Evaluation of the Characteristics of Short Acquisition Times Using the Clear Adaptive Low-Noise Method and Advanced Intelligent Clear-IQ Engine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Image Reconstruction
2.3. Analyses of Phantom Images
2.4. Analysis of Phantom Images
3. Results
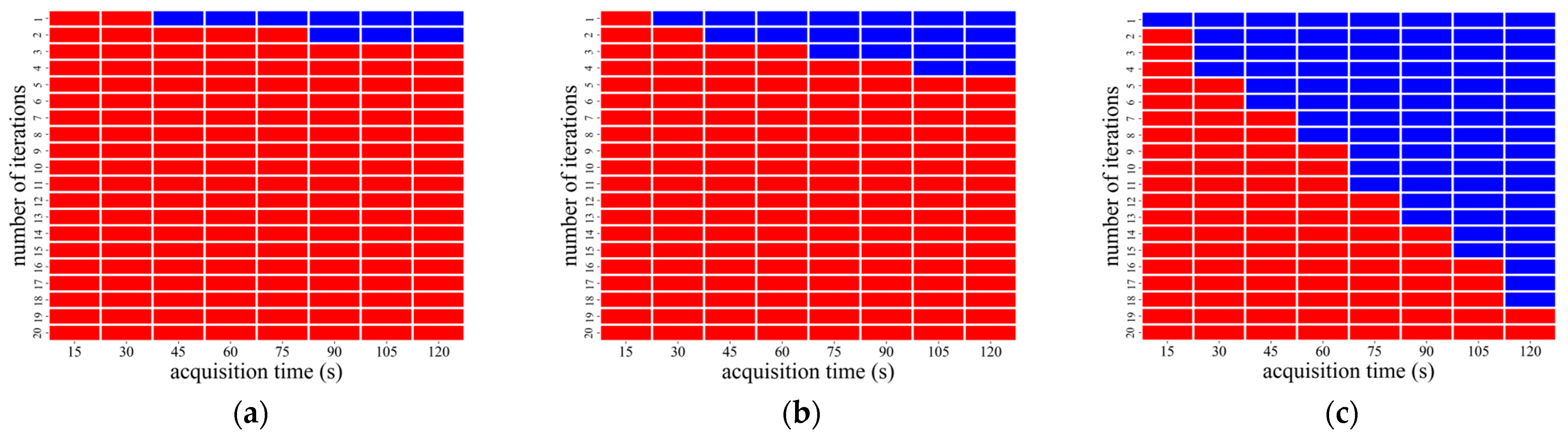
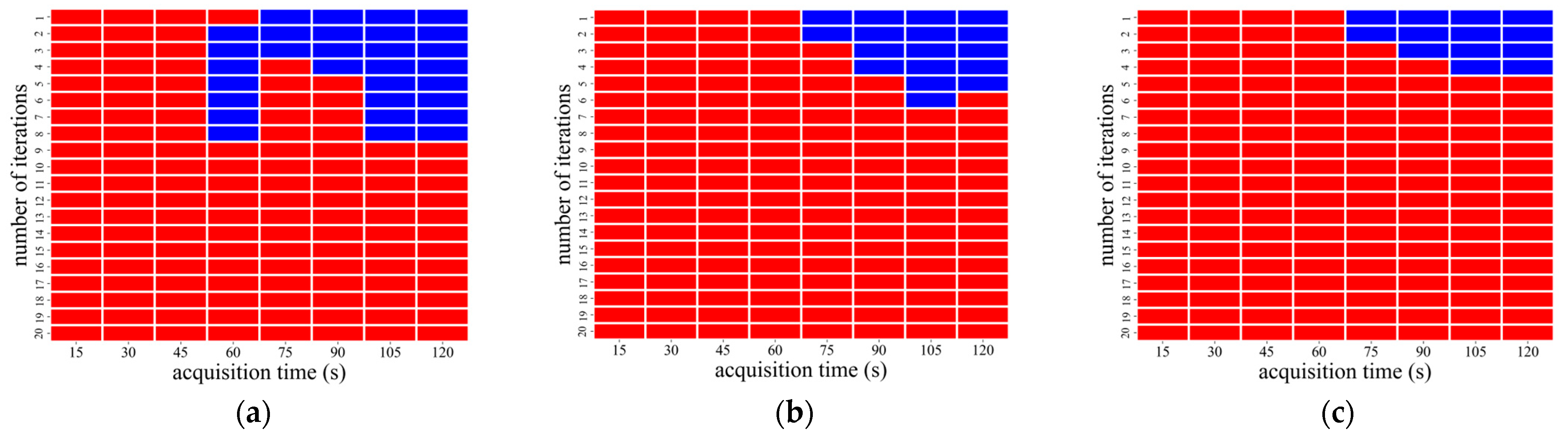
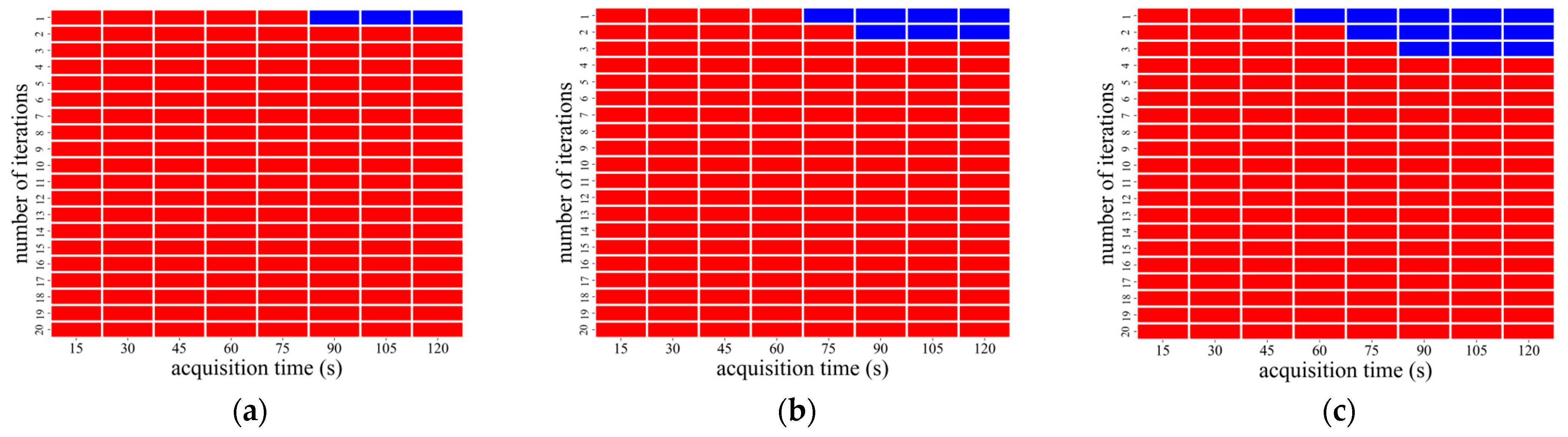
3.1. Image Analysis Using OSEM Reconstruction
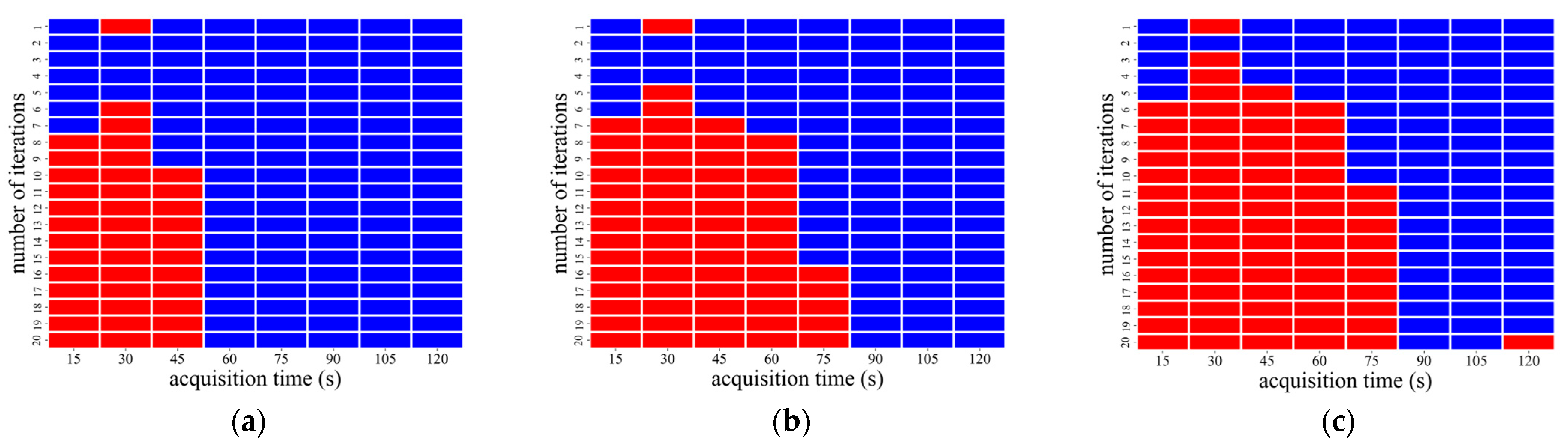
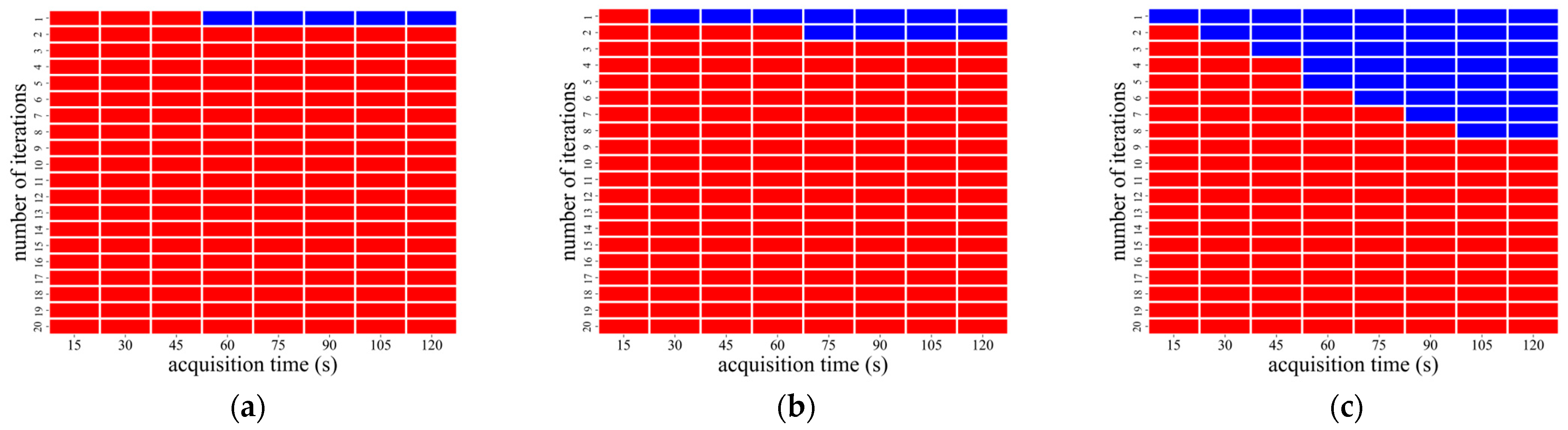
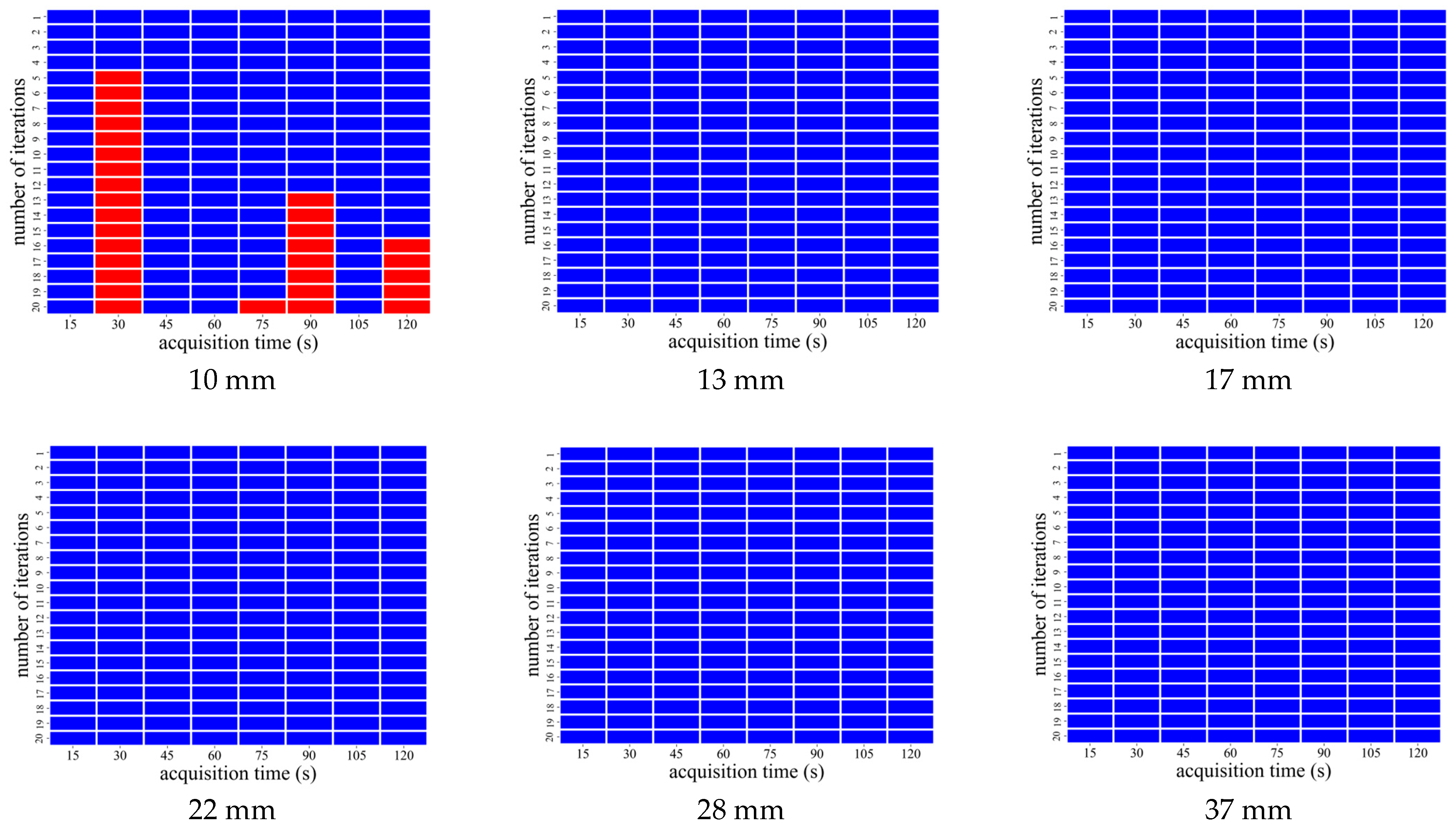
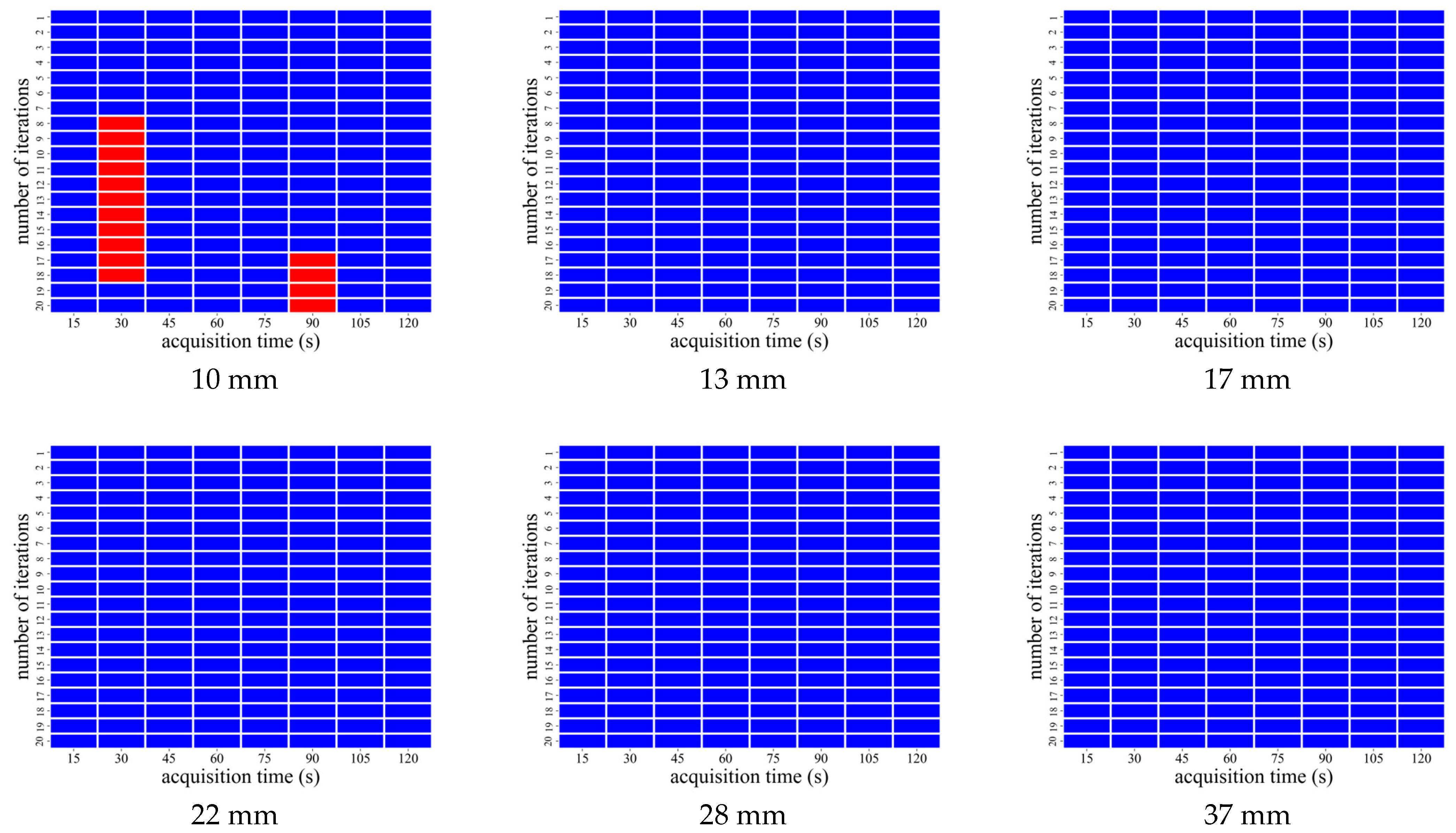
3.2. AiCE Reconstruction
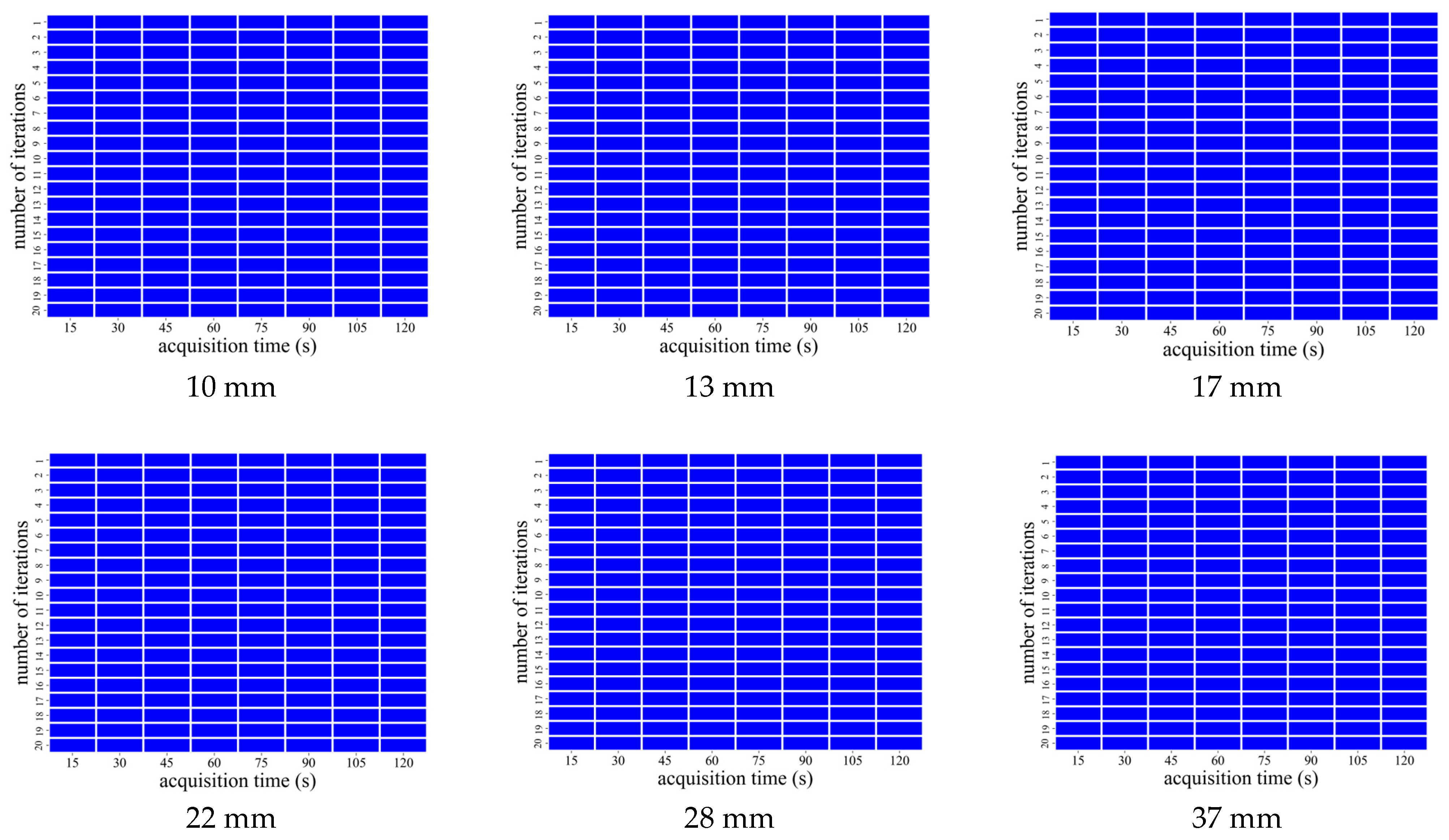
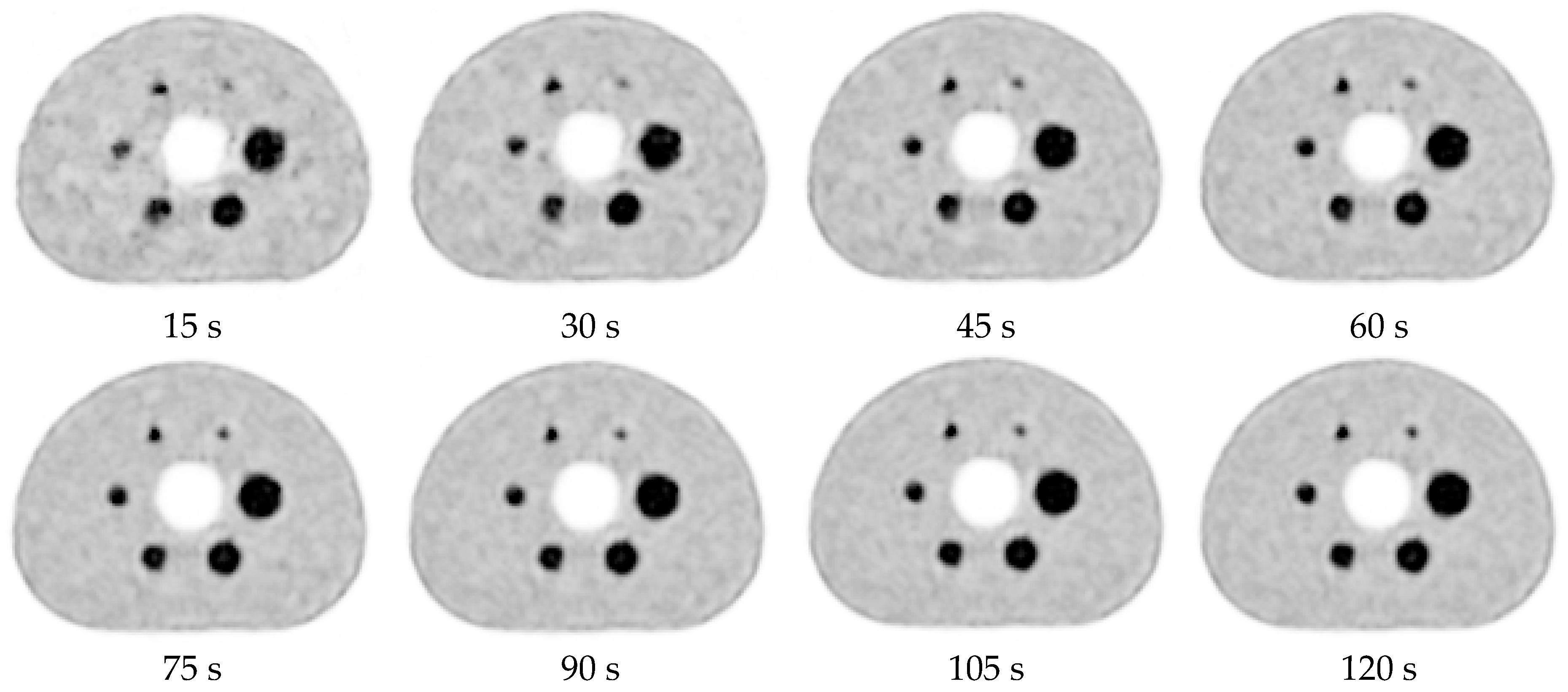
3.3. Visual Evaluation with AiCE Reconstruction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukukita, H.; Suzuki, K.; Matsumoto, K.; Terauchi, T.; Daisaki, H.; Ikari, Y.; Shimada, N.; Senda, M. Japanese guideline for the oncology FDG-PET/CT data acquisition protocol: Synopsis of Version 2.0. Ann. Nucl. Med. 2014, 28, 693–705. [Google Scholar] [CrossRef]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the use of 18F-FDG PET in oncology. J. Nucl. Med. 2008, 49, 480–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, S.; Qi, M.; Chen, W.; Kong, Q.; Zhang, J.; Song, S. Investigation of PET image quality with acquisition time/bed and enhancement of lesion quantification accuracy through deep progressive learning. EJNMMI Phys. 2024, 11, 7. [Google Scholar] [CrossRef]
