Cannabidiol Mediates Beneficial Effects on the Microvasculature of Murine Hearts with Regard to Irradiation-Induced Inflammation and Early Signs of Fibrosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
- CBD treatment starting simultaneous with irradiation does not reduce the expression of inflammatory markers on heart ECs
- CBD treatment starting 2 weeks before irradiation reduced the expression of inflammatory markers on heart ECs
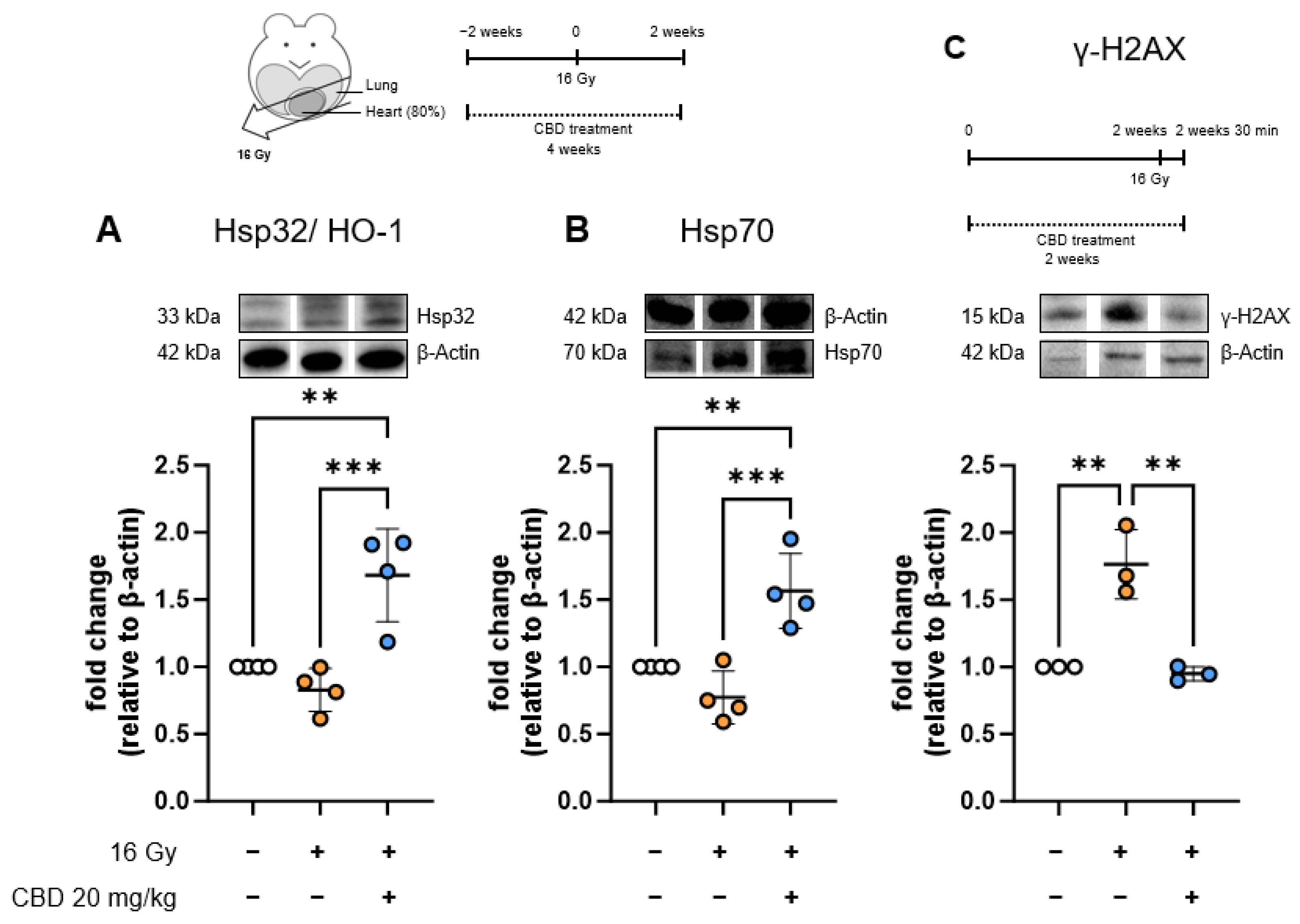
- The anti-inflammatory effect of CBD treatment starting 2 weeks before irradiation is associated with increased stress protein synthesis in heart ECs
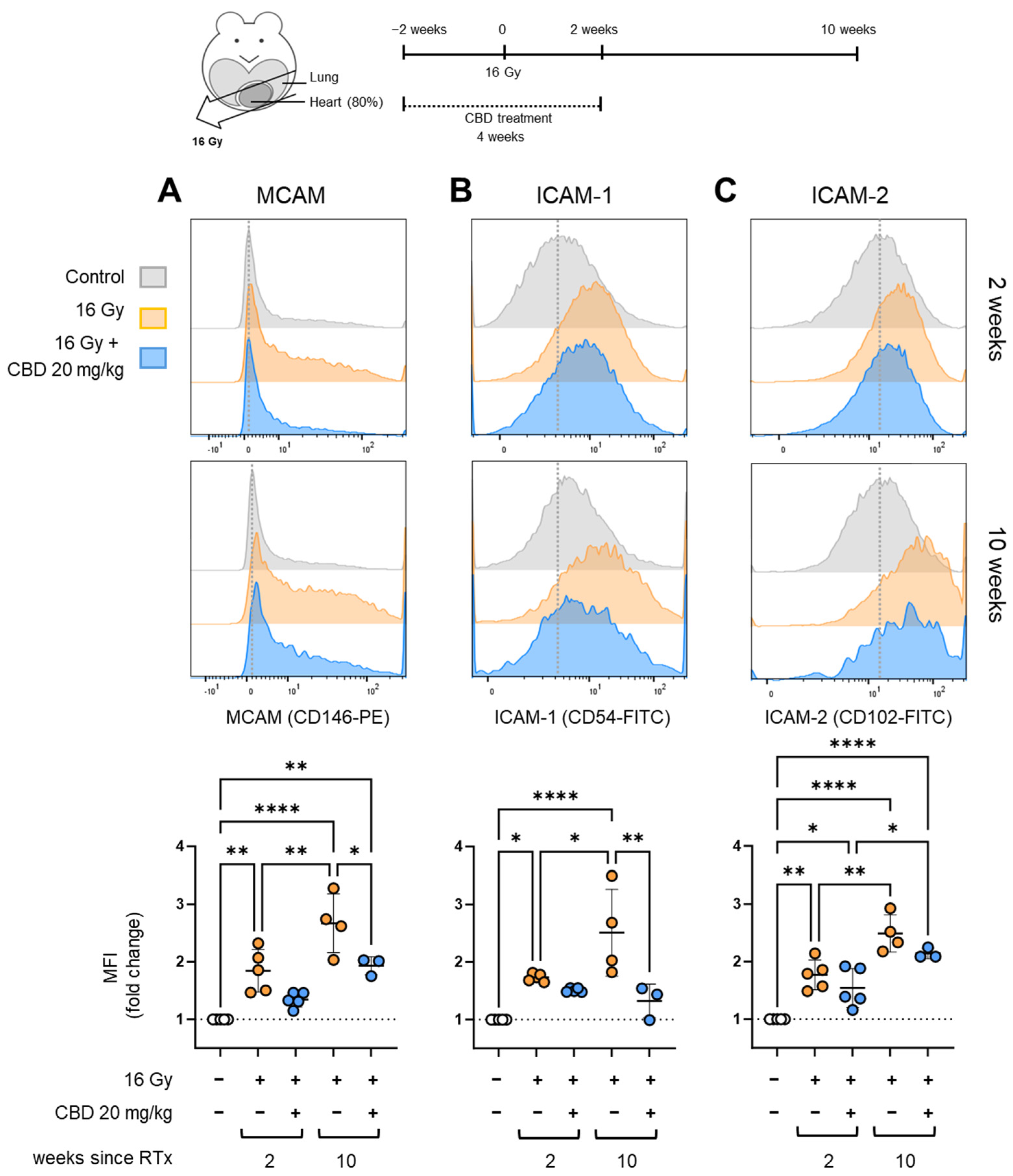
- CBD treatment starting 2 weeks before irradiation increases VCAM-1 on heart ECs
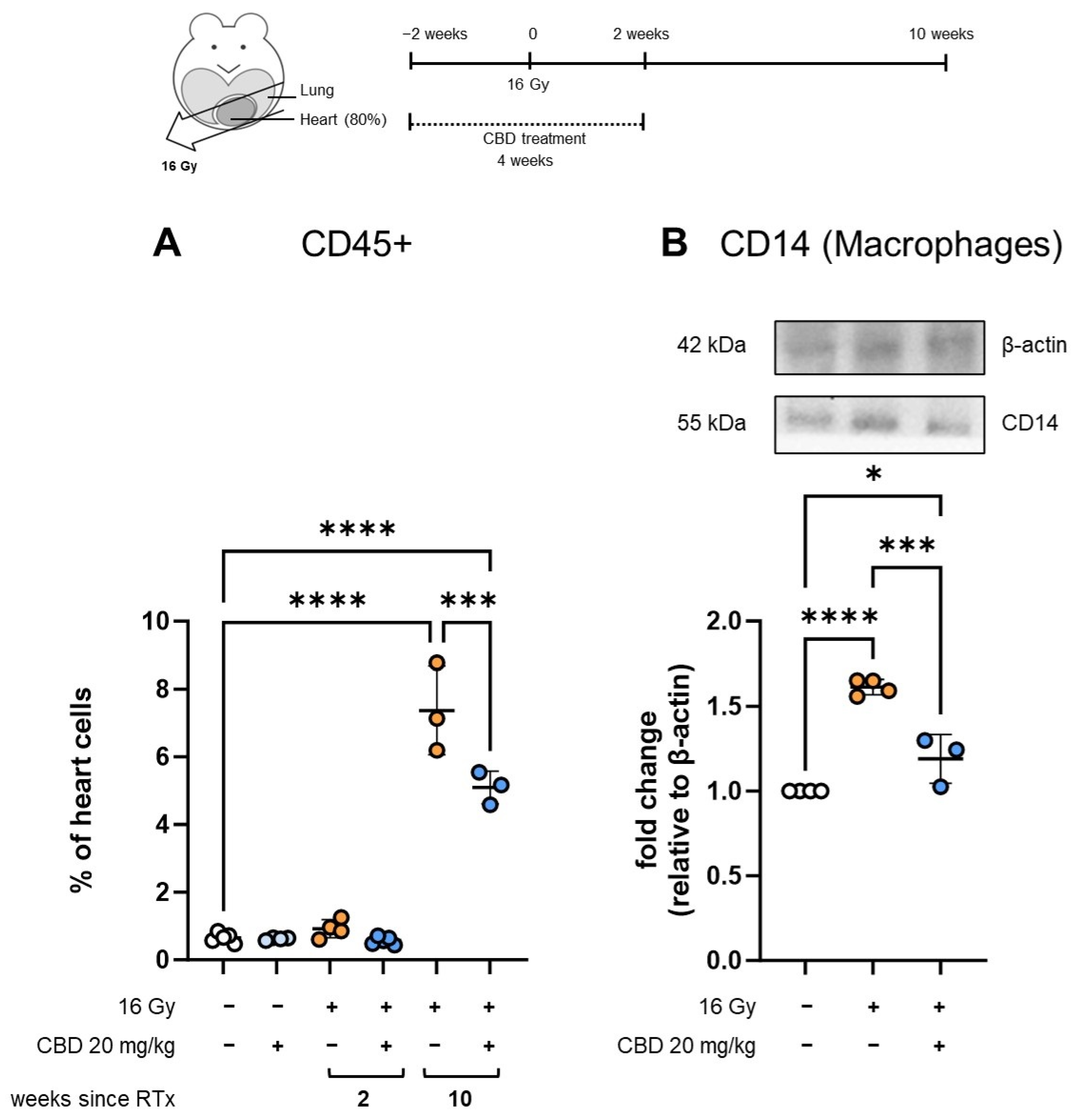
- CBD treatment starting 2 weeks before irradiation reduces immune cell infiltration into the heart
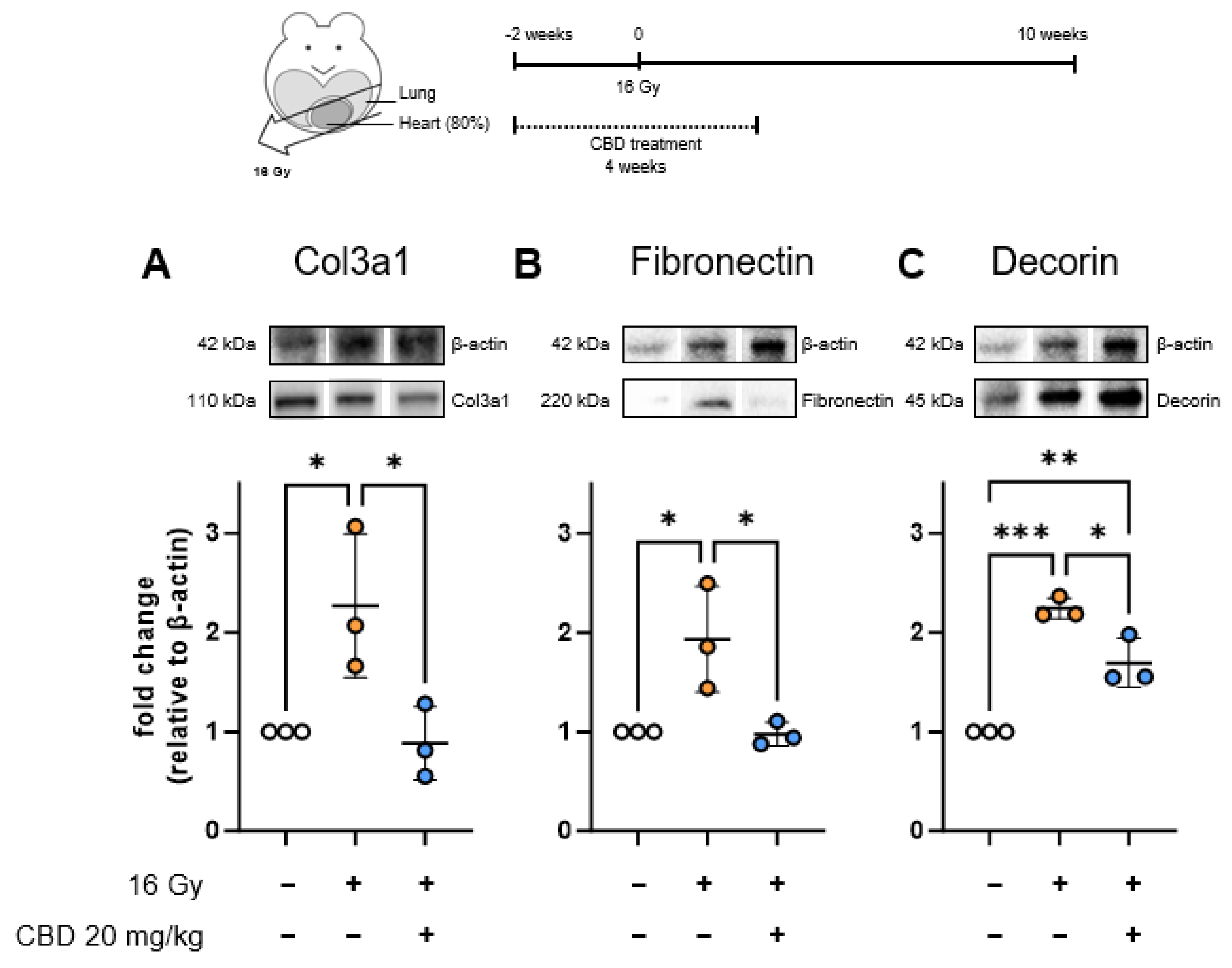
- CBD treatment starting 2 weeks before irradiation reduces the expression of early fibrosis markers in the heart
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| CAM | Cell adhesion molecule |
| HO-1 | Heme-oxygenase-1 |
| Hsp | Heat shock protein |
| ICAM | Intercellular adhesion molecule |
| Ig | Immunoglobulin |
| mAb | Monoclonal antibody |
| MCAM | Melanoma cell adhesion molecule |
| PVDF | Polyvinylidene fluoride |
| RIHD | Radiation-induced heart disease |
| SARRP | Small Animal Radiation Research Platform |
| SD | Standard deviation |
| TNF-α | Tumor necrosis factor alpha |
| VCAM-1 | Vascular cell adhesion molecule 1 |
Appendix A
Appendix A.1
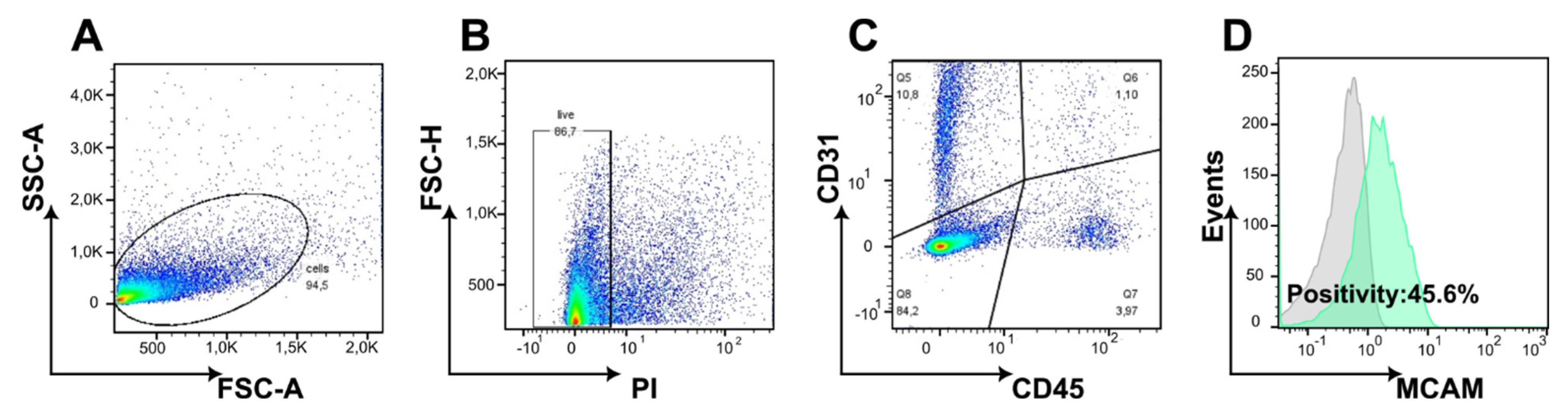
Appendix A.2
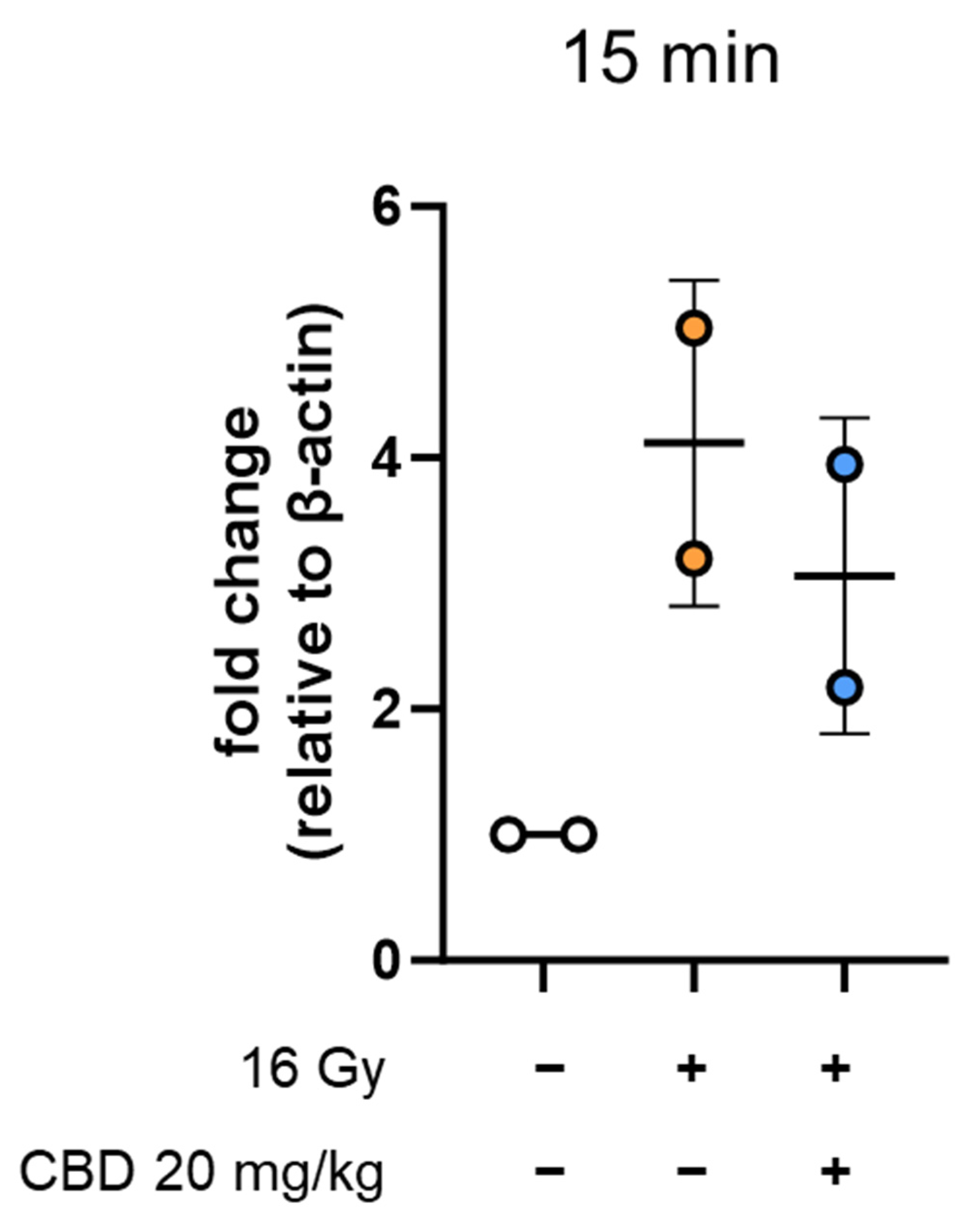
References
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Rakotomalala, A.; Escande, A.; Furlan, A.; Meignan, S.; Lartigau, E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the "Six Rs" of Radiotherapy. Front. Endocrinol. 2021, 12, 742215. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef]
