An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems
Abstract
1. Introduction
2. Properties of Frequently Found Glucocorticoids in Water
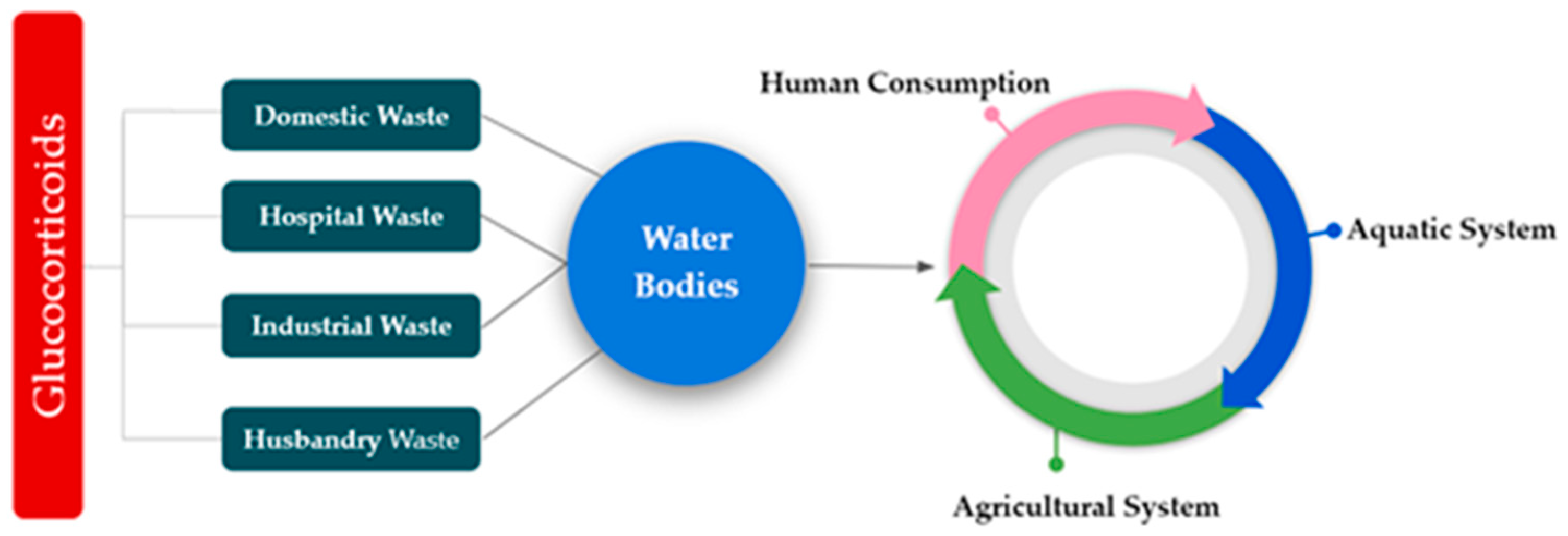
3. Glucocorticoids’ Sources and Pathways in Environment
4. Adverse Effects of Glucocorticoids’ in Natural Environment
5. Glucocorticoids Compounds Removal Methods Efficiency
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sossalla, N.A.; Nivala, J.; Escher, B.I.; Reemtsma, T.; Schlichting, R.; van Afferden, M.; Müller, R.A. Resilience of Micropollutant and Biological Effect Removal in an Aerated Horizontal Flow Treatment Wetland. Water 2020, 12, 3050. [Google Scholar]
- Aborkhees, G.; Raina-Fulton, R.; Thirunavokkarasu, O. Determination of Endocrine Disrupting Chemicals in Water and Wastewater Samples by Liquid Chromatography-Negative Ion Electrospray Ionization-Tandem Mass Spectrometry. Molecules 2020, 25, 3906. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Shen, X.; Zhang, A.; Yan, S.; Li, X.; Miruka, A.C.; Wu, S.; Guo, Y.; Ognier, S. Degradation of glucocorticoids in aqueous solution by dielectric barrier discharge: Kinetics, mechanisms, and degradation pathways. Chem. Eng. J. 2019, 374, 412–428. [Google Scholar] [CrossRef]
- Shah, S.M.; Wahba, M.; Yu, L.; Achari, G.; Habibi, H.R. Health Impact Assessment of Sulfolane on Embryonic Development of Zebrafish (Danio rerio). Toxics 2019, 7, 42. [Google Scholar] [CrossRef]
- EPA, U. Endocrine Disruptor Screening Program Weight-of-Evidence: Evaluating Results of EDSP Tier 1 Screening to Identify the Need for Tier 2 Testing; Office of Chemical Safety and Pollution Prevention: Washington, WA, USA, 2011. [Google Scholar]
- Andersson, A.-M.; Skakkebaek, N.E. Exposure to exogenous estrogens in food: Possible impact on human development and health. Eur. J. Endocrinol. 1999, 140, 477–485. [Google Scholar] [CrossRef]
- Plotan, M.; Frizzell, C.; Robinson, V.; Elliott, C.T.; Connolly, L. Endocrine disruptor activity in bottled mineral and flavoured water. Food Chem. 2013, 136, 1590–1596. [Google Scholar] [CrossRef]
- Brotons, J.A.; Olea-Serrano, M.F.; Villalobos, M.; Pedraza, V.; Olea, N. Xenoestrogens Released from Lacquer Coatings in Food Cans. Environ. Health Perspect. 1995, 103, 608–612. [Google Scholar]
- Fisher, J.S. Environmental anti-androgens and male reproductive health: Focus on phthalates and testicular dysgenesis syndrome. Reproduction 2004, 127, 305–315. [Google Scholar] [CrossRef]
- Bourguignon, J.-P.; Parent, A.-S. Early homeostatic disturbances of human growth and maturation by endocrine disrupters. Curr. Opin. Pediatr. 2010, 22, 470–477. [Google Scholar] [CrossRef]
- Zama, A.M.; Uzumcu, M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: An ovarian perspective. Front. Neuroendocr. 2010, 31, 420–439. [Google Scholar] [CrossRef]
- Hryniewicka, M.; Starczewska, B.; Gołębiewska, A. Determination of Budesonide and Sulfasalazine in Water and Wastewater Samples Using DLLME-SFO-HPLC-UV Method. Water 2019, 11, 1581. [Google Scholar] [CrossRef]
- Allijn, I.E.; Oldenkamp, R.; Storm, G.; Ragas, A.M.J.; Schiffelers, R.M. Environmental impact of switching from the synthetic glucocorticoid prednisolone to the natural alkaloid berberine. PLoS ONE 2018, 13, e0199095. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. Effective Strategies for Monitoring and Regulating Chemical Mixtures and Contaminants Sharing Pathways of Toxicity. Int. J. Environ. Res. Public Health 2015, 12, 10549–10557. [Google Scholar] [CrossRef]
- Paragliola, R.M.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Treatment with Synthetic Glucocorticoids and the Hypothalamus-Pituitary-Adrenal Axis. Int. J. Mol. Sci. 2017, 18, 2201. [Google Scholar] [CrossRef]
- Iglesias, A.; Nebot, C.; Vázquez, B.I.; Coronel-Olivares, C.; Abuín, C.M.F.; Cepeda, A. Monitoring the Presence of 13 Active Compounds in Surface Water Collected from Rural Areas in Northwestern Spain. Int. J. Environ. Res. Public Health 2014, 11, 5251–5272. [Google Scholar] [CrossRef]
- Yazdan, M.M.S.; Ahad, T.; Jahan, I.; Mazumder, M. Review on the Evaluation of the Impacts of Wastewater Disposal in Hydraulic Fracturing Industry in the United States. Technologies 2020, 8, 67. [Google Scholar] [CrossRef]
- Yazdan, M.M.S.; Rahaman, A.Z.; Noor, F.; Duti, B.M. Establishment of Co-Relation between Remote Sensing Based Trmm Data and Ground Based Precipitation Data in North-East Region of Bangladesh. In Proceedings of the 2nd International Conference on Civil Engineering for Sustainable Development (ICCESD-2014), KUET, Khulna, Bangladesh, 17–19 April 2014; pp. 14–16. [Google Scholar]
- Lecomte, S.; Habauzit, D.; Charlier, T.D.; Pakdel, F. Emerging Estrogenic Pollutants in the Aquatic Environment and Breast Cancer. Genes 2017, 8, 229. [Google Scholar] [CrossRef]
- Hasona, N.A.; AlRashidi, A.A.; Aldugieman, T.Z.; Alshdokhi, A.M.; Ahmed, M.Q. Vitis vinifera Extract Ameliorate Hepatic and Renal Dysfunction Induced by Dexamethasone in Albino Rats. Toxics 2017, 5, 11. [Google Scholar] [CrossRef]
- Øverli, Ø.; Kotziana, S.; Winberg, S. Effects of Cortisol on Aggression and Locomotor Activity in Rainbow Trout. Horm. Behav. 2002, 42, 53–61. [Google Scholar] [CrossRef]
