Polysaccharides: The Sustainable Foreground in Energy Storage Systems
Abstract
:1. Introduction
2. Plant-Derived Polysaccharides
2.1. Lignocellulosic Biomass (Cellulose, Lignin, and Hemicelluloses)
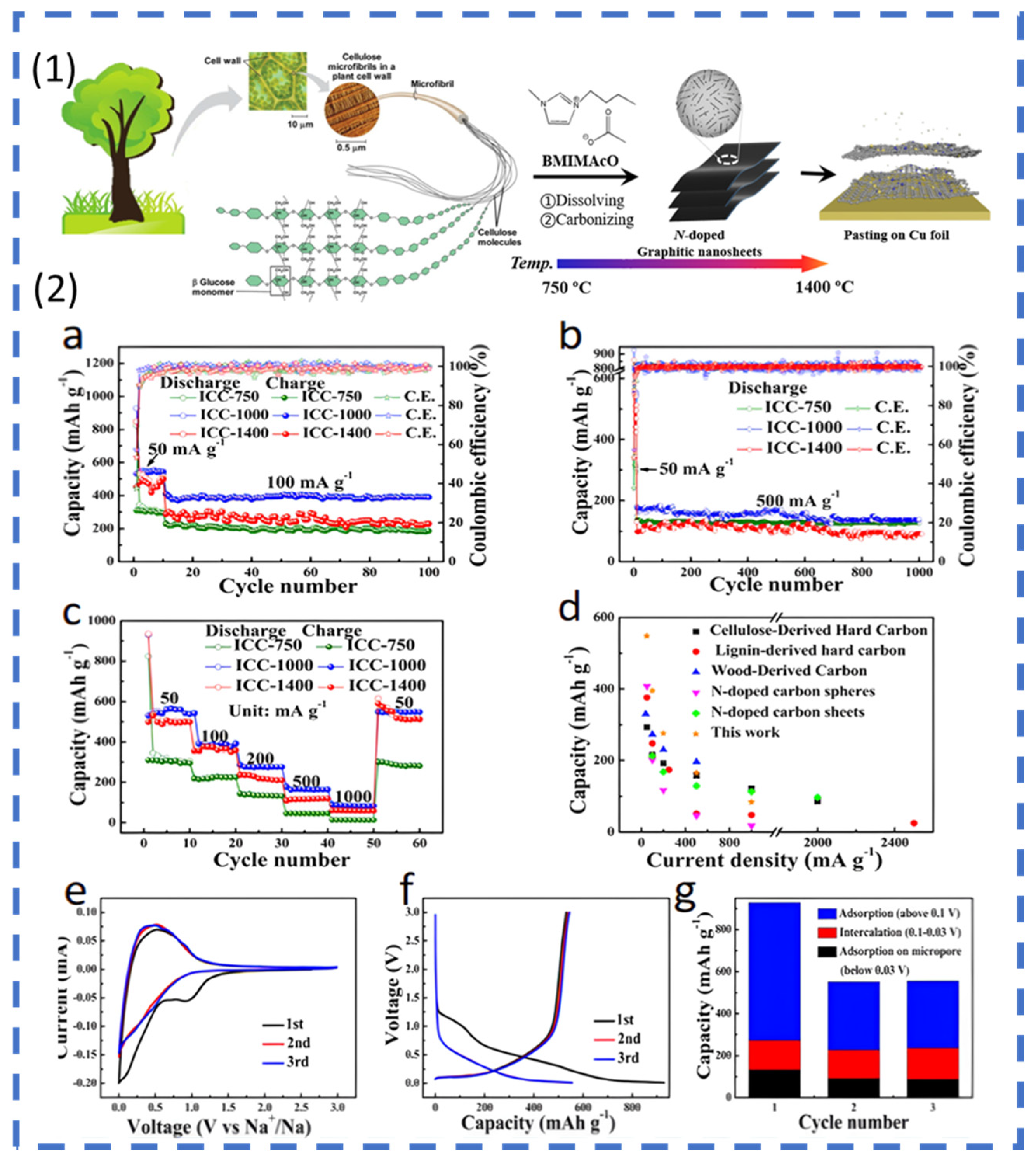
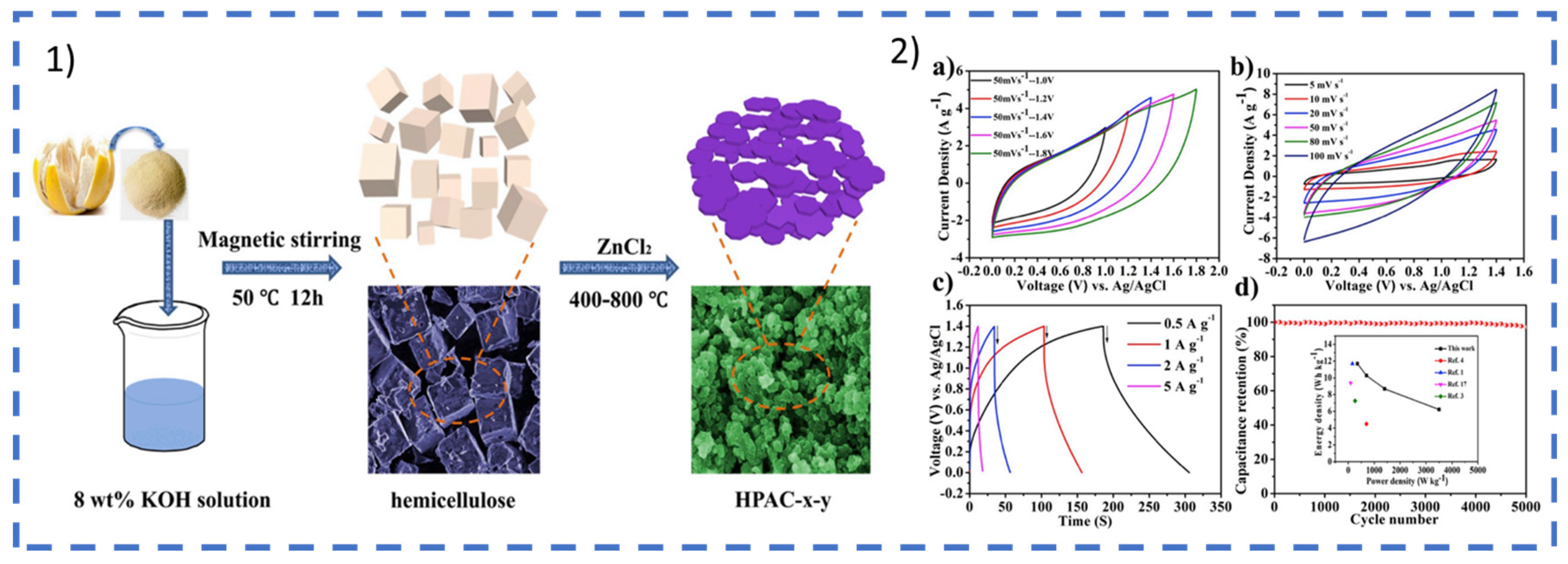
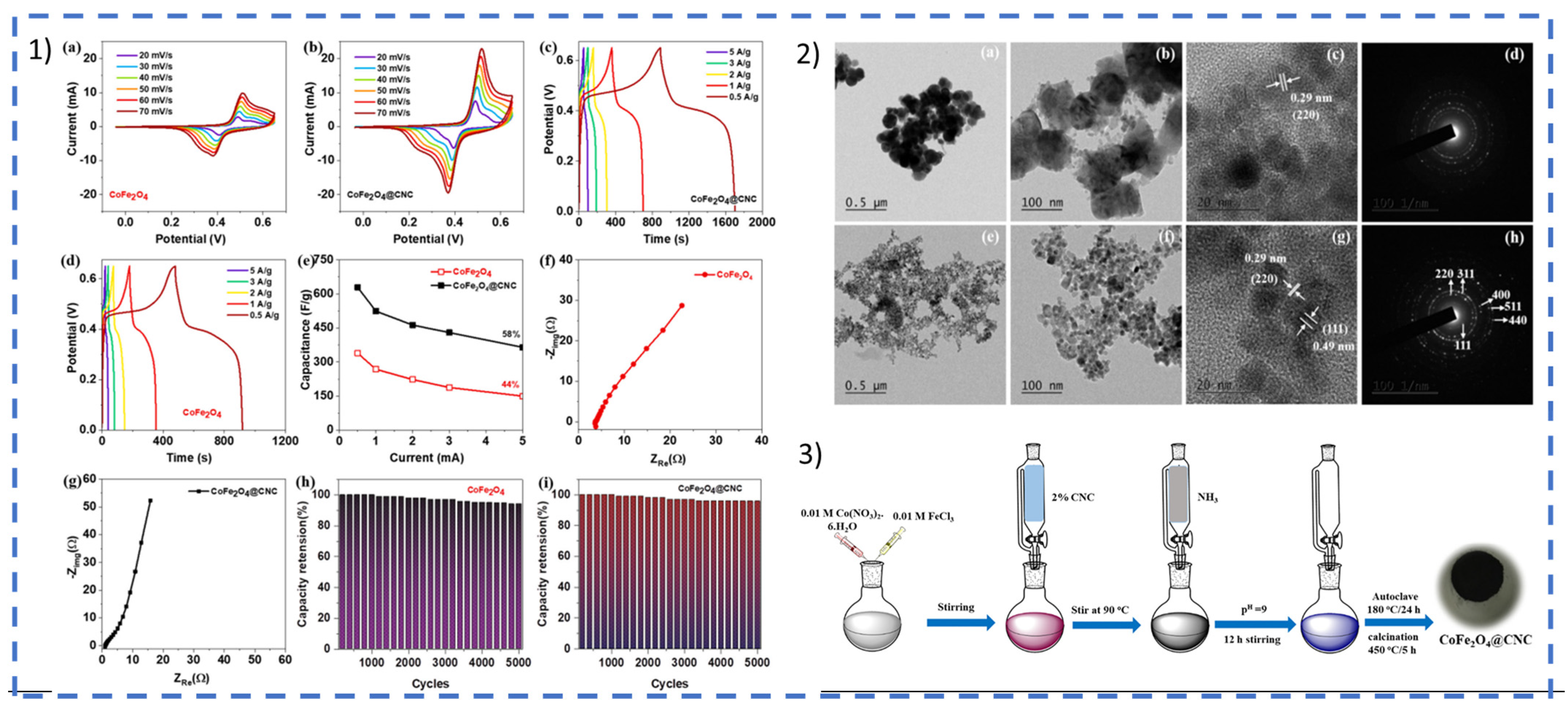
2.1.1. ESSs Based on Lignocellulosic Biomass
Active Material (Carbon Precursors)
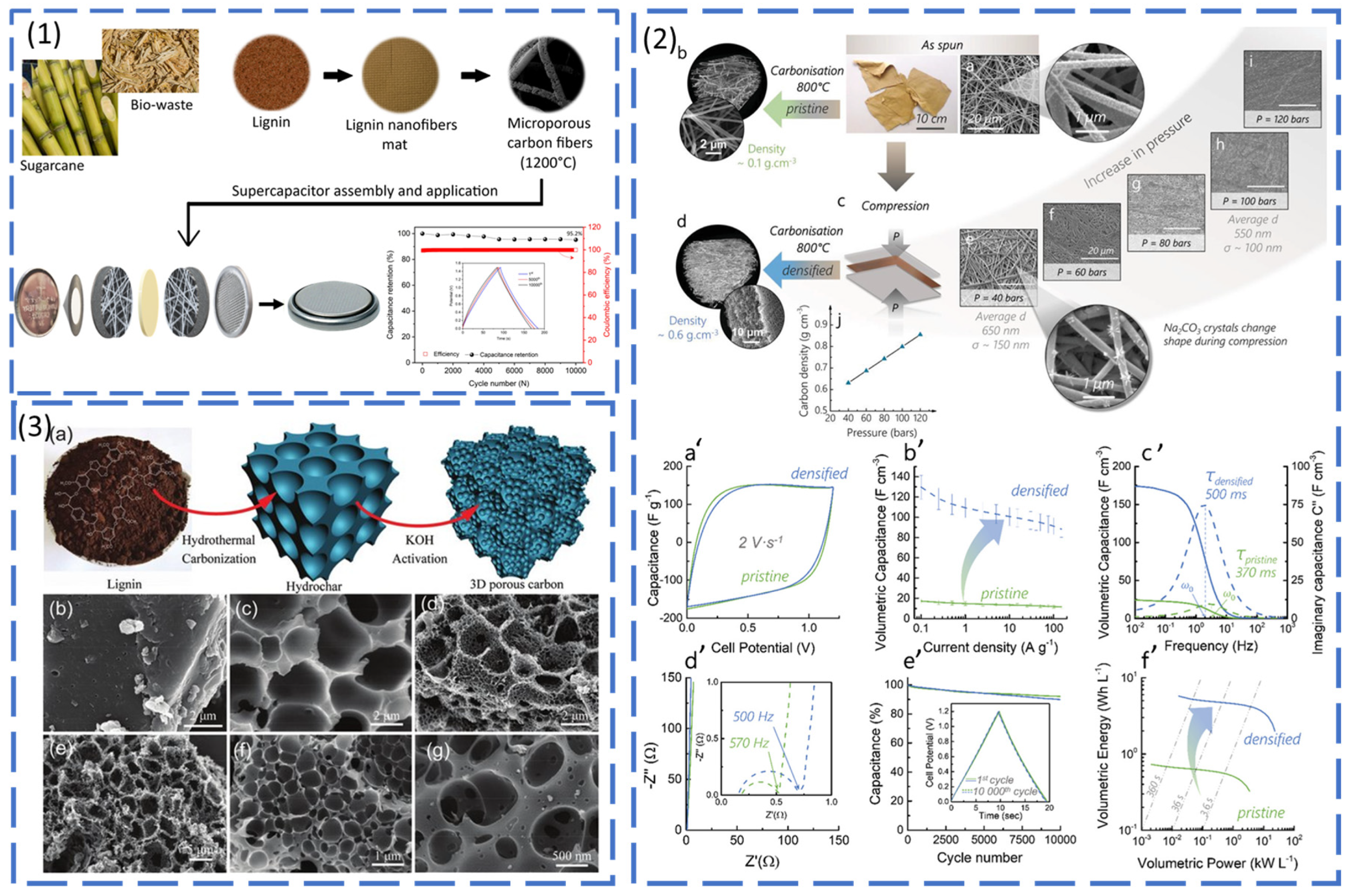
Binders
Electrolytes
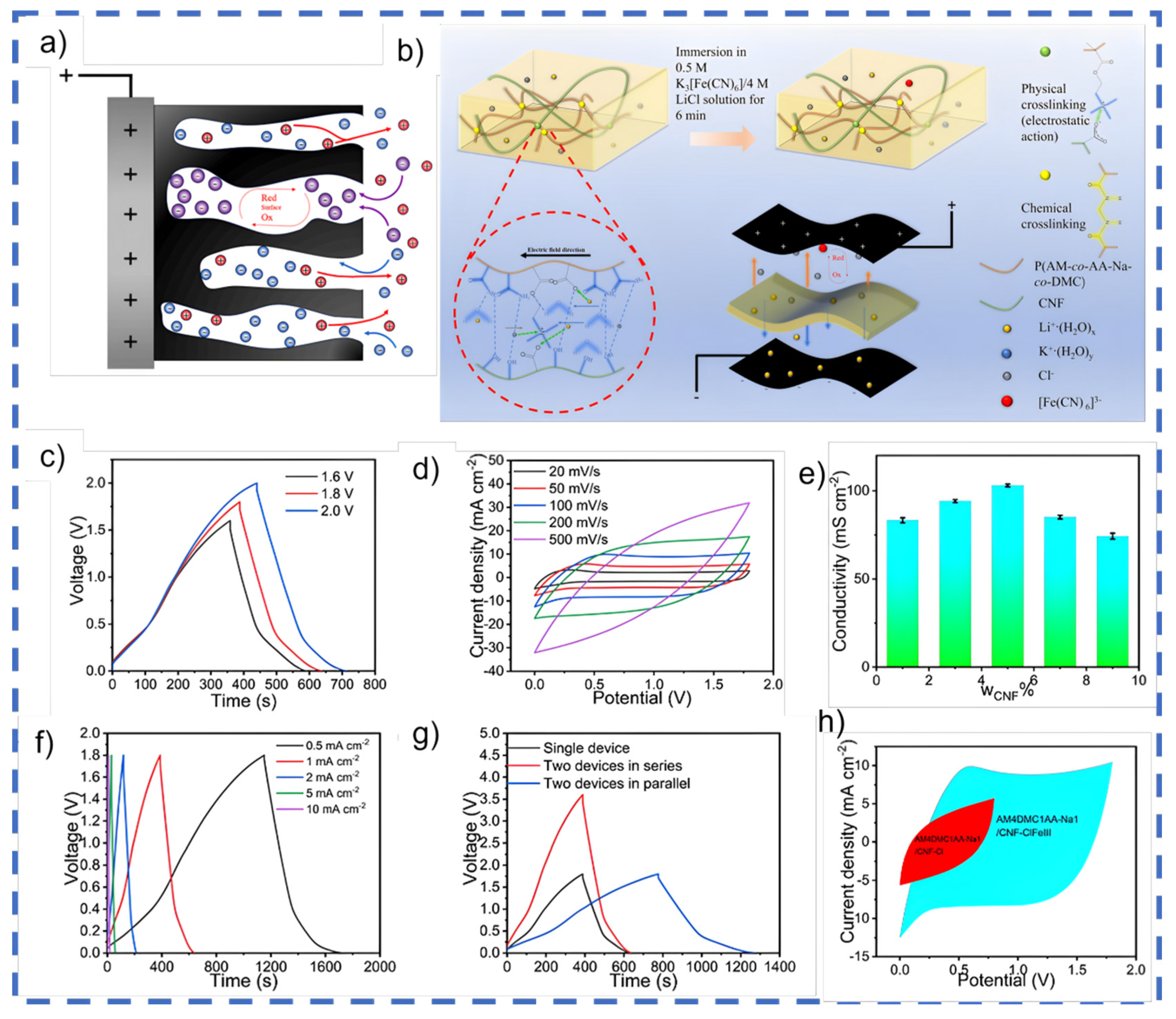
Separators
| Ref | Polysaccharide (Modified Form) | Functionality | Energy Density | Power Density | Specific Capacity/ Specific Capacitance | ESS |
|---|---|---|---|---|---|---|
| [46] | Lignin/Nanocellulose | Active material | 11.7 W h kg−1 | 349.9 W kg−1 | 216.2 F g−1 at 0.5 A g−1 | SC |
| [47] | Lignin (lignin carbon nanocomposite | Cathode | 23 W h kg−1 | 610 W kg−1 | 75 mAh g−1 | Zn–lignin BAT |
| [39] | Lignin (heteroatom-doped) | Porous carbon | - | - | 348 F g−1 at 1 A g−1 | SC |
| [49] | Lignin (lignin carbon nanofiber) | Active material | 1.77 W h kg−1 | 156 kW kg−1 | 11.95 F g−1 | SC |
| [85] | Lignin (lignin carbon nanofiber) | Active material | 2245 μW h cm−3 | 60 kW kg−1 | - | SC |
| [51] | Lignin (lignin carbon nanofiber) | Active material | 6 W h L−1 | - | 130 F cm−3 | SC |
| [86] | Lignin (lignin carbon nanofiber) | Active material | 37.1 W h kg−1 | 400 W kg−1 | 442.2 F g−1 | SC |
| [50] | Lignin (lignin carbon nanofiber) | Anode | - | - | 1783.8 mA h g−1 at 0.2 C | Li-ion BAT |
| 453.2 mA h g1 at 0.2 C | Na-ion BAT | |||||
| [87] | Lignin (lignin carbon nanofiber) | Anode | - | - | 330 mA h g1 at 100 mA g−1 | Na-ion BAT |
| [56] | Commercial biochar pellet (bio-graphite) | Anode | - | - | 293 mA h g−1 at 20 mA g−1 | Li-ion BAT |
| [88] | Biochar from olive tree and spent malt rootlets | Electrocatalysts | - | - | - | Zn-air BAT |
| [89] | Biochar(tetra-heteroatom-doped N, O, S, Cl) | Active carbon | 30.2 W h kg−1 | 164.0 W kg−1 | 638 F g−1 at 0.5 A g−1 | SC |
| [60] | IL-induced microcrystalline cellulose biochar | Anode | - | - | 391 mA h g−1 at 100 mA g−1 | Na-ion BAT |
| [61] | Chiral nematic nanocrystalline cellulose-based biochar | Anode | - | - | 314 mA h g−1 at 0.1 C | Na-ion BAT |
| [62] | Hemicellulose (porous AC) | Porous active material | 11.7 W h kg−1 | 349.9 W kg−1 | 302.4 F g−1 at 0.5 A g−1 | SC |
| [63] | Soybean dreg | Honeycomb-like carbonaceous material | 23.4 W h kg−1 | 225 W kg−1 | 281.4 F g–1 at 0.5 A g–1 | Quasi-solid-state SC |
| 247.6 F cm–3 at 0.5 A g–1 | ||||||
| [14] | Biomass (forestry waste) | Anode | - | - | 370 mA h g–1 at 100 mA g–1 | Li-ion BAT |
| Anode | 147.7 mA h g–1 at 50 mA g–1 | Na-ion BAT | ||||
| [28] | Cellulose (HCs) | Anode | - | - | 334.9 mA h g−1 at 30 mA g−1 | Na-ion BAT |
| [90] | Glucose, sucrose, maltose, cellulose, glycogen, and amylopectin (HCs) | Anode | - | - | 353 and 290 mA h g−1 at 25 mA g−1 | Na and K-ion BATs |
| [66] | Renewable cotton (HCs) | Anode | - | - | 315 mA h g−1 at 0.1 C | Na-ion BAT |
| [67] | Cellulose (HCs) | Anode | - | - | 310 mA h g−1 at C/10 rate | Na-ion BAT |
| [68] | Regenerated spherical cellulose (HCs) | Anode | - | - | 300 mA h g−1 at 0.1 C | Li-ion BAT |
| [71] | Cellulose nanofibers | Binder | 2.9 Wh kg−1 | 21.3 kW kg−1 | 268.4 F g−1 at 5 A g−1 | SC |
| [72] | Cellulose nanocrystals (nanocomposite) | Template for electrode | 17.9 Wh kg−1 | 530.2 W kg−1 | 629 F g−1 at 0.5 A g−1 | SC |
| [74] | Cellulose | Hydrogel electrolyte | 20.4 Wh kg−1 | 194 W kg−1 | 255 F g−1 | Flexible SC |
| [75] | Cellulose (cellulose-polyacrylamide double network) | Hydrogel electrolyte | 0.09 mWh m−2 | 4.5 mW cm−2 | 989 mF cm−2 at 2 mA cm−2 | Flexible solid-state SC |
| [27] | PAM–hydroxy propyl cellulose hydrogel | Porous carbon electrode | 17.2 W h kg–1 | 550 W kg–1 | 102.5 F g–1 at 0.5 A g–1 | SC |
