Development of a Predictive Model for Mild Cognitive Impairment in Parkinson’s Disease with Normal Cognition Using Kernel-Based C5.0 Machine Learning Blending: Preliminary Research †
Abstract
1. Introduction
2. Method
2.1. Data Source
2.2. Measurement
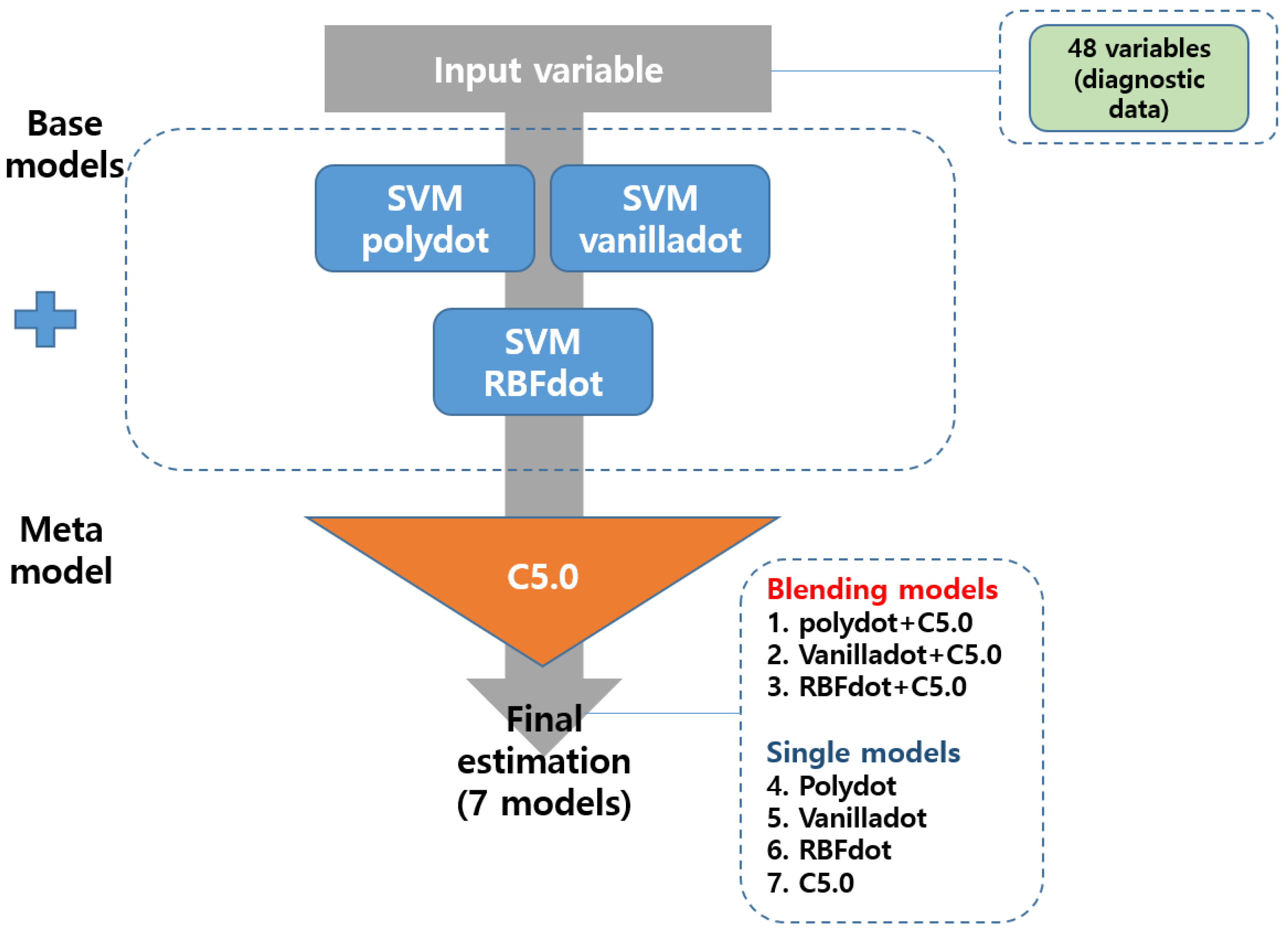
2.3. Model Blending Based on Machine Learning
3. Results
3.1. General Characteristics of Subjects
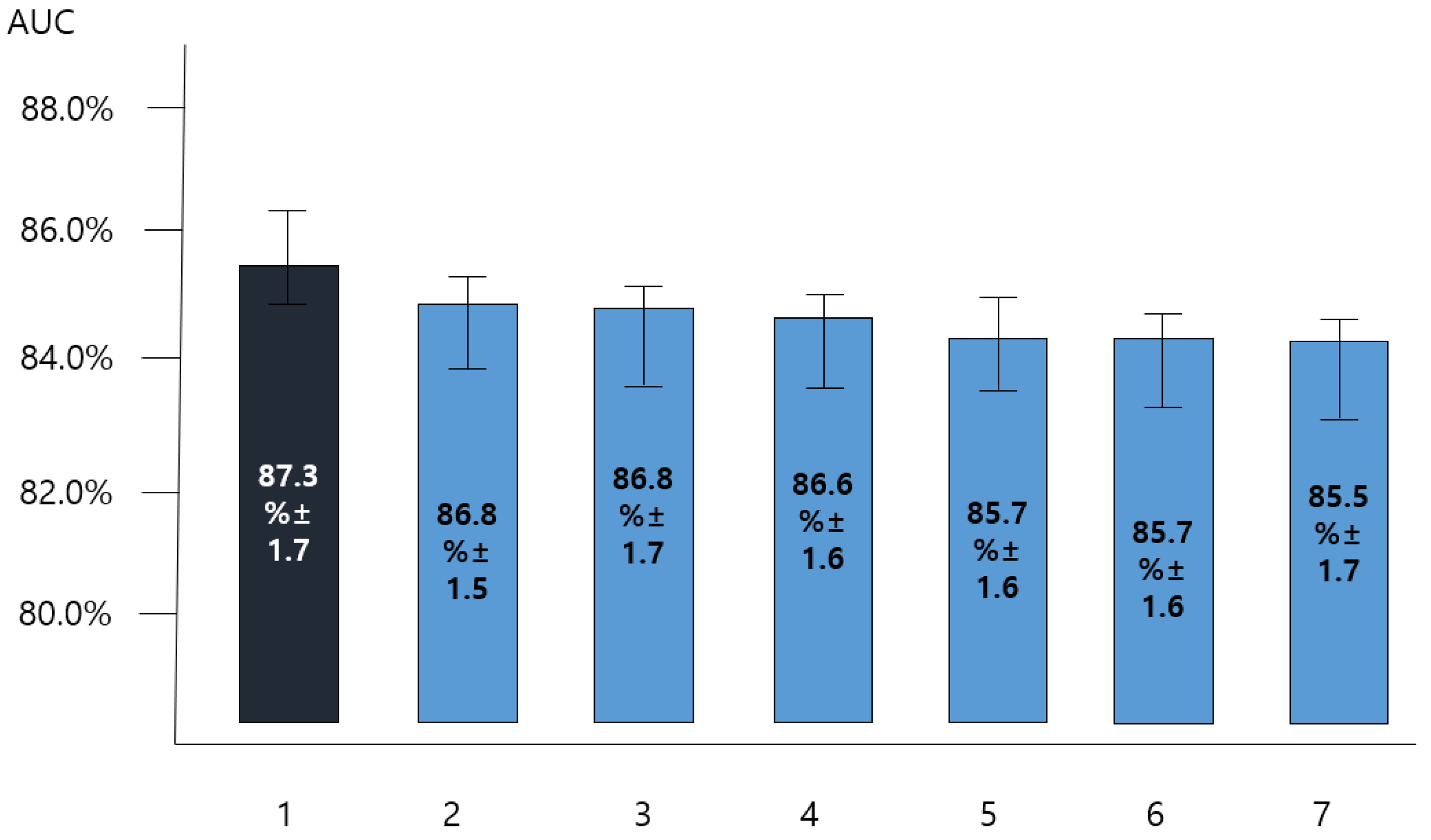
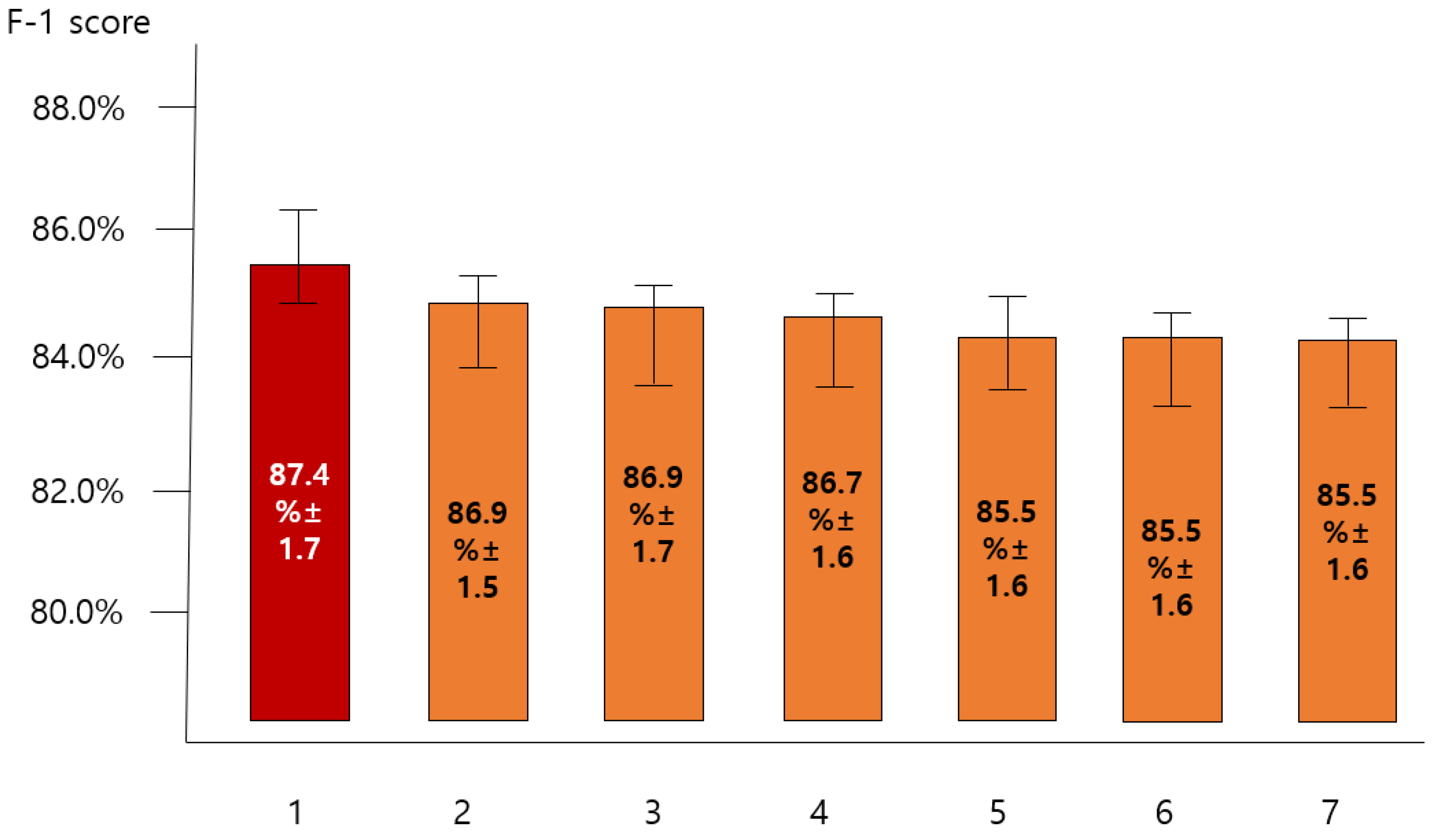
3.2. Comparing the Predictive Performance of Single Model and That of Blending Model
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brookmeyer, R.; Gray, S.; Kawas, C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health 1998, 88, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Gustavson, D.E.; Elman, J.A.; Sanderson-Cimino, M.; Franz, C.E.; Panizzon, M.S.; Jak, A.J.; Reynolds, C.A.; Neale, M.C.; Lyons, M.J.; Kremen, W. Extensive memory testing improves prediction of progression to MCI in late middle age. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12004. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Lee, Z.; Kwon, L.N.; Chun, H.W. Medical health records-based Mild Cognitive Impairment (MCI) prediction for effective dementia care. Int. J. Environ. Res. Public Health 2021, 18, 9223. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Cicero, C.E.; Baschi, R.; Davì, M.; Luca, A.; Restivo, V.; Zangara, C.; Fierro, B.; Zappia, M.; Nicoletti, A. Mild cognitive impairment in Parkinson’s disease: The Parkinson’s disease cognitive study (PACOS). J. Neurol. 2018, 265, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.E.; Ekman, U.; Forsgren, L.; Elgh, E. Cognitive function in the early phase of Parkinson’s disease, a five-year follow-up. Acta Neurol. Scand. 2015, 132, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Baiano, C.; Barone, P.; Trojano, L.; Santangelo, G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov. Disord. 2020, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.C.; Chan, L.L.; Tan, L.C.; Tan, E.K. Mild cognitive impairment in Parkinson’s disease: A distinct clinical entity? Transl. Neurodegener. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Horta, S.; Kulisevsky, J. Mild cognitive impairment in Parkinson’s disease. J. Neural Transm. 2019, 126, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Roheger, M.; Kalbe, E.; Liepelt-Scarfone, I. Progression of cognitive decline in Parkinson’s disease. J. Parkinsons Dis. 2018, 8, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zokaei, N.; Husain, M. Working Memory in Alzheimer’s Disease and Parkinson’s Disease. In Processes of Visuospatial Attention and Working Memory; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Biundo, R.; Weis, L.; Pilleri, M.; Facchini, S.; Formento-Dojot, P.; Vallelunga, A.; Antonini, A. Diagnostic and screening power of neuropsychological testing in detecting mild cognitive impairment in Parkinson’s disease. J. Neural Transm. 2013, 120, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H. Application of machine learning technique to distinguish Parkinson’s disease dementia and Alzheimer’s dementia: Predictive power of Parkinson’s disease-related non-motor symptoms and neuropsychological profile. J. Pers. Med. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewski, A.W.; Chudzik, A.; Szlufik, S.; Habela, P.; Koziorowski, D.M. Comparison of different data mining methods to determine disease progression in dissimilar groups of Parkinson’s patients. Fundam. Inform. 2020, 176, 167–181. [Google Scholar] [CrossRef]
