The Hepatitis B Virus PreS1/HBsAg Ratio Is a Predictive Marker for the Occurrence of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
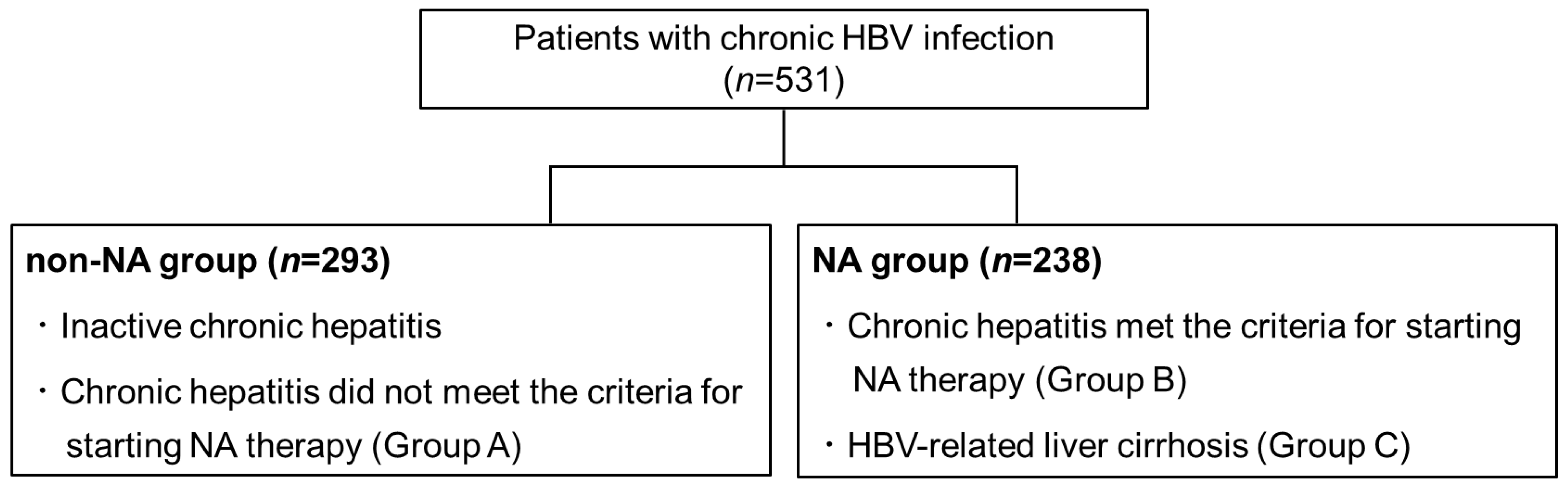
2.1. Patients