- Larsson, D.J.; Hällman, H.; Förlin, L. More Male Fish Embryos near a Pulp Mill. Environ. Toxicol. Chem. Int. J. 2000, 19, 2911–2917. [Google Scholar]
- Liu, S.; Ying, G.-G.; Zhao, J.-L.; Zhou, L.-J.; Yang, B.; Chen, Z.-F.; Lai, H.-J. Occurrence and fate of androgens, estrogens, glucocorticoids and progestagens in two different types of municipal wastewater treatment plants. J. Environ. Monit. 2012, 14, 482–491. [Google Scholar] [CrossRef]
- Runnalls, T.J.; Margiotta-Casaluci, L.; Kugathas, S.; Sumpter, J.P. Pharmaceuticals in the Aquatic Environment: Steroids and Anti-Steroids as High Priorities for Research. Hum. Ecol. Risk Assess. Int. J. 2010, 16, 1318–1338. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Huetos, O.; Ramos, M.; De Pozuelo, M.M.; Reuvers, T.B.A.; Andrés, M.S. Determination of dexamethasone in feed by TLC and HPLC. Analyst 1999, 124, 1583–1587. [Google Scholar] [CrossRef]
- Morren, M.A.; Dooms-Goossens, A. Contact allergy to corticosteroids. Diagnosis and management. Clin. Rev. Allergy Immunol. 1996, 14, 199–208. [Google Scholar] [CrossRef]
- Jacob, S.E.; Steele, T. Corticosteroid classes: A quick reference guide including patch test substances and cross-reactivity. J. Am. Acad. Dermatol. 2006, 54, 723–727. [Google Scholar] [CrossRef]
- Torres, M.J.; Canto, G. Hypersensitivity reactions to corticosteroids. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 273–279. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhou, G.-J.; Liu, S.-S.; Yue, W.-Z.; Yu, S.; Sun, K.-F.; Cheng, H.; Ying, G.-G.; Xu, X.-R. Occurrence, source analysis and risk assessment of androgens, glucocorticoids and progestagens in the Hailing Bay region, South China Sea. Sci. Total. Environ. 2015, 536, 99–107. [Google Scholar] [CrossRef]
- Arnon, S.; Dahan, O.; Elhanany, S.; Cohen, K.; Pankratov, I.; Gross, A.; Ronen, Z.; Baram, S.; Shore, L.S. Transport of Testosterone and Estrogen from Dairy-Farm Waste Lagoons to Groundwater. Environ. Sci. Technol. 2008, 42, 5521–5526. [Google Scholar] [CrossRef]
- Scarth, J.; Akre, C. Presence and Metabolism of Endogenous Steroid Hormones in Meat-producing Animals; Royal Society of Chemistry (RSC): London, UK, 2009; pp. 48–96. [Google Scholar]
- Liu, S.; Ying, G.-G.; Zhou, L.-J.; Zhang, R.-Q.; Chen, Z.-F.; Lai, H.-J. Steroids in a typical swine farm and their release into the environment. Water Res. 2012, 46, 3754–3768. [Google Scholar] [CrossRef]
- Kolodziej, E.; Gray, J.L.; Sedlak, D.L. Quantification of Steroid Hormones with Pheromonal Properties in Municipal Wastewater Effluent. Environ. Toxicol. Chem. 2003, 22, 2622–2629. [Google Scholar] [CrossRef]
- Orlando, E.F.; Kolok, A.S.; Binzcik, G.A.; Gates, J.L.; Horton, M.K.; Lambright, C.S.; Gray, L.E., Jr.; Soto, A.M.; Guillette, L.J., Jr. Endocrine-Disrupting Effects of Cattle Feedlot Effluent on an Aquatic Sentinel Species, the Fathead Minnow. Environ. Health Perspect. 2004, 112, 353–358. [Google Scholar]
- Chang, H.; Hu, J.; Shao, B. Occurrence of Natural and Synthetic Glucocorticoids in Sewage Treatment Plants and Receiving River Waters. Environ. Sci. Technol. 2007, 41, 3462–3468. [Google Scholar] [CrossRef]
- Piram, A.; Salvador, A.; Gauvrit, J.-Y.; Lantéri, P.; Faure, R. Development and optimisation of a single extraction procedure for the LC/MS/MS analysis of two pharmaceutical classes residues in sewage treatment plant. Talanta 2008, 74, 1463–1475. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Hu, J. Determination and Source Apportionment of Five Classes of Steroid Hormones in Urban Rivers. Environ. Sci. Technol. 2009, 43, 7691–7698. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, S.; Chang, H.; Hu, J. Behaviors of Glucocorticoids, Androgens and Progestogens in a Municipal Sewage Treatment Plant: Comparison to Estrogens. Environ. Sci. Technol. 2011, 45, 2725–2733. [Google Scholar] [CrossRef]
- Ammann, A.A.; Macikova, P.; Groh, K.J.; Schirmer, K.; Suter, M.J. LC-MS/MS Determination of Potential Endocrine Disruptors of Cortico Signalling in Rivers and Wastewaters. Anal. Bioanal. Chem. 2014, 406, 7653–7665. [Google Scholar]
- Isobe, T.; Sato, K.; Joon-Woo, K.; Tanabe, S.; Suzuki, G.; Nakayama, K. Determination of natural and synthetic glucocorticoids in effluent of sewage treatment plants using ultrahigh performance liquid chromatography-tandem mass spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 14127–14135. [Google Scholar] [CrossRef]
- Weizel, A.; Schluesener, M.P.; Dierkes, G.; Ternes, T.A. Occurrence of Glucocorticoids, Mineralocorticoids, and Progestogens in Various Treated Wastewater, Rivers, and Streams. Environ. Sci. Technol. 2018, 52, 5296–5307. [Google Scholar] [CrossRef]
- Stavreva, D.A.; George, A.; Klausmeyer, P.; Varticovski, L.; Sack, D.A.; Voss, T.C.; Schiltz, R.L.; Blazer, V.S.; Iwanowicz, L.R.; Hager, G.L. Prevalent Glucocorticoid and Androgen Activity in US Water Sources. Sci. Rep. 2012, 2, 1–8. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Verebey, Z.; Sharma, V.; Kovacsics, L.; Fekete, J. Simultaneous determination of corticosteroids, androgens, and progesterone in river water by liquid chromatography–tandem mass spectrometry. Chemosphere 2010, 78, 972–979. [Google Scholar] [CrossRef]