- Beach, T.A.; Groves, A.M.; Williams, J.P.; Finkelstein, J.N. Modeling radiation-induced lung injury: Lessons learned from whole thorax irradiation. Int. J. Radiat. Biol. 2020, 96, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, X.; Yu, M.; Lin, B.; Yu, C. Radiation-induced liver injury and hepatocyte senescence. Cell. Death Discov. 2021, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Drost, L.; Yee, C.; Lam, H.; Zhang, L.; Wronski, M.; McCann, C.; Lee, J.; Vesprini, D.; Leung, E.; Chow, E. A Systematic Review of Heart Dose in Breast Radiotherapy. Clin. Breast Cancer 2018, 18, e819–e824. [Google Scholar] [CrossRef]
- Wollschläger, D.; Karle, H.; Stockinger, M.; Bartkowiak, D.; Bührdel, S.; Merzenich, H.; Wiegel, T.; Blettner, M.; Schmidberger, H. Radiation dose distribution in functional heart regions from tangential breast cancer radiotherapy. Radiother. Oncol. 2016, 119, 65–70. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Devarakonda, S.; Thorsell, A.; Hedenström, P.; Rezapour, A.; Heden, L.; Banerjee, S.; Johansson, M.E.; Birchenough, G.; Morén, A.T.; Gustavsson, K.; et al. Low-grade intestinal inflammation two decades after pelvic radiotherapy. EBioMedicine 2023, 94, 104691. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef] [PubMed]
- Moisander, M.; Skyttä, T.; Kivistö, S.; Huhtala, H.; Nikus, K.; Virtanen, V.; Kellokumpu-Lehtinen, P.L.; Raatikainen, P.; Tuohinen, S. Radiotherapy-induced diffuse myocardial fibrosis in early-stage breast cancer patients—Multimodality imaging study with six-year follow-up. Radiat. Oncol. 2023, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Hector, S.; Trott, K.R. Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data? Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 10–18. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Cannabinoids as anticancer drugs: Current status of preclinical research. Br. J. Cancer 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Sievert, W.; Stangl, S.; Steiger, K.; Multhoff, G. Improved Overall Survival of Mice by Reducing Lung Side Effects After High-Precision Heart Irradiation Using a Small Animal Radiation Research Platform. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Sievert, W.; Trott, K.R.; Azimzadeh, O.; Tapio, S.; Zitzelsberger, H.; Multhoff, G. Late proliferating and inflammatory effects on murine microvascular heart and lung endothelial cells after irradiation. Radiother. Oncol. 2015, 117, 376–381. [Google Scholar] [CrossRef]