2.2. Quantification of PreS1 and HBsAg Level
2.3. Measurement of Other HBV-Related Markers
2.4. Liver Fibrosis Indices
2.5. Statistical Analysis
3. Results
3.1. A Comparison of the Initial Values between the NA Group and the Non-NA Group
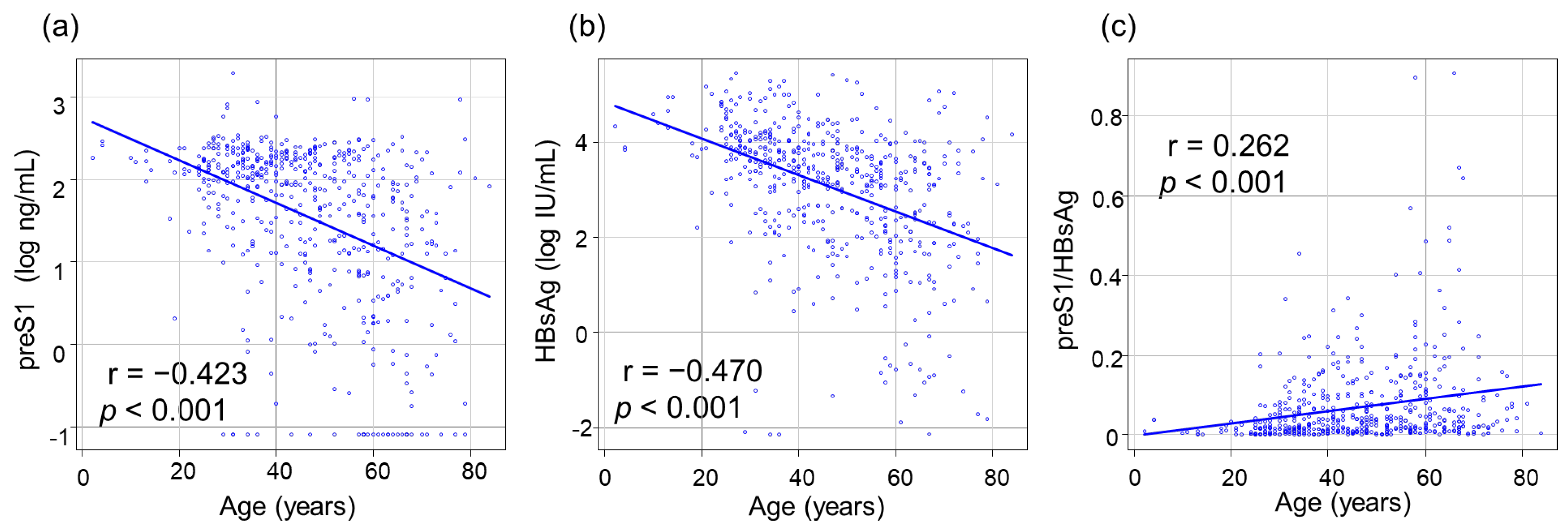
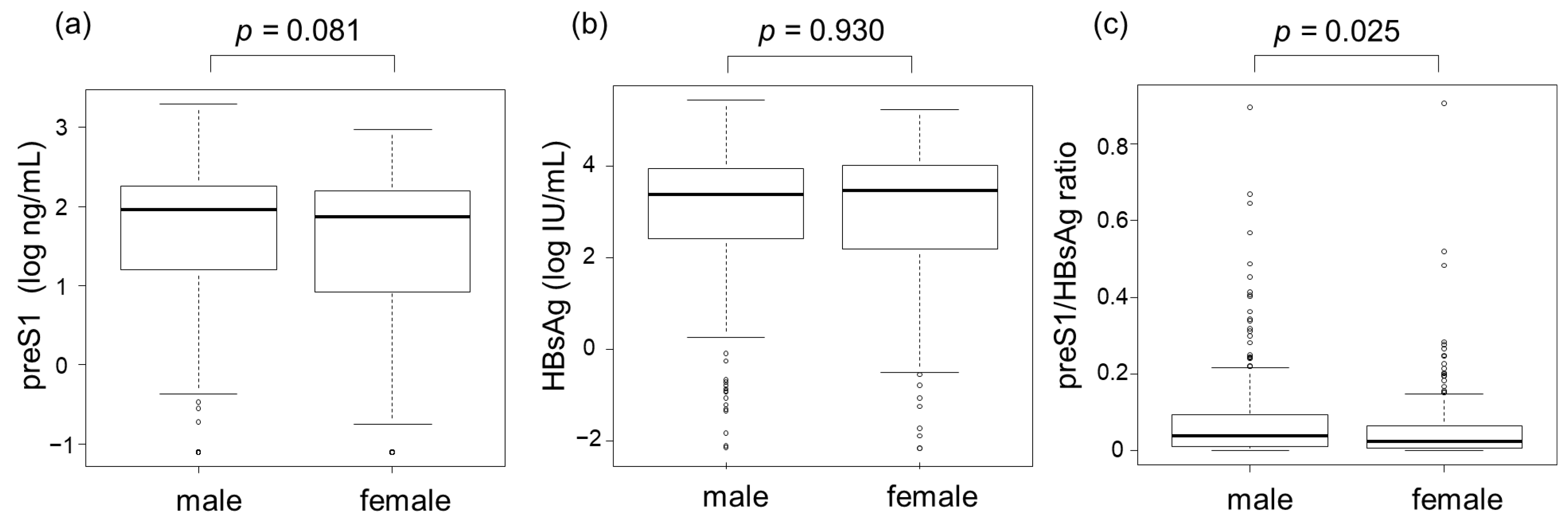
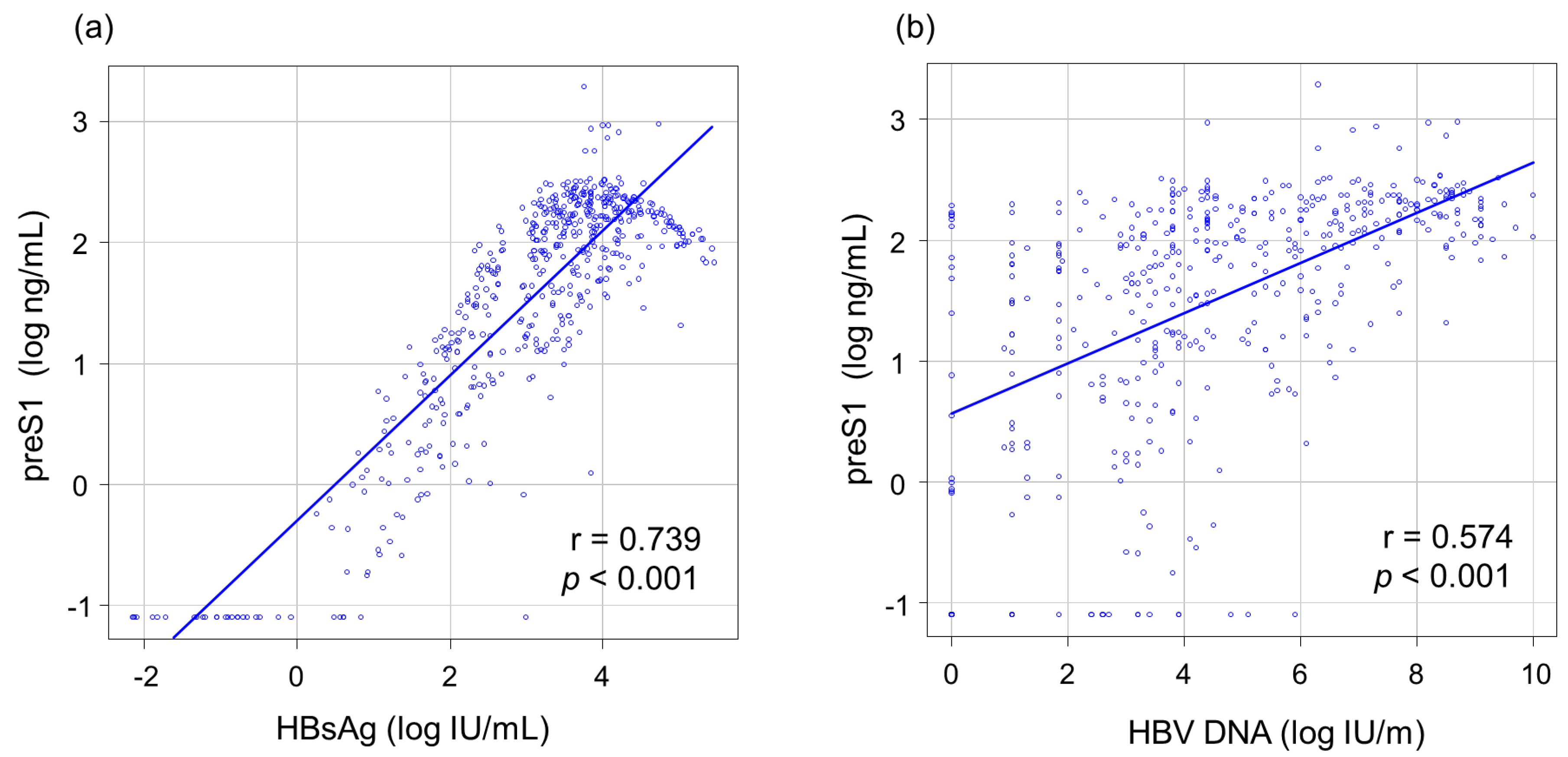
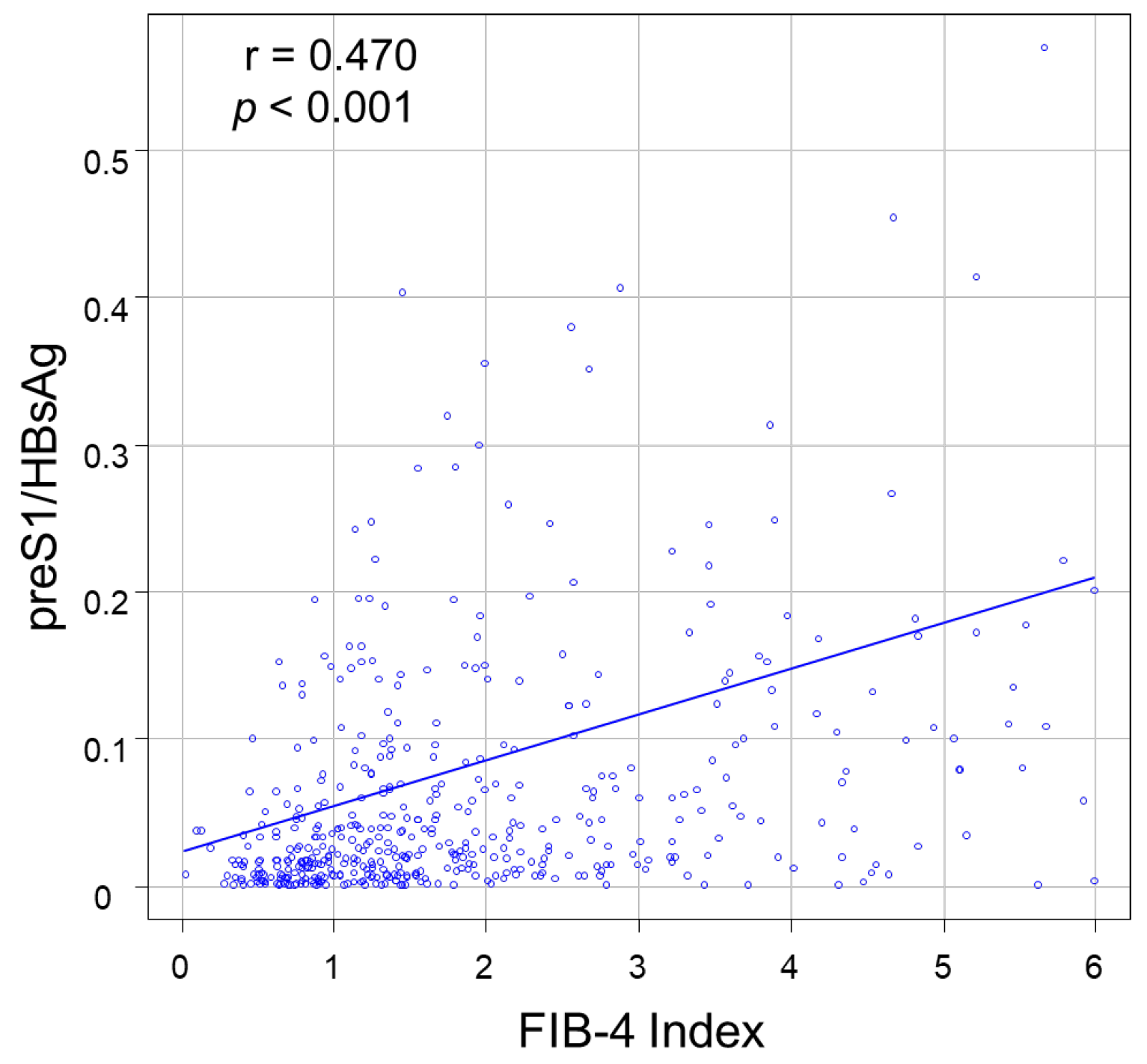