- Macikova, P.; Groh, K.; Ammann, A.A.; Schirmer, K.; Suter, M.J.-F. Endocrine Disrupting Compounds Affecting Corticosteroid Signaling Pathways in Czech and Swiss Waters: Potential Impact on Fish. Environ. Sci. Technol. 2014, 48, 12902–12911. [Google Scholar] [CrossRef]
- Singh, N.; Rieder, M.J.; Tucker, M.J. Mechanisms of Glucocorticoid-Mediated Anti-Inflammatory and Immunosuppressive Action. Paed Perinat. Drug Ther. 2004, 6, 107–115. [Google Scholar]
- Newton, R.; Leigh, R.; Giembycz, M.A. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacol. Ther. 2010, 125, 286–327. [Google Scholar] [CrossRef]
- Croxtall, J.D.; Van Hal, P.T.W.; Choudhury, Q.; Gilroy, D.W.; Flower, R.J. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br. J. Pharmacol. 2002, 135, 511–519. [Google Scholar] [CrossRef]
- Smoak, K.A.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech. Ageing Dev. 2004, 125, 697–706. [Google Scholar] [CrossRef]
- Coopman, S.; Degreef, H.; Dooms-Goossens, A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br. J. Dermatol. 1989, 121, 27–34. [Google Scholar] [CrossRef]
- Galon, J.; Franchimont, D.; Hiroi, N.; Frey, G.; Boettner, A.; Ehrhart-Bornstein, M.; O’Shea, J.J.; Chrousos, G.P.; Bornstein, S.R. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002, 16, 61–71. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Talaber, G.; Jondal, M.; Okret, S. Extra-adrenal glucocorticoid synthesis: Immune regulation and aspects on local organ homeostasis. Mol. Cell. Endocrinol. 2013, 380, 89–98. [Google Scholar] [CrossRef]
- Bornstein, S.; Ziegler, C.; Krug, A.; Kanczkowski, W.; Rettori, V.; McCann, S.; Wirth, M.; Zacharowski, K. The Role of Toll-like Receptors in the Immune-Adrenal Crosstalk. Ann. N. Y. Acad. Sci. 2006, 1088, 307–318. [Google Scholar] [CrossRef]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef]
- Pemberton, P.A.; Stein, P.E.; Pepys, M.B.; Potter, J.M.; Carrell, R.W. Hormone binding globulins undergo serpin conformational change in inflammation. Nat. Cell Biol. 1988, 336, 257–258. [Google Scholar] [CrossRef]
- Woodward, M.J.; De Boer, J.; Heidorn, S.; Hubank, M.; Kioussis, D.; Williams, O.; Brady, H.J.M. Tnfaip8 is an essential gene for the regulation of glucocorticoid-mediated apoptosis of thymocytes. Cell Death Differ. 2009, 17, 316–323. [Google Scholar] [CrossRef]
- Patrick, G. History of Cortisone and Related Compounds. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure:function relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J. Cellular Processing of the Glucocorticoid Receptor Gene and Protein: New Mechanisms for Generating Tissue-specific Actions of Glucocorticoids. J. Biol. Chem. 2011, 286, 3177–3184. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Libert, C. On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic 2011, 13, 364–374. [Google Scholar] [CrossRef]
- Luisi, B.F.; Xu, W.X.; Otwinowski, Z.; Freedman, L.P.; Yamamoto, K.R.; Sigler, P.B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nat. Cell Biol. 1991, 352, 497–505. [Google Scholar] [CrossRef]
- Ratman, D.; Berghe, W.V.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef]
- Biddie, S.; John, S.; Sabo, P.J.; Thurman, R.E.; Johnson, T.A.; Schiltz, R.L.; Miranda, T.B.; Sung, M.-H.; Trump, S.; Lightman, S.; et al. Transcription Factor AP1 Potentiates Chromatin Accessibility and Glucocorticoid Receptor Binding. Mol. Cell 2011, 43, 145–155. [Google Scholar] [CrossRef]
- Tuckermann, J.P.; Reichardt, H.M.; Arribas, R.; Richter, K.H.; Schütz, G.; Angel, P. The DNA Binding-Independent Function of the Glucocorticoid Receptor Mediates Repression of Ap-1–Dependent Genes in Skin. J. Cell Biol. 1999, 147, 1365–1370. [Google Scholar] [CrossRef]
- Belfroid, A.; Van der Horst, A.; Vethaak, A.; Schäfer, A.; Rijs, G.; Wegener, J.; Cofino, W. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci. Total Environ. 1999, 225, 101–108. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Ying, G.-G.; Kookana, R.; Ru, Y.-J. Occurrence and fate of hormone steroids in the environment. Environ. Int. 2002, 28, 545–551. [Google Scholar] [CrossRef]
- Thomas, K.V.; Hurst, M.R.; Matthiessen, P.; McHugh, M.; Smith, A.; Waldock, M.J. An Assessment of in Vitro Androgenic Activity and the Identification of Environmental Androgens in United Kingdom Estuaries. Environ. Toxicol. Chem. Int. J. 2002, 21, 1456–1461. [Google Scholar]
- Peck, M.; Gibson, R.W.; Kortenkamp, A.; Hill, E.M. Sediments Are Major Sinks of Steroidal Estrogens in Two United Kingdom Rivers. Environ. Toxicol. Chem. 2004, 23, 945–952. [Google Scholar] [CrossRef]
- Yu, Z.; Xiao, B.; Huang, W.; Peng, P. Sorption of Steroid Estrogens to Soils and Sediments. Environ. Toxicol. Chem. 2004, 23, 531–539. [Google Scholar] [CrossRef]
- Labadie, P.; Budzinski, H. Determination of Steroidal Hormone Profiles along the Jalle d’Eysines River (near Bordeaux, France). Environ. Sci. Technol. 2005, 39, 5113–5120. [Google Scholar] [CrossRef]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Holsboer, F.; Barden, N. Antidepressants and Hypothalamic-Pituitary-Adrenocortical Regulation. Endocr. Rev. 1996, 17, 187–205. [Google Scholar]