- Bardin, N.; Blot-Chabaud, M.; Despoix, N.; Kebir, A.; Harhouri, K.; Arsanto, J.P.; Espinosa, L.; Perrin, P.; Robert, S.; Vely, F.; et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Halai, K.; Whiteford, J.; Ma, B.; Nourshargh, S.; Woodfin, A. ICAM-2 facilitates luminal interactions between neutrophils and endothelial cells in vivo. J. Cell. Sci. 2014, 127 Pt 3, 620–629. [Google Scholar]
- Muller, W.A. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am. J. Pathol. 2014, 184, 886–896. [Google Scholar] [CrossRef]
- Anil, S.M.; Peeri, H.; Koltai, H. Medical Cannabis Activity Against Inflammation: Active Compounds and Modes of Action. Front. Pharmacol. 2022, 13, 908198. [Google Scholar] [CrossRef]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal. Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef] [PubMed]
- Böckmann, S.; Hinz, B. Cannabidiol Promotes Endothelial Cell Survival by Heme Oxygenase-1-Mediated Autophagy. Cells 2020, 9, 1703. [Google Scholar] [CrossRef]
- Khomenko, I.P.; Bakhtina, L.Y.; Zelenina, O.M.; Kruglov, S.V.; Manukhina, E.B.; Bayda, L.A.; Malyshev, I.Y. Role of heat shock proteins HSP70 and HSP32 in the protective effect of adaptation of cultured HT22 hippocampal cells to oxidative stress. Bull. Exp. Biol. Med. 2007, 144, 174–177. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Min, J.K.; Kim, Y.M.; Kim, S.W.; Kwon, M.C.; Kong, Y.Y.; Hwang, I.K.; Won, M.H.; Rho, J.; Kwon, Y.G. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: Induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J. Immunol. 2005, 175, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wu, R.; Wang, S.; Zhuang, L.; Chen, P.; Li, S.; Zhu, Q.; Li, H.; Lin, Y.; Li, M.; et al. Elucidating the changes in the heterogeneity and function of radiation-induced cardiac macrophages using single-cell RNA sequencing. Front. Immunol. 2024, 15, 1363278. [Google Scholar] [CrossRef]