| [76] | Cellulose nanofibers | Hydrogel electrolyte | 139.65 mWh cm−2 | 0.88 mW cm−2 | 396.30 mF cm−2 | Flexible SC |
| [77] | Carboxymethylated cellulose nanofibers (NC-ionogel) | GPE | - | - | 160 F g−1 | SC |
| [70] | MXene-bacterial cellulose | Flexible electrodes | 15μW h cm−2 | 41 μW cm−2 | 346 mF cm−2 | SC |
| [30] | Bacterial cellulose (N/O/S-doped) | Carbon electrode | 5.14 Wh/kg | 5000 W/kg | 268.2 F/g at 0.5 A/g | SC |
| [29] | Bacterial Cellulose/MXene | Mesoporous Electrodes | 9.63 Wh kg–1 | 250 W kg–1. | 594 F g–1 at 1 A g–1 | SC |
| [78] | Cellulose acetate (nanocomposite polyvinyl alcohol/cellulose acetate) | GPE | 0.39 Wh kg−1 | 2.84 W kg−1 | 26.23 F g−1 (Electrode) | SC |
| 8.01 F g−1 (EDLC) | ||||||
| [79] | CMC | Hydrogel electrolyte | 63.3 Wh kg−1 | - | 309 F g−1 | Quasi-solid-state SC |
| [81] | Cellulose acetate and oxidized carboxymethyl cellulose | Solid and GPE | - | - | 230 mA h g−1 | Li-ion BAT |
| [83] | Cellulose | Porous membrane separator | - | - | 947 mAh g−1 | LiS BAT |
| [84] | Phosphorylated CNF | Separator | - | - | 5.37 mA h cm−2 | LiS BAT |
| [7] | Cellulose | Separator | 33.4 Wh kg−1 | 46 W kg−1 | 25 mAh g−1 | Li structural BAT |
| [3] | Rice husk | Porous carbon | - | - | 1032 mA h g−1 at 0.1 C | Li–S BAT |
2.2. Seaweed Polysacharides: Alginate, Agar and Gums
2.2.1. ESSs Based on Alginate
Electrolytes
Separators

Active Material (Carbon Precursors)
2.2.2. ESSs Based on Agar and Gums
Active Material (Carbon Precursors)
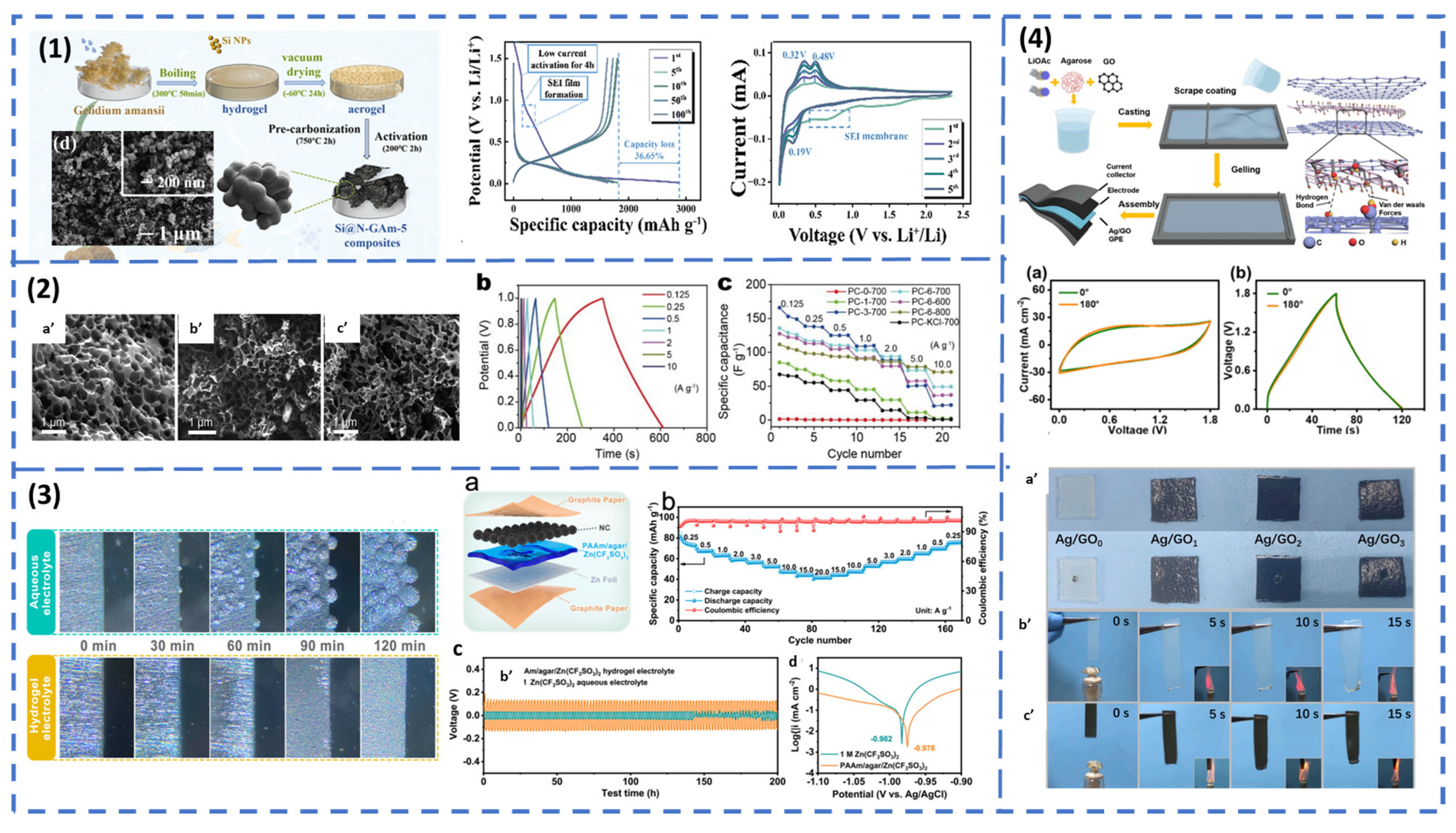
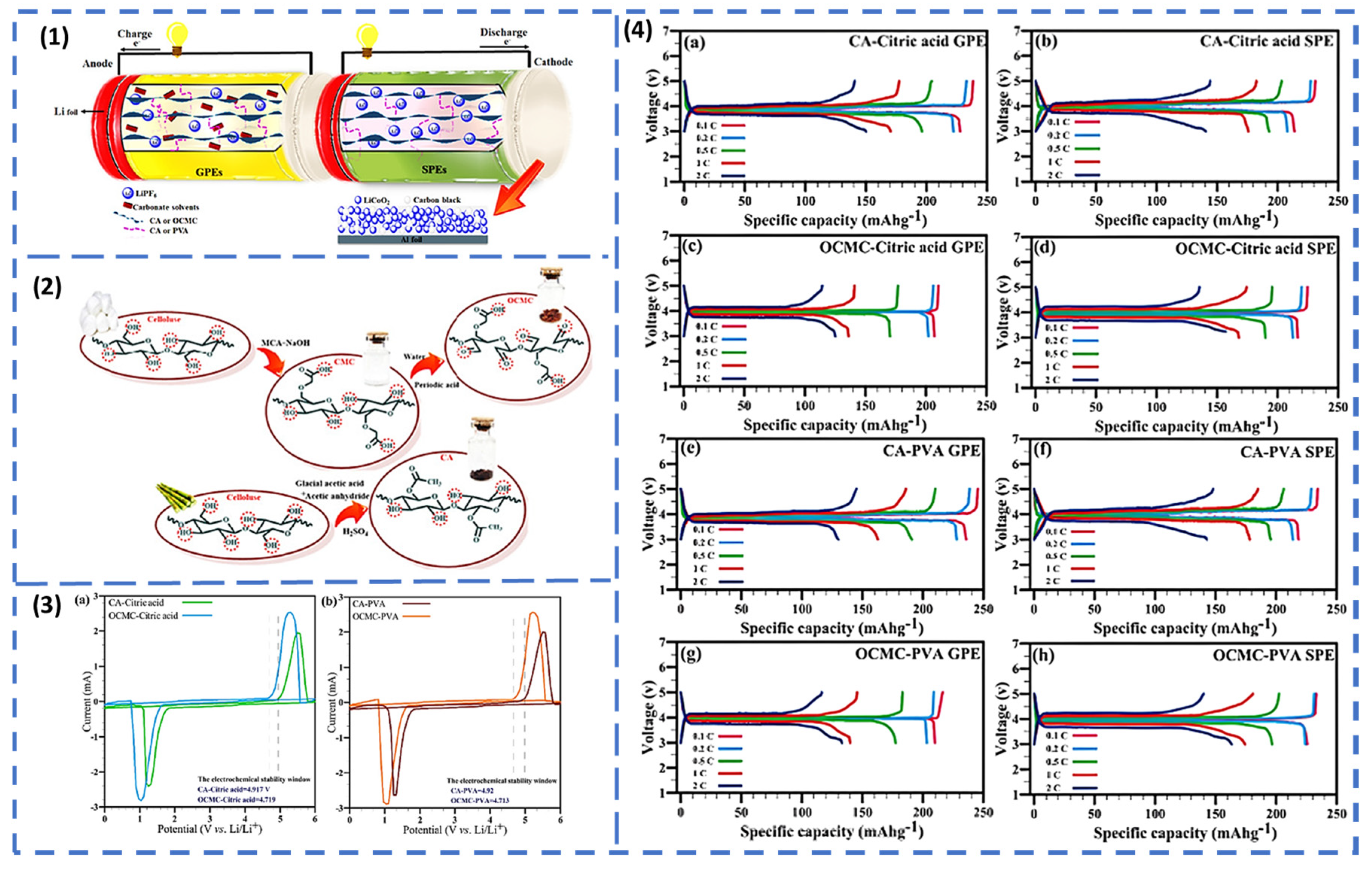
Electrolytes
Binders
| Ref | Polysaccharide (Modified Form) | Functionality | Energy Density | Power Density | Specific Capacity/ Specific Capacitance | ESS |
|---|---|---|---|---|---|---|
| [4] | Kelp (sodium alginate) | Hydrogel electrolyte, membrane separator and binder | 8 W h kg−1 | 25 W kg−1 | 277 F g−1 and 88.2 F cm−3 | solid-state SC |
| 2.5 W h L−1 | 8 W L−1 | |||||
| [19] | Alginate (mussel-inspired catechol conjugation) | Binder | - | - | 600 mAh g −1 | Li-ion BAT |
| [98] | Alginate (alginate/PEDOT:PSS) | Hydrogel electrolyte | 40.08 Wh kg−1 | 400.35 W kg−1 | 356 F g−1 at 100 mV/s g−1 | Flexible solid-state SC |
| [102] | Sodium alginate (sodium alginate/attapulgite) | Separator | - | - | 115 mAh g−1 at 5 C | Li-ion BAT |
| [103] | Sodium alginate/PMIA | Separator | - | - | 101.1 mAh g−1 at 2 C | Li-ion BAT |
| [104] | Alginate fiber-grafted polyetheramine | Separator | - | - | 100 mAh g–1 at 2 C | Li-Metal BAT |
| [105] | Cellulose and calcium Alginate fiber | Separator | - | - | 160 mAh/g at 0.5 C | Li-ion BAT |
| [106] | Alginate | Separator | - | - | 151 mAh g−1 at 1 C | Li BAT |
| [111] | Sodium alginate | porous carbon aerogel | 28 W h kg−1 | 400 W kg−1 | 204 F g−1 at 0.2 A g−1 | SC |
| [110] | Alginate nanofibers from seaweed (N-doped graphitic nanofibers) | Electrocatalyst and anode material | - | - | 625 mAh g−1 at 1 A g−1 and 197 F g−1 at 1 A g−1 | Li-ion BAT and SC |
| [108] | Sodium alginate /melamine fibers | Electrode/gel electrolyte /separator | 20.87 Wh kg−1 | 4000 W kg−1 | 441.80 F g−1 at 0.5 A/g | Solid-state SC |
| [113] | Alginate (polyurea-crosslinked nitrogen/metal co-doped alginate) | Carbon aerogel | 6.5 Wh kg−1 | 37.5 W kg−1 | 95.3 F g−1 at 1 A/g | SC |
| [114] | Phenolic resin/ammonium alginate | Coral-like carbon structure | 9.82 Wh kg−1 | 115 W kg−1 | 282.8 F g−1 at 0.5 A g−1 | SC |
| [117] | Co3O4 nanoparticles anchored carbon aerogel | Carbon aerogel | - | - | 779 mAh g–1 | Li-ion BAT |
| [118] | FeSe nanoparticles/carbon nanofiber aerogels | Carbon nanofiber aerogels | - | - | 313 mA h g−1 at 2000 mA g−1 | Na-ion BAT |
| [132] | Agar-PVA/GO | Gel electrolyte | - | 123.7 mW/cm2 | 595.8 mA h g−1 | Zn-Air BAT |
| [133] | Agar/PAM | Hydrogel electrolyte | - | - | 300 mAh g−1 at 0.1 A g−1 | Zinc-ion BAT |