- Halpenny, C.M.; Kocan, R.M.; Winton, J.R.; Perry, J.A.; Hershberger, P.K. Elevated Temperature Exacerbates Ichthyophonus Infections in Buffalo Sculpin. Fish Health Newsl. 2002, 17–20. [Google Scholar]
- Gilmour, K.M.; Dibattista, J.D.; Thomas, J.B. Physiological Causes and Consequences of Social Status in Salmonid Fish. Integr. Comp. Biol. 2005, 45, 263–273. [Google Scholar] [CrossRef]
- Frerichs, V.A.; Tornatore, K.M. Determination of the glucocorticoids prednisone, prednisolone, dexamethasone, and cortisol in human serum using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2004, 802, 329–338. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.; Lester, J.N.; Cartmell, E. Assessing potential modifications to the activated sludge process to improve simultaneous removal of a diverse range of micropollutants. Water Res. 2014, 62, 180–192. [Google Scholar] [CrossRef]
- Maeng, S.K.; Choi, B.G.; Lee, K.T.; Song, K.G. Influences of solid retention time, nitrification and microbial activity on the attenuation of pharmaceuticals and estrogens in membrane bioreactors. Water Res. 2013, 47, 3151–3162. [Google Scholar] [CrossRef]
- Gulde, R.; Helbling, D.E.; Scheidegger, A.; Fenner, K. PH-Dependent Biotransformation of Ionizable Organic Micropollutants in Activated Sludge. Environ. Sci. Technol. 2014, 48, 13760–13768. [Google Scholar]
- Sathyamoorthy, S.; Chandran, K.; Ramsburg, A. Biodegradation and Cometabolic Modeling of Selected Beta Blockers during Ammonia Oxidation. Environ. Sci. Technol. 2013, 47, 12835–12843. [Google Scholar] [CrossRef]
- Helbling, D.E.; Johnson, D.R.; Honti, M.; Fenner, K. Micropollutant Biotransformation Kinetics Associate with WWTP Process Parameters and Microbial Community Characteristics. Environ. Sci. Technol. 2012, 46, 10579–10588. [Google Scholar]
- Xue, W.; Wu, C.; Xiao, K.; Huang, X.; Zhou, H.; Tsuno, H.; Tanaka, H. Elimination and fate of selected micro-organic pollutants in a full-scale anaerobic/anoxic/aerobic process combined with membrane bioreactor for municipal wastewater reclamation. Water Res. 2010, 44, 5999–6010. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of Pharmaceutical and Personal Care Products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef]
- Zupanc, M.; Kosjek, T.; Petkovšek, M.; Dular, M.; Kompare, B.; Širok, B.; Blažeka, Ž.; Heath, E. Removal of pharmaceuticals from wastewater by biological processes, hydrodynamic cavitation and UV treatment. Ultrason. Sonochem. 2013, 20, 1104–1112. [Google Scholar] [CrossRef]
- Majewsky, M.; Gallé, T.; Zwank, L.; Fischer, K. Influence of microbial activity on polar xenobiotic degradation in activated sludge systems. Water Sci. Technol. 2010, 62, 701–707. [Google Scholar] [CrossRef][Green Version]
- Falås, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.A.; Joss, A. Tracing the limits of organic micropollutant removal in biological wastewater treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef]
- Irwin, L.K.; Gray, S.; Oberdörster, E. Vitellogenin induction in painted turtle, Chrysemys picta, as a biomarker of exposure to environmental levels of estradiol. Aquat. Toxicol. 2001, 55, 49–60. [Google Scholar] [CrossRef]
- Andrási, N.; Molnár, B.; Dobos, B.; Vasanits-Zsigrai, A.; Záray, G.; Molnár-Perl, I. Determination of steroids in the dissolved and in the suspended phases of wastewater and Danube River samples by gas chromatography, tandem mass spectrometry. Talanta 2013, 115, 367–373. [Google Scholar] [CrossRef]
- Aufartová, J.; Mahugo-Santana, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Nováková, L.; Solich, P. Determination of steroid hormones in biological and environmental samples using green microextraction techniques: An overview. Anal. Chim. Acta 2011, 704, 33–46. [Google Scholar] [CrossRef]
- Caldas, S.; Arias, J.; Rombaldi, C.; Mello, L.; Cerqueira, M.B.R.; Martins, A.; Primel, E. Occurrence of Pesticides and PPCPs in Surface and Drinking Water in Southern Brazil: Data on 4-Year Monitoring. J. Braz. Chem. Soc. 2018, 30, 71–80. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Endocrine-disrupting compounds: Occurrence, detection methods, effects and promising treatment pathways—A critical review. J. Environ. Chem. Eng. 2021, 9, 104558. [Google Scholar] [CrossRef]
- Kassotis, C.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Liu, H.; Ru, J.; Qu, J.; Dai, R.; Wang, Z.; Hu, C. Removal of persistent organic pollutants from micro-polluted drinking water by triolein embedded absorbent. Bioresour. Technol. 2009, 100, 2995–3002. [Google Scholar] [CrossRef]
- Jones-Lepp, T.L.; Stevens, R. Pharmaceuticals and personal care products in biosolids/sewage sludge: The interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 2006, 387, 1173–1183. [Google Scholar] [CrossRef]
- Zuo, Y.; Lin, Y. Solvent Effects on the Silylation-Gas Chromatography–Mass Spectrometric Determination of Natural and Synthetic Estrogenic Steroid Hormones, Comment on Formation of Chlorinated Estrones via Hypochlorous Disinfection of Wastewater Effluent Containing Estrone by Hideyuki Nakamura, Ryoko Kuruto-Niwa, Mitsuo Uchida and Yoshiyasu Terao [Chemosphere 66 (2007) 1441–1448]. Chemosphere 2007, 7, 1175–1176. [Google Scholar]