- Na, K.; Oh, B.C.; Jung, Y. Multifaceted role of CD14 in innate immunity and tissue homeostasis. Cytokine Growth Factor Rev. 2023, 74, 100–107. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68–69, 490–506. [Google Scholar] [CrossRef]
- Meagher, P.B.; Lee, X.A.; Lee, J.; Visram, A.; Friedberg, M.K.; Connelly, K.A. Cardiac Fibrosis: Key Role of Integrins in Cardiac Homeostasis and Remodeling. Cells 2021, 10, 770. [Google Scholar] [CrossRef]
- Dong, Y.; Zhong, J.; Dong, L. The Role of Decorin in Autoimmune and Inflammatory Diseases. J. Immunol. Res. 2022, 2022, 1283383. [Google Scholar] [CrossRef]
- Bauer, L.; Alkotub, B.; Ballmann, M.; Hasanzadeh Kafshgari, M.; Rammes, G.; Multhoff, G. Cannabidiol (CBD) Protects Lung Endothelial Cells from Irradiation-Induced Oxidative Stress and Inflammation In Vitro and In Vivo. Cancers 2024, 16, 3589. [Google Scholar] [CrossRef] [PubMed]
- Szyller, J.; Bil-Lula, I. Heat Shock Proteins in Oxidative Stress and Ischemia/Reperfusion Injury and Benefits from Physical Exercises: A Review to the Current Knowledge. Oxid. Med. Cell. Longev. 2021, 2021, 6678457. [Google Scholar] [CrossRef] [PubMed]
- Moloney, T.C.; Hoban, D.B.; Barry, F.P.; Howard, L.; Dowd, E. Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012, 21, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Issan, Y.; Kornowski, R.; Aravot, D.; Shainberg, A.; Laniado-Schwartzman, M.; Sodhi, K.; Abraham, N.G.; Hochhauser, E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS ONE 2014, 9, e92246. [Google Scholar] [CrossRef]
- Amrani, M.; Corbett, J.; Boateng, S.Y.; Dunn, M.J.; Yacoub, M.H. Yacoub, Kinetics of induction and protective effect of heat-shock proteins after cardioplegic arrest. Ann. Thorac. Surg. 1996, 61, 1407–1411. [Google Scholar] [CrossRef]
