- Review
Lactic Acid Bacteria: From Bioprocessing to Nanomedicine
- Maryam Rezvani,
- Maria Manconi and
- Nejat Düzgüneş
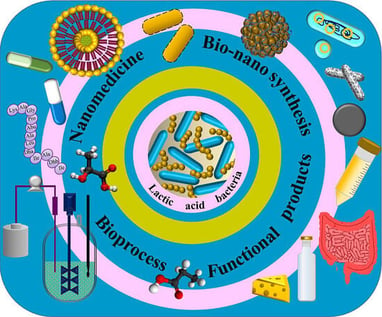
Background/Objectives: Lactic acid bacteria have long been recognized as pivotal microorganisms in food fermentation and health promotion. However, their significance has recently grown due to innovative applications in various fields, particularly at the intersection of biotechnology and nanotechnology. This study aimed to provide a comprehensive overview of these emerging applications. Methods: The latest scientific literature was drawn from online databases and thoroughly reviewed. The new nomenclature system based on the post-2020 reclassification was used for reports. Results: The current study highlighted the evolving role of lactic acid bacteria, beyond their traditional use as starter cultures for food fermentation, in newer challenges, including the production of high-value bioactive compounds through bioprocessing under optimal conditions to enhance the yield, underlining the involved genes and pathways. Furthermore, this review addressed the beneficial effects of lactic acid bacteria as probiotics, postbiotics, and paraprobiotics in the treatment of various diseases and disorders, their application in the production of functional foods, and the encapsulation of their bioproducts to produce advanced health-promoting functional ingredients. The potential use of lactic acid bacteria to synthesize metallic nanoparticles, minicells, and carbon dots was also explored, promising significant advancements in nanomedicine. Conclusions: This review could open a new horizon for leveraging the potential of lactic acid bacteria in biotechnology, food science, and nanomedicine. The multilateral perspective offered here would provide a foundation for future research and development to exploit the capabilities of lactic acid bacteria across these innovative fields.
27 January 2026