- Chan, C.; Fulton, R.; Barnett, R.; Feng, D.D.; Meikle, S. Postreconstruction nonlocal means filtering of whole-body PET with an anatomical prior. IEEE. Trans Med. Imaging 2014, 33, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y. Effect of low-dose 18F-FDG on image quality in digital PET/CT. Res. Sq. 2022, 11, 1–19. [Google Scholar] [CrossRef]
- Buades, B.; Coll, J.; Morel, M. A non-local algorithm for image denoising. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05), San Diego, CA, USA, 20–25 June 2005; Volume 2, pp. 60–65. [Google Scholar] [CrossRef]
- Chan, C.; Meikle, S.; Fulton, R.; Tian, G.J.; Cai, W.; Feng, D.D. A non-local post-filtering algorithm for PET incorporating anatomical knowledge. In Proceedings of the IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009; pp. 2728–2732. [Google Scholar] [CrossRef]
- Dutta, J.; Leahy, R.M.; Li, Q. Non-local means denoising of dynamic PET images. PLoS ONE 2013, 8, e81390. [Google Scholar] [CrossRef]
- Inomata, T.; Sato, K.; Ibaraki, M.; Kominami, M.; Kinoshita, F.; Shinohara, Y.; Kato, M.; Kinoshita, T. Evaluation of low-dose whole-body FDG PET with SiPM-based PET/CT scanner: Visual and semi-quantitative analyses using random sampling from full-dose scan data. Jpn. J. Radiol. Technol. 