3.2. Correlations between PreS1 Level and Other HBV Markers
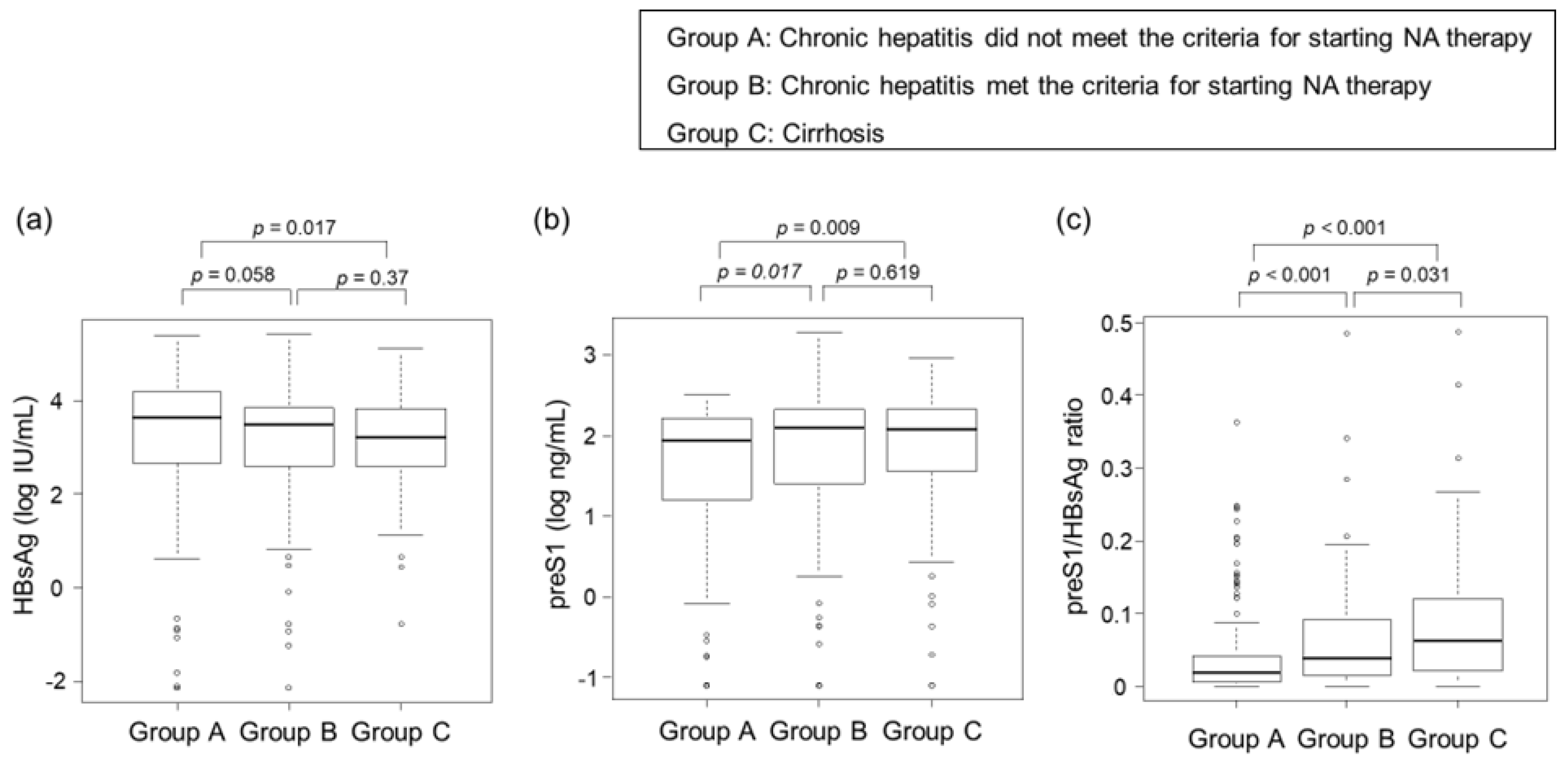
3.3. Correlations between Clinical Stage of CHB Infection and HBsAg, PreS1 Level, and PreS1/HBsAg Ratio
