E.K.; Conlan, B.F.; Saska, I.; Daly, N.L.; van der Weerden, N.L.; Craik, D.J.; et al. Efficient Backbone Cyclization of Linear Peptides by a Recombinant Asparaginyl Endopeptidase. Nat. Commun. 2015, 6, 10199. [Google Scholar] [CrossRef]
- Tang, T.M.S.; Cardella, D.; Lander, A.J.; Li, X.; Escudero, J.S.; Tsai, Y.-H.; Luk, L.Y.P. Use of an Asparaginyl Endopeptidase for Chemo-Enzymatic Peptide and Protein Labeling. Chem. Sci. 2020, 11, 5881–5888. [Google Scholar] [CrossRef]
- Yang, R.; Wong, Y.H.; Nguyen, G.K.T.; Tam, J.P.; Lescar, J.; Wu, B. Engineering a Catalytically Efficient Recombinant Protein Ligase. J. Am. Chem. Soc. 2017, 139, 5351–5358. [Google Scholar] [CrossRef]
- Rehm, F.B.H.; Tyler, T.J.; Xie, J.; Yap, K.; Durek, T.; Craik, D.J. Asparaginyl Ligases: New Enzymes for the Protein Engineer’s Toolbox. ChemBioChem 2021, 22, 2079–2086. [Google Scholar] [CrossRef]
- Rehm, F.B.H.; Harmand, T.J.; Yap, K.; Durek, T.; Craik, D.J.; Ploegh, H.L. Site-Specific Sequential Protein Labeling Catalyzed by a Single Recombinant Ligase. J. Am. Chem. Soc. 2019, 141, 17388–17393. [Google Scholar] [CrossRef]
- Rehm, F.B.H.; Tyler, T.J.; de Veer, S.J.; Craik, D.J.; Durek, T. Enzymatic C-to-C Protein Ligation. Angew. Chem. Int. Ed. 2022, 61, e202116672. [Google Scholar] [CrossRef]
- Rehm, F.B.H.; Tyler, T.J.; Yap, K.; Durek, T.; Craik, D.J. Improved Asparaginyl-Ligase-Catalyzed Transpeptidation via Selective Nucleophile Quenching. Angew. Chem. Int. Ed. 2021, 60, 4004–4008. [Google Scholar] [CrossRef]
- Tang, J.; Hao, M.; Liu, J.; Chen, Y.; Wufuer, G.; Zhu, J.; Zhang, X.; Zheng, T.; Fang, M.; Zhang, S.; et al. Author Correction: Design of a Recombinant Asparaginyl Ligase for Site-Specific Modification Using Efficient Recognition and Nucleophile Motifs. Commun. Chem. 2024, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Simon Tang, T.M.; Mason, J.M. Intracellular Application of an Asparaginyl Endopeptidase for Producing Recombinant Head-to-Tail Cyclic Proteins. JACS Au 2023, 3, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-C.; Zhang, Y.-N.; Zhang, H.; Chen, Y.; Cui, Z.-H.; Zhu, W.-J.; Fang, G.-M. Asparaginyl Endopeptidase-Mediated Peptide Cyclization for Phage Display. Org. Lett. 2024, 26, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Fottner, M.; Heimgärtner, J.; Gantz, M.; Mühlhofer, R.; Nast-Kolb, T.; Lang, K. Site-Specific Protein Labeling and Generation of Defined Ubiquitin-Protein Conjugates Using an Asparaginyl Endopeptidase. J. Am. Chem. Soc. 2022, 144, 13118–13126. [Google Scholar] [CrossRef]
- Wanka, V.; Fottner, M.; Cigler, M.; Lang, K. Genetic Code Expansion Approaches to Decipher the Ubiquitin Code. Chem. Rev. 2024, 124, 11544–11584. [Google Scholar] [CrossRef]
- de Veer, S.J.; Craik, D.J.; Rehm, F.B.H. Highly Efficient Transpeptidase-Catalyzed Isopeptide Ligation. J. Am. Chem. Soc. 2025, 147, 557–565. [Google Scholar] [CrossRef]
- Gundemir, S.; Colak, G.; Tucholski, J.; Johnson, G.V.W. Transglutaminase 2: A Molecular Swiss Army Knife. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 406–419. [Google Scholar] [CrossRef]
- Keillor, J.W.; Clouthier, C.M.; Apperley, K.Y.P.; Akbar, A.; Mulani, A. Acyl Transfer Mechanisms of Tissue Transglutaminase. Bioorg. Chem. 2014, 57, 186–197. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Clarke, D.D.; Waelsch, H. An Enzymically Catalyzed Incorporation of Amines into Proteins. Biochim. Biophys. Acta 1957, 25, 451–452. [Google Scholar] [CrossRef]
