Assessing the Genotoxic Impact of Ni-Cr Alloys in Dental Prosthodontics: A Preliminary Comparative Analysis with and Without Beryllium
Abstract
1. Introduction
- -
- Cell proliferation assay: This measures the growth rate of cells to determine if the alloys affect normal cell division, an indicator of possible toxicity or carcinogenic effects.
- -
- In vitro micronucleus assay: This test assesses the appearance of micronuclei in mammalian cells, which are indicators of chromosomal damage or chromosome loss. The test is performed on cultures of human lymphocytes extracted from peripheral blood, providing a sensitive and relevant method for the assessment of genotoxicity.
2. Materials and Methods
2.1. Ethical Compliance
2.2. Study Design
2.3. Sample Preparation
2.3.1. Types of Dental Alloys Used
2.3.2. Milling and Powder Preparation
2.3.3. Sample Sterilization
2.4. Experimental Group Classification
2.4.1. Biological Model—Human Lymphocyte Cultures
2.4.2. Experimental Groups and Alloy Concentrations
2.5. Sample Processing and Testing Methods
2.5.1. Lymphocyte Culture and Incubation
2.5.2. Exposure to Metal Powders
2.5.3. Culture Termination and Cell Fixation
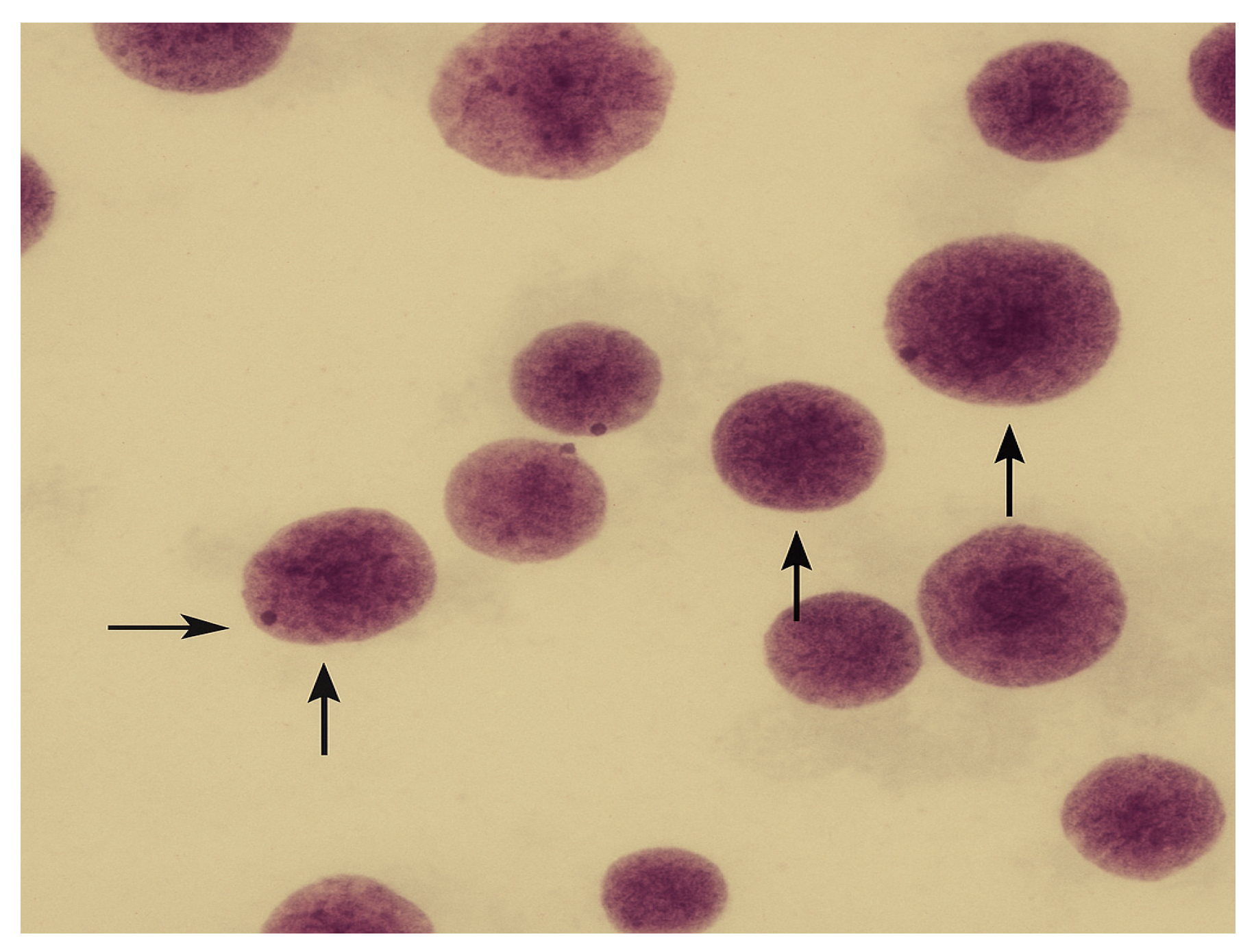
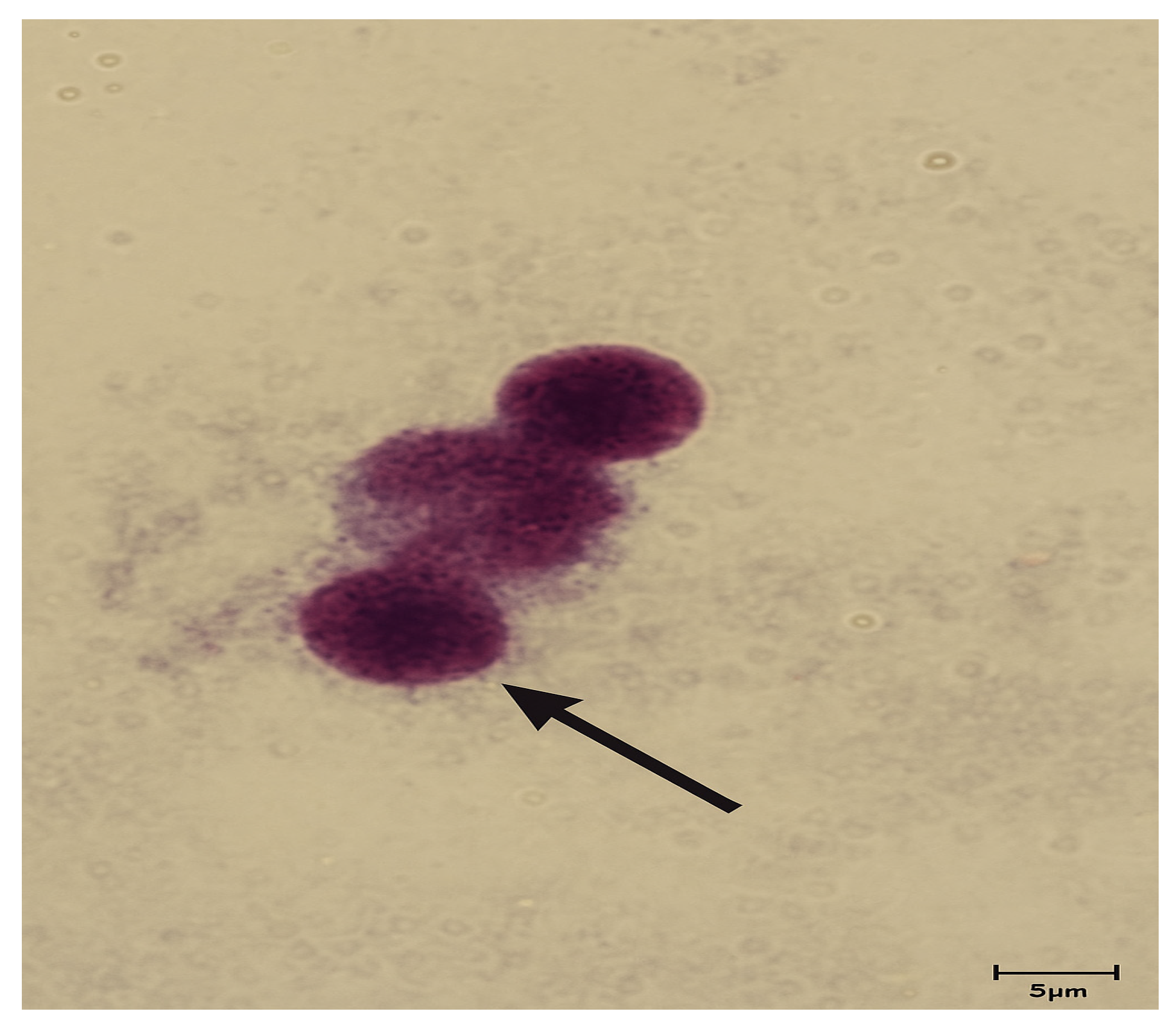
2.5.4. Micronucleus Assay
2.5.5. Staining and Microscopic Analysis
2.6. Statistical Analysis
3. Results
3.1. Cell Proliferation Assay
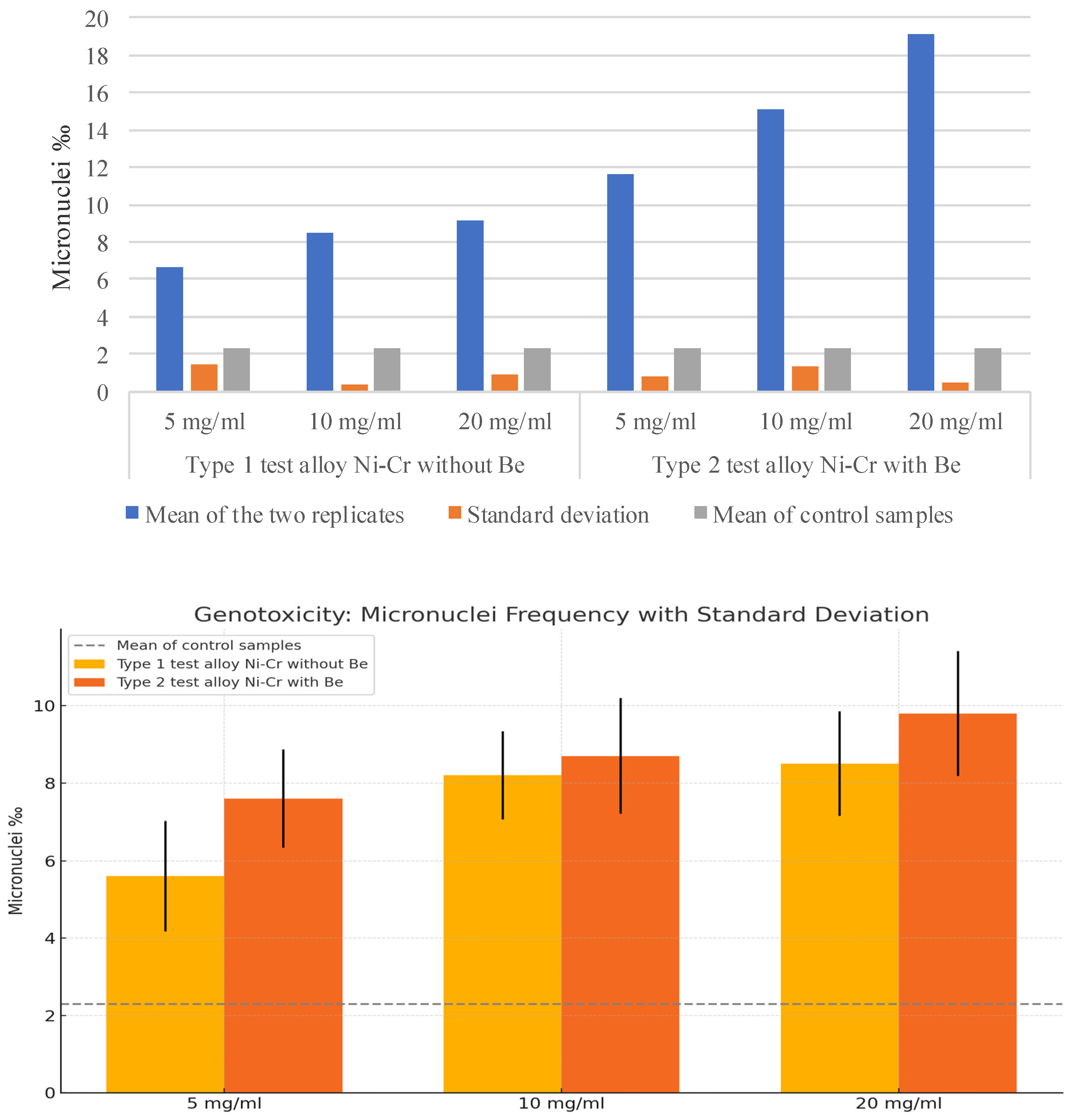
3.2. Micronucleus Assay
4. Discussion
5. Conclusions