2023, 79, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Yamagiwa, K.; Tsuchiya, J.; Yokoyama, K.; Watanabe, R.; Kimura, K.; Kishino, M.; Tateishi, U. Enhancement of 18F-fluorodeoxyglucose PET image quality by deep-learning-based image reconstruction using advanced intelligent clear-IQ engine in semiconductor-based PET/CT scanners. Diagnostics 2022, 12, 2500. [Google Scholar] [CrossRef]
- Li, M.; Cui, X.; Yue, H.; Ma, C.; Li, K.; Chai, L.; Ge, M.; Li, H.; Ng, Y.L.; Zhou, Y.; et al. The efficacy of short acquisition time using 18F-FDG total-body PET/CT for the identification of pediatric epileptic foci. EJNMMI Res. 2024, 14, 21. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Hu, P.C.; Wu, R.Z.; Gu, Y.S.; Chen, S.G.; Yu, H.J.; Wang, X.Q.; Song, J.; Shi, H.C. The image quality, lesion detectability, and acquisition time of 18F-FDG total-body PET/CT in oncological patients. Eur. J. Nucl. Med. Mol Imaging 2020, 47, 2507–2515. [Google Scholar] [CrossRef]
- Alberts, I.; Sachpekidis, C.; Prenosil, G.; Viscione, M.; Bohn, K.P.; Mingels, C.; Shi, K.; Ashar-Oromieh, A.; Rominger, A. Digital PET/CT allows for shorter acquisition protocols or reduced radiopharmaceutical dose in [18F]-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sonni, I.; Baratto, L.; Park, S.; Hatami, N.; Srinivas, S.; Davidzon, G.; Gambhir, S.S.; Iagaru, A. Initial experience with a SiPM-based PET/CT scanner: Influence of acquisition time on image quality. EJNMMI Phys. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Pilz, J.; Hehenwarter, L.; Zimmermann, G.; Rendl, G.; Schweighofer-Zwink, G.; Beheshti, M.; Pirich, C. Feasibility of equivalent performance of 3D TOF [18F]-FDG PET/CT with reduced acquisition time using clinical and semiquantitative parameters. EJNMMI Res. 2021, 11, 44. [Google Scholar] [CrossRef]