- Deng, B.; He, X.; Wang, Z.; Kang, J.; Zhang, G.; Li, L.; Kang, X. HSP70 protects PC12 cells against TBHP-induced apoptosis and oxidative stress by activating the Nrf2/HO-1 signaling pathway. In Vitro Cell. Dev. Biol. Anim. 2024, 60, 868–878. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Bidmon-Fliegenschnee, B.; Lederhuber, H.C.; Csaicsich, D.; Pichler, J.; Herzog, R.; Memaran-Dadgar, N.; Huber, W.D.; Aufricht, C.; Kratochwill, K. Overexpression of Hsp70 confers cytoprotection during gliadin exposure in Caco-2 cells. Pediatr. Res. 2015, 78, 358–364. [Google Scholar] [CrossRef]
- Mazzei, L.; Docherty, N.G.; Manucha, W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones 2015, 20, 893–906. [Google Scholar] [CrossRef]
- Wittmann, A.; Bartels, A.; Alkotub, B.; Bauer, L.; Kafshgari, M.H.; Multhoff, G. Chronic inflammatory effects of in vivo irradiation of the murine heart on endothelial cells mimic mechanisms involved in atherosclerosis. Strahlenther. Onkol. 2023, 199, 1214–1224. [Google Scholar] [CrossRef]
- Van Buul, J.D.; Van Rijssel, J.; Van Alphen, F.P.; van Stalborch, A.M.; Mul, E.P.; Hordijk, P.L.I. ICAM-1 clustering on endothelial cells recruits VCAM-1. J. Biomed. Biotechnol. 2010, 2010, 120328. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef]
- Sumagin, R.I.H. Sarelius, Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J. Immunol. 2010, 184, 5242–5252. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.R.; Wu, Y.; Zacchi, L.F.; Ta, H.T. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: Drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc. Res. 2023, 119, 2278–2293. [Google Scholar] [CrossRef] [PubMed]
- Shamseddine, A.; Patel, S.H.; Chavez, V.; Moore, Z.R.; Adnan, M.; Di Bona, M.; Li, J.; Dang, C.T.; Ramanathan, L.V.; Oeffinger, K.C.; et al. Innate immune signaling drives late cardiac toxicity following DNA-damaging cancer therapies. J. Exp. Med. 2023, 220, e20220809. [Google Scholar] [CrossRef]
- Fajardo, L.F.; Stewart, J.R. Experimental radiation-induced heart disease. I. Light microscopic studies. Am. J. Pathol. 1970, 59, 299–316. [Google Scholar]
- Dreyfuss, A.D.; Goia, D.; Shoniyozov, K.; Shewale, S.V.; Velalopoulou, A.; Mazzoni, S.; Avgousti, H.; Metzler, S.D.; Bravo, P.E.; Feigenberg, S.J.; et al. A Novel Mouse Model of Radiation-Induced Cardiac Injury Reveals Biological and Radiological Biomarkers of Cardiac Dysfunction with Potential Clinical Relevance. Clin. Cancer Res. 2021, 27, 2266–2276. [Google Scholar] [CrossRef]