- Carballa, M.; Omil, F.; Lema, J. Comparison of predicted and measured concentrations of selected pharmaceuticals, fragrances and hormones in Spanish sewage. Chemosphere 2008, 72, 1118–1123. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gomez, M.; Ternes, T. Behavior of Pharmaceuticals, Cosmetics and Hormones in a Sewage Treatment Plant. Water Res. 2004, 38, 2918–2926. [Google Scholar]
- Nagarnaik, P.; Mills, M.; Boulanger, B. Concentrations and mass loadings of hormones, alkylphenols, and alkylphenol ethoxylates in healthcare facility wastewaters. Chemosphere 2010, 78, 1056–1062. [Google Scholar] [CrossRef]
- Rodriguez-Navas, C.; Björklund, E.; Halling-Sørensen, B.; Hansen, M. Biogas final digestive byproduct applied to croplands as fertilizer contains high levels of steroid hormones. Environ. Pollut. 2013, 180, 368–371. [Google Scholar] [CrossRef]
- Sayles, G.; Marsh, T. Biological Fate of Estrogenic Compounds Associated with Sewage Treatment: A Review. In Proceedings of the Effective Risk Management of Endocrine Disrupting Chemicals Workshop, Cincinnati, OH, USA, 18–19 September 2001; pp. 18–19. [Google Scholar]
- Lai, K.M.; Johnson, K.L.; Scrimshaw, M.D.; Lester, J.N. Binding of Waterborne Steroid Estrogens to Solid Phases in River and Estuarine Systems. Environ. Sci. Technol. 2000, 34, 3890–3894. [Google Scholar] [CrossRef]
- Lester, J.N.; Edge, D.R. Sewage and sewage sludge treatment. Pollution 2007, 113–144. [Google Scholar] [CrossRef]
- Mastrup, M.; Jensen, R.L.; Schäfer, A.I.; Khan, S. Fate Modeling-an Important Tool for Water Recycling. In Recent Advances in Water Recycling Technologies; Schäfer, A.I., Sherman, P., Waite, T.D., Eds.; Libraries Australia: Brisbane, Australia, 2001; pp. 103–112. [Google Scholar]
- Dong, Z.; Senn, D.; Moran, R.E.; Shine, J.P. Prioritizing environmental risk of prescription pharmaceuticals. Regul. Toxicol. Pharmacol. 2013, 65, 60–67. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Zhou, Z.; Sharma, V.K. Occurrence, Transportation, Monitoring and Treatment of Emerging Micro-Pollutants in Waste Water—A Review from Global Views. Microchem. J. 2013, 110, 292–300. [Google Scholar]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Ratola, N.; Cincinelli, A.; Alves, A.; Katsoyiannis, A. Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. J. Hazard. Mater. 2012, 239–240, 1–18. [Google Scholar] [CrossRef]
- Garnaga, G. Integrated assessment of pollution in the Baltic Sea. Ekologija 2013, 58, 58. [Google Scholar] [CrossRef]
- Ribeiro, C.; Ribeiro, A.R.; Tiritan, M.E.; De Voogt, P. Priority Substances and Emerging Organic Pollutants in Portuguese Aquatic Environment: A Review. Rev. Environ. Contam. Toxicol. 2015, 238, 1–44. [Google Scholar] [CrossRef]
- Conley, J.M.; Evans, N.; Cardon, M.C.; Rosenblum, L.; Iwanowicz, L.R.; Hartig, P.C.; Schenck, K.M.; Bradley, P.M.; Wilson, V.S. Occurrence and In Vitro Bioactivity of Estrogen, Androgen, and Glucocorticoid Compounds in a Nationwide Screen of United States Stream Waters. Environ. Sci. Technol. 2017, 51, 4781–4791. [Google Scholar] [CrossRef]
- D’Ascenzo, G.; Di Corcia, A.; Gentili, A.; Mancini, R.; Mastropasqua, R.; Nazzari, M.; Samperi, R. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci. Total Environ. 2003, 302, 199–209. [Google Scholar] [CrossRef]
- McNeil, P.L.; Nebot, C.; Sloman, K.A. Physiological and Behavioral Effects of Exposure to Environmentally Relevant Concentrations of Prednisolone During Zebrafish (Danio rerio) Embryogenesis. Environ. Sci. Technol. 2016, 50, 5294–5304. [Google Scholar] [CrossRef]
- Grdulska, A.; Kowalik, R. Pharmaceuticals in Water and Wastewater-Overview (Farmaceutyki w Wodach i Ściekach). Struct. Environ. 2020, 12, 79–84. [Google Scholar]
- Lalone, C.A.; Villeneuve, D.L.; Olmstead, A.W.; Medlock, E.K.; Kahl, M.D.; Jensen, K.M.; Durhan, E.J.; Makynen, E.A.; Blanksma, C.A.; Cavallin, J.E.; et al. Effects of a glucocorticoid receptor agonist, dexamethasone, on fathead minnow reproduction, growth, and development. Environ. Toxicol. Chem. 2012, 31, 611–622. [Google Scholar] [CrossRef]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef]
- Willi, R.A.; Salgueiro-González, N.; Carcaiso, G.; Fent, K. Glucocorticoid mixtures of fluticasone propionate, triamcinolone acetonide and clobetasol propionate induce additive effects in zebrafish embryos. J. Hazard. Mater. 2019, 374, 101–109. [Google Scholar] [CrossRef]
- Kokate, T.G.; Svensson, B.E.; Rogawski, M.A. Anticonvulsant activity of neurosteroids: Correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J. Pharmacol. Exp. Ther. 1994, 270, 1223–1229. [Google Scholar]
- Reddy, D.S.; Rogawski, M.A. Stress-Induced Deoxycorticosterone-Derived Neurosteroids Modulate GABAA Receptor Function and Seizure Susceptibility. J. Neurosci. 2002, 22, 3795–3805. [Google Scholar]