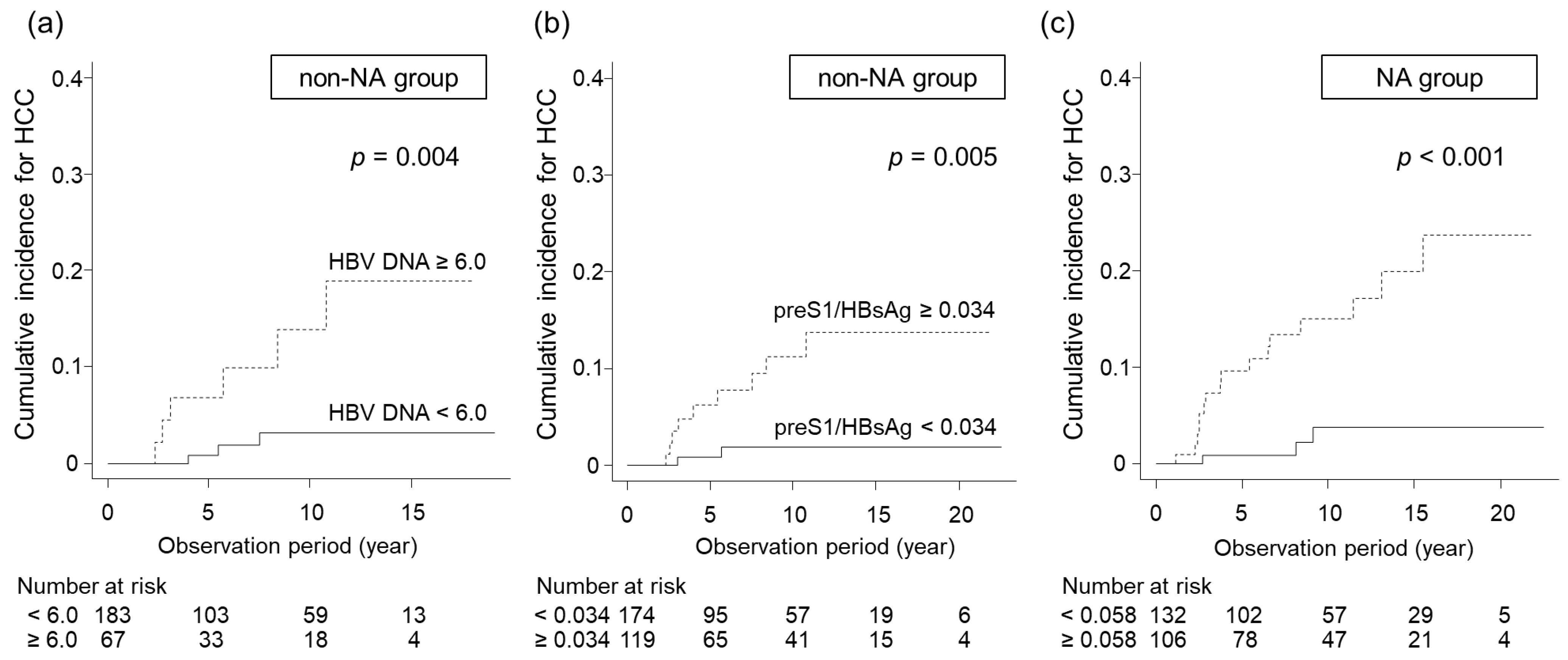
3.4. Identification of Predictive Factors for HCC Development in Non-NA and NA Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 13 May 2024).
