Predominance of Genotype 5 Hepatitis Delta Virus Infection in a Portuguese Hepatology Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Population
2.2. Sample Collection, HDV RNA Detection and Genotype Assessment
2.3. Epidemiological and Clinical Characterization of Participants
2.4. Statistical Analysis
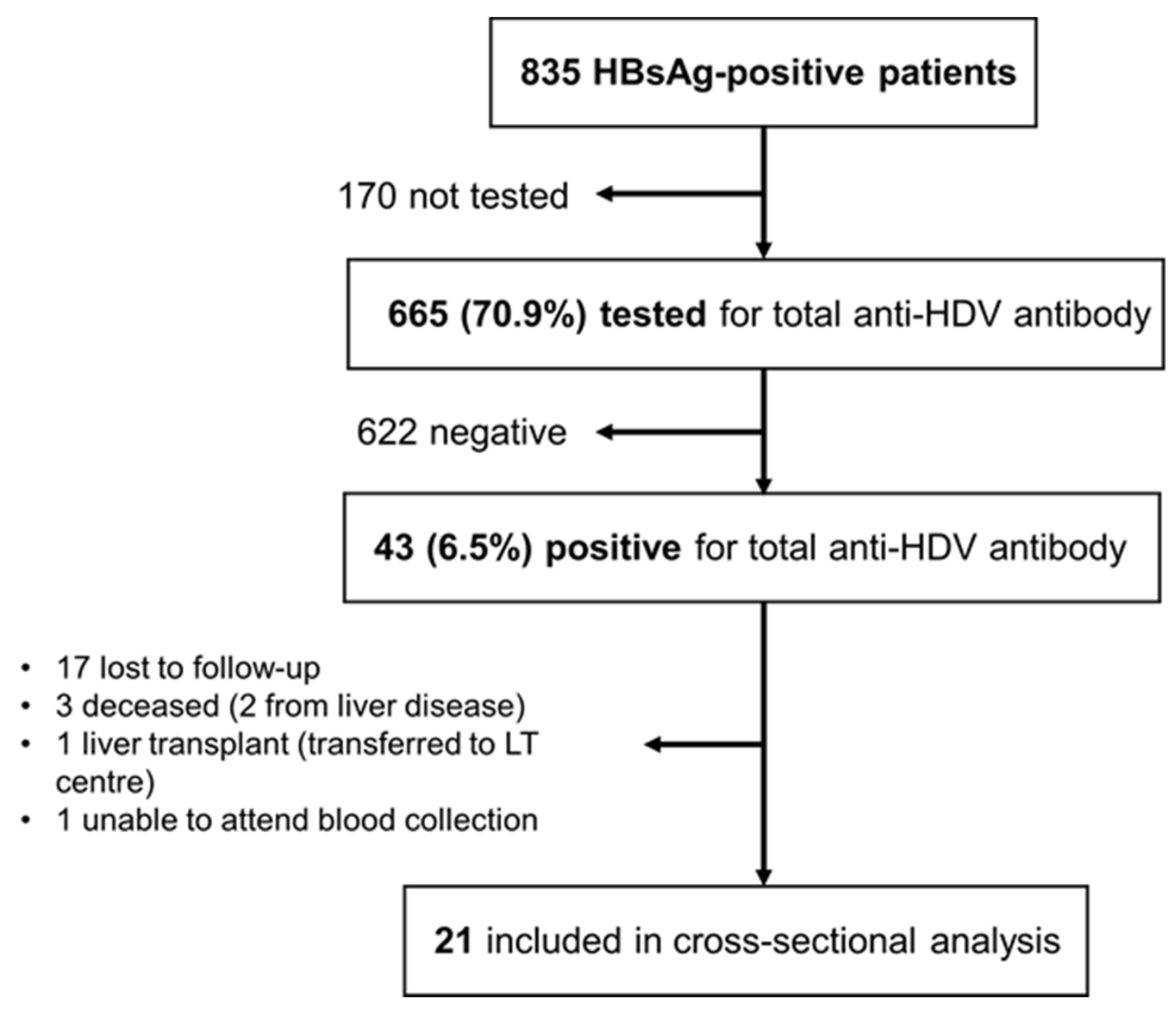
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunetto, M.R.; Ricco, G.; Negro, F.; Wedemeyer, H.; Yurdaydin, C.; Asselah, T.; Papatheodoridis, G.; Gheorghe, L.; Agarwal, K.; Farci, P.; et al. EASL Clinical Practice Guidelines on hepatitis delta virus. J. Hepatol. 2023, 79, 433–460. [Google Scholar] [CrossRef]
- Fattovich, G. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. Gut 2000, 46, 420–426. [Google Scholar] [CrossRef]
- Alfaiate, D.; Clément, S.; Gomes, D.; Goossens, N.; Negro, F. Chronic hepatitis D and hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. J. Hepatol. 2020, 73, 533–539. [Google Scholar] [CrossRef]
- Hughes, S.A.; Wedemeyer, H.; Harrison, P.M. Hepatitis delta virus. Lancet 2011, 378, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Da, B.L.; Heller, T.; Koh, C. Hepatitis D infection: From initial discovery to current investigational therapies. Gastroenterol. Rep. 2019, 7, 231–245. [Google Scholar] [CrossRef]
- Wranke, A.; Heidrich, B.; Deterding, K.; Hupa-Breier, K.L.; Kirschner, J.; Bremer, B.; Cornberg, M.; Wedemeyer, H. Clinical long-term outcome of hepatitis D compared to hepatitis B monoinfection. Hepatol Int. 2023, 17, 1359–1367. [Google Scholar] [CrossRef]
- Wranke, A.; Heidrich, B.; Deterding, K.; Hupa-Breier, K.L.; Kirschner, J.; Bremer, B.; Cornberg, M.; Wedemeyer, H. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Shen, D.-T.; Ji, D.-Z.; Han, P.-C.; Zhang, W.-M.; Ma, J.-F.; Chen, W.-S.; Goyal, H.; Pan, S.; Xu, H.-G. Prevalence and burden of hepatitis D virus infection in the global population: A systematic review and meta-analysis. Gut 2019, 68, 512–521. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, S.; Ou, X.; Li, S.; Ma, Z.; Wang, W.; Peppelenbosch, M.P.; Liu, J.; Pan, Q. Estimating the global prevalence, disease progression, and clinical outcome of hepatitis delta virus infection. J. Infect. Dis. 2020, 221, 1677–1687. [Google Scholar] [CrossRef]
- Geneva: World Health Organization. HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 13 June 2024).
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. 2024. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 13 June 2024).