- Fragoso Costa, P.; Jentzen, W.; Brahmer, A.; Mavroeidi, I.A.; Zarrad, F.; Umutlu, L.; Fendler, W.P.; Rischpler, C.; Herrmann, K.; Conti, M.; et al. Phantom-based acquisition time and image reconstruction parameter optimisation for oncologic FDG PET/CT examinations using a digital system. BMC Cancer 2022, 22, 899. [Google Scholar] [CrossRef]
- Alqahtani, M.M.; Willowson, K.P.; Constable, C.; Fulton, R.; Kench, P.L. Optimization of 99mTc whole-body SPECT/CT image quality: A phantom study. J. Appl Clin. Med Phys. 2022, 23, e13528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Q.; Li, C.; Song, Y.; Zhang, Y.; Chen, J.C. Optimization of spatial resolution and image reconstruction parameters for the small-animal Metis™ PET/CT system. Electronics 2022, 11, 1542. [Google Scholar] [CrossRef]
- Emura, R. [[PET] 6. Overview of clinical applications of artificial intelligence in PET]. Jpn. J. Radiol. Technol. 2023, 79, 595–606. [Google Scholar] [CrossRef]
- Shehata, M.A.; Saad, A.M.; Kamel, S.; Stanietzky, N.; Roman-Colon, A.M.; Morani, A.C.; Elsayes, K.M.; Jensen, C.T. Deep-learning CT reconstruction in clinical scans of the abdomen: A systematic review and meta-analysis. Abdom. Radiol. 2023, 48, 2724–2756. [Google Scholar] [CrossRef]