- Pincus, J.H.; Waelsch, H. The Specificity of Transglutaminase: II. Structural Requirements of the Amine Substrate. Arch. Biochem. Biophys. 1968, 126, 44–52. [Google Scholar] [CrossRef]
- Griffin, M.; Casadio, R.; Bergamini, C.M. Transglutaminases: Nature’s Biological Glues. Biochem. J. 2002, 368, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Aaron, L.; Torsten, M. Microbial Transglutaminase: A New Potential Player in Celiac Disease. Clin. Immunol. 2019, 199, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, X.; Ye, J.; Rao, S.; Zhou, J.; Du, G.; Liu, S. Enhanced Thermostability and Catalytic Activity of Streptomyces Mobaraenesis Transglutaminase by Rationally Engineering Its Flexible Regions. J. Agric. Food Chem. 2023, 71, 6366–6375. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yang, P.; Zhou, J.; Du, G.; Liu, S. Efficient Production of a Thermostable Mutant of Transglutaminase by Streptomyces Mobaraensis. J. Agric. Food Chem. 2024, 72, 4207–4216. [Google Scholar] [CrossRef]
- Marx, C.K.; Hertel, T.C.; Pietzsch, M. Random Mutagenesis of a Recombinant Microbial Transglutaminase for the Generation of Thermostable and Heat-Sensitive Variants. J. Biotechnol. 2008, 136, 156–162. [Google Scholar] [CrossRef]
- Buettner, K.; Hertel, T.C.; Pietzsch, M. Increased Thermostability of Microbial Transglutaminase by Combination of Several Hot Spots Evolved by Random and Saturation Mutagenesis. Amino Acids 2012, 42, 987–996. [Google Scholar] [CrossRef]
- Yokoyama, K.; Utsumi, H.; Nakamura, T.; Ogaya, D.; Shimba, N.; Suzuki, E.; Taguchi, S. Screening for Improved Activity of a Transglutaminase from Streptomyces Mobaraensis Created by a Novel Rational Mutagenesis and Random Mutagenesis. Appl. Microbiol. Biotechnol. 2010, 87, 2087–2096. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Zhao, B.; Wang, H.; Rao, S.; Du, G.; Zhou, J.; Chen, J.; Liu, S. Significantly Improving the Thermostability and Catalytic Efficiency of Streptomyces Mobaraenesis Transglutaminase through Combined Rational Design. J. Agric. Food Chem. 2021, 69, 15268–15278. [Google Scholar] [CrossRef]
- Malešević, M.; Migge, A.; Hertel, T.C.; Pietzsch, M. A Fluorescence-Based Array Screen for Transglutaminase Substrates. ChemBioChem 2015, 16, 1169–1174. [Google Scholar] [CrossRef]
- Wang, X.; Xu, K.; Fu, H.; Chen, Q.; Zhao, B.; Zhao, X.; Zhou, J. Enhancing Substrate Specificity of Microbial Transglutaminase for Precise Nanobody Labeling. Synth. Syst. Biotechnol. 2025, 10, 185–193. [Google Scholar] [CrossRef]
- Siegel, M.; Khosla, C. Transglutaminase 2 Inhibitors and Their Therapeutic Role in Disease States. Pharmacol. Ther. 2007, 115, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Sadiki, A.; Liu, S.; Vaidya, S.R.; Kercher, E.M.; Lang, R.T.; McIsaac, J.; Spring, B.Q.; Auclair, J.R.; Zhou, Z.S. Site-Specific Conjugation of Native Antibody: Transglutaminase-Mediated Modification of a Conserved Glutamine While Maintaining the Primary Sequence and Core Fc Glycan via Trimming with an Endoglycosidase. Bioconjug. Chem. 2024, 35, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dickgiesser, S.; Rieker, M.; Mueller-Pompalla, D.; Schröter, C.; Tonillo, J.; Warszawski, S.; Raab-Westphal, S.; Kühn, S.; Knehans, T.; Könning, D.; et al. Site-Specific Conjugation of Native Antibodies Using Engineered Microbial Transglutaminases. Bioconjug. Chem. 2020, 31, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Spidel, J.L.; Vaessen, B.; Albone, E.F.; Cheng, X.; Verdi, A.; Kline, J.B. Site-Specific Conjugation to Native and Engineered Lysines in Human Immunoglobulins by Microbial Transglutaminase. Bioconjug. Chem. 2017, 28, 2471–2484. [Google Scholar] [CrossRef]