- Reddy, D.S. Pharmacology of Endogenous Neuroactive Steroids. Crit. Rev. Neurobiol. 2004, 15, 197–234. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Koide, E.M.; Imbery, J.J.; Helbing, C.C. Contaminant and Environmental Influences on Thyroid Hormone Action in Amphibian Metamorphosis. Front. Endocrinol. 2019, 10, 276. [Google Scholar]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Herrera-Melián, J.A.; Rodríguez-Rodríguez, R.; Ojeda-González, Z.; Landívar-Andrade, V.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Pharmaceutical and personal care product residues in a macrophyte pond-constructed wetland treating wastewater from a university campus: Presence, removal and ecological risk assessment. Sci. Total Environ. 2020, 703, 135596. [Google Scholar] [CrossRef]
- Zwart, N.; Jonker, W.; Ten Broek, R.; de Boer, J.; Somsen, G.; Kool, J.; Hamers, T.; Houtman, C.J.; Lamoree, M.H. Identification of Mutagenic and Endocrine Disrupting Compounds in Surface Water and Wastewater Treatment Plant Effluents Using High-Resolution Effect-Directed Analysis. Water Res. 2020, 168, 115204. [Google Scholar]
- Willi, R.A.; Faltermann, S.; Hettich, T.; Fent, K. Active Glucocorticoids Have a Range of Important Adverse Developmental and Physiological Effects on Developing Zebrafish Embryos. Environ. Sci. Technol. 2017, 52, 877–885. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Owen, S.F.; Huerta, B.; Rodríguez-Mozaz, S.; Kugathas, S.; Barceló, D.; Rand-Weaver, M.; Sumpter, J.P. Internal Exposure Dynamics Drive the Adverse Outcome Pathways of Synthetic Glucocorticoids in Fish. Sci. Rep. 2016, 6, 1–13. [Google Scholar]
- Wiley, J.W.; Higgins, G.; Athey, B.D. Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis. Neurogastroenterol. Motil. 2016, 28, 12–25. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, M.Y.; Choi, C.S.; Sohn, Y.W.; Park, B.R.; Choi, M.-G.; Nah, Y.-H.; Choi, S.C. The effect of chronic variable stress on bowel habit and adrenal function in rats. J. Gastroenterol. Hepatol. 2008, 23, 1840–1846. [Google Scholar] [CrossRef]
- Bradford, K.; Shih, W.; Videlock, E.J.; Presson, A.P.; Naliboff, B.D.; Mayer, E.A.; Chang, L. Association Between Early Adverse Life Events and Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2012, 10, 385–390.e3. [Google Scholar] [CrossRef]
- Larauche, M.; Mulak, A.; Taché, Y. Stress and visceral pain: From animal models to clinical therapies. Exp. Neurol. 2012, 233, 49–67. [Google Scholar] [CrossRef]
- Larauche, M.; Mulak, A.; Taché, Y. Stress-Related Alterations of Visceral Sensation: Animal Models for Irritable Bowel Syndrome Study. J. Neurogastroenterol. Motil. 2011, 17, 213–234. [Google Scholar] [CrossRef]
- Restivo, V.; Kidd, K.A.; Surette, M.G.; Servos, M.R.; Wilson, J.Y. Rainbow darter (Etheostoma caeruleum) from a river impacted by municipal wastewater effluents have altered gut content microbiomes. Sci. Total Environ. 2021, 751, 141724. [Google Scholar] [CrossRef]
- Umland, S.P.; Schleimer, R.P.; Johnston, S. Review of the Molecular and Cellular Mechanisms of Action of Glucocorticoids for Use in Asthma. Pulm. Pharmacol. Ther. 2002, 15, 35–50. [Google Scholar] [CrossRef]
- Miyamoto, A.; Kitaichi, Y.; Uchikura, K. Degradation of Corticosteroids during Activated Sludge Processing. Chem. Pharm. Bull. 2014, 62, 72–76. [Google Scholar] [CrossRef]
- Jia, A.; Wu, S.; Daniels, K.; Snyder, S.A. Balancing the Budget: Accounting for Glucocorticoid Bioactivity and Fate during Water Treatment. Environ. Sci. Technol. 2016, 50, 2870–2880. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, J.D.; Lee, W.-Y.; Chung, B.C.; Choi, M.H. Quantitative metabolic profiling of 21 endogenous corticosteroids in urine by liquid chromatography–triple quadrupole-mass spectrometry. Anal. Chim. Acta 2009, 632, 101–108. [Google Scholar] [CrossRef]
- Leet, J.K.; Sassman, S.; Amberg, J.J.; Olmstead, A.W.; Lee, L.S.; Ankley, G.T.; Sepúlveda, M.S. Environmental hormones and their impacts on sex differentiation in fathead minnows. Aquat. Toxicol. 2015, 158, 98–107. [Google Scholar] [CrossRef]
- Pollock, M.S.; Dubé, M.G.; Schryer, R. Investigating the link between pulp mill effluent and endocrine disruption: Attempts to explain the presence of intersex fish in the Wabigoon River, Ontario, Canada. Environ. Toxicol. Chem. 2010, 29, 952–965. [Google Scholar] [CrossRef]
- Adams, N.R. Clover phytoestrogens in sheep in Western Australia. Pure Appl. Chem. 1998, 70, 1855–1862. [Google Scholar] [CrossRef][Green Version]
- Panter, G.; Thompson, R.; Sumpter, J. Adverse reproductive effects in male fathead minnows (Pimephales promelas) exposed to environmentally relevant concentrations of the natural oestrogens, oestradiol and oestrone. Aquat. Toxicol. 1998, 42, 243–253. [Google Scholar] [CrossRef]
- De Alda, M.J.L.; Cruz, S.D.; Petrovic, M.; Barceló, D. Liquid chromatography–(tandem) mass spectrometry of selected emerging pollutants (steroid sex hormones, drugs and alkylphenolic surfactants) in the aquatic environment. J. Chromatogr. A 2003, 1000, 503–526. [Google Scholar] [CrossRef]
- Lopez, J. Endocrine-Disrupting Chemical Pollution: Why the EPA Should Regulate These Chemicals under the Clean Water Act. Sustain. Dev. L. Pol’y 2009, 10, 19. [Google Scholar]