| [121] | Agarose/K2C2O4 | Porous carbon | 18.9 Wh kg−1 at 0.125 A g−1 | 20 kW kg−1 at 10 A g−1 | 166.0 F g−1 at 0.125 A g−1 | SC |
| [123] | Agarose (O/N) | Carbon aerogel | 51.8 Wh kg−1 | 443 W kg−1 | 174 mA h g−1 | K-ion BAT and SC |
| [125] | Seaweed/Si N-doped | Biochar-anode | - | - | 1111.61 mA h g–1 at 1 A g–1 | Li-ion BAT |
| [127] | Lamellar agarose/graphene oxide | Gel polymer electrolyte | - | - | 791.67 mF cm−2 at 5 mA cm−2 | Solid-state SC |
| [128] | Guar gum/vinyl acetate | Anode | - | - | - | Lead-acid BAT |
| [129] | Agar | Electrolyte additive | 2912 Wh kg−1 | - | 2366.86 mA h g−1 | Aluminum-air BAT |
| [130] | Gum arabic | Gel polymer electrolyte | - | - | 0.2 mA cm−2 with 0.2 mA h cm−2 | Zinc-ion BAT |
| [20] | Xanthum gum (Millipede inspired) | Structured binder | - | - | 2150 mA h g−1 | BAT |
| [126] | PAM/agar/Zn(CF3SO3)2) | Cathode/hydrogel electrolyte | 61.3 Wh kg−1 | - | 92.8 mA h g–1 73.4 mA h g–1 | Zinc-ion hybrid solid-state SC |
| [134] | Sodium alginate and pectin | Solid blend biopolymer electrolyte (1.26 × 10–7 S cm–1 at 303 K) | - | - | - | ESS |
| [135] | Potato starch and guar gum (mass loading—7.0 mg cm−2) | Binder | - | - | 20 F g−1 at 10 A g−1 | SC |
2.3. Starch
2.3.1. ESSs Based on Starch
Binders
Electrolytes
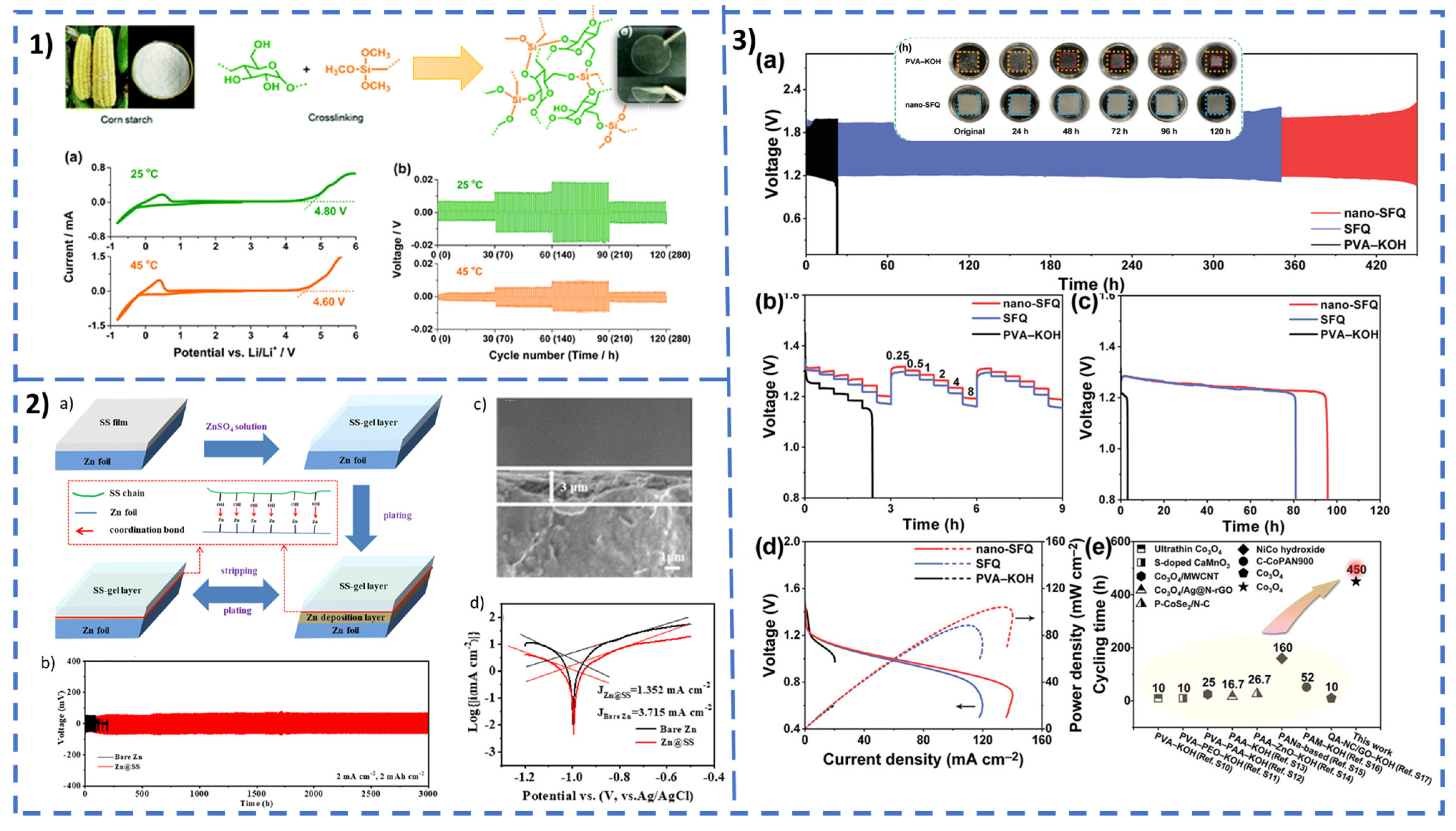
Separators
Active Material (Carbon Precursors)
| Ref | Polysaccharide (Modified Form) | Functionality | Energy Density | Power Density | Specific Capacity/ Specific Capacitance | ESS |
|---|---|---|---|---|---|---|
| [141] | Starch | Binder | 12 Wh kg −1 | 100 kW kg−1 | - | SC |
| [146] | Chitosan/potato starch | Electrolyte | 3.17 Wh kg−1 | 855 W kg−1 | 16.1 F g−1 | SC |
| [148] | LiCl/starch | Hydrogel electrolyte | 28 Wh kg−1 | - | 62.3 F g−1 | SC |
| [149] | Starch–lithium acetate and titania | Solid polymer electrolyte | - | - | 119.77 F g−1 | solid-state SC |
| [151] | Starch | Anode (hydroxyl layer) | - | - | 115 mAh g−1 at 1.0 A g−1 | zinc-ion BAT |
| [153] | Starch/Al2(SO4)3·18H2O | Gel electrolyte | - | - | 193 mA h g−1 (Al|MoO3) | Al-ion BAT |
| 140 mA h g−1 (Al|MnO2) | ||||||
| [155] | Potato Starch | Separator | 8 Wh kg−1 | 1 kW kg−1 | 30 F g−1 | EDLC |
| [159] | Starch | Porous cathode | - | - | 3978 mAh/g at 1 C | Li-O2 BAT |
| [160] | Starch–magnesium nitrate | Porous carbon | - | - | 229 F g–1 at 1 A g–1 | SC |
| [161] | Starch | Biocarbon | 60.16 Wh kg−1 (@140 W kg−1) | 24,590 W kg−1 (@51.24 Wh kg−1) | 192 F g–1 | SC |
| [164] | Corn starch | Porous carbon | 24.5 Wh kg–1 | 695 W kg−1 | 372 F g–1 at 0.5 A g–1 | SC |
2.4. Pectin
2.4.1. ESSs Based on Pectin
Electrolytes
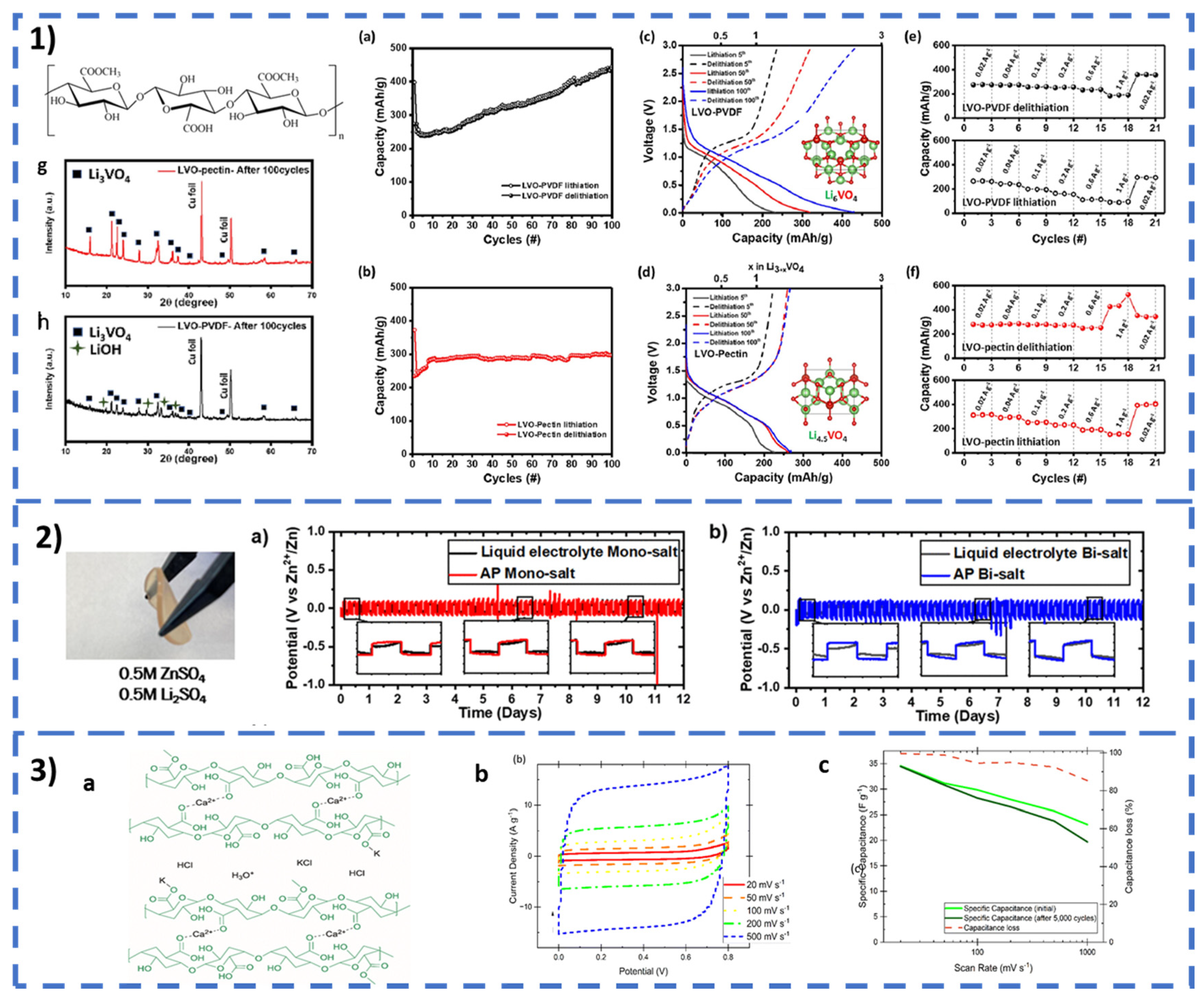
Binders
| Ref | Polysaccharide (Modified Form) | Functionality | Energy Density | Power Density | Specific Capacity/ Specific Capacitance | ESS |
|---|---|---|---|---|---|---|
| [165] | Apple pectin | Hydrogel Electrolyte (>10–3 S cm–1) | - | - | - | ESS |
| [166] | Pectin–Mg(NO3)2 | Electrolyte (10−4 S cm−1) | - | - | - | ESS |
| [167] | Pectin–ZnCl2 | Electrolyte (4.49 × 10−3 S/cm) | - | - | - | Zn-ion BAT |
| [169] | Pectin doped with NH4X (X=Cl, Br) | Electrolyte (NH4Cl—4.52 × 10−4 S cm−1 NH4Br—1.07 × 10−3 S cm−1) | - | - | - | Solid-state devices |
| [170] | Pectin–ammonium thiocyanate (NH4SCN) | Electrolyte (1.5 × 10−3 S cm−1) | - | - | - | ESS |
| [171] | Pectin–Li3VO4 | Anode | 230 mAh g−1 at 0.02 A g−1 | Li-ion BAT | ||
| 400 mAh g−1 1 A g−1 | ||||||
| [173] | Pectin/PEG/graphite | Electrode and electrolyte | - | 0.86 mW cm−2 | 11.48 mF cm−2 | SC |
2.5. Sugars (Dextran and Glucose)
2.5.1. ESSs Based on Dextran
Electrolytes
2.5.2. ESSs Based on Glucose
Active Material (Carbon Precursors)

| Ref | Polysaccharide (Modified Form) | Functionality | Energy Density | Power Density | Specific Capacity/ Specific Capacitance | ESS |