- Lambert, J.M.; Morris, C.Q. Antibody–Drug Conjugates (ADCs) for Personalized Treatment of Solid Tumors: A Review. Adv. Ther. 2017, 34, 1015–1035. [Google Scholar] [CrossRef]
- Bolzati, C.; Spolaore, B. Enzymatic Methods for the Site-Specific Radiolabeling of Targeting Proteins. Molecules 2021, 26, 3492. [Google Scholar] [CrossRef]
- Jeger, S.; Zimmermann, K.; Blanc, A.; Grünberg, J.; Honer, M.; Hunziker, P.; Struthers, H.; Schibli, R. Site-Specific and Stoichiometric Modification of Antibodies by Bacterial Transglutaminase. Angew. Chem. Int. Ed. 2010, 49, 9995–9997. [Google Scholar] [CrossRef]
- Dick, L.W., Jr.; Qiu, D.; Mahon, D.; Adamo, M.; Cheng, K.-C. C-Terminal Lysine Variants in Fully Human Monoclonal Antibodies: Investigation of Test Methods and Possible Causes. Biotechnol. Bioeng. 2008, 100, 1132–1143. [Google Scholar] [CrossRef]
- Kuraishi, C.; Sakamoto, J.; Yamazaki, K.; Susa, Y.; Kuhara, C.; Soeda, T. Production of Restructured Meat Using Microbial Transglutaminase without Salt or Cooking. J. Food Sci. 1997, 62, 488–490. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, X.; Zhang, J.; Shi, P.; Cao, C.; Zhang, H.; Liu, Q.; Kong, B. Underlying Mechanisms and Combined Effects of Transglutaminase and κ-Carrageenan on the Quality Profiles and In Vitro Digestibility of Frankfurters. Food Hydrocoll. 2024, 147, 109344. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Transglutaminase in Foods and Biotechnology. Int. J. Mol. Sci. 2023, 24, 12402. [Google Scholar] [CrossRef] [PubMed]
- Zinina, O.; Merenkova, S.; Galimov, D.; Okuskhanova, E.; Rebezov, M.; Khayrullin, M.; Anichkina, O. Effects of Microbial Transglutaminase on Technological, Rheological, and Microstructural Indicators of Minced Meat with the Addition of Plant Raw Materials. Int. J. Food Sci. 2020, 2020, 8869401. [Google Scholar] [CrossRef] [PubMed]
- Baugreet, S.; Kerry, J.P.; Brodkorb, A.; Gomez, C.; Auty, M.; Allen, P.; Hamill, R.M. Optimisation of Plant Protein and Transglutaminase Content in Novel Beef Restructured Steaks for Older Adults by Central Composite Design. Meat Sci. 2018, 142, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Chau, V.; Tobias, J.W.; Bachmair, A.; Marriott, D.; Ecker, D.J.; Gonda, D.K.; Varshavsky, A. A Multiubiquitin Chain Is Confined to Specific Lysine in a Targeted Short-Lived Protein. Science 1989, 243, 1576–1583. [Google Scholar] [CrossRef]
- Berndsen, C.E.; Wolberger, C. New Insights into Ubiquitin E3 Ligase Mechanism. Nat. Struct. Mol. Biol. 2014, 21, 301–307. [Google Scholar] [CrossRef]
- Ramachandran, S.; Ciulli, A. Building Ubiquitination Machineries: E3 Ligase Multi-Subunit Assembly and Substrate Targeting by PROTACs and Molecular Glues. Curr. Opin. Struct. Biol. 2021, 67, 110–119. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Toma-Fukai, S.; Shimizu, T. Structural Diversity of Ubiquitin E3 Ligase. Molecules 2021, 26, 6682. [Google Scholar] [CrossRef]