- López-Fernández, R.; Martínez, L.; Villaverde, S. Membrane bioreactor for the treatment of pharmaceutical wastewater containing corticosteroids. Desalination 2012, 300, 19–23. [Google Scholar] [CrossRef]
- Han, J.; Meng, S.; Dong, Y.; Hu, J.; Gao, W. Capturing hormones and bisphenol A from water via sustained hydrogen bond driven sorption in polyamide microfiltration membranes. Water Res. 2013, 47, 197–208. [Google Scholar] [CrossRef]
- Wojnarowicz, P.; Yang, W.; Zhou, H.; Parker, W.J.; Helbing, C.C. Changes in hormone and stress-inducing activities of municipal wastewater in a conventional activated sludge wastewater treatment plant. Water Res. 2014, 66, 265–272. [Google Scholar] [CrossRef]
- Baronti, C.; Curini, R.; D’Ascenzo, G.; Di Corcia, A.; Gentili, A.; Samperi, R. Monitoring Natural and Synthetic Estrogens at Activated Sludge Sewage Treatment Plants and in a Receiving River Water. Environ. Sci. Technol. 2000, 34, 5059–5066. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography–mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci. Total Environ. 2012, 419, 208–215. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Peirovi, R.; Ebrahimi, A.A. A Review on the Importance of Hormones Monitoring and Their Removal in Conventional Wastewater Treatment Systems. J. Environ. Health Sustain. Dev. 2017, 2, 310–318. [Google Scholar]
- Nuzzo, J.B. The Biological Threat to U.S. Water Supplies: Toward a National Water Security Policy. Biosecur. Bioterror. Biodef. Strat. Pr. Sci. 2006, 4, 147–159. [Google Scholar] [CrossRef]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic Review of Toxicity Removal by Advanced Wastewater Treatment Technologies via Ozonation and Activated Carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.-G.; Zhao, J.-L.; Liu, S.; Zhou, L.-J.; Chen, F. Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate(VI) treatment of secondary wastewater effluents. Water Res. 2012, 46, 2194–2204. [Google Scholar] [CrossRef]
- Pendergast, M.T.M.; Nygaard, J.M.; Ghosh, A.K.; Hoek, E.M. Using nanocomposite materials technology to understand and control reverse osmosis membrane compaction. Desalination 2010, 261, 255–263. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Fabrication of Catalytic Membranes for the Treatment of Drinking Water Using Combined Ozonation and Ultrafiltration. Environ. Sci. Technol. 2005, 39, 7656–7661. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of Pollutants from Surface Water and Groundwater by Nanofiltration: Overview of Possible Applications in the Drinking Water Industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar]
- Humplik, T.; Lee, J.; O’Hern, S.C.; Fellman, B.A.; Baig, M.A.; Hassan, S.F.; Atieh, M.; Rahman, F.; Laoui, T.; Karnik, R.; et al. Nanostructured materials for water desalination. Nanotechnol. 2011, 22, 292001. [Google Scholar] [CrossRef]
- WHO. Economic and Health Effects of Increasing Coverage of Low Cost Household Drinking-Water Supply and Sanitation Interventions to Countries off-Track to Meet MDG Target 10: Background Document to the “Human Development Report 2006"; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- WHO/UNICEF. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Pretali, L.; Albini, A.; Cantalupi, A.; Maraschi, F.; Nicolis, S.; Sturini, M. TiO2-Photocatalyzed Water Depollution, a Strong, yet Selective Depollution Method: New Evidence from the Solar Light Induced Degradation of Glucocorticoids in Freshwaters. Appl. Sci. 2021, 11, 2486. [Google Scholar] [CrossRef]
- Díez, A.M.; Ribeiro, A.S.; Sanromán, M.A.; Pazos, M. Optimization of photo-Fenton process for the treatment of prednisolone. Environ. Sci. Pollut. Res. 2018, 25, 27768–27782. [Google Scholar] [CrossRef]
- Klauson, D.; Pilnik-Sudareva, J.; Pronina, N.; Budarnaja, O.; Krichevskaya, M.; Käkinen, A.; Juganson, K.; Preis, S. Aqueous photocatalytic oxidation of prednisolone. Open Chem. 2013, 11, 1620–1633. [Google Scholar] [CrossRef]
- Romao, J.; Hamdy, M.S.; Mul, G.; Baltrusaitis, J. Photocatalytic decomposition of cortisone acetate in aqueous solution. J. Hazard. Mater. 2015, 282, 208–215. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Sun, W.; Ren, D.; Li, X.; Li, X.; Liu, Y.; Li, Q.; Pan, X. Occurrence, Removal, and Fate of Progestogens, Androgens, Estrogens, and Phenols in Six Sewage Treatment Plants around Dianchi Lake in China. Environ. Sci. Pollut. Res. 2014, 21, 12898–12908. [Google Scholar]
- Chang, S.; Waite, T.D.; Ong, P.E.A.; Schäfer, A.I.; Fane, A.G. Assessment of Trace Estrogenic Contaminants Removal by Coagulant Addition, Powdered Activated Carbon Adsorption and Powdered Activated Carbon/Microfiltration Processes. J. Environ. Eng. 2004, 130, 736–742. [Google Scholar] [CrossRef][Green Version]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in Wastewater: Fate and Removal Processes. Phys. Chem. Wastewater Treat. Resour. Recovery 2017, 3, 75–117. [Google Scholar] [CrossRef]
| Compounds | CAS | Molecular Weight (g/mole) | log Kow | Formula | Structure |
|---|---|---|---|---|---|
| Cortisol | 152-58-9 | 362.5 | 3.5 | C21H30O5 | 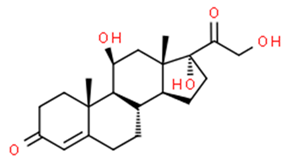 |