|---|---|---|---|---|---|---|
| [18] | Dextran | Electrolyte additive | - | - | 3400 mAh cm−2 at 5 mA cm−2 | Zn-ion BAT |
| 400 mAh cm−2 at 5 mA cm−2 | ||||||
| [179] | Glucose | Biochar electrode | - | 101.2 mW cm at 139.4 mA cm−3 | - | Zn-air BAT |
| [177] | Glucose-derived N/O co-doped AC | Electrode | - | - | 417 F g−1 at 0.5 A/g | SC |
| [175] | HTC glucose-derived AC | Electrode | 2.6 Wh L−1 | 0.64 kW L−1 | 240 F g−1 at 0.1 A/g | SC |
| Pyrolyzed gucose-derived AC | 2.7 Wh L−1 | 0.14 kW L−1 | 220 F g−1 at 0.1 A/g | |||
| [181] | D-gucose-derived HCs | Anode | - | - | 250 mA h g−1 at 50 mA g−1 | Na-ion BAT |
| [182] | HTC gucose-derived hollow carbon nanospheres | Anode | - | - | 200 mA h g −1 at 50 mA g −1 | Na-ion BAT |
3. Animal-Derived Polysaccharides
3.1. Chitin

3.1.1. ESSs Based on Chitin
Separators
Active Material (Carbon Precursors)
3.2. Chitosan
3.2.1. ESSs Based on Chitosan
Separators
Electrolytes
Active Material (Carbon Precursors)
Binders
| Ref | Polysaccharide (Modified Form) | Functionality | Energy Density | Power Density | Specific Capacity/ Specific Capacitance | ESS |
|---|---|---|---|---|---|---|
| [186] | Carboxymethylated chitin-IL | SPE (1.16 × 10−3 S·cm−1) | - | - | - | SC |
| [191] | Chitin nanofibers | Separators | - | - | 251.9 mAh g−1 at 0.15 A g−1 | Zinc-ion BAT |
| [192] | Cyanoethyl-chitin nanofiber | Separators (0.33 mS/cm) | - | - | ~230 mA h g−1 at 0.2 C | Li-ion BAT |
| [205] | Cyanoethyl-chitin nanofiber | Separators | - | - | 155.1 mA h g−1 at 0.2 C | Li-ion BAT |
| [193] | Chitin | Porous Carbon | 4.9 W h kg–1 | 11.3 W kg–1 | 173–177 F g–1 at 0.05 A g–1 | ESS |
| [194] | Chitosan–carboxymethylcellulose | Hydrogels Electrolyte (0.19 S∙cm−1) | - | 85 mW∙cm−2 | 1026 mA∙h g−1 | Zinc–air BAT |
| [195] | Chitosan nanofiber | Separators (0.68 mS cm−1) | - | - | 117 mAh g−1 at 1 C | Li-ion BAT |
| [197] | Chitosan–avocado Starch | Hydrogels Electrolyte (0.61 S·cm−1) | - | 90 mW·cm−2 | 618 mA h·g−1 | Zinc–air BAT |
| [190] | Carboxylated chitosan and PAM | Hydrogel electrolyte (2.59 × 10−2 S cm−1) | 14.28 Wh kg−1 | 449.98 W kg−1 | 37.28 F g−1 at 0.2 A g−1 | Solid-state SC |
| [198] | Chitosan–N, O self-co-doped | Porous carbon | 28.74 Wh kg− 1 | 399.92 W kg− 1 | 287.94 F g− 1 at 1.0 A g− 1 | ESS |
| [188] | Chitosan/reduced graphene oxide | Electrode material | 8.4 Wh kg−1 | 50 W kg−1 | 274 F g−1 at 0.5 A g−1 | SC |
| [199] | Chitosan-derived carbon aerogel | Active electrode material | 65 Wh kg−1 | 1500 W kg−1 | 209 F g−1 at 1.0 A g−1 | SC |
| [201] | Calcium gluconate and chitosan | N, O co-doped biochar | 16.23 Wh∙kg−1 | 152.16 W·kg−1 | 386.3 F∙g−1, 320.6 F∙cm−3 at 0.5 A·g−1 | SC |
| [202] | Lignin-modified chitosan xerogel | N,P,O-doped porous carbon electrode material | 58 Wh/kg | 375 W/kg | 534.9 F/g at 1 A/g | SC |
| 11.5 Wh/kg | 4500 W/kg | |||||
| [189] | Chitosan | Nitrogen self-doped carbon anode | - | - | 518 mAh g−1 at 200 mA g−1 | Li-ion BAT |
| [203] | CNC-reinforced chitosan | Hard carbon anode | - | - | 285 mAh g−1 at 25 mA g−1 | Na-ion BAT |
| [5] | Chitosan | Binder | - | - | 342.3 mAh g−1 at 2 C | Li-ion BAT |
| [204] | Chitosan–methanesulfonic acid | Binder | - | - | 212 mA h g−1 at 2 C | Li-S BAT |
| [109] | Chitosan | Binder | - | - | 625.76 mAh g−1 at 0.05 C | Li-ion BAT |
4. Hybrids and Composites
5. Conclusions and Future Perspectives
- −
- Polysaccharides, being biodegradable and biocompatible, can fend against the challenges of electronic wastes. The innate structure of polysaccharides with strong hydrogen bonding and numerous functional groups is of great advantage when it comes to their use in ESSs. Tuning the functional groups to derive desired conductive and mechanical properties can render the final material or device flexible and transparent and meet the commercial requirements.