- Jeong, Y.; Oh, A.-R.; Jung, Y.H.; Gi, H.; Kim, Y.U.; Kim, K. Targeting E3 Ubiquitin Ligases and Their Adaptors as a Therapeutic Strategy for Metabolic Diseases. Exp. Mol. Med. 2023, 55, 2097–2104. [Google Scholar] [CrossRef]
- Wang, X.S.; Cotton, T.R.; Trevelyan, S.J.; Richardson, L.W.; Lee, W.T.; Silke, J.; Lechtenberg, B.C. The Unifying Catalytic Mechanism of the RING-between-RING E3 Ubiquitin Ligase Family. Nat. Commun. 2023, 14, 168. [Google Scholar] [CrossRef]
- Lechtenberg, B.C.; Rajput, A.; Sanishvili, R.; Dobaczewska, M.K.; Ware, C.F.; Mace, P.D.; Riedl, S.J. Structure of a HOIP/E2~ubiquitin Complex Reveals RBR E3 Ligase Mechanism and Regulation. Nature 2016, 529, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Timms, R.T.; Mena, E.L.; Leng, Y.; Li, M.Z.; Tchasovnikarova, I.A.; Koren, I.; Elledge, S.J. Author Correction: Defining E3 Ligase–Substrate Relationships through Multiplex CRISPR Screening. Nat. Cell Biol. 2024, 26, 305. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ciulli, A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discov. 2021, 26, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, Present and Future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef]
- Moreau, K.; Coen, M.; Zhang, A.X.; Pachl, F.; Castaldi, M.P.; Dahl, G.; Boyd, H.; Scott, C.; Newham, P. Proteolysis-Targeting Chimeras in Drug Development: A Safety Perspective. Br. J. Pharmacol. 2020, 177, 1709–1718. [Google Scholar] [CrossRef]
- Ye, P.; Chi, X.; Cha, J.-H.; Luo, S.; Yang, G.; Yan, X.; Yang, W.-H. Potential of E3 Ubiquitin Ligases in Cancer Immunity: Opportunities and Challenges. Cells 2021, 10, 3309. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, D.; Wang, Y. The PROTAC Technology in Drug Development. Cell Biochem. Funct. 2019, 37, 21–30. [Google Scholar] [CrossRef]
- Li, R.; Liu, M.; Yang, Z.; Li, J.; Gao, Y.; Tan, R. Proteolysis-Targeting Chimeras (PROTACs) in Cancer Therapy: Present and Future. Molecules 2022, 27, 8828. [Google Scholar] [CrossRef]
- Pasieka, A.; Diamanti, E.; Uliassi, E.; Laura Bolognesi, M. Click Chemistry and Targeted Degradation: A Winning Combination for Medicinal Chemists? ChemMedChem 2023, 18, e202300422. [Google Scholar] [CrossRef]
- Yang, C.; Tripathi, R.; Wang, B. Click Chemistry in the Development of PROTACs. RSC Chem. Biol. 2024, 5, 189–197. [Google Scholar] [CrossRef]
- Chang, M.; Gao, F.; Pontigon, D.; Gnawali, G.; Xu, H.; Wang, W. Bioorthogonal PROTAC Prodrugs Enabled by On-Target Activation. J. Am. Chem. Soc. 2023, 145, 14155–14163. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hou, B.; Zhu, Q.; Yang, L.; Jiang, X.; Zou, Z.; Li, X.; Xu, T.; Zheng, M.; Chen, Y.-H.; et al. Author Correction: Engineered Bioorthogonal POLY-PROTAC Nanoparticles for Tumour-Specific Protein Degradation and Precise Cancer Therapy. Nat. Commun. 2022, 13, 4978. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Zhao, X.; Dai, Y.; Zhang, X.; Zhang, Q.; Wu, Y.; Hu, D.; Li, J. ClickRNA-PROTAC for Tumor-Selective Protein Degradation and Targeted Cancer Therapy. J. Am. Chem. Soc. 2024, 146, 27382–27391. [Google Scholar] [CrossRef] [PubMed]
- Dragovich, P.S. Degrader-Antibody Conjugates. Chem. Soc. Rev. 2022, 51, 3886–3897. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Xie, Y.; Wang, Y. What Influences the Activity of Degrader−Antibody Conjugates (DACs). Eur. J. Med. Chem. 2024, 268, 116216. [Google Scholar] [CrossRef]