- Mori, M.; Fujioka, T.; Hara, M.; Katsuta, L.; Yashima, Y.; Yamaga, E.; Yamagiwa, K.; Tsuchiya, J.; Hayashi, K.; Kumaki, Y.; et al. Deep Learning-Based Image Quality Improvement in Digital Positron Emission Tomography for Breast Cancer. Diagnostics 2023, 13, 794. [Google Scholar] [CrossRef]
- Tsuchiya, J.; Yokoyama, K.; Yamagiwa, K.; Watanabe, R.; Kimura, K.; Kishino, M.; Chan, C.; Asma, E.; Tateishi, U. Deep learning-based image quality improvement of 18F-fluorodeoxyglucose positron emission tomography: A retrospective observational study. EJNMMI Phys. 2021, 8, 31. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; Sinaasappel, M.; Dekker, M.C.; Stokkel, M.P.M.; de Wit-van der Veen, B.J. 177 Lutetium SPECT/CT: Evaluation of collimator, photopeak and scatter correction. J. Appl. Clin. Med. Phys. 2020, 21, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, D.; Dinter, D.J.; Sadick, M.; Brade, J.; Schoenberg, O.S.; Büsing, K. The impact of acquisition time on image quality in whole-body 18F-FDG PET/CT for cancer staging. J. Nucl. Med. Technol. 2012, 40, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Akamatsu, G.; Kasahara, Y.; Mitsumoto, K.; Baba, S.; Tsutsui, Y.; Himuro, K.; Mikasa, S.; Kidera, D.; Sasaki, M. Improvement in PET/CT image quality in overweight patients with PSF and TOF. Ann. Nucl. Med. 2015, 29, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, G.; Ishikawa, K.; Mitsumoto, K.; Taniguchi, T.; Ohya, N.; Baba, S.; Abe, K.; Sasaki, M. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J. Nucl. Med. 2012, 53, 1716–1722. [Google Scholar] [CrossRef]