6. Study Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wataha, J.C. Alloys for prosthodontic restorations. J. Prosthet. Dent. 2002, 87, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Bidanda, B.; Bártolo, P. Metallic and Ceramic Biomaterials: Current and Future Developments. In Bio-Materials and Prototyping Applications in Medicine; Bártolo, P., Bidanda, B., Eds.; Springer: Boston, MA, USA, 2008; pp. 1–14. [Google Scholar]
- Bezzon, O.L.; Ribeiro, R.F.; Rollo, J.M.; Crosara, S. Castability and resistance of ceramometal bonding in Ni-Cr and Ni-Cr-Be alloys. J. Prosthet. Dent. 2001, 85, 299–304. [Google Scholar] [CrossRef]
- Cheng, T.-P.; Tsai, W.-T.; Lin, J.-H.C.; Lee, J.-T. The effect of beryllium on the corrosion resistance of nickel-chromium dental alloys. J. Mater. Sci. Mater. Med. 1990, 1, 211–218. [Google Scholar] [CrossRef]
- Cohen, S.; Vaidyanathan, T.; Schulman, A. The effect of limited beryllium additions on a Ni-Cr alloy. J. Prosthet. Dent. 1988, 60, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Vineeth, G.; Alla, R.K.; Srinivasa Raju, D.; Suresh Sajjan, M.C.; Ramaraju, A.V.; Yeleti, H. Casting alloys: The saga of their existence and the recipe of their blend. Int. J. Dent. Mater. 2019, 1, 25–35. [Google Scholar] [CrossRef]
- Geis-Gerstorfer, J.; Pässler, K. Studies on the influence of BE content on the corrosion behavior and mechanical properties of Ni-25Cr-10Mo alloys. Dent. Mater. 1993, 9, 177–181. [Google Scholar] [CrossRef]
- Bezzon, O.L.; Mattos, M.d.G.d.; Ribeiro, R.F.; Rollo, J.M.A. Effect of beryllium on the castability and resistance of ceramometal bonds in nickel-chromium alloys. J. Prosthet. Dent. 1998, 80, 570–574. [Google Scholar] [CrossRef]