- −
- Strong hydrogen bonding promotes ionic conductivity and water retention in hydrogel structures and establishes a wider electrochemical stability window. The presence of functional groups also allows the formation of hybrid structures with metal ions and other conducting polymers. Nanostructured polysaccharides further improve mechanical strength and maintain electrolyte stability. The presence of amorphous regions in polysaccharides facilitates better ionic mobility.
- −
- The flammability of organic solvents in batteries is a major concern. Alginate-based electrolytes have exhibited superior flame retardancy and thermal stability. SPEs based on PEO and alginate salts have been reported to exhibit self-extinguishing properties.
- −
- The complex network structures of polysaccharides make them good raw materials for carbonaceous materials. Polysaccharides with self-doped heteroatoms, high porosity and hierarchical structures are greatly useful as electrode materials of supercapacitors and batteries.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirst, E. The Rôle of Sugars as Energy Reserves in Nature. J. R. Soc. Arts 1966, 114, 290–307. [Google Scholar]
- Wang, L.; Wang, M.; Wise, M.J.; Liu, Q.; Yang, T.; Zhu, Z.; Li, C.; Tan, X.; Tang, D.; Wang, W. Recent Progress in the Structure of Glycogen Serving as a Durable Energy Reserve in Bacteria. World J. Microbiol. Biotechnol. 2020, 36, 14. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, M.K.; Peng, H.-J.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M.-M. Porous Carbon Derived from Rice Husks as Sustainable Bioresources: Insights into the Role of Micro-/mesoporous Hierarchy in Hosting Active Species for Lithium–sulphur Batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef]
- Zeng, J.; Wei, L.; Guo, X. Bio-Inspired High-Performance Solid-State Supercapacitors with the Electrolyte, Separator, Binder and Electrodes Entirely from Kelp. J. Mater. Chem. A 2017, 5, 25282–25292. [Google Scholar] [CrossRef]
- Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zhang, L.; Zheng, H. Chitosan, a New and Environmental Benign Electrode Binder for Use with Graphite Anode in Lithium-Ion Batteries. Electrochim. Acta 2013, 105, 378–383. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Zeng, J. Biopolymer-based Gel Electrolytes for Electrochemical Energy Storage: Advances and Prospects. Prog. Mater. Sci. 2024, 144, 101264. [Google Scholar] [CrossRef]
- Chaudhary, R.; Xu, J.; Xia, Z.; Asp, L.E. Unveiling the Multifunctional Carbon Fiber Structural Battery. Adv. Mater. 2024, 36, 2409725. [Google Scholar] [CrossRef]
- Song, Z.; Miao, L.; Lv, Y.; Gan, L.; Liu, M. Versatile Carbon Superstructures for Energy Storage. J. Mater. Chem. A 2023, 11, 12434–12455. [Google Scholar] [CrossRef]
- Ahmed, M.M.S.; Hasan, M.J.; Chowdhury, M.S.; Rahman, M.K.; Islam, M.S.; Hossain, M.S.; Islam, M.A.; Hossain, N.; Mobarak, M.H. Prospects and Challenges of Energy Storage Materials: A Comprehensive Review. Chem. Eng. J. Adv. 2024, 20, 100657. [Google Scholar] [CrossRef]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Basha, D.B.; Hussain, S.; Uddin, I.; Gondal, M.A. Critical Review on Recent Developments in Conducting Polymer Nanocomposites for Supercapacitors. Synth. Met. 2023, 295, 117326. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, Z.; Han, T.; Yang, H.; Asfaw, H.D.; Gond, R.; Younesi, R.; Jönsson, P.G.; Yang, W. From Waste Biomass to Hard Carbon Anodes: Predicting the Relationship between Biomass Processing Parameters and Performance of Hard Carbons in Sodium-Ion Batteries. Processes 2023, 11, 764. [Google Scholar] [CrossRef]
- Pham, H.D.; Mahale, K.; Hoang, T.M.L.; Mundree, S.G.; Gomez-Romero, P.; Dubal, D.P. Dual Carbon Potassium-Ion Capacitors: Biomass-Derived Graphene-like Carbon Nanosheet Cathodes. ACS Appl. Mater. Interfaces 2020, 12, 48518–48525. [Google Scholar] [CrossRef]
- Simões dos Reis, G.; Mayandi Subramaniyam, C.; Cárdenas, A.D.; Larsson, S.H.; Thyrel, M.; Lassi, U.; García-Alvarado, F. Facile Synthesis of Sustainable Activated Biochars with Different Pore Structures as Efficient Additive-Carbon-Free Anodes for Lithium- and Sodium-Ion Batteries. ACS Omega 2022, 7, 42570–42581. [Google Scholar] [CrossRef]
- Wen, Y.; Chi, L.; Wenelska, K.; Wen, X.; Chen, X.; Mijowska, E. Eucalyptus Derived Heteroatom-Doped Hierarchical Porous Carbons as Electrode Materials in Supercapacitors. Sci. Rep. 2020, 10, 14631. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.L.; Sezali, N.A.A.; Tsai, C.-K.; Doong, R.-A. Tuning the Surface Charge of Rice Straw-Derived Cellulose Nanofibril Membrane Separator for Electrochemical Performance Enhancement of Supercapacitors. J. Power Sources 2024, 614, 234965. [Google Scholar] [CrossRef]
- Tian, J.; Kong, Y.; Qian, S.; Zhang, Z.; Xia, Y.; Li, Z. Mechanically Robust Multifunctional Starch Films Reinforced by Surface-Tailored Nanofibrillated Cellulose. Compos. Part B Eng. 2024, 275, 111339. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Wu, J.; Zheng, Z.; Yu, Z.; She, F.; Lai, L.; Li, H.; Chen, Y.; Wei, L. Dextran: A Multifunctional and Universal Electrolyte Additive for Aqueous Zn Ion Batteries. Adv. Energy Mater. 2023, 13, 2301743. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Kim, J.; Lee, I.; Kim, S.; Jeong, Y.K.; Hong, S.; Ryu, J.H.; Kim, T.-S.; Park, J.-K.; Lee, H.; et al. Mussel-Inspired Adhesive Binders for High-Performance Silicon Nanoparticle Anodes in Lithium-Ion Batteries. Adv. Mater. 2013, 25, 1571–1576. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Kwon, T.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J.W. Millipede-Inspired Structural Design Principle for High Performance Polysaccharide Binders in Silicon Anodes. Energy Environ. Sci. 2015, 8, 1224–1230. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent Advances in Biopolymer-Based Hydrogel Electrolytes for Flexible Supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef] [PubMed]
- Wisińska, N.H.; Skunik-Nuckowska, M.; Garbacz, P.; Dyjak, S.; Wieczorek, W.; Kulesza, P.J. Polysaccharide-Based Hydrogel Electrolytes Enriched with Poly(norepinephrine) for Sustainable Aqueous Electrochemical Capacitors. J. Environ. Chem. Eng. 2023, 11, 109346. [Google Scholar] [CrossRef]
- Aziz, S.B.; Murad, A.R.; Abdulwahid, R.T.; Aziz, D.M.; Abdalrahman, A.A.; Abdullah, R.M.; Kadir, M.F.Z.; Abdullah, O.G.; Halim, N.A.; Hassan, J. Plasticised Chitosan: Dextran Polymer Blend Electrolyte for Energy Harvesting Application: Tuning the Ion Transport and EDLC Charge Storage Capacity through TiO2 Dispersion. Int. J. Biol. Macromol. 2024, 273, 133203. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Amaral, A.J.R.; Budtova, T.; Wever, P.D.; Groth, T.; Heinze, T.; Höfte, H.; Huber, A.; Ikkala, O.; Kapuśniak, J.; et al. The European Polysaccharide Network of Excellence (EPNOE) Research Roadmap 2040: Advanced Strategies for Exploiting the Vast Potential of Polysaccharides as Renewable Bioresources. Carbohydr. Polym. 2024, 326, 121633. [Google Scholar] [CrossRef]
- Renewables 2024—Analysis. IEA 2024. Available online: https://www.iea.org/reports/renewables-2024 (accessed on 17 December 2024).
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A Comprehensive Review of Biomass Based Thermochemical Conversion Technologies Integrated with CO2 Capture and Utilisation within BECCS Networks. Resour. Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- Velychkivska, N.; Golunova, A.; Panda, A.; Shinde, P.A.; Ma, R.; Ariga, K.; Yamauchi, Y.; Hill, J.P.; Labuta, J.; Shrestha, L.K. Ultrahigh Surface Area Hierarchically Porous Carbon Materials from Polyacrylamide–Cellulose Hydrogel for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2024, 7, 2906–2917. [Google Scholar] [CrossRef]
- Qin, L.; Xu, S.; Lu, Z.; Wang, L.; Chen, L.; Zhang, D.; Tian, J.; Wei, T.; Chen, J.; Guo, C. Cellulose as a Novel Precursor to Construct High-Performance Hard Carbon Anode toward Enhanced Sodium-Ion Batteries. Diam. Relat. Mater. 2023, 136, 110065. [Google Scholar] [CrossRef]
- Luo, Y.; Que, W.; Tang, Y.; Kang, Y.; Bin, X.; Wu, Z.; Yuliarto, B.; Gao, B.; Henzie, J.; Yamauchi, Y. Regulating Functional Groups Enhances the Performance of Flexible Microporous MXene/Bacterial Cellulose Electrodes in Supercapacitors. ACS Nano 2024, 18, 11675–11687. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Li, J.; Cao, D. Carbonization of Bacterial Cellulose with Structure Retention and Nitrogen/Sulfur/Oxygen Doping for Application in Supercapacitors Electrode. Chem. Eng. J. 2024, 495, 153590. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic Biomass as Sustainable Feedstock and Materials for Power Generation and Energy Storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.; Zhang, X.; Si, C. Lignin-Containing Cellulose Nanomaterials: Preparation and Applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Ye, M.; Tang, Y.; Wen, Z.; Liu, X.; Chao Li, C. Renewable Lignin and Its Macromolecule Derivatives: An Emerging Platform toward Sustainable Electrochemical Energy Storage. Green Chem. 2023, 25, 4154–4179. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Y.; Chen, T.; Qi, J.; Xu, T.; Du, H.; Si, C. Lignocellulosic Materials for Energy Storage Devices. Ind. Crops Prod. 2023, 203, 117174. [Google Scholar] [CrossRef]
- Muddasar, M.; Culebras, M.; Collins, M.N. Lignin and Its Carbon Derivatives: Synthesis Techniques and Their Energy Storage Applications. Mater. Today Sustain. 2024, 28, 100990. [Google Scholar] [CrossRef]
- Li, W.; Shi, J. Lignin-Derived Carbon Material for Electrochemical Energy Storage Applications: Insight into the Process-Structure-Properties-Performance Correlations. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin Derived Carbon Materials: Current Status and Future Trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Zhang, W.; Li, J.; Zhang, B.; Si, C. Lignin-Derived 0–3 Dimensional Carbon Materials: Synthesis, Configurations and Applications. Ind. Crops Prod. 2023, 204, 117342. [Google Scholar] [CrossRef]
- Demir, M.; Tessema, T.-D.; Farghaly, A.A.; Nyankson, E.; Saraswat, S.K.; Aksoy, B.; Islamoglu, T.; Collinson, M.M.; El-Kaderi, H.M.; Gupta, R.B. Lignin-Derived Heteroatom-Doped Porous Carbons for Supercapacitor and CO2 Capture Applications. Int. J. Energy Res. 2018, 42, 2686–2700. [Google Scholar] [CrossRef]
- Alemany-Molina, G.; Martínez-Sánchez, B.; Morallón, E.; Cazorla-Amorós, D. The Role of Oxygen Heteroatoms in the Surface (Electro)chemistry of Carbon Materials. Carbon Reports 2022, 1, 162–174. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; E, J.; Xue, Y. A Comprehensive Review on Lignin Pyrolysis: Mechanism, Modeling and the Effects of Inherent Metals in Biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jin, M.-H.; Park, J.-H.; Lee, Y.-J.; Choi, Y.-C. Flexible Synthetic Strategies for Lignin-Derived Hierarchically Porous Carbon Materials. ACS Sustain. Chem. Eng. 2018, 6, 10454–10462. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-Based Electrodes for Energy Storage Application. Ind. Crops Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Wang, H.; Fu, F.; Huang, M.; Feng, Y.; Han, D.; Xi, Y.; Xiong, W.; Yang, D.; Niu, L. Lignin-Based Materials for Electrochemical Energy Storage Devices. Nano Mater. Sci. 2023, 5, 141–160. [Google Scholar] [CrossRef]
- Madhu, R.; Periasamy, A.P.; Schlee, P.; Hérou, S.; Titirici, M.-M. Lignin: A Sustainable Precursor for Nanostructured Carbon Materials for Supercapacitors. Carbon N. Y. 2023, 207, 172–197. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, C.; Huang, C.; Deng, Y.; Zou, X.; Ma, W.; Fang, G.; Ragauskas, A.J. Cellulose Regulated Lignin/cellulose-Based Carbon Materials with Hierarchical Porous Structure for Energy Storage. Adv. Compos. Hybrid Mater. 2024, 7, 51. [Google Scholar] [CrossRef]