| Cortisone | 53-06-5 | 360.4 | 1.5 | C21H28O5 | 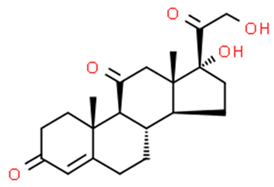 |
| Dexamethasone | 50-02-2 | 392.5 | 1.8 | C22H29FO5 |  |
| Prednisolone | 53-03-2 | 360.5 | 1.6 | C21H28O5 | 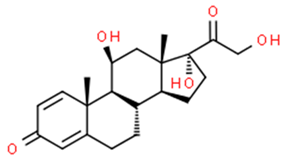 |
| Prednisone | 50-24-8 | 358.4 | 1.4 | C21H28O5 | 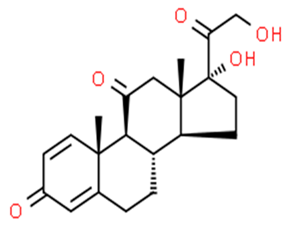 |
| 6α-methylprednisolone | 83-43-2 | 374.5 | 2.0 | C22H30O5 |  |
| Compounds | Effluent Concentration (ng/L) | Toxicity and Impacts | Sources | References |
|---|---|---|---|---|
| Prednisolone or beclomethasone | 0.7–1.7 | Studies showed a significant increase of plasma glucose levels in fathead minnow; also number of leukocytes in the peripheral blood was decreased and fold changed in the transcripts of more genes. | Europe: United Kingdom, The Netherlands, Spain, Switzerland, Hungary, Wastewater in France, Hospital Wastewater in Netherlands, Surface water in Spain, (Czech and Slovak republics Sewage and River water) | [13,113,114,115] |
| Cortisol | 100–145 | It was reported that cortisol suppress immune function in fish. The exposure of zebrafish to cortisol (145 ng/L) could cause the accelerated hatching, increased significant level of heart rate, detoriorate the muscle contractions, and genetic expression changes. | Asia: Japan (Ehime Prefecture), China (Sewage Treatment Plants (STPs) and Receiving River Waters Beijing), India, Malaysia, | [21,55,77,115,116,117] |
| Dexamethasone (Betamethasone) | >0.1–1.7 | Dexamethasone or betamethasone affected adversely on the reproduction, growth, and development in fathead minnow (Pimephales promelas); this could affect the development, reproduction, growth and mRNA expression of amphibians and fish. | Oceania: Australia (River water and municipal sewage) | [20,26,118,119,120,121,122] |
| Prendnisone | 0.2–100 | Several stuies have showed that the presense of prendnisone made increase of se rum free amino acid levels significantly in common carp (Cyprinus carpio), morphological changes with swimming behavior, and adverse effects on physiology of zebrafish at the exposure concentration of 100 ng/L. | New Zealand: New Zealand (municipal sewage) | [23,42,123,124] |
| Cortisone | 1.3–433 | It has been reported that unlike cortisol, unexpected exposure (91 ng/L) could cause the accelerated hatching, increased significant level of heart rate, detoriorate the muscle contractions, and genetic expression changes in Zebra fish | North America: USA, Drinking water in Canada, Mexico. | [58,116,120,124,125] |
| 6α-methylprednisolone | 60–91 | Serum free amino acid levels was increased in common carp (Cyprinus carpio) due to 6α-methylprednisolone. | South America: Wastewater in Uruguay and Brazil, shallow lakes system Argentina | [113,115,123,124] |
| Process | Removal Efficiency | References |
|---|---|---|
| Adsorption with nano particles, e.g., Fe (VI) nanoparticle adsorption | Highly Effective (80–99%) | [95] |
| Adsorption with activated carbon | Highly Effective (98%) | [150,151,152] |
| Sorption | Effective (98%) | [108,117] |
| Photocatalysis (µg /liter levels) | Effective (>95%) | [158,159,160,161,162] |
| Chlorination | Activated sludge systems combined with chlorination in tertiary treatment has been effective (95%) | [108] |
| Combination of reverse osmosis and micro-filtration | Depends on the concentration of GCs.(56–90%) | [163] |
| Advanced oxidation processes (Ozone, UV/H2O2, photo-Fenton processes) | Highly Effective (<90%) | [151] |
| Combination of ozonation and granular activated carbon (GAC) | Moderately Effective (70–85%) | [151,163] |
| Ultrafiltration | Not Effective (~8%) but for hydrophobic membranes (such as Cortisone) its efficiency goes beyond 80% | [154] |
| Activated sludge systems with UV disinfection | Not Effective (49%) | [163] |
| Combination of membrane filtration, ultra-filtration | Depends on the filtration type, size, and effluent concentration | [95] |
| Attached growth process | Varies between compounds, Moderately Effective | [135,150] |
| Microfiltration membranes | Not Effective (<18% unless combines with activated carbon or ultrafiltration) | [153,154,164] |
| Coagulation and flocculation | Not Effective (<10%) | [108,117,145,149,165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdan, M.M.S.; Ahad, M.T.; Mallick, Z.; Mallick, S.P.; Jahan, I.; Mazumder, M. An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems. Pollutants 2021, 1, 141-155. https://doi.org/10.3390/pollutants1030012
Yazdan MMS, Ahad MT, Mallick Z, Mallick SP, Jahan I, Mazumder M. An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems. Pollutants. 2021; 1(3):141-155. https://doi.org/10.3390/pollutants1030012
Chicago/Turabian StyleYazdan, Munshi Md. Shafwat, Md Tanvir Ahad, Zayed Mallick, Synthia Parveen Mallick, Ishrat Jahan, and Mozammel Mazumder. 2021. "An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems" Pollutants 1, no. 3: 141-155. https://doi.org/10.3390/pollutants1030012
APA StyleYazdan, M. M. S., Ahad, M. T., Mallick, Z., Mallick, S. P., Jahan, I., & Mazumder, M. (2021). An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems. Pollutants, 1(3), 141-155. https://doi.org/10.3390/pollutants1030012