- Registry Aftsad. Toxicological Profile for Beryllium. 2003. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp4.pdf (accessed on 1 March 2025).
- Agency, U.S.E.P. Toxicological Review of Beryllium and Compounds. 2008. Available online: https://iris.epa.gov/static/pdfs/0012tr.pdf (accessed on 1 March 2025).
- UKEA. A Review of the Toxicity of Beryllium in Air 2008. Available online: https://assets.publishing.service.gov.uk/media/5a7c6f8ded915d696ccfcc2f/scho0508bobo-e-e.pdf (accessed on 26 December 2024).
- Annangi, B.; Bonassi, S.; Marcos, R.; Hernández, A. Biomonitoring of humans exposed to arsenic, chromium, nickel, vanadium, and complex mixtures of metals by using the micronucleus test in lymphocytes. Mutat. Res. Mol. Mech. Mutagen. 2016, 770, 140–161. [Google Scholar] [CrossRef]
- Apostoli, P.; Porru, S.; Alessio, L. Behaviour of urinary beryllium in general population and in subjects with low-level occupational exposure. Med. Lav. 1989, 80, 390–396. [Google Scholar]
- Halder, A.; De, M. Increase in DNA damage in lymphocytes and micronucleus frequency in buccal cells in silica-exposed workers. Indian J. Occup. Environ. Med. 2012, 16, 34–37. [Google Scholar] [CrossRef]
- Nishioka, H. Mutagenic activities of metal compounds in bacteria. Mutat. Res. 1975, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.; Ishidate, M.; Stoner, G.; Morgan, M.; Ratpan, F.; Callander, R. Studies on the genotoxicity of beryllium sulphate in vitro and in vivo. Mutat. Res. Toxicol. 1990, 240, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Fritzenschaf, H.; Kohlpoth, M.; Rusche, B.; Schiffmann, D. Testing of known carcinogens and noncarcinogens in the Syrian hamster embryo (SHE) micronucleus test in vitro; correlations with in vivo micronucleus formation and cell transformation. Mutat. Res. Toxicol. 1993, 319, 47–53. [Google Scholar] [CrossRef]