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol. Res. 2020, 50, 892–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, X.; Zhu, L.; Liu, J.; Qiu, Y.; Li, Y.; Liu, Y.; Xue, R.; Zhan, J.; Jiang, S.; et al. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone. Aliment. Pharmacol. Ther. 2023, 57, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Yan, X.; Xue, R.; Zhan, J.; Jiang, S.; Geng, Y.; Liu, Y.; Mao, M.; Xia, J.; et al. Presence of liver inflammation in Asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis. Hepatol. Commun. 2022, 6, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Li, X.; Le, M.H.; Le, A.K.; Yeo, Y.H.; Trinh, H.N.; Zhang, J.; Li, J.; Wong, C.; Wong, C.; et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin. Gastroenterol. Hepatol. 2022, 20, 1803–1812.e5. [Google Scholar] [CrossRef]
- Choi, G.H.; Kim, G.A.; Choi, J.; Han, S.; Lim, Y.S. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation. Aliment. Pharmacol. Ther. 2019, 50, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.S.; Gill, U.S.; Litwin, S.; Zhou, Y.; Peri, S.; Pop, O.; Hong, M.L.; Naik, S.; Quaglia, A.; Bertoletti, A.; et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 2016, 151, 986–998.e4. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Z.; Sun, Z.; Zhao, J.; Zhu, D.; Wang, Y.; Fu, S.; Guo, J.; Liu, L.; Su, L.; et al. Vaccines targeting preS1 domain overcome immune tolerance in hepatitis B virus carrier mice. Hepatology 2017, 66, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection—Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Klingmüller, U.; Schaller, H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J. Virol. 1993, 67, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M. The association between hepatocarcinogenesis and intracellular alterations due to hepatitis B virus infection. Liver Int. 2021, 41, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012, 1, e00049. [Google Scholar] [CrossRef]
- Park, J.-H.; Iwamoto, M.; Yun, J.-H.; Uchikubo-Kamo, T.; Son, D.; Jin, Z.; Yoshida, H.; Ohki, M.; Ishimoto, N.; Mizutani, K.; et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 2022, 606, 1027–1031. [Google Scholar] [CrossRef]
- Nishida, Y.; Imamura, M.; Teraoka, Y.; Morio, K.; Fujino, H.; Ono, A.; Nakahara, T.; Murakami, E.; Yamauchi, M.; Kawaoka, T.; et al. Serum preS1 and HBsAg ratio reflects liver fibrosis and predicts the development of hepatocellular carcinoma in chronic hepatitis B patients. J. Viral Hepat. 2021, 28, 1304–1311. [Google Scholar] [CrossRef]
- Nihon Arukoru Igakkai/Japenese Medical Society of Alcohol Studies. Guidelines for conflict of interest in clinical research. Jpn. J. Alcohol Stud. Drug Depend. 2013, 48, 339–357. (In Japanese) [Google Scholar]
- Hatooka, H.; Shimomura, Y.; Imamura, M.; Teraoka, Y.; Morio, K.; Fujino, H.; Ono, A.; Nakahara, T.; Murakami, E.; Yamauchi, M.; et al. Construction of an anti-hepatitis B virus preS1 antibody and usefulness of preS1 measurement for chronic hepatitis B patients: Anti-HBV PreS1 antibody. J. Infect. 2022, 84, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Lin, W.L.; Hung, J.H.; Huang, W. Association of the hepatitis B virus large surface protein with viral infectivity and endoplasmic reticulum stress-mediated liver carcinogenesis. Cells 2020, 9, 2052. [Google Scholar] [CrossRef]