- Kumar, D.; Franco, L.R.; Abdou, N.; Shu, R.; Martinelli, A.; Araujo, C.M.; Gladisch, J.; Gueskine, V.; Crispin, R.; Khan, Z. Water-in-Polymer Salt Electrolyte for Long-Life Rechargeable Aqueous Zinc-Lignin Battery. ENERGY Environ. Mater. 2024, 8, e12752. [Google Scholar] [CrossRef]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-Based Electrospun Carbon Nanofibers. Front. Mater. 2019, 62. [Google Scholar] [CrossRef]
- Dias, Y.J.; Silva, V.D.; Pourdeyhimi, B.; Medeiros, E.S.; Yarin, A.L. Freestanding Carbon Nanofibers Derived from Biopolymer (Kraft Lignin) as Ultra-Microporous Electrodes for Supercapacitors. Batteries 2023, 9, 566. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lu, Z.; Liu, J.; Bai, L.; Dong, J.; Nan, D. Constructing Porous Lignin-Based Carbon Nanofiber Anodes with Flexibility for High-Performance Lithium/sodium-Ion Batteries. Mater. Today Sustain. 2022, 20, 100234. [Google Scholar] [CrossRef]
- Hérou, S.; Bailey, J.J.; Kok, M.; Schlee, P.; Jervis, R.; Brett, D.J.L.; Shearing, P.R.; Ribadeneyra, M.C.; Titirici, M. High-Density Lignin-Derived Carbon Nanofiber Supercapacitors with Enhanced Volumetric Energy Density. Adv. Sci. 2021, 8, 2100016. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Sun, X.; Wang, F.; Yang, R. Enzymatic Hydrolysis Lignin Derived Hierarchical Porous Carbon for Supercapacitors in Ionic Liquids with High Power and Energy Densities. Green Chem. 2017, 19, 2595–2602. [Google Scholar] [CrossRef]
- Say, M.G.; Brett, C.J.; Edberg, J.; Roth, S.V.; Söderberg, L.D.; Engquist, I.; Berggren, M. Scalable Paper Supercapacitors for Printed Wearable Electronics. ACS Appl. Mater. Interfaces 2022, 14, 55850–55863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, R.; Sun, R.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose Structured Paper-Based Lithium Metal Batteries. ACS Appl. Energy Mater. 2018, 1, 4341–4350. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, X.; Xu, W.; Chen, W.; Zhao, K.; Wang, Y.; Hong, S.; Wu, Q.; Li, M.-C.; et al. Synergic Effect of Dendrite-Free and Zinc Gating in Lignin-Containing Cellulose Nanofibers-MXene Layer Enabling Long-Cycle-Life Zinc Metal Batteries. Adv. Sci. 2022, 9, 2202380. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, Y.; Han, T.; Yang, H.; Gond, R.; Subasi, Y.; Asfaw, H.D.; Younesi, R.; Jönsson, P.G.; Yang, W. Bio-Based Anode Material Production for Lithium–ion Batteries through Catalytic Graphitization of Biochar: The Deployment of Hybrid Catalysts. Sci. Rep. 2024, 14, 3966. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar Production Techniques Utilizing Biomass Waste-Derived Materials and Environmental Applications – A Review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, C.; Fu, L. Biochar-Based Materials for Electroanalytical Applications: An Overview. Green Anal. Chem. 2023, 7, 100081. [Google Scholar] [CrossRef]
- Bartoli, M.; Piovano, A.; Elia, G.A.; Meligrana, G.; Pedraza, R.; Pianta, N.; Tealdi, C.; Pagot, G.; Negro, E.; Triolo, C.; et al. Pristine and Engineered Biochar as Na-Ion Batteries Anode Material: A Comprehensive Overview. Renew. Sustain. Energy Rev. 2024, 194, 114304. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, Z.; Shang, Q.; Han, J.; Li, L.; Chen, J.; Fakudze, S.; Tian, Z.; Liu, C. Ionic Liquid-Induced Low Temperature Graphitization of Cellulose-Derived Biochar for High Performance Sodium Storage. Surf. Coatings Technol. 2021, 412, 127034. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Guo, Z.; Jensen, A.C.S.; Feng, J.; Luo, H.; Ding, F.; Lu, Y.; Hu, Y.-S.; Titirici, M.-M. Achieving High Initial Coulombic Efficiency for Competent Na Storage by Microstructure Tailoring from Chiral Nematic Nanocrystalline Cellulose. Carbon Energy 2022, 4, 914–923. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Chang, Z.; Yan, S.; Liu, S.; Han, S. A New Method of Synthesizing Hemicellulose-Derived Porous Activated Carbon for High-Performance Supercapacitors. Microporous Mesoporous Mater. 2020, 292, 109707. [Google Scholar] [CrossRef]
- Li, Z.; Bai, Z.; Mi, H.; Ji, C.; Gao, S.; Pang, H. Biowaste-Derived Porous Carbon with Tuned Microstructure for High-Energy Quasi-Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 13127–13135. [Google Scholar] [CrossRef]
- Saju, S.K.; Chattopadhyay, S.; Xu, J.; Alhashim, S.; Pramanik, A.; Ajayan, P.M. Hard Carbon Anode for Lithium-, Sodium-, and Potassium-Ion Batteries: Advancement and Future Perspective. Cell Reports Phys. Sci. 2024, 5, 101851. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, Z.; Jin, G.; Tu, J.; Dhmees, A.S.; Tang, Y.; Sun, D.; Zhang, R.; Wang, H. Understanding the Relationship of Closed Pore Structure in Biomass- Derived Hard Carbon with Cellulose Regulating Strategy. Small 2024, n/a, 2407341. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Titirici, M.-M.; Chen, L.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Simone, V.; Boulineau, A.; de Geyer, A.; Rouchon, D.; Simonin, L.; Martinet, S. Hard Carbon Derived from Cellulose as Anode for Sodium Ion Batteries: Dependence of Electrochemical Properties on Structure. J. Energy Chem. 2016, 25, 761–768. [Google Scholar] [CrossRef]
- Fischer, J.; Wolfram, L.; Oswald, S.; Fischer, S.; Mikhailova, D. Carbons Derived from Regenerated Spherical Cellulose as Anodes for Li-Ion Batteries at Elevated Temperatures. ChemPhysChem 2024, 25, e202300833. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Bijoy, T.K. A Comprehensive Review of Current and Emerging Binder Technologies for Energy Storage Applications. ACS Appl. Energy Mater. 2023, 6, 11773–11794. [Google Scholar] [CrossRef]
- Weng, M.; Zhou, J.; Ye, Y.; Qiu, H.; Zhou, P.; Luo, Z.; Guo, Q. Self-Chargeable Supercapacitor Made with MXene-Bacterial Cellulose Nanofiber Composite for Wearable Devices. J. Colloid Interface Sci. 2023, 647, 277–286. [Google Scholar] [CrossRef]
- Mian, M.M.; Kamana, I.M.L.; An, X.; Abbas, S.C.; Ahommed, M.S.; He, Z.; Ni, Y. Cellulose Nanofibers as Effective Binders for Activated Biochar-Derived High-Performance Supercapacitors. Carbohydr. Polym. 2023, 301, 120353. [Google Scholar] [CrossRef]
- Palem, R.R.; Ramesh, S.; Rabani, I.; Shimoga, G.; Bathula, C.; Kim, H.S.; Seo, Y.-S.; Kim, H.-S.; Lee, S.-H. Microstructurally Assembled Transition Metal Oxides with Cellulose Nanocrystals for High-Performance Supercapacitors. J. Energy Storage 2022, 50, 104712. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-Conductive Macromolecular Gels and Membranes for Solid Lithium Cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Xia, T.; Li, Q.; Ao, C.; Wang, Q.; Zhang, W.; Lu, C.; Deng, Y. Hollow Polypyrrole/cellulose Hydrogels for High-Performance Flexible Supercapacitors. Energy Storage Mater. 2020, 31, 135–145. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Chen, J.; Wang, Y.; Cheng, Z.; Chen, X.; Guo, M. A Cellulose-Based Interpenetrating Network Hydrogel Electrolyte for Flexible Solid-State Supercapacitors. Cellulose 2023, 30, 2399–2412. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Xu, P.; Xu, J.; Gao, Y.; Gao, G. Cellulose Nanofiber Hydrogel with High Conductivity Electrolytes for High Voltage Flexible Supercapacitors. Carbohydr. Polym. 2024, 326, 121654. [Google Scholar] [CrossRef]
- González-Gil, R.M.; Borràs, M.; Chbani, A.; Abitbol, T.; Fall, A.; Aulin, C.; Aucher, C.; Martínez-Crespiera, S. Sustainable and Printable Nanocellulose-Based Ionogels as Gel Polymer Electrolytes for Supercapacitors. Nanomaterials 2022, 12, 273. [Google Scholar] [CrossRef]
- Rahim, A.A.; Shamsuri, N.A.; Adam, A.A.; Aziz, M.F.; Hamsan, M.H.; Rusdi, H.; Siong, S.O.J.; Noor, I.M.; Kadir, M.F.Z.; Shukur, M.F. Characterization of Nanocomposite Polyvinyl Alcohol/cellulose Acetate Blend Gel Polymer Electrolytes for Supercapacitor Application. J. Energy Storage 2024, 97, 112964. [Google Scholar] [CrossRef]
- Lin, X.; Wang, M.; Zhao, J.; Wu, X.; Xie, J.; Yang, J. Super-Tough and Self-Healable All-Cellulose-Based Electrolyte for Fast Degradable Quasi-Solid-State Supercapacitor. Carbohydr. Polym. 2023, 304, 120502. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Zhou, X.; Gui, S.; Wei, L.; Yang, H.; Li, H.; Guo, X. Ionic Conduction in Polymer-Based Solid Electrolytes. Adv. Sci. 2023, 10, 2201718. [Google Scholar] [CrossRef]
- Hadad, S.; Hamrahjoo, M.; Dehghani, E.; Salami-Kalajahi, M.; Eliseeva, S.N.; Moghaddam, A.R.; Roghani-Mamaqani, H. Cellulose-Based Solid and Gel Polymer Electrolytes with Super High Ionic Conductivity and Charge Capacity for High Performance Lithium Ion Batteries. Sustain. Mater. Technol. 2022, 33, e00503. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-Based Separators for Lithium Batteries: Source, Preparation and Performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Yu, B.-C.; Park, K.; Jang, J.-H.; Goodenough, J.B. Cellulose-Based Porous Membrane for Suppressing Li Dendrite Formation in Lithium–Sulfur Battery. ACS Energy Lett. 2016, 1, 633–637. [Google Scholar] [CrossRef]
- Li, Z.; Qian, P.; Li, H.; Xiao, H.; Chen, J.; Li, G. Phosphorylated Cellulose Nanofibers Establishing Reliable Ion-Sieving Barriers for Durable Lithium-Sulfur Batteries. J. Energy Chem. 2024, 92, 619–628. [Google Scholar] [CrossRef]
- Schlee, P.; Herou, S.; Jervis, R.; Shearing, P.R.; Brett, D.J.L.; Baker, D.; Hosseinaei, O.; Tomani, P.; Murshed, M.M.; Li, Y.; et al. Free-Standing Supercapacitors from Kraft Lignin Nanofibers with Remarkable Volumetric Energy Density. Chem. Sci. 2019, 10, 2980–2988. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841. [Google Scholar] [CrossRef]
- Mushtaq, M.; Beaucamp, A.; Collins, M.N.; Kennedy, T. Sustainable Lignin-Based 3D Porous Carbon Nanofibers As a Na-Ion Battery Anode. ECS Meet. Abstr. 2023, MA2023-02, 742. [Google Scholar] [CrossRef]
- Kottis, T.; Soursos, N.; Govatsi, K.; Sygellou, L.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D.; Lianos, P. Biochar from Olive Tree Twigs and Spent Malt Rootlets as Electrodes in Zn-Air Batteries. J. Colloid Interface Sci. 2024, 665, 10–18. [Google Scholar] [CrossRef]
- Xue, C.-F.; Lin, Y.; Zhao, W.; Wu, T.; Wei, Y.-Y.; Li, X.-H.; Yan, W.-J.; Hao, X.-G. Green Preparation of High Active Biochar with Tetra-Heteroatom Self-Doped Surface for Aqueous Electrochemical Supercapacitor with Boosted Energy Density. J. Energy Storage 2024, 90, 111872. [Google Scholar] [CrossRef]
- Yamamoto, H.; Muratsubaki, S.; Kubota, K.; Fukunishi, M.; Watanabe, H.; Kim, J.; Komaba, S. Synthesizing Higher-Capacity Hard-Carbons from Cellulose for Na- and K-Ion Batteries. J. Mater. Chem. A 2018, 6, 16844–16848. [Google Scholar] [CrossRef]
- Prakoso, T.; Devianto, H.; Rustamaji, H.; Wulan, P.P.D.K.; Gozan, M. Nanocarbon Material and Chemicals from Seaweed for Energy Storage Components. In Chemical Substitutes from Agricultural and Industrial By-Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 59–86. ISBN 978-3-527-84114-1. [Google Scholar]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative Binders for Sustainable Electrochemical Energy Storage – the Transition to Aqueous Electrode Processing and Bio-Derived Polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, J.; Jiang, Q.; Wang, H.; Huang, H.; Rao, P.; Mao, J. A High-Performance Alginate Hydrogel Binder for Aqueous Zn−Ion Batteries. ChemPhysChem 2022, 23, e202200106. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Manjunatha, R.; Kumar, R.; Mitra, S. A Facile Bottom-Up Approach to Construct Hybrid Flexible Cathode Scaffold for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 33775–33785. [Google Scholar] [CrossRef]