- Dény, P. Hepatitis Delta Virus Genetic Variability: From Genotypes I, II, III to Eight Major Clades? Curr. Top. Microbiol. Immunol. 2006, 307, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wranke, A.; Borzacov, L.M.P.; Parana, R.; Lobato, C.; Hamid, S.; Ceausu, E.; Dalekos, G.N.; Rizzetto, M.; Turcanu, A.; Niro, G.A.; et al. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: The Hepatitis Delta International Network (HDIN). Liver Int. 2018, 38, 842–850. [Google Scholar] [CrossRef]
- Roulot, D.; Brichler, S.; Layese, R.; BenAbdesselam, Z.; Zoulim, F.; Thibault, V.; Scholtes, C.; Roche, B.; Castelnau, C.; Poynard, T.; et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J. Hepatol. 2020, 73, 1046–1062. [Google Scholar] [CrossRef]
- Spaan, M.; Carey, I.; Bruce, M.; Shang, D.; Horner, M.; Dusheiko, G.; Agarwal, K. Hepatitis delta genotype 5 is associated with favourable disease outcome and better response to treatment compared to genotype 1. J. Hepatol. 2020, 72, 1097–1104. [Google Scholar] [CrossRef]
- Rizzetto, M.; Hamid, S.; Negro, F. The changing context of hepatitis D. J. Hepatol. 2021, 74, 1200–1211. [Google Scholar] [CrossRef]
- Wranke, A.; Heidrich, B.; Ernst, S.; Calle Serrano, B.; Caruntu, F.A.; Curescu, M.G.; Yalcin, K.; Gürel, S.; Zeuzem, S.; Erhardt, A.; et al. Anti-HDV IgM as a Marker of Disease Activity in Hepatitis Delta. PLoS ONE. 2014, 9, e101002. [Google Scholar] [CrossRef]
- Zachou, K.; Yurdaydin, C.; Drebber, U.; Dalekos, G.N.; Erhardt, A.; Cakaloglu, Y.; Degertekin, H.; Gurel, S.; Zeuzem, S.; Bozkaya, H.; et al. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int. 2010, 30, 430–437. [Google Scholar] [CrossRef]
- Ebik, B.; Cangul, M.S.; Yalçin, K. What does quantitative HBsAg level mean in chronic hepatitis D infection? Eur. J. Gastroenterol. Hepatol. 2023, 35, 320–326. [Google Scholar] [CrossRef]
- Palom, A.; Rando-Segura, A.; Vico, J.; Pacín, B.; Vargas, E.; Barreira-Díaz, A.; Rodríguez-Frías, F.; Riveiro-Barciela, M.; Esteban, R.; Buti, M. Implementation of anti-HDV reflex testing among HBsAg-positive individuals increases testing for hepatitis D. JHEP Rep. 2022, 4, 100547. [Google Scholar] [CrossRef]
- Razavi, H.A.; Buti, M.; Terrault, N.A.; Zeuzem, S.; Yurdaydin, C.; Tanaka, J.; Aghemo, A.; Akarca, U.S.; Al Masri, N.M.; Alalwan, A.M.; et al. Hepatitis D double reflex testing of all hepatitis B carriers in low-HBV- and high-HBV/HDV-prevalence countries. J. Hepatol. 2023, 79, 576–580. [Google Scholar] [CrossRef]