- Ghobadi, G.; Van Der Veen, S.; Bartelds, B.; De Boer, R.A.; Dickinson, M.G.; De Jong, J.R.; Faber, H. Physiological interaction of heart and lung in thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e639–e646. [Google Scholar] [CrossRef]
- Hernandez, D.; Cheng, C.Y.; Hernandez-Villafuerte, K.; Schlander, M. Survival and comorbidities in lung cancer patients: Evidence from administrative claims data in Germany. Oncol. Res. 2022, 30, 173–185. [Google Scholar] [CrossRef]
- Ribeiro, A.; Almeida, V.I.; Costola-de-Souza, C.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Gimenes-Junior, J.A.; Akamine, A.T.; Crippa, J.A.; Tavares-de-Lima, W.; et al. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015, 37, 35–41. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Ramos-González, M.; Lozano, O.; Jerjes-Sánchez, C.; García-Rivas, G. Therapeutic Applications of Cannabinoids in Cardiomyopathy and Heart Failure. Oxid. Med. Cell. Longev. 2020, 2020, 4587024. [Google Scholar] [CrossRef]
- Tayo, B.; Taylor, L.; Sahebkar, F.; Morrison, G. A Phase I, Open-Label, Parallel-Group, Single-Dose Trial of the Pharmacokinetics, Safety, and Tolerability of Cannabidiol in Subjects with Mild to Severe Renal Impairment. Clin. Pharmacokinet. 2020, 59, 747–755. [Google Scholar] [CrossRef]
- Dahlgren, M.K.; Lambros, A.M.; Smith, R.T.; Sagar, K.A.; El-Abboud, C.; Gruber, S.A. Clinical and cognitive improvement following full-spectrum, high-cannabidiol treatment for anxiety: Open-label data from a two-stage, phase 2 clinical trial. Commun. Med. 2022, 2, 139. [Google Scholar] [CrossRef]
- Tang, Y.; Tonkovich, K.L.; Rudisill, T.M. The Effectiveness and Safety of Cannabidiol in Non-seizure-related Indications: A Systematic Review of Published Randomized Clinical Trials. Pharmaceut. Med. 2022, 36, 353–385. [Google Scholar] [CrossRef]
