Comparative Analysis of Gene Expression in Periodontal Ligament Stem Cells Exposed to Biodentine and Bio-C Repair: Implications for Cementogenesis—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. PDLSCs Isolation and Culture
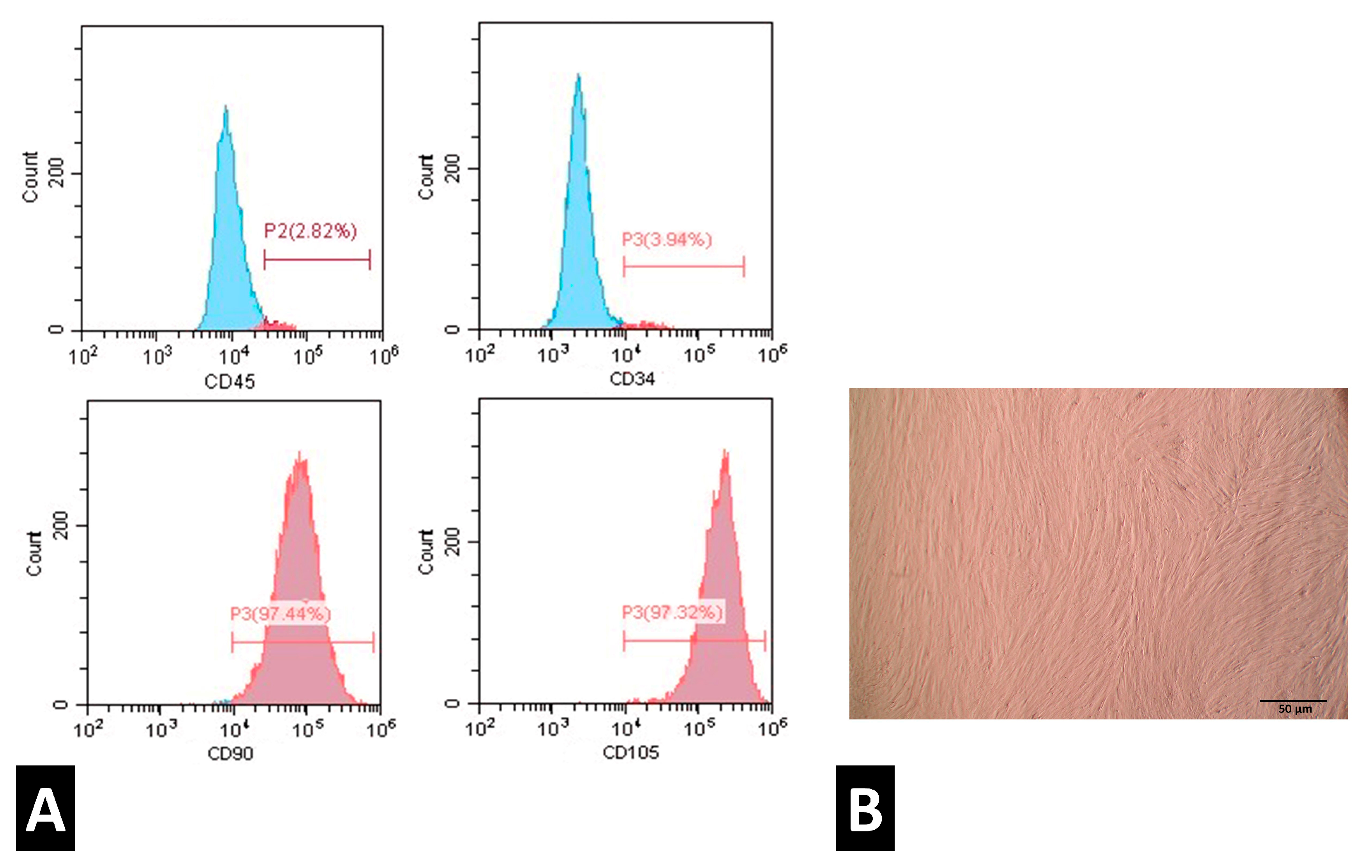
2.2. Characterization of PDLSCs
2.3. Material Extracts and Preparation
2.4. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Statistical Analysis
3. Results
3.1. PDLSCs Characterization
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncan, H.F. Present status and future directions—Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.N.; Perez Alfayate, R.; Prichard, J. Vital Pulp Therapy an Insight Over the Available Literature and Future Expecta-tions. Eur. Endod. J. 2020, 5, 46–53. [Google Scholar] [PubMed]
- Wuersching, S.N.; Diegritz, C.; Hickel, R.; Huth, K.C.; Kollmuss, M. A comprehensive in vitro comparison of the biological and physicochemical properties of bioactive root canal sealers. Clin. Oral Investig. 2022, 26, 6209–6222. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.-J.; Patel, K.D.; Knowles, J.C.; Lee, J.-H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef]
- Inada, R.N.H.; Silva, E.C.A.; Lopes, C.S.; Queiroz, M.B.; Torres, F.F.E.; da Silva, G.F.; Cerri, P.S.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Biocompatibility, bioactivity, porosity, and sealer/dentin interface of bioceramic ready-to-use sealers using a dentin-tube model. Sci. Rep. 2024, 14, 16768. [Google Scholar] [CrossRef]
- Estivalet, M.S.; de Araújo, L.P.; Immich, F.; da Silva, A.F.; Ferreira, N.d.S.; da Rosa, W.L.O.; Piva, E. Bioactivity Potential of Bioceramic-Based Root Canal Sealers: A Scoping Review. Life 2022, 12, 1853. [Google Scholar] [CrossRef]
- de Sousa Reis, M.; Scarparo, R.K.; Steier, L.; de Figueiredo, J.A.P. Periradicular inflammatory response, bone resorption, and ce-mentum repair after sealing of furcation perforation with mineral trioxide aggregate (MTA Angelus™) or Biodentine™. Clin. Oral Investig. 2019, 23, 4019–4027. [Google Scholar] [CrossRef]
- Estrela, C.; Cintra, L.T.A.; Duarte, M.A.H.; Rossi-Fedele, G.; Gavini, G.; Sousa-Neto, M.D. Mechanism of action of Bioactive Endodontic Materials. Braz. Dent. J. 2023, 34, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Song, W.; Ye, L.; Yang, C.; Xing, Y.; Yuan, Z. Clinical application of calcium silicate-based bioceramics in endodontics. J. Transl. Med. 2023, 21, 853. [Google Scholar] [CrossRef]
- Jung, M.-K.; Park, S.-C.; Kim, Y.-J.; Park, J.-T.; Knowles, J.C.; Park, J.-H.; Dashnyam, K.; Jun, S.-K.; Lee, H.-H.; Lee, J.-H. Premixed Calcium Silicate-Based Root Canal Sealer Reinforced with Bioactive Glass Nanoparticles to Improve Biological Properties. Pharmaceutics 2022, 14, 1903. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, Y.; Wang, Q.; Dong, M.; Yang, M.; Chen, W.; Wang, S.; Zhang, H.; Zheng, S.; Cao, C.Y.; et al. A strontium and amorphous calcium phosphate dipped premixed injectable calcium silicate-based ceramic for dental root canal sealing. Ceram. Int. 2021, 47, 33738–33750. [Google Scholar] [CrossRef]