- Le Gal, F.; Brichler, S.; Sahli, R.; Chevret, S.; Gordien, E. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology 2016, 64, 1483–1494. [Google Scholar] [CrossRef]
- Zauli, D.; Crespi, C.; Bianchi, F.B.; Craxi, A.; Pisi, E. Autoimmunity in Chronic Liver Disease Caused by Hepatitis Delta Virus. J. Clin. Pathol. 1986, 39, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Obermayer-Straub, P.; Manns, M.P. Hepatitis C and D, Retroviruses and Autoimmune Manifestations. J. Autoimmun. 2001, 16, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Hermanussen, L.; Lampalzer, S.; Bockmann, J.H.; Ziegler, A.E.; Piecha, F.; Dandri, M.; Pischke, S.; Haag, F.; Lohse, A.W.; Lütgehetmann, M.; et al. Non-organ-specific autoantibodies with unspecific patterns are a frequent para-infectious feature of chronic hepatitis D. Front. Med. 2023, 10, 1169096. [Google Scholar] [CrossRef]
- Zachou, K.; Yurdaydin, C.; Drebber, U.; Schlaphoff, V.; Dienes, H.; Manns, M.; Wedemeyer, H.; Dalekos, G. Impact of organ and non-organ-specific autoantibodies on the treatment outcome of patients with hepatitis D virus infection. J. Hepatol. 2015, 62, S531–S532. [Google Scholar] [CrossRef]
- Vergani, D.; Mieli-Vergani, G. Autoimmune manifestations in viral hepatitis. Semin. Immunopathol. 2013, 35, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, L.; Wedemeyer, H. Interferon-based treatment of chronic hepatitis D. Liver Int. 2023, 43, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, F.; Velosa, J.; Carneiro de Moura, M. Infecção com o agente delta na doença hepática crónica e carcinoma hepatocelular. Um achado pouco frequente em Portugal. J. Médico. 1985, 118, 405–409. [Google Scholar]
- Velosa, J.; Ramalho, F.; Serejo, F.; Marinho, R.; Carneiro de Moura, M. Hepatite crónica: O espectro etiológico. Acta Med. Port. 1993, 6, 233–238. [Google Scholar] [PubMed]
- Ramalho, F.; Carvalho, G.; Bonino, F.; Baptista, A.; Carneiro de Moura, M. Clinical and epidemiological significance of hepatitis delta virus (HDV) infection in chronic HBV carriers in Portugal. Prog. Clin. Biol. Res. 1987, 234, 409–417. [Google Scholar] [PubMed]
- Carneiro de Moura, M.; Gameiro, R.; Matos, L.; Macedo, G.; Areias, J.; Carvalho, A.; Moneiro, E.; Rodrigues, B.; Marcelino, M. Hepatite crónica B em Portugal: Estudo multicêntrico de 735 doentes observados em Hospitais do Serviço Nacional de Saúde. In Proceedings of the “8ª Reunião da Associação Portuguesa Para o Estudo do Fígado”, Cascais, Portugal, 3–6 April 2008. [Google Scholar]