- Dearborn, J.T.; Nelvagal, H.R.; Rensing, N.R.; Takahashi, K.; Hughes, S.M.; Wishart, T.M.; Cooper, J.D.; Wong, M.; Sands, M.S. Effects of chronic cannabidiol in a mouse model of naturally occurring neuroinflammation, neurodegeneration, and spontaneous seizures. Sci. Rep. 2022, 12, 11286. [Google Scholar] [CrossRef] [PubMed]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef]
- Suzuki, A.; Andrade, R.J.; Bjornsson, E.; Lucena, M.I.; Lee, W.M.; Yuen, N.A.; Hunt, C.M.; Freston, J.W. Freston, Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: Unified list based on international collaborative work. Drug Saf. 2010, 33, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; Armento, G.; Spalato Ceruso, M.; Catania, G.; Leakos, M.; Santini, D.; Minotti, G.; Tonini, G. Drug-induced hepatotoxicity in cancer patients—implication for treatment. Expert Opin. Drug Saf. 2016, 15, 1219–1238. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, E.; Blessing, E.; Hinz, B. Non-Psychoactive Phytocannabinoids Inhibit Inflammation-Related Changes of Human Coronary Artery Smooth Muscle and Endothelial Cells. Cells 2023, 12, 2389. [Google Scholar] [CrossRef]
- Frodella, C.M.; Liu, L.; Tan, W.; Pruett, S.B.; Kaplan, B.L. The mechanism by which cannabidiol (CBD) suppresses TNF-alpha secretion involves inappropriate localization of TNF-alpha converting enzyme (TACE). Cell. Immunol. 2024, 397–398, 104812. [Google Scholar] [CrossRef]
- Walls, G.M.; O’Kane, R.; Ghita, M.; Kuburas, R.; McGarry, C.K.; Cole, A.J.; Jain, S.; Butterworth, K.T. Butterworth, Murine models of radiation cardiotoxicity: A systematic review and recommendations for future studies. Radiother. Oncol. 2022, 173, 19–31. [Google Scholar] [CrossRef] [PubMed]


| Antibody (Clone) | Dilution | Company |
|---|---|---|
| CD31-APC (MEC 13.3) | 1:4 | BD Biosciences |
| CD34-FITC (RAM34) | 1:10 | eBioscience (ThermoFisher Scientific) |
| CD45-Vio Blue (REA737) | 1:10 | Miltenyi Biotec |
| CD54 (ICAM-1)-FITC (3E2) | no | BD Biosciences |
| CD61 (Integrin β-3)-FITC (HMβ3-1) | no | BD Biosciences |
| CD102 (ICAM-2)-FITC (3C4 mIC2/4) | 1:10 | BD Biosciences |
| CD105 (Endoglin)-PE MJ7/18) | 1:4 | eBioscience (ThermoFisher Scientific) |