- Wang, H.C.; Huang, W.; Lai, M.D.; Su, I.J. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006, 97, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M.; Hiraga, N.; Zhang, Y.; Yamashita, M.; Sato, O.; Oka, N.; Shiraishi, K.; Izaki, Y.; Makokha, G.N.; Uchida, T.; et al. Endoplasmic reticulum-mediated induction of interleukin-8 occurs by hepatitis B virus infection and contributes to suppression of interferon responsiveness in human hepatocytes. Virology 2018, 525, 48–61. [Google Scholar] [CrossRef]
- Tsushima, K.; Tsuge, M.; Hiraga, N.; Uchida, T.; Murakami, E.; Makokha, G.N.; Kurihara, M.; Nomura, M.; Hiyama, Y.; Fujino, H.; et al. Comparison of intracellular responses between HBV genotype A and C infection in human hepatocyte chimeric mice. J. Gastroenterol. 2019, 54, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Hildt, E.; Saher, G.; Bruss, V.; Hofschneider, P.H. The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology 1996, 225, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Hildt, E.; Hofschneider, P.H. The PreS2 activators of the hepatitis B virus: Activators of tumour promoter pathways. Recent Results Cancer Res. 1998, 154, 315–329. [Google Scholar] [PubMed]
- Tsai, H.W.; Lee, Y.P.; Yen, C.J.; Cheng, K.H.; Huang, C.J.; Huang, W. The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery. Int. J. Mol. Sci. 2022, 23, 5376. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; He, W.; Zhou, Z.; Diao, Y.; Sui, J.; Li, W. PreS1-targeting chimeric antigen receptor T cells diminish HBV infection in liver humanized FRG mice. Virology 2023, 586, 23–34. [Google Scholar] [CrossRef]
| Factors | Overall (n = 531) | Non-NA Group (n = 293) | NA Group (n = 238) | p-Value ** |
|---|---|---|---|---|
| Age (yr) * | 46 (34–59) | 44 (32–58) | 48 (38–60) | <0.001 |
| Gender (male/female) | 337/194 | 176/117 | 161/77 | 0.090 |
| Platelet count (×104/µL) * | 17.6 (13.6–21.7) | 18.9 (15.7–22.3) | 16.3 (11.9–20.2) | <0.001 |
| Total bilirubin (mg/dL) * | 0.8 (0.6–1.1) | 0.8 (0.6–1.0) | 0.8 (0.6–1.2) | 0.070 |
| AST (U/L) * | 35 (22–70) | 27 (20–45) | 57 (32–96) | <0.001 |
| ALT (U/L) * | 42 (21–105) | 28 (18–63) | 62 (34–166) | <0.001 |
| Albumin (g/dL) * | 4.3 (4–4.6) | 4.4 (4.2–4.7) | 4.2 (3.8–4.4) | <0.001 |
| Prothrombin activity (%) * | 90 (80–99) | 95 (86–104) | 83 (6–117) | <0.001 |
| HBsAg (log IU/mL) * | 3.4 (2.3–4.0) | 3.4 (2.1–4.0) | 3.4 (2.6–3.8) | 0.600 |
| preS1 (ng/mL) * | 84 (13–175) | 55 (6.7–135) | 122 (33–216) | <0.001 |
| preS1/HBsAg * | 0.03 (0.01–0.09) | 0.02 (0.01–0.07) | 0.05 (0.02–0.1) | <0.001 |
| HBeAg (positive/negative/unmeasured) | 76/200/255 | 28/124/141 | 48/76/114 | <0.001 |
| HBV DNA (log IU/mL) * | 4.4 (3.1–6.7) | 3.8 (2.2–6.1) | 5.4 (3.8–7.1) | <0.001 |
| FIB-4 index * | 1.5 (0.9–2.8) | 1.3 (0.8–1.9) | 2.2 (1.2–4.2) | <0.001 |
| AFP | 4.4 (2.5–9.6) | 2.5 (2.5–6.3) | 7.3 (2.7–14) | <0.001 |