- Gamelas, V.; Saraiva, R.; Silva, M.J.; Corte-Real, R.; Calinas, F. Aspectos epidemiológicos da infecção por vírus da hepatite delta em Portugal. In Proceedings of the “Congresso Português de Hepatologia 2021”, Fátima, Portugal, 27–29 May 2021. [Google Scholar]
- Garrido, I.; Liberal, R.; Teixeira, S.; Koch, C.; Macedo, G. Hepatite delta em Portugal. In Proceedings of the “Congresso Português de Hepatologia 2022”, Fátima, Portugal, 28–30 April 2022. [Google Scholar]
- Ivaniushina, V.; Radjef, N.; Alexeeva, M.; Gault, E.; Semenov, S.; Salhi, M.; Kiselev, O.; Dény, P. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J. Gen. Virol. 2001, 82, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Usman, Z.; Velkov, S.; Protzer, U.; Roggendorf, M.; Frishman, D.; Karimzadeh, H. HDVdb: A Comprehensive Hepatitis D Virus Database. Viruses 2020, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.F.; Carvalho, R.; Correia, F.P.; Branco, J.C.; Nuno Costa, M.; Martins, A. Autoimmune Hepatitis Induced by Hepatitis Delta Virus: A Conundrum. GE Port. J. Gastroenterol. 2024, 31, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Shearer, D.; Child, H.; Razavi-Shearer, K.; Voeller, A.; Razavi, H.; Buti, M.; Tacke, F.; Terrault, N.; Zeuzem, S.; Abbas, Z.; et al. Adjusted estimate of the prevalence of hepatitis delta virus in 25 countries and territories. J. Hepatol. 2024, 80, 232–242. [Google Scholar] [CrossRef]
- Degasperi, E.; Anolli, M.P.; Jachs, M.; Reiberger, T.; de Lédinghen, V.; Metivier, S.; D’Offizi, G.; De Maria, F.; Schramm, C.; Schmidt, H.; et al. WED-391 Long-term virological and clinical outcomes of patients with HDV-related compensated cirrhosis treated with bulevirtide monotherapy for up to 120 weeks: A retrospective multicenter european study (SAVE-D). J. Hepatol. 2024, 80, S793–S794. [Google Scholar] [CrossRef]
- Lampertico, P.; Roulot, D.; Wedemeyer, H. Bulevirtide with or without pegIFNα for patients with compensated chronic hepatitis delta: From clinical trials to real-world studies. J. Hepatol. 2022, 77, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Gigi, E.; Lagopoulos, V.; Liakos, A. Management of autoimmune hepatitis induced by hepatitis delta virus. World J. Gastroenterol. 2024, 30, 799–805. [Google Scholar] [CrossRef] [PubMed]
| Area and Country of Origin | HDV Seroprevalence–Positive/Total (%) |
|---|---|
| Europe | 10/258 (3.9%) |