- Tripuraneni, S.C.; Namburi, S.K. Evaluation of genotoxicity of recycled Ni-Cr dental casting alloys: An in vitro study. J. Appl. Biomater. Biomech. 2008, 6, 47–54. [Google Scholar] [PubMed]
- Ribeiro, D.A.; Yujra, V.Q.; De Moura, C.F.G.; Handan, B.A.; Viana, M.D.B.; Yamauchi, L.Y.; Castelo, P.M.; Aguiar, O. Genotoxicity Induced by Dental Materials: A Comprehensive Review. Anticancer. Res. 2017, 37, 4017–4024. [Google Scholar] [CrossRef][Green Version]
- Altas, E.; Gurhan, I.D.; Urkmez, A.S. Influence of processing flaws on cytotoxicity and genotoxicity of Co-Cr and Ni-Cr based dental crowns and bridges. In Proceedings of the 10th International Workshop on Biomedical Engineering, Kos, Greece, 5–7 October 2011; pp. 1–4. [Google Scholar] [CrossRef]
- OCED Test No. 487: In Vitro Mammalian Cell Micronucleus Test Paris: 21. Publishing. 2014. Available online: https://www.oecd.org/en/publications/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264264861-en.html (accessed on 1 March 2025).
- Ye, C.J.; Sharpe, Z.; Alemara, S.; Mackenzie, S.; Liu, G.; Abdallah, B.; Horne, S.; Regan, S.; Heng, H.H. Micronuclei and Genome Chaos: Changing the System Inheritance. Genes 2019, 10, 366. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Bashabsheh, O.M.; Darmani, H. Elements released from dental casting alloys and their cytotoxic effects. Int. J. Prosthodont. 2002, 15, 473–478. [Google Scholar]