- Sun, L.; Fu, S.; Wang, F. Decision tree SVM model with fisher feature selection for speech emotion recognition. Eurasip J. Audio Speech Music Process. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, H. Development of a Predictive Model for Mild Cognitive Impairment in Parkinson’s Disease with Normal Cognition Using Kernel-Based C5.0 Machine Learning Blending: Preliminary Research. Eng. Proc. 2021, 11, 18. https://doi.org/10.3390/ASEC2021-11147
Byeon H. Development of a Predictive Model for Mild Cognitive Impairment in Parkinson’s Disease with Normal Cognition Using Kernel-Based C5.0 Machine Learning Blending: Preliminary Research. Engineering Proceedings. 2021; 11(1):18. https://doi.org/10.3390/ASEC2021-11147
Chicago/Turabian StyleByeon, Haewon. 2021. "Development of a Predictive Model for Mild Cognitive Impairment in Parkinson’s Disease with Normal Cognition Using Kernel-Based C5.0 Machine Learning Blending: Preliminary Research" Engineering Proceedings 11, no. 1: 18. https://doi.org/10.3390/ASEC2021-11147
APA StyleByeon, H. (2021). Development of a Predictive Model for Mild Cognitive Impairment in Parkinson’s Disease with Normal Cognition Using Kernel-Based C5.0 Machine Learning Blending: Preliminary Research. Engineering Proceedings, 11(1), 18. https://doi.org/10.3390/ASEC2021-11147