| Portugal | 7/232 (3.0%) |
| Central and Eastern Europe | 3/25 (12.0%) |
| Romania | 1/14 (7.1%) |
| Ukraine | 2/6 (33.3%) |
| Bulgaria | 0/2 (0.0%) |
| Croatia | 0/1 (0.0%) |
| Latvia | 0/1 (0.0%) |
| Russia | 0/1 (0.0%) |
| Luxembourg | 0/1 (0.0%) |
| Africa | 32/387 (8.3%) |
| Guinea-Bissau | 29/143 (20.3%) |
| Cape Verde | 1/99 (1.0%) |
| Angola | 0/84 (0.0%) |
| São Tomé and Príncipe | 0/51 (0.0%) |
| Mozambique | 0/5 (0.0%) |
| Senegal | 1/3 (33.3%) |
| Equatorial Guinea | 1/1 (100%) |
| Gambia | 0/1 (0.0%) |
| Asia | 0/12 (0.0%) |
| China | 0/5 (0.0%) |
| Pakistan | 0/3 (0.0%) |
| India | 0/2 (0.0%) |
| Nepal | 0/1 (0.0%) |
| Bangladesh | 0/1 (0.0%) |
| South America | 1/8 (12.5%) |
| Brazil | 1/7 (14.3%) |
| Paraguay | 0/1 (0.0%) |
| Total | 43/665 (6.5%) |
| Age—M ± SD years | 41.2 ± 9.9 |
| Male—n (%) | 12 (57.1%) |
| HBeAg-negative at diagnosis—n (%) | 18 (85.7%) |
| Anti-HDV IgM positive—n (%) | 1 (4.8%) |
| Cirrhosis *—n (%) | 7 (33.3%) |
| Median liver stiffness (kPa) | 7.8 |
| Treatment with nucleos(t)ide analogs—n (%) | 15 (71.4%) |
| Previous treatment with peginterferon **—n (%) | 7 (33.3%) |
| HDV RNA positivity—n (%) | 8 (38.1%) |
| HDV genotype 1/genotype 5—n (%) | 1 (12.5%)/7 (87.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, M.F.; Coelho, H.; Branco, J.C.e.; Bragança, S.; Alexandrino, G.; Costa, M.N.; Carvalho, R.; Pádua, E.; Martins, A. Predominance of Genotype 5 Hepatitis Delta Virus Infection in a Portuguese Hepatology Unit. Livers 2024, 4, 388-397. https://doi.org/10.3390/livers4030028
Cardoso MF, Coelho H, Branco JCe, Bragança S, Alexandrino G, Costa MN, Carvalho R, Pádua E, Martins A. Predominance of Genotype 5 Hepatitis Delta Virus Infection in a Portuguese Hepatology Unit. Livers. 2024; 4(3):388-397. https://doi.org/10.3390/livers4030028
Chicago/Turabian StyleCardoso, Mariana Ferreira, Henrique Coelho, Joana Carvalho e Branco, Sofia Bragança, Gonçalo Alexandrino, Mariana Nuno Costa, Rita Carvalho, Elizabeth Pádua, and Alexandra Martins. 2024. "Predominance of Genotype 5 Hepatitis Delta Virus Infection in a Portuguese Hepatology Unit" Livers 4, no. 3: 388-397. https://doi.org/10.3390/livers4030028
APA StyleCardoso, M. F., Coelho, H., Branco, J. C. e., Bragança, S., Alexandrino, G., Costa, M. N., Carvalho, R., Pádua, E., & Martins, A. (2024). Predominance of Genotype 5 Hepatitis Delta Virus Infection in a Portuguese Hepatology Unit. Livers, 4(3), 388-397. https://doi.org/10.3390/livers4030028