- Rahmim, A.; Qi, J.; Sossi, V. Resolution modeling in PET imaging: Theory, practice, benefits, and pitfalls. Med. Phys. 2013, 40, 064301. [Google Scholar] [CrossRef]
- Nakamura, A.; Tanizaki, Y.; Takeuchi, M.; Ito, S.; Sano, Y.; Sato, M.; Kanno, T.; Okada, H.; Torizuka, T.; Nishizawa, S. Impact of point spread function correction in standardized uptake value quantitation for positron emission tomography images: A study based on phantom experiments and clinical images. Jpn. J. Radiol. Technol. 2014, 70, 542–548. [Google Scholar] [CrossRef]
- Weyts, K.; Lasnon, C.; Ciappuccini, R.; Lequesne, J.; Corroyer-Dulmont, A.; Quak, E.; Clarisse, B.; Roussel, L.; Bardet, S.; Jaudet, C. Artificial intelligence-based PET denoising could allow a two-fold reduction in [18F]FDG PET acquisition time in digital PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3750–3760. [Google Scholar] [CrossRef]
- Trägårdh, E.; Minarik, D.; Almquist, H.; Bitzén, U.; Garpered, S.; Hvittfelt, E.; Olsson, B.; Oddstig, J. Impact of acquisition time and penalizing factor in a block-sequential regularized expectation maximization reconstruction algorithm on a Si-photomultiplier-based PET-CT system for 18F-FDG. EJNMMI Res. 2019, 9, 64. [Google Scholar] [CrossRef]






| Scintillator | LYSO |
|---|---|
| Size of scintillator | 4.1 × 4.1 × 20 mm |