- Tanvir, A.H.; Khaleque, A.; Kim, G.-H.; Yoo, W.-Y.; Kim, Y.-Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Primus, C.; Kulild, J.C. Tricalcium silicate cement sealers: Do the potential benefits of bioactivity justify the drawbacks? J. Am. Dent. Assoc. 2022, 153, 750–760. [Google Scholar] [CrossRef]
- Alchawoosh, A.; Hashimoto, K.; Kawashima, N.; Noda, S.; Nozaki, K.; Okiji, T. Hydraulic calcium silicate-based root canal sealers mitigate proinflammatory cytokine synthesis and promote osteogenesis in vitro. J. Dent. Sci. 2023, 18, 1731–1739. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Weir, M.D.; Schneider, A.; Ma, T.; Oates, T.W.; Xu, H.H.K.; Zhang, K.; Bai, Y. Periodontal ligament stem cell-based bioactive constructs for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1071472. [Google Scholar] [CrossRef]
- López-García, S.; Lozano, A.; García-Bernal, D.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Moraleda, J.M.; Murcia, L.; Rodríguez-Lozano, F.J. Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells. J. Clin. Med. 2019, 8, 1216. [Google Scholar] [CrossRef]
- Di Vito, A.; Bria, J.; Antonelli, A.; Mesuraca, M.; Barni, T.; Giudice, A.; Chiarella, E. A Review of Novel Strategies for Human Periodontal Ligament Stem Cell Ex Vivo Expansion: Are They an Evidence-Based Promise for Regenerative Periodontal Therapy? Int. J. Mol. Sci. 2023, 24, 7798. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef]
- Mohebichamkhorami, F.; Fattahi, R.; Niknam, Z.; Aliashrafi, M.; Naeimi, S.K.; Gilanchi, S.; Zali, H. Periodontal ligament stem cells as a promising therapeutic target for neural damage. Stem Cell Res. Ther. 2022, 13, 273. [Google Scholar] [CrossRef]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, J.; Wang, C.; Zhong, D.; Xie, L.; Fang, H. Immunomodulatory Properties of Stem Cells in Periodontitis: Current Status and Future Prospective. Stem Cells Int. 2020, 2020, 9836518. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Guerrero-Gironés, J.; Pecci-Lloret, M.R.; Melo, M. Biological interactions between calcium silicate-based endodontic biomaterials and periodontal ligament stem cells: A systematic review of in vitro studies. Int. Endod. J. 2021, 54, 2025–2043. [Google Scholar] [CrossRef]
- Souza, T.A.; Bezerra, M.M.; Silva, P.G.B.; Costa, J.J.N.; Carneiro, R.F.L.A.; Barcelos, J.O.F.; Vasconcelos, B.C.; Chaves, H.V. Bone morphogenetic proteins in biomineralization of two endodontic restorative cements. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 348–357. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: www.iso.org/standard/75769.html (accessed on 4 March 2025).
- Saber, S.M.; Gomaa, S.M.; Elashiry, M.M.; El-Banna, A.; Schäfer, E. Comparative biological properties of resin-free and resin-based calcium silicate-based endodontic repair materials on human periodontal ligament stem cells. Clin. Oral Investig. 2023, 27, 6757–6768. [Google Scholar] [CrossRef]