- Alp, G.; Çakmak, G.; Sert, M.; Burgaz, Y. Corrosion potential in artificial saliva and possible genotoxic and cytotoxic damage in buccal epithelial cells of patients who underwent Ni-Cr based porcelain-fused-to-metal fixed dental prostheses. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 19–26. [Google Scholar] [CrossRef]
- Ellakany, P.; AlGhamdi, M.A.; Alshehri, T.; Abdelrahman, Z. Cytotoxicity of Commercially Pure Titanium (cpTi), Silver-Palladium (Ag-Pd), and Nickel-Chromium (Ni-Cr) Alloys Commonly Used in the Fabrication of Dental Prosthetic Restorations. Cureus 2022, 14, e31679. [Google Scholar] [CrossRef]
- Morán-Martínez, J.; Luna, K.M.-D.; Betancourt-Martínez, N.; Carranza-Rosales, P.; Contreras-Martínez, J.; López-Meza, M.; Rodríguez-Villarreal, O. Genotoxicity in oral epithelial cells in children caused by nickel in metal crowns. Genet. Mol. Res. 2013, 12, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Dragus, L.; Ghergic, D.L.; Comaneanu, R.M.; Bechir, A.; Coman, C.; Botoaca, O. In vitro comparative tests about the biocompatibility of some dental alloys. Rev. Chim. 2019, 70, 610–613. [Google Scholar] [CrossRef]
- Zhihong, C.; Yezhen, L.; Zhiyuan, G.; Zhongqiao, Y.; Xiaoxue, L.; Yifeng, R.; Weikun, F.; Jiliang, H. Comparison of the cytogenotoxicity induced by five different dental alloys using four in vitro assays. Dent. Mater. J. 2011, 30, 861–868. [Google Scholar] [CrossRef]
- Union, E. Consolidated Text: Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices. EUR-Lex—Access to European Union Law 2007. Available online: https://eur-lex.europa.eu/eli/dir/1993/42/oj/eng (accessed on 1 March 2025).