| DCP | 17 (13–22) | 17 (14–22) | 17 (13–22) | 0.755 |
| nucleotide/nucleoside analog | ADV:2, | |||
| LAM:79, | ||||
| ETV:143, | ||||
| TDF:8, | ||||
| TAF:6 |
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| p-Value * | HR (95% CI) | p-Value ** | |
| Age, ≥44/<44 | 0.054 | ||
| Gender, male/female | 0.116 | ||
| Platelet count (×104/µL), <17.3/≥17.3 | 0.343 | ||
| Total bilirubin (mg/dL), <0.7/≥0.7 | 0.271 | ||
| AST (U/L), ≥38/<38 | <0.001 | ||
| ALT (U/L), ≥33/<33 | 0.002 | ||
| Albumin (g/dL), <4.3/≥4.3 | 0.110 | ||
| Prothrombin activity (%), <89/≥89 | 0.144 | ||
| HBsAg (log IU/mL), <3.8/≥3.8 | 0.061 | ||
| preS1 (ng/mL), ≥105/<105 | 0.037 | ||
| preS1/HBsAg, ≥0.034/<0.034 | 0.005 | 15.830 (1.854–135.200) | 0.012 |
| HBeAg, positive/negative | 0.512 | ||
| HBV DNA (log IU/mL), ≥6.0/<6.0 | 0.004 | 10.94 (1.932–61.980) | <0.001 |
| FIB-4 index, ≥1.55/<1.55 | 0.007 | 1.581 (0.328–7.632) | 0.568 |
| AFP, ≥5.9/<5.9 | <0.001 | 2.998(0.471–19.090) | 0.245 |
| DCP, ≥18/<18 | 0.936 | ||
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| p-Value * | HR (95% CI) | p-Value ** | |
| Age, ≥47/<47 | 0.012 | ||
| Gender, male/female | 0.154 | ||
| Platelet count (×104/µL), <12.0/≥12.0 | 0.014 | ||
| Total bilirubin (mg/dL), <0.9/≥0.9 | 0.084 | ||
| AST (U/L), ≥71/<71 | 0.190 | ||
| ALT (U/L), ≥111/<111 | 0.142 | ||
| Albumin (g/dL), <4.1/≥4.1 | 0.103 | ||
| Prothrombin activity (%), <92/≥92 | 0.308 | ||
| HBsAg (log IU/mL), <3.5/≥3.5 | 0.002 | ||
| preS1 (ng/mL), ≥171.29/<170.29 | 0.102 | ||
| preS1/HBsAg, ≥0.058/<0.058 | <0.001 | 4.249 (1.179–15.310) | 0.027 |
| HBeAg, positive/negative | 0.406 | ||
| HBV DNA (log IU/mL), ≥4.9/<4.9 | 0.144 | ||
| FIB-4 index, ≥2.75/<2.75 | 0.044 | 1.049 (0.367–3.001) | 0.929 |
| AFP, ≥9.7/<9.7 | 0.019 | 3.249 (1.010–10.450) | 0.058 |
| DCP, ≥16/<16 | 0.884 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosaka, M.; Fujino, H.; Tsuge, M.; Uchikawa, S.; Ono, A.; Murakami, E.; Kawaoka, T.; Miki, D.; Hayes, C.N.; Oka, S. The Hepatitis B Virus PreS1/HBsAg Ratio Is a Predictive Marker for the Occurrence of Hepatocellular Carcinoma. Livers 2024, 4, 364-376. https://doi.org/10.3390/livers4030026
Kosaka M, Fujino H, Tsuge M, Uchikawa S, Ono A, Murakami E, Kawaoka T, Miki D, Hayes CN, Oka S. The Hepatitis B Virus PreS1/HBsAg Ratio Is a Predictive Marker for the Occurrence of Hepatocellular Carcinoma. Livers. 2024; 4(3):364-376. https://doi.org/10.3390/livers4030026
Chicago/Turabian StyleKosaka, Masanari, Hatsue Fujino, Masataka Tsuge, Shinsuke Uchikawa, Atsushi Ono, Eisuke Murakami, Tomokazu Kawaoka, Daiki Miki, C. Nelson Hayes, and Shiro Oka. 2024. "The Hepatitis B Virus PreS1/HBsAg Ratio Is a Predictive Marker for the Occurrence of Hepatocellular Carcinoma" Livers 4, no. 3: 364-376. https://doi.org/10.3390/livers4030026
APA StyleKosaka, M., Fujino, H., Tsuge, M., Uchikawa, S., Ono, A., Murakami, E., Kawaoka, T., Miki, D., Hayes, C. N., & Oka, S. (2024). The Hepatitis B Virus PreS1/HBsAg Ratio Is a Predictive Marker for the Occurrence of Hepatocellular Carcinoma. Livers, 4(3), 364-376. https://doi.org/10.3390/livers4030026