- Alhazmi, Y.A.; Aljabri, M.Y.; Raafat, S.N.; Gomaa, S.M.; Shamel, M. Exploring the Effects of Low-Level Laser Therapy on the Cyto-compatibility and Osteo/Odontogenic Potential of Gingival-Derived Mesenchymal Stem Cells: Preliminary Report. Appl. Sci. 2023, 13, 8490. [Google Scholar] [CrossRef]
- Raghavendra, S.S.; Jadhav, G.R.; Gathani, K.M.; Kotadia, P. Bioceramics in endodontics—A review. J. Istanb. Univ. Fac. Dent. 2017, 51, 128–137. [Google Scholar] [CrossRef]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Arroyo, R.; López, S.; Romo, E.; Montoya, G.; Hoz, L.; Pedraza, C.; Garfias, Y.; Arzate, H. Carboxy-Terminal Cementum Protein 1-Derived Peptide 4 (cemp1-p4) Promotes Mineralization through wnt/β-catenin Signaling in Human Oral Mucosa Stem Cells. Int. J. Mol. Sci. 2020, 21, 1307. [Google Scholar] [CrossRef]
- Gauthier, P.; Yu, Z.; Tran, Q.T.; Bhatti, F.-U.; Zhu, X.; Huang, G.T.-J. Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment. Cell Tissue Res. 2017, 368, 227. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Q.; Ma, X.; Zhong, Y.; Tang, H.; Mai, S. The mechanism of biomineralization: Progress in mineralization from in-tracellular generation to extracellular deposition. Jpn. Dent. Sci. Rev. 2023, 59, 181–190. [Google Scholar] [CrossRef] [PubMed]
- López-García, S.; Sánchez-Bautista, S.; García-Bernal, D.; Lozano, A.; Forner, L.; Sanz, J.L.; Murcia, L.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Premixed calcium silicate-based ceramic sealers promote osteo-genic/cementogenic differentiation of human periodontal ligament stem cells: A microscopy study. Microsc. Res. Tech. 2024, 87, 1584–1597. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar]
- Fan, C.; Ji, Q.; Zhang, C.; Xu, S.; Sun, H.; Li, Z. TGF-β induces periodontal ligament stem cell senescence through increase of ROS production. Mol. Med. Rep. 2019, 20, 3123–3130. [Google Scholar] [CrossRef]
- Pitaru, S.; Pritzki, A.; Bar-Kana, I.; Grosskopf, A.; Savion, N.; Narayanan, A.S. Bone morphogenetic protein 2 induces the expression of cementum attachment protein in human periodontal ligament clones. Connect. Tissue Res. 2002, 43, 257–264. [Google Scholar] [CrossRef]
- Rahman, S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The Role of Interleukin 6 in Periodontitis and Its Complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef]
- Kerkis, I.; da Silva, Á.P.; Araldi, R.P. The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neurological conditions. Front. Immunol. 2024, 15, 1400533. [Google Scholar] [CrossRef]
- Ancuța, C.; Chirieac, R.; Ancuța, E.; Țănculescu, O.; Solomon, S.M.; Fătu, A.M.; Doloca, A.; Iordache, C. Exploring the Role of Inter-leukin-6 Receptor Inhibitor Tocilizumab in Patients with Active Rheumatoid Arthritis and Periodontal Disease. J. Clin. Med. 2021, 10, 878. [Google Scholar] [CrossRef]
- Huang, J.; Cai, X.; Ou, Y.; Zhou, Y.; Wang, Y. Resolution of inflammation in periodontitis: A review. Int. J. Clin. Exp. Pathol. 2018, 11, 4283–4295. [Google Scholar] [PubMed]
- Kaigler, D.; A Cirelli, J.; Giannobile, W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFβ Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef]
- Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium—Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. [Google Scholar] [CrossRef]
- Bezerra, M.M.; de Lima, V.; Alencar, V.B.; Vieira, I.B.; Brito, G.A.C.; Ribeiro, R.A.; Rocha, F.A.C. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 2000, 71, 1009–1014. [Google Scholar] [CrossRef]
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An Evidence-Based Update on the Molecular Mechanisms Underlying Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 3829. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Aslam, N.; Jafar, H.; Abu Harfil, Z.; Awidi, A. Biocompatibility of Biodentine™ ® with Periodontal Ligament Stem Cells: In Vitro Study. Dent. J. 2020, 8, 17. [Google Scholar] [CrossRef]
- Pedrosa, M.d.S.; Alves, T.; Rahhal, J.G.; Nogueira, F.N.; Sipert, C.R. Cytotoxicity of reparative endodontic cements on human periodontal ligament stem cells. Pesqui. Bras. Odontopediatr. Clin. Integr. 2022, 22, e210114. [Google Scholar] [CrossRef]
- López-García, S.; Rodríguez-Lozano, F.J.; Sanz, J.L.; Forner, L.; Pecci-Lloret, M.P.; Lozano, A.; Murcia, L.; Sánchez-Bautista, S.; Oñate-Sánchez, R.E. Biological properties of Ceraputty as a retrograde filling material: An in vitro study on hPDLSCs. Clin. Oral Investig. 2023, 27, 4233–4243. [Google Scholar] [CrossRef]
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palumbo, G.; Piccininni, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Alhawsawi, B.F.; Alghamdi, A.; Aldobaikhi, S.O.; Alanazi, M.H.; Alahmad, F.A. The Management of Root Perforation: A Review of the Literature. Cureus 2024, 16, e72296. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.L.; Freitas, G.A.N.; Ribeiro, M.T.H.; Lemus, N.X.A.; Soares, C.J.; Moura, C.C.G. Effects of different calcium-silicate based ma-terials on fracture resistance of immature permanent teeth with replacement root resorption and osteoclastogenesis. Restor. Dent. Endod. 2023, 48, e21. [Google Scholar] [CrossRef] [PubMed]
- de Toubes, K.S.; Tonelli, S.Q.; Girelli, C.F.M.; Azevedo, C.G.d.S.; Thompson, A.C.T.; Nunes, E.; Silveira, F.F. Bio-C Repair—A New Bioceramic Material for Root Perforation Management: Two Case Reports. Braz. Dent. J. 2021, 32, 104–110. [Google Scholar] [CrossRef]

| Bioactive Material | Introduction | Mode of Application | Benefits/Uses |
|---|---|---|---|
| Calcium Hydroxide | Introduced in the 1930s; widely used in pulp therapy and endodontics. | Applied as a pulp-capping material or intracanal medicament. | Antibacterial properties, stimulates reparative dentin formation, promotes healing. |
| Mineral Trioxide Aggregate (MTA) | Developed in the 1990s as a root repair and pulp-capping material. | Used for pulp capping, apexification, perforation repair, and root-end filling. | Superior sealing ability, biocompatibility, promotes hydroxyapatite formation, stimulates tissue healing. |
| Biodentine | A calcium silicate-based material introduced as a dentin substitute in 2010. | Used in direct/indirect pulp capping, root repair, and dentin replacement. | Shorter setting time than MTA, promotes dentin bridge formation, excellent biocompatibility. |