- Maneiro, M.; Forte, N.; Shchepinova, M.M.; Kounde, C.S.; Chudasama, V.; Baker, J.R.; Tate, E.W. Antibody–PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chem. Biol. 2020, 15, 1306–1312. [Google Scholar] [CrossRef]
- Pillow, T.H.; Adhikari, P.; Blake, R.A.; Chen, J.; Del Rosario, G.; Deshmukh, G.; Figueroa, I.; Gascoigne, K.E.; Kamath, A.V.; Kaufman, S.; et al. Antibody Conjugation of a Chimeric BET Degrader Enables in Vivo Activity. ChemMedChem 2020, 15, 17–25. [Google Scholar] [CrossRef]
- Cotton, A.D.; Nguyen, D.P.; Gramespacher, J.A.; Seiple, I.B.; Wells, J.A. Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. J. Am. Chem. Soc. 2021, 143, 593–598. [Google Scholar] [CrossRef]
- Gramespacher, J.A.; Cotton, A.D.; Burroughs, P.W.W.; Seiple, I.B.; Wells, J.A. Roadmap for Optimizing and Broadening Antibody-Based PROTACs for Degradation of Cell Surface Proteins. ACS Chem. Biol. 2022, 17, 1259–1268. [Google Scholar] [CrossRef]
- Kane, P.M.; Yamashiro, C.T.; Wolczyk, D.F.; Neff, N.; Goebl, M.; Stevens, T.H. Protein Splicing Converts the Yeast TFP1 Gene Product to the 69-kdDSubunit of the Vacuolar H+-Adenosine Triphosphatase. Science 1990, 250, 651–657. [Google Scholar] [CrossRef]
- Hirata, R.; Ohsumk, Y.; Nakano, A.; Kawasaki, H.; Suzuki, K.; Anraku, Y. Molecular Structure of a Gene, VMA1, Encoding the Catalytic Subunit of H(+)-Translocating Adenosine Triphosphatase from Vacuolar Membranes of Saccharomyces Cerevisiae. J. Biol. Chem. 1990, 265, 6726–6733. [Google Scholar] [CrossRef] [PubMed]
- Perler, F.B.; Davis, E.O.; Dean, G.E.; Gimble, F.S.; Jack, W.E.; Neff, N.; Noren, C.J.; Thorner, J.; Belfort, M. Protein Splicing Elements: Inteins and Exteins--a Definition of Terms and Recommended Nomenclature. Nucleic Acids Res. 1994, 22, 1125. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhong, B.; Dai, Z. Protein Splicing of Inteins: A Powerful Tool in Synthetic Biology. Front. Bioeng. Biotechnol. 2022, 10, 810180. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Nasker, S.S.; Mehra, A.; Panda, S.; Nayak, S. Inteins in Science: Evolution to Application. Microorganisms 2020, 8, 2004. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.W.; Belfort, M.; Lennon, C.W. Inteins—Mechanism of Protein Splicing, Emerging Regulatory Roles, and Applications in Protein Engineering. Front. Microbiol. 2023, 14, 1305848. [Google Scholar] [CrossRef]
- Friedel, K.; Popp, M.A.; Matern, J.C.J.; Gazdag, E.M.; Thiel, I.V.; Volkmann, G.; Blankenfeldt, W.; Mootz, H.D. A Functional Interplay between Intein and Extein Sequences in Protein Splicing Compensates for the Essential Block B Histidine. Chem. Sci. 2019, 10, 239–251. [Google Scholar] [CrossRef]
- Volkmann, G.; Mootz, H.D. Recent Progress in Intein Research: From Mechanism to Directed Evolution and Applications. Cell Mol. Life Sci. 2012, 70, 1185–1206. [Google Scholar] [CrossRef]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B.H. Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef]
- Pinto, F.; Thornton, E.L.; Wang, B. An Expanded Library of Orthogonal Split Inteins Enables Modular Multi-Peptide Assemblies. Nat. Commun. 2020, 11, 1529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).