| Detector | SiPM |
| Axial FOV | 270 mm |
| Diameter of the detector ring | 825.4 mm |
| CaLM | Acquisition Time (s) | Iteration Number |
|---|---|---|
| Standard | 75–120 | 1 |
| Strong | 75 | 1–2 |
| 90–120 | 1–3 |
| CaLM | Acquisition Time (s) | Iteration Number |
|---|---|---|
| Mild | 60–120 | 1 |
| Standard | 45 and 60 | 1 |
| 75–120 | 1 and 2 | |
| Strong | 15 and 45 | 1 |
| 60 | 1–5 | |
| 75 | 1–6 | |
| 90 | 1–7 | |
| 105 and 120 | 1–8 |
| Acquisition Time (s) | Iteration Number | CV < 10.0 | RC ≥ 38 | N10mm < 5.6 | QH10mm/N10mm > 2.8 | |
|---|---|---|---|---|---|---|
| AiCE | 15 | - | 11.7 | 31.5 | 7.7 | 1.9 |
| 30 | - | 9.6 | 32.6 | 6.7 | 2.0 | |
| 45 | - | 8.8 | 38.5 | 6.1 | 2.9 | |
| 60 | - | 8.6 | 41.8 | 6.0 | 3.6 | |
| 75 | - | 7.9 | 50.7 | 5.8 | 4.8 | |
| 90 | - | 7.9 | 54.9 | 5.9 | 5.2 | |
| 105 | - | 7.5 | 57.2 | 5.6 | 5.7 | |
| 120 | - | 7.4 | 56.9 | 5.7 | 5.7 |
| Average Score (5-Point Scale) | |||
|---|---|---|---|
| Acquisition Time (s) | 10 mm Sphere Visualization | Overall Image Quality | Image Noise |
| 15 | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.33 ± 0.47 |
| 30 | 2.00 ± 0.00 | 2.67 ± 0.47 | 2.00 ± 0.00 |
| 45 | 3.33 ± 0.47 | 3.00 ± 0.82 | 2.67 ± 0.47 |
| 60 | 3.33 ± 0.47 | 3.67 ± 0.47 | 3.00 ± 0.82 |
| 75 | 4.00 ± 0.82 | 4.00 ± 0.82 | 3.67 ± 0.47 |
| 90 | 3.67 ± 0.47 | 4.33 ± 0.47 | 4.33 ± 0.47 |
| 105 | 4.00 ± 0.82 | 4.33 ± 0.47 | 4.67 ± 0.47 |