| Bio-C Repair | A newer ready-to-use bioceramic material developed for root repair. | Applied as a repair material for root perforations, apexification, and retrograde fillings. | Cytocompatibility, biomineralization, cementogenic potential, ease of application. |
| Calcium Silicate-Based Sealers | Emerging class of root canal sealers in the 2010s. | Used as sealers in root canal treatment. | Release of calcium ions, hydroxyapatite formation, excellent sealing ability, bioactivity. |
| TheraCal LC | Light-cured resin-modified calcium silicate material introduced in the 2010s. | Applied for direct/indirect pulp capping. | Controlled calcium release, quick setting, ease of handling, promotes dentin bridge formation. |
| Bioceramic Putty | Introduced as a premixed calcium silicate-based material for endodontic repairs. | Used in perforation repair, root-end filling, and apexification procedures. | Antibacterial, high bioactivity, promotes tissue regeneration, convenient premixed form. |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
|
|
| Material | Composition | Company | Form |
|---|---|---|---|
| Biodentine | Powder: Tricalcium silicate, zirconium oxide, calcium carbonate, iron oxide. The liquid is made up of water with some calcium chloride and plasticizer additions. | Septodont, Saint Maur-des-Fosses, France. | Powder and liquid |
| Bio-C Repair | Tricalcium silicate, calcium oxide, zirconium oxide, iron oxide, silicon dioxide, dispersing agent. | Angelus, Londrina, PR, Brazil. | Ready for use syringe. |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| CEMP1 | GGGCACATCAAGCACTGACAG | CCCTTAGGAAGTGGCTGTCCAG |
| CAP | TTTTTCTGGTCGCGTGGACT | TCACCAGCAACTCCAACAGG |
| TGF-β1 | GGATACCAACTATTGCTTCAGCT | AGGCTCCAAATGTAGGGGCAGGG |
| BMP2 | TGTATCGCAGGCACTCAGGTCA | CCACTCGTTTCTGGTAGTTCTTC |
| β-actin | TCCGTCGCCGGTCCACACCC | TCACCAACTGGGACGATATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, M.M.; Al Ankily, M.; Meer, M.; Shamel, M. Comparative Analysis of Gene Expression in Periodontal Ligament Stem Cells Exposed to Biodentine and Bio-C Repair: Implications for Cementogenesis—An In Vitro Study. Oral 2025, 5, 19. https://doi.org/10.3390/oral5010019
Bakr MM, Al Ankily M, Meer M, Shamel M. Comparative Analysis of Gene Expression in Periodontal Ligament Stem Cells Exposed to Biodentine and Bio-C Repair: Implications for Cementogenesis—An In Vitro Study. Oral. 2025; 5(1):19. https://doi.org/10.3390/oral5010019
Chicago/Turabian StyleBakr, Mahmoud M., Mahmoud Al Ankily, Mohammed Meer, and Mohamed Shamel. 2025. "Comparative Analysis of Gene Expression in Periodontal Ligament Stem Cells Exposed to Biodentine and Bio-C Repair: Implications for Cementogenesis—An In Vitro Study" Oral 5, no. 1: 19. https://doi.org/10.3390/oral5010019
APA StyleBakr, M. M., Al Ankily, M., Meer, M., & Shamel, M. (2025). Comparative Analysis of Gene Expression in Periodontal Ligament Stem Cells Exposed to Biodentine and Bio-C Repair: Implications for Cementogenesis—An In Vitro Study. Oral, 5(1), 19. https://doi.org/10.3390/oral5010019