| CD106 (VCAM-1)-PE (M/K-2) | 1:2 | Invitrogen (ThermoFisher Scientific) |
| CD144 (VE-cadherin)-PE (11D4.1) | 1:4 | BD Biosciences |
| CD146 (MCAM)-PE (ME-9F1) | 1:4 | BD Biosciences |
| Antibody (Dilution) | Company |
|---|---|
| β-Actin (1:10,000) | Sigma-Aldrich |
| CD14 | Invitrogen |
| Col3a1 | Santa Cruz Biotechnology |
| Decorin | Abcam |
| Fibronectin | Invitrogen |
| Heme-oxygenase-1 (HO-1) | Cell Signaling Technology |
| Hsp70 | multimmune GmbH |
| γ-H2AX (1:5000) | Abcam |
| HRP-conjugated rabbit anti-mouse Ig (1:2000) | Dako-Agilent |
| HRP-conjugated swine anti-rabbit Ig | BD Biosciences |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, L.; Alkotub, B.; Ballmann, M.; Hachani, K.; Jin, M.; Hasanzadeh Kafshgari, M.; Rammes, G.; Pockley, A.G.; Multhoff, G. Cannabidiol Mediates Beneficial Effects on the Microvasculature of Murine Hearts with Regard to Irradiation-Induced Inflammation and Early Signs of Fibrosis. Radiation 2025, 5, 17. https://doi.org/10.3390/radiation5020017
Bauer L, Alkotub B, Ballmann M, Hachani K, Jin M, Hasanzadeh Kafshgari M, Rammes G, Pockley AG, Multhoff G. Cannabidiol Mediates Beneficial Effects on the Microvasculature of Murine Hearts with Regard to Irradiation-Induced Inflammation and Early Signs of Fibrosis. Radiation. 2025; 5(2):17. https://doi.org/10.3390/radiation5020017
Chicago/Turabian StyleBauer, Lisa, Bayan Alkotub, Markus Ballmann, Khouloud Hachani, Mengyao Jin, Morteza Hasanzadeh Kafshgari, Gerhard Rammes, Alan Graham Pockley, and Gabriele Multhoff. 2025. "Cannabidiol Mediates Beneficial Effects on the Microvasculature of Murine Hearts with Regard to Irradiation-Induced Inflammation and Early Signs of Fibrosis" Radiation 5, no. 2: 17. https://doi.org/10.3390/radiation5020017
APA StyleBauer, L., Alkotub, B., Ballmann, M., Hachani, K., Jin, M., Hasanzadeh Kafshgari, M., Rammes, G., Pockley, A. G., & Multhoff, G. (2025). Cannabidiol Mediates Beneficial Effects on the Microvasculature of Murine Hearts with Regard to Irradiation-Induced Inflammation and Early Signs of Fibrosis. Radiation, 5(2), 17. https://doi.org/10.3390/radiation5020017