| 120 | 4.67 ± 0.47 | 4.33 ± 0.94 | 4.67 ± 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogasawara, R.; Irikawa, A.; Watanabe, Y.; Harada, T.; Hosokawa, S.; Koyama, K.; Tsuda, K.; Kimura, T.; Okuda, K.; Takahashi, Y. Evaluation of the Characteristics of Short Acquisition Times Using the Clear Adaptive Low-Noise Method and Advanced Intelligent Clear-IQ Engine. Radiation 2025, 5, 18. https://doi.org/10.3390/radiation5020018
Ogasawara R, Irikawa A, Watanabe Y, Harada T, Hosokawa S, Koyama K, Tsuda K, Kimura T, Okuda K, Takahashi Y. Evaluation of the Characteristics of Short Acquisition Times Using the Clear Adaptive Low-Noise Method and Advanced Intelligent Clear-IQ Engine. Radiation. 2025; 5(2):18. https://doi.org/10.3390/radiation5020018
Chicago/Turabian StyleOgasawara, Ryosuke, Akiko Irikawa, Yuya Watanabe, Tomoya Harada, Shota Hosokawa, Kazuya Koyama, Keisuke Tsuda, Toru Kimura, Koichi Okuda, and Yasuyuki Takahashi. 2025. "Evaluation of the Characteristics of Short Acquisition Times Using the Clear Adaptive Low-Noise Method and Advanced Intelligent Clear-IQ Engine" Radiation 5, no. 2: 18. https://doi.org/10.3390/radiation5020018
APA StyleOgasawara, R., Irikawa, A., Watanabe, Y., Harada, T., Hosokawa, S., Koyama, K., Tsuda, K., Kimura, T., Okuda, K., & Takahashi, Y. (2025). Evaluation of the Characteristics of Short Acquisition Times Using the Clear Adaptive Low-Noise Method and Advanced Intelligent Clear-IQ Engine. Radiation, 5(2), 18. https://doi.org/10.3390/radiation5020018