- Strupp, C. Beryllium metal I. experimental results on acute oral toxicity, local skin and eye effects, and genotoxicity. Ann. Occup. Hyg. 2011, 55, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Beryllium: Genotoxicity and carcinogenicity. Mutat. Res. Mol. Mech. Mutagen. 2003, 533, 99–105. [Google Scholar] [CrossRef]
- Jiménez-Lamana, J.; Godin, S.; Aragonès, G.; Bladé, C.; Szpunar, J.; Łobinski, R. Nickel Nanoparticles Induce the Synthesis of a Tumor-Related Polypeptide in Human Epidermal Keratinocytes. Nanomaterials 2020, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wang, Y.-F.; Huang, W.-R.; Huang, Y.-T. Nickel induces oxidative stress and genotoxicity in human lymphocytes. Toxicol. Appl. Pharmacol. 2003, 189, 153–159. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Sawicka, E.; Jurkowska, K.; Piwowar, A. Chromium (III) and chromium (VI) as important players in the induction of genotoxicity—Current view. Ann. Agric. Environ. Med. 2021, 28, 1–10. [Google Scholar] [CrossRef]
- Otelea, M.; Handra, C.; Rascu, A. Registered cases of occupational n-hexane intoxication in Bucharest. Romanian J. Leg. Med. 2015, 23, 279–284. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.-C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome—A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef] [PubMed]





| Substance Type | Cytotoxicity Depends on the Concentration of the Substance in the Culture Medium | ||
|---|---|---|---|
| 5 mg/mL | 10 mg/mL | 20 mg/mL | |
| Type 1 test alloy Ni-Cr without beryllium | 40% | 49.2% | 58.2% |
| Type 2 test alloy Ni-Cr with beryllium | 56.2% | 57.4% | 60% |
| Substance Type | The Concentration of Substances in the Culture Medium | ||
|---|---|---|---|
| 5 mg/mL | 10 mg/mL | 20 mg/mL | |
| Type 1 Ni-Cr test alloy without beryllium Replica 1 | 5.6‰ | 8.2‰ | 8.5‰ |
| Replica 2 | 7.6‰ | 8.7‰ | 9.8‰ |
| Negative control Replica 1 | 2.6‰ | ||
| Replica 2 | 2‰ | ||
| Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Range | Minimum | Maximum | Mean | Std. Error | Std. Deviation | Variance | p-Value | |
| FB.R1.5 mg/mL | 10 | 4 | 4 | 8 | 5.60 | 0.452 | 1.430 | 2.044 | <0.05 |
| FB.R1.10 mg/mL | 10 | 3 | 7 | 10 | 8.20 | 0.359 | 1.135 | 1.289 | <0.01 |
| FB.R1.20 mg/mL | 10 | 4 | 6 | 10 | 8.50 | 0.428 | 1.354 | 1.833 | <0.01 |
| Valid N (listwise) | 10 | ||||||||
| Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Range | Minimum | Maximum | Mean | Std. Error | Std. Deviation | Variance | p-Value | |
| FB.R2.5 mg/mL | 10 | 4 | 6 | 10 | 7.60 | 0.400 | 1.265 | 1.600 | <0.05 |
| FB.R2.10 mg/mL | 10 | 4 | 7 | 11 | 8.70 | 0.473 | 1.494 | 2.233 | <0.01 |
| FB.R2.20 mg/mL | 10 | 6 | 89 | 14 | 9.80 | 0.512 | 1.619 | 2.622 | <0.001 |
| Valid N (listwise) | 10 | ||||||||
| Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Range | Minimum | Maximum | Mean | Std. Error | Std. Deviation | Variance | p-Value | |
| CB.R1.5 mg/mL | 10 | 5 | 9 | 14 | 12.20 | 0.611 | 1.932 | 3.733 | <0.05 |
| CB.R1.10 mg/mL | 10 | 5 | 14 | 19 | 16.00 | 0.683 | 2.160 | 4.667 | <0.01 |
| CB.R1.20 mg/mL | 10 | 7 | 14 | 21 | 18.80 | 0.827 | 2.616 | 6.844 | <0.001 |
| Valid N (listwise) | 10 | ||||||||
| Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Range | Minimum | Maximum | Mean | Std. Error | Std. Deviation | Variance | p-Value | |
| CB.R2.5 mg/mL | 10 | 4 | 9 | 13 | 11.00 | 0.422 | 1.333 | 1.778 | <0.05 |
| CB.R2.10 mg/mL | 10 | 7 | 11 | 18 | 14.10 | 0.690 | 2.183 | 4.767 | <0.01 |
| CB.R2.20 mg/mL | 10 | 9 | 13 | 22 | 19.40 | 0.763 | 2.413 | 5.822 | <0.001 |
| Valid N (listwise) | 10 | ||||||||
| Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Range | Minimum | Maximum | Mean | Std. Error | Std. Deviation | Variance | p-Value | |
| M.R1 | 10 | 3 | 1 | 4 | 2.60 | 0.340 | 1.075 | 1.156 | <0.05 |
| M.R2 | 10 | 4 | 0 | 4 | 2.00 | 0.365 | 1.155 | 1.333 | <0.05 |
| Valid N (listwise) | 10 | ||||||||
| Experimental Condition | Mean | Std. Error | Std. Deviation | Variance | p-Value |
|---|---|---|---|---|---|
| FB.R1.5 mg/mL | 5.6 | 0.452 | 1.43 | 2.044 | <0.05 |
| FB.R1.10 mg/mL | 8.2 | 0.359 | 1.135 | 1.289 | <0.01 |
| FB.R1.20 mg/mL | 8.5 | 0.428 | 1.354 | 1.833 | <0.01 |
| FB.R2.5 mg/mL | 7.6 | 0.4 | 1.265 | 1.6 | <0.05 |
| FB.R2.10 mg/mL | 8.7 | 0.473 | 1.494 | 2.233 | <0.01 |
| FB.R2.20 mg/mL | 9.8 | 0.512 | 1.619 | 2.622 | <0.001 |
| CB.R1.5 mg/mL | 12.2 | 0.611 | 1.932 | 3.733 | <0.05 |
| CB.R1.10 mg/mL | 16.0 | 0.683 | 2.16 | 4.667 | <0.01 |
| CB.R1.20 mg/mL | 18.8 | 0.827 | 2.616 | 6.844 | <0.001 |
| CB.R2.5 mg/mL | 11.0 | 0.422 | 1.333 | 1.778 | <0.05 |
| CB.R2.10 mg/mL | 14.1 | 0.69 | 2.183 | 4.767 | <0.01 |
| CB.R2.20 mg/mL | 19.4 | 0.763 | 2.413 | 5.822 | <0.001 |
| M.R1 | 2.6 | 0.34 | 1.075 | 1.156 | <0.05 |
| M.R2 | 2.0 | 0.365 | 1.155 | 1.333 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caministeanu, F.; Perieanu, V.S.; Popa, A.S.; Manolescu, L.S.C.; Stetiu, A.A.; Costea, R.C.; Burlibasa, M.; Vorovenci, A.; Costea, R.M.; Serbanescu, C.M.; et al. Assessing the Genotoxic Impact of Ni-Cr Alloys in Dental Prosthodontics: A Preliminary Comparative Analysis with and Without Beryllium. Oral 2025, 5, 32. https://doi.org/10.3390/oral5020032
Caministeanu F, Perieanu VS, Popa AS, Manolescu LSC, Stetiu AA, Costea RC, Burlibasa M, Vorovenci A, Costea RM, Serbanescu CM, et al. Assessing the Genotoxic Impact of Ni-Cr Alloys in Dental Prosthodontics: A Preliminary Comparative Analysis with and Without Beryllium. Oral. 2025; 5(2):32. https://doi.org/10.3390/oral5020032
Chicago/Turabian StyleCaministeanu, Florentina, Viorel Stefan Perieanu, Andrei Sabin Popa, Loredana Sabina Cornelia Manolescu, Andreea Angela Stetiu, Radu Catalin Costea, Mihai Burlibasa, Andrei Vorovenci, Raluca Mariana Costea, Cristina Maria Serbanescu, and et al. 2025. "Assessing the Genotoxic Impact of Ni-Cr Alloys in Dental Prosthodontics: A Preliminary Comparative Analysis with and Without Beryllium" Oral 5, no. 2: 32. https://doi.org/10.3390/oral5020032
APA StyleCaministeanu, F., Perieanu, V. S., Popa, A. S., Manolescu, L. S. C., Stetiu, A. A., Costea, R. C., Burlibasa, M., Vorovenci, A., Costea, R. M., Serbanescu, C. M., Dragus, A. C., Stetiu, M. A., Malita, M. A., & Burlibasa, L. (2025). Assessing the Genotoxic Impact of Ni-Cr Alloys in Dental Prosthodontics: A Preliminary Comparative Analysis with and Without Beryllium. Oral, 5(2), 32. https://doi.org/10.3390/oral5020032