- Kucinskis, G.; Kruze, B.; Korde, P.; Sarakovskis, A.; Viksna, A.; Hodakovska, J.; Bajars, G. Enhanced Electrochemical Properties of Na0.67MnO2 Cathode for Na-Ion Batteries Prepared with Novel Tetrabutylammonium Alginate Binder. Batteries 2022, 8, 6. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Liu, B.; Li, Z.; Zhang, J.; Yang, G.; Hiralal, P.; Jin, S.; Zhou, H. Flexible and Anti-Freezing Zinc-Ion Batteries Using a Guar-Gum/sodium-Alginate/ethylene-Glycol Hydrogel Electrolyte. Energy Storage Mater. 2021, 41, 599–605. [Google Scholar] [CrossRef]
- Badawi, N.M.; Bhatia, M.; Ramesh, S.; Ramesh, K.; Kuniyil, M.; Shaik, M.R.; Khan, M.; Shaik, B.; Adil, S.F. Self-Healing, Flexible and Smart 3D Hydrogel Electrolytes Based on Alginate/PEDOT:PSS for Supercapacitor Applications. Polymers 2023, 15, 571. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly Stretchable, Compressible and Arbitrarily Deformable All-Hydrogel Soft Supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Tao, F.; Qin, L.; Wang, Z.; Pan, Q. Self-Healable and Cold-Resistant Supercapacitor Based on a Multifunctional Hydrogel Electrolyte. ACS Appl. Mater. Interfaces 2017, 9, 15541–15548. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, Z.; Wang, D.; Yuan, H.; Zhang, H.; Tan, Y. A Flame Retarded Polymer-Based Composite Solid Electrolyte Improved by Natural Polysaccharides. Compos. Commun. 2021, 26, 100774. [Google Scholar] [CrossRef]
- Song, Q.; Li, A.; Shi, L.; Qian, C.; Feric, T.G.; Fu, Y.; Zhang, H.; Li, Z.; Wang, P.; Li, Z.; et al. Thermally Stable, Nano-Porous and Eco-Friendly Sodium Alginate/attapulgite Separator for Lithium-Ion Batteries. Energy Storage Mater. 2019, 22, 48–56. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Chen, Z.; Sun, Y.; Duan, C.; Li, C.; Yan, J.; Wu, X.; Kawi, S. Facile Fabrication of PMIA Composite Separator with Bi-Functional Sodium-Alginate Coating Layer for Synergistically Increasing Performance of Lithium-Ion Batteries. J. Colloid Interface Sci. 2023, 648, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, T.; Wang, S.; Wang, C.; Li, D.; Xia, Y. Alginate Fiber-Grafted Polyetheramine-Driven High Ion-Conductive and Flame-Retardant Separator and Solid Polymer Electrolyte for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 56780–56789. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, Z.; Shi, R.; Quan, F.; Wang, B.; Ma, X.; Ji, Q.; Tian, X.; Xia, Y. Preparation and Properties of an Alginate-Based Fiber Separator for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 38175–38182. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhang, J.; Chai, J.; Ma, J.; Yue, L.; Dong, T.; Zang, X.; Liu, Z.; Zhang, B.; Cui, G. Sustainable and Superior Heat-Resistant Alginate Nonwoven Separator of LiNi0.5Mn1.5O4/Li Batteries Operated at 55 °C. ACS Appl. Mater. Interfaces 2017, 9, 3694–3701. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y. Sodium Alginate-Based Functional Materials toward Sustainable Applications: Water Treatment and Energy Storage. Ind. Eng. Chem. Res. 2023, 62, 11279–11304. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Shang, M.; Zhang, M.; Zhao, X.; Liu, S.; Liu, X.; Liu, S.; Yi, X. N, O Co-Doped Carbon Aerogel Derived from Sodium Alginate/melamine Composite for All-Solid-State Supercapacitor. Appl. Surf. Sci. 2023, 608, 155109. [Google Scholar] [CrossRef]
- Hamzelui, N.; Linhorst, M.; Martin Nyenhuis, G.; Haneke, L.; Eshetu, G.G.; Placke, T.; Winter, M.; Moerschbacher, B.M.; Figgemeier, E. Chitosan as Enabling Polymeric Binder Material for Silicon-Graphite-Based Anodes in Lithium-Ion Batteries. Energy Technol. 2023, 11, 2201239. [Google Scholar] [CrossRef]
- Li, D.; Lv, C.; Liu, L.; Xia, Y.; She, X.; Guo, S.; Yang, D. Egg-Box Structure in Cobalt Alginate: A New Approach to Multifunctional Hierarchical Mesoporous N-Doped Carbon Nanofibers for Efficient Catalysis and Energy Storage. ACS Cent. Sci. 2015, 1, 261–269. [Google Scholar] [CrossRef]
- Li, X.; Lai, L.; Wu, F.; Xie, W.; Liu, J. Hierarchical Porous Carbon Aerogel Derived from Sodium Alginate for High Performance Electrochemical Capacitor Electrode. Processes 2023, 11, 3355. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, F.; Jia, C.; Mu, K.; Yu, M.; Lv, Y.; Shao, Z. Nitrogen and Oxygen-Codoped Carbon Nanospheres for Excellent Specific Capacitance and Cyclic Stability Supercapacitor Electrodes. Chem. Eng. J. 2017, 330, 1166–1173. [Google Scholar] [CrossRef]
- Samartzis, N.; Athanasiou, M.; Raptopoulos, G.; Paraskevopoulou, P.; Ioannides, T. Electrochemical Energy Storage in Nitrogen/Metal-Doped Carbon Aerogels Derived from Polyurea-Crosslinked Alginate Aerogels. ChemNanoMat 2023, 9, e202300028. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Y.; Li, X.; Jiao, T.; Dong, X.; Zhang, R.; Liang, P. Coral-like Interconnected Porous Carbon Derived from Phenolic Resin/ammonium Alginate Composite for High-Rate Supercapacitor. J. Power Sources 2023, 573, 232933. [Google Scholar] [CrossRef]
- Thong, Y.J.; Beh, J.H.; Lai, J.C.; Lim, T.H. Synthesis and Characterization of Alginate-Based Sol–Gel Synthesis of Lithium Nickel Phosphate with Surface Area Control. Ind. Eng. Chem. Res. 2019, 58, 625–631. [Google Scholar] [CrossRef]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon Aerogel Based Materials for Secondary Batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, Z.; Yin, L. Co3O4/Carbon Aerogel Hybrids as Anode Materials for Lithium-Ion Batteries with Enhanced Electrochemical Properties. ACS Appl. Mater. Interfaces 2013, 5, 8337–8344. [Google Scholar] [CrossRef]
- Lv, C.; Liu, H.; Li, D.; Chen, S.; Zhang, H.; She, X.; Guo, X.; Yang, D. Ultrafine FeSe Nanoparticles Embedded into 3D Carbon Nanofiber Aerogels with FeSe/Carbon Interface for Efficient and Long-Life Sodium Storage. Carbon N. Y. 2019, 143, 106–115. [Google Scholar] [CrossRef]
- Nie, Z.; Peng, K.; Lin, L.; Yang, J.; Cheng, Z.; Gan, Q.; Chen, Y.; Feng, C. A Conductive Hydrogel Based on Nature Polymer Agar with Self-Healing Ability and Stretchability for Flexible Sensors. Chem. Eng. J. 2023, 454, 139843. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Cheong, J.Y. Recent Advances in the Multifunctional Natural Gum-Based Binders for High-Performance Rechargeable Batteries. Energies 2022, 15, 8552. [Google Scholar] [CrossRef]
- Hwang, S.; Zhou, J.; Tang, T.; Goossens, K.; Bielawski, C.W.; Geng, J. Agarose-Based Hierarchical Porous Carbons Prepared with Gas-Generating Activators and Used in High-Power Density Supercapacitors. Energy & Fuels 2021, 35, 19775–19783. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, K.; Jiang, K.; Zhang, F.; Zhu, G.; Xu, H. Progress of Synthetic Strategies and Properties of Heteroatoms-Doped (N, P, S, O) Carbon Materials for Supercapacitors. J. Energy Storage 2022, 56, 105995. [Google Scholar] [CrossRef]
- Xie, K.; Liu, X.; Li, H.; Fang, L.; Xia, K.; Yang, D.; Zou, Y.; Zhang, X. Heteroatom Tuning in Agarose Derived Carbon Aerogel for Enhanced Potassium Ion Multiple Energy Storage. Carbon Energy 2024, 6, e427. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, J.; Yu, H.; Wang, L.; Song, Y. Si@nitrogen-Doped Porous Carbon Derived from Covalent Organic Framework for Enhanced Li-Storage. J. Colloid Interface Sci. 2023, 634, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Sun, C.; Pan, J.; Gao, C.; Zhang, R.; Jia, F.; Wang, F.; Wang, Q. Seaweed─Modification of Si by Natural Nitrogen-Doped Porous Biochar for High-Efficiency Lithium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 11389–11399. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wu, D.; Liu, Z.; Mi, H.; Liao, Y.; Wu, M.; Cui, H.; Li, X.; Wu, T.; Bai, Z. Natural Polysaccharide Strengthened Hydrogel Electrolyte and Biopolymer Derived Carbon for Durable Aqueous Zinc Ion Storage. ACS Appl. Mater. Interfaces 2022, 14, 23452–23464. [Google Scholar] [CrossRef]
- Lv, L.; Hui, B.; Zhang, X.; Zou, Y.; Yang, D. Lamellar Agarose/graphene Oxide Gel Polymer Electrolyte Network for All-Solid-State Supercapacitor. Chem. Eng. J. 2023, 452, 139443. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Ali, I.H.; Younas, M.; Gupta, B. Inhibition of Hydrogen Evolution and Corrosion Protection of Negative Electrode of Lead-Acid Battery by Natural Polysaccharide Composite: Experimental and Surface Analysis. J. Energy Storage 2023, 57, 106272. [Google Scholar] [CrossRef]
- Lee, W.-H.; Choi, S.-R.; Kim, J.-G. Effect of Agar as Electrolyte Additive on the Aluminum-Air Batteries. J. Electrochem. Soc. 2020, 167, 110503. [Google Scholar] [CrossRef]
- Wu, K.; Cui, J.; Yi, J.; Liu, X.; Ning, F.; Liu, Y.; Zhang, J. Biodegradable Gel Electrolyte Suppressing Water-Induced Issues for Long-Life Zinc Metal Anodes. ACS Appl. Mater. Interfaces 2022, 14, 34612–34619. [Google Scholar] [CrossRef]
- Hu, S.; Cai, Z.; Huang, T.; Zhang, H.; Yu, A. A Modified Natural Polysaccharide as a High-Performance Binder for Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 4311–4317. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Guo, Y.; Liu, P.; Han, X.; Wu, D. Agar-PVA/GO Double Network Gel Electrolyte for High Performance Flexible Zinc-Air Batteries. Mater. Today Chem. 2023, 29, 101384. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, W.; Zhou, X.; Zhang, X.; Guo, W.; Shi, X.; Xiong, Y.; Zhu, Y. Agar-Based Hydrogel Polymer Electrolyte for High-Performance Zinc-Ion Batteries at All Climatic Temperatures. iScience 2023, 26, 106437. [Google Scholar] [CrossRef]
- Jansi, R.; Vinay, B.; Revathy, M.S.; Sasikumar, P.; Marasamy, L.; Janani, A.; Haldhar, R.; Kim, S.-C.; Almarhoon, Z.M.; Hossain, M.K. Synergistic Blends of Sodium Alginate and Pectin Biopolymer Hosts as Conducting Electrolytes for Electrochemical Applications. ACS Omega 2024, 9, 13906–13916. [Google Scholar] [CrossRef] [PubMed]
- Ruschhaupt, P.; Varzi, A.; Passerini, S. Natural Polymers as Green Binders for High-Loading Supercapacitor Electrodes. ChemSusChem 2020, 13, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Huang, J.; Tu, T.; Li, L. Cost-Trivial Material Contributes Greatly: A Review of the Application of Starch in Energy Storage Systems. J. Energy Storage 2023, 73, 109060. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Chapter 5 Starch Gelatinization. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2008; Volume 55, pp. 221–268. [Google Scholar]
- Cai, T.; Sun, H.; Qiao, J.; Zhu, L.; Zhang, F.; Zhang, J.; Tang, Z.; Wei, X.; Yang, J.; Yuan, Q.; et al. Cell-Free Chemoenzymatic Starch Synthesis from Carbon Dioxide. Science 2021, 373, 1523–1527. [Google Scholar] [CrossRef]
- Biswal, A.K.; Chakraborty, S.; Saha, J.; Panda, P.K.; Pradhan, S.K.; Behera, P.K.; Misra, P.K. Process Optimization, Fabrication, and Characterization of a Starch-Based Biodegradable Film Derived from an Underutilized Crop. ACS Food Sci. Technol. 2024, 4, 1844–1863. [Google Scholar] [CrossRef]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef]
- Varzi, A.; Passerini, S. Enabling High Areal Capacitance in Electrochemical Double Layer Capacitors by Means of the Environmentally Friendly Starch Binder. J. Power Sources 2015, 300, 216–222. [Google Scholar] [CrossRef]
- Jin, B.; Wang, D.; Song, L.; Cai, Y.; Ali, A.; Hou, Y.; Chen, J.; Zhang, Q.; Zhan, X. Biomass-Derived Fluorinated Corn Starch Emulsion as Binder for Silicon and Silicon Oxide Based Anodes in Lithium-Ion Batteries. Electrochim. Acta 2021, 365, 137359. [Google Scholar] [CrossRef]
- Rohan, R.; Kuo, T.-C.; Chiou, C.-Y.; Chang, Y.-L.; Li, C.-C.; Lee, J.-T. Low-Cost and Sustainable Corn Starch as a High-Performance Aqueous Binder in Silicon Anodes via in Situ Cross-Linking. J. Power Sources 2018, 396, 459–466. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E.; Durairaj, R. Utilisation of Corn Starch in Production of “green” Polymer Electrolytes. Mater. Res. Innov. 2011, 15, s13–s18. [Google Scholar] [CrossRef]
- Abdulwahid, R.T.; Aziz, S.B.; Kadir, M.F.Z. Environmentally Friendly Plasticized Electrolyte Based on Chitosan (CS): Potato Starch (PS) Polymers for EDLC Application: Steps toward the Greener Energy Storage Devices Derived from Biopolymers. J. Energy Storage 2023, 67, 107636. [Google Scholar] [CrossRef]
- Song, A.; Huang, Y.; Zhong, X.; Cao, H.; Liu, B.; Lin, Y.; Wang, M.; Li, X. Gel Polymer Electrolyte with High Performances Based on Pure Natural Polymer Matrix of Potato Starch Composite Lignocellulose. Electrochim. Acta 2017, 245, 981–992. [Google Scholar] [CrossRef]
- El Sharkawy, H.M.; Ismail, A.A.M.; Allam, N.K. Environmentally Benign Natural Hydrogel Electrolyte Enables a Wide Operating Potential Window for Energy Storage Devices. ACS Sustain. Chem. Eng. 2024, 12, 3517–3526. [Google Scholar] [CrossRef]
- Ong, A.C.W.; Shamsuri, N.A.; Zaine, S.N.A.; Panuh, D.; Shukur, M.F. Nanocomposite Polymer Electrolytes Comprising Starch-Lithium Acetate and Titania for All-Solid-State Supercapacitor. Ionics 2021, 27, 853–865. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Liu, K.; Liu, Y.; Liu, J.; Wang, X. Unique Starch Polymer Electrolyte for High Capacity All-Solid-State Lithium Sulfur Battery. Green Chem. 2016, 18, 3796–3803. [Google Scholar] [CrossRef]
- Wang, K.; Su, T.-T.; Shao, C.-Y.; Ren, W.-F.; Sun, R.-C. Dynamically Responsive and Ionic Conductive Gel Coatings to Realize the Stable Circulation of Zinc Metal Anodes for Zinc-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 16225–16237. [Google Scholar] [CrossRef]
- Fan, X.; Wang, H.; Liu, X.; Liu, J.; Zhao, N.; Zhong, C.; Hu, W.; Lu, J. Functionalized Nanocomposite Gel Polymer Electrolyte with Strong Alkaline-Tolerance and High Zinc Anode Stability for Ultralong-Life Flexible Zinc–Air Batteries. Adv. Mater. 2023, 35, 2209290. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sasirajan Little Flower, S.R.; Hanamantrao, D.P.; Kasiviswanathan, K.; Sesu, D.C.; Muthu, K.; Elumalai, V.; Vediappan, K. Starch Gel Electrolyte and Its Interaction with Trivalent Aluminum for Aqueous Aluminum-Ion Batteries: Enhanced Low Temperature Electrochemical Performance. Small 2024, 20, 2402245. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Li, X.; Liao, T.; Zhai, J.; Wang, X.; Huo, K. Biomass-Based Functional Separators for Rechargeable Batteries. Batter. Energy 2024, 3, 20240015. [Google Scholar] [CrossRef]
- Jeżowski, P.; Menzel, J.; Baranowska, H.M.; Kowalczewski, P.Ł. Microwaved-Assisted Synthesis of Starch-Based Biopolymer Membranes for Novel Green Electrochemical Energy Storage Devices. Materials 2023, 16, 7111. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.P.; Barros, S.C.; Alves, R.; Esperança, J.M.S.S.; Navarro-Segarra, M.; Sabaté, N.; Silva, M.M.; Esquivel, J.P. Biopolymer Electrolyte Membranes (BioPEMs) for Sustainable Primary Redox Batteries. Adv. Sustain. Syst. 2020, 4, 1900110. [Google Scholar] [CrossRef]
- Budarin, V.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; White, R.J. Carbonaceous Materials. U.S. Patent US8790548B2, 15 March 2007. [Google Scholar]
- Clark, J.H.; Budarin, V.; Deswarte, F.E.I.; Hardy, J.J.E.; Kerton, F.M.; Hunt, A.J.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Rodriguez, A.; et al. Green Chemistry and the Biorefinery: A Partnership for a Sustainable Future. Green Chem. 2006, 8, 853–860. [Google Scholar] [CrossRef]
- Uriburu-Gray, M.; Pinar-Serrano, A.; Cavus, G.; Knipping, E.; Aucher, C.; Conesa-Cabeza, A.; Satti, A.; Amantia, D.; Martínez-Crespiera, S. Mesoporous Carbons from Polysaccharides and Their Use in Li-O2 Batteries. Nanomaterials 2020, 10, 2036. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Aoki, Y.; Habazaki, H. Starch-Derived Hierarchical Porous Carbon with Controlled Porosity for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7292–7303. [Google Scholar] [CrossRef]
- Kang, S.; Kim, B.; Lee, S.; Baek, J.; Yoo, J. Tailoring Porosity of Starch-Derived Biocarbon for Enhanced Supercapacitor Performance. Mater. Technol. 2024, 39, 2338628. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Guo, Z.; Song, Z.; Lin, Y.; Lin, W.; Zheng, L.; Huang, Z.; Hong, Z.; Titirici, M.-M. Sustainable and Scalable Fabrication of High-Performance Hard Carbon Anode for Na-Ion Battery. J. Power Sources 2023, 557, 232534. [Google Scholar] [CrossRef]
- Mahmud, E.; Islam, M.R. Improved Electrochemical Performance of Bio-Derived Plasticized Starch/ Reduced Graphene Oxide/ Molybdenum Disulfide Ternary Nanocomposite for Flexible Energy Storage Applications. Sci. Rep. 2023, 13, 20967. [Google Scholar] [CrossRef]
- Liu, M.-C.; Lu, C.; Xu, Y.; Hu, Y.-X.; Li, J.; Zhang, H.; Zhang, Y.-S.; Zhang, B.-M.; Kong, L.-B.; Liu, W.-W.; et al. Three-Dimensional Interconnected Reticular Porous Carbon From Corn Starch By a Sample Sol–Gel Method Toward High-Performance Supercapacitors With Aqueous and Ionic Liquid Electrolytes. ACS Sustain. Chem. Eng. 2019, 7, 18690–18699. [Google Scholar] [CrossRef]
- Chelfouh, N.; Coquil, G.; Rousselot, S.; Foran, G.; Briqueleur, E.; Shoghi, F.; Caradant, L.; Dollé, M. Apple Pectin-Based Hydrogel Electrolyte for Energy Storage Applications. ACS Sustain. Chem. Eng. 2022, 10, 15802–15812. [Google Scholar] [CrossRef]
- Kiruthika, S.; Malathi, M.; Selvasekarapandian, S.; Tamilarasan, K.; Moniha, V.; Manjuladevi, R. Eco-Friendly Biopolymer Electrolyte, Pectin with Magnesium Nitrate Salt, for Application in Electrochemical Devices. J. Solid State Electrochem. 2019, 23, 2181–2193. [Google Scholar] [CrossRef]
- Eswaragomathy, S.; Selvanayagam, S.; Selvasekarapandian, S.; Muniraj Vignesh, N.; Aafrin Hazaana, S.; Meera Naachiyar, R. Preparation of Pectin Biopolymer Electrolyte for Zinc-Ion Battery Application. Ionics 2023, 29, 2329–2340. [Google Scholar] [CrossRef]
- Wilson, N.W.; Botte, G.G. Novel Biopolymer Pectin-Based Hydrogel Electrolytes for Sustainable Energy Storage. Mater. Adv. 2024, 5, 7312–7326. [Google Scholar] [CrossRef]
- Vijaya, N.; Selvasekarapandian, S.; Sornalatha, M.; Sujithra, K.S.; Monisha, S. Proton-Conducting Biopolymer Electrolytes Based on Pectin Doped with NH4X (X=Cl, Br). Ionics 2017, 23, 2799–2808. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Shanthi, C.; Selvasekarapandian, S.; Manjuladevi, R.; Perumal, P.; Chrishtopher Selvin, P. Synthesis and Characterization of Pectin-Based Biopolymer Electrolyte for Electrochemical Applications. Ionics 2019, 25, 203–214. [Google Scholar] [CrossRef]
- Su, Y.-H.; Chung, C.-Y.; Chen, Y.-R.; Wu, F.-Y.; Lin, Y.-H.; Chi, P.-W.; Wu, P.M.; Paul, T.; Lin, H.-E.; Chang-Liao, K.-S.; et al. A Green Recyclable Li3VO4-Pectin Electrode Exhibiting Pseudocapacitive Effect as an Advanced Anode for Lithium-Ion Battery. J. Energy Storage 2023, 72, 108454. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chen, W.-M.; Chen, Y.-R.; Chen, L.-Y.; Su, Y.-H.; Chi, P.-W.M.; Wu, P.; Chang-Liao, K.-S.; Tang, H.-Y.; Wu, M.-K. Enhanced Fast Charging Capabilities in Natural Graphite/iron Cross-Linked Pectin Electrodes for Lithium-Ion Batteries. Mater. Adv. 2024, 5, 6820–6829. [Google Scholar] [CrossRef]
- Harikumar, M.E.; Batabyal, S.K. Fabrication of Pectin Biopolymer-Based Biocompatible Freestanding Electrodes for Supercapacitor Applications. Polym. Adv. Technol. 2023, 34, 2890–2902. [Google Scholar] [CrossRef]
- Olawoye, B.; Jolayemi, O.S.; Origbemisoye, B.A.; Oluwajuyitan, T.D.; Popoola-Akinola, O. Hydrolysis of Starch. In Starch: Advances in Modifications, Technologies and Applications; Sharanagat, V.S., Saxena, D.C., Kumar, K., Kumar, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 83–101. ISBN 978-3-031-35843-2. [Google Scholar]
- Sevilla, M.; Yu, L.; Ania, C.O.; Titirici, M.-M. Supercapacitive Behavior of Two Glucose-Derived Microporous Carbons: Direct Pyrolysis versus Hydrothermal Carbonization. ChemElectroChem 2014, 1, 2138–2145. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black Perspectives for a Green Future: Hydrothermal Carbons for Environment Protection and Energy Storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Cai, X.; Xiao, Y.; Sun, W.; Yang, F. Glucose-Derived Activated Carbons for Supercapacitors: Comparison between Single O Doping and N/O Co-Doping. Electrochim. Acta 2022, 406, 139861. [Google Scholar] [CrossRef]
- Wortmann, M.; Keil, W.; Diestelhorst, E.; Westphal, M.; Haverkamp, R.; Brockhagen, B.; Biedinger, J.; Bondzio, L.; Weinberger, C.; Baier, D.; et al. Hard Carbon Microspheres with Bimodal Size Distribution and Hierarchical Porosity via Hydrothermal Carbonization of Trehalose. RSC Adv. 2023, 13, 14181–14189. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Ji, Y.; Chun, Y.; Lee, J.H.; Yoo, H.Y.; Kim, S.; Lee, J.H.; Park, C.; Kim, S.W. Eco-Friend Air Electrode for Flexible Quasi-Solid-State Electrolyte Zinc-Air Battery Based on Spherical Glucose Biochar Derived from Biorefinery Process. Mater. Today Sustain. 2023, 23, 100456. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and Structural Differences between Glucose, Cellulose and Lignocellulosic Biomass Derived Hydrothermal Carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Väli, R.; Tooming, T.; Thomberg, T.; Jänes, A.; Lust, E. Novel D-Glucose Derived Hard Carbon Anode for Sodium-Ion Batteries. ECS Meet. Abstr. 2016, MA2016-01, 512. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, R.J.; Yu, L.; Titirici, M.-M.; Antonietti, M.; Maier, J. Hollow Carbon Nanospheres with Superior Rate Capability for Sodium-Based Batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and Application of Chitin and Its Derivatives: A Review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Vinodh, R.; Sasikumar, Y.; Kim, H.-J.; Atchudan, R.; Yi, M. Chitin and Chitosan Based Biopolymer Derived Electrode Materials for Supercapacitor Applications: A Critical Review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Nishi, N.; Noguchi, J.; Tokura, S.; Shiota, H. Studies on Chitin. I. Acetylation of Chitin. Polym. J. 1979, 11, 27–32. [Google Scholar] [CrossRef]
- Latifi, M.; Ahmad, A.; Kaddami, H.; Hasyareeda Hassan, N.; Dieden, R.; Habibi, Y. Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes. Polymers 2020, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, L.; Zhao, B.; Gu, X.; Li, T.; Huang, L.; Wu, Q.; Yu, S.; Liu, S. Sandwich Construction of Chitosan/reduced Graphene Oxide Composite as Additive-Free Electrode Material for High-Performance Supercapacitors. Carbohydr. Polym. 2021, 255, 117397. [Google Scholar] [CrossRef]
- Xia, W.; Cheng, M.; Hu, J.; Liu, Q.; Wei, T.; Wang, R.; Li, W.; Liu, B. Facile and Controllable Synthesis of Nitrogen Self-Doped Chitosan-Derived Carbon for High-Performance Li-Ion Batteries. Carbon Lett. 2024, 34, 85–94. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Zhao, L.; Hadi, M.K.; Sambasivam, S.; Zhou, Q.; Ran, F. A Renewable Hydrogel Electrolyte Membrane Prepared by Carboxylated Chitosan and Polyacrylamide for Solid-State Supercapacitors with Wide Working Temperature Range. J. Power Sources 2023, 560, 232704. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Zhang, J.; Li, M.; Tan, F.; Xue, X.; Sui, Z.; Zou, Y.; Zhang, X.; Zhang, W.; et al. Biomass Chitin Nanofiber Separators Proactively Stabilizing Zinc Anodes for Dendrite-Free Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2405957. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, X.; Chen, K.; Cheng, J.; Xiong, F.; Zhang, X.; Hou, Z.; Ma, X.; Zi, Z. Fluorinated Polymer Coated Cyanoethyl-Chitin Nanofiber Composite Separators for High Performance Lithium Ion Batteries. Scr. Mater. 2024, 242, 115951. [Google Scholar] [CrossRef]
- Itoi, H.; Saeki, G.; Usami, T.; Takagi, S.; Suzuki, H.; Ishii, T.; Iwata, H.; Ohzawa, Y. Activation-Free Synthesis of Chitin-Derived Porous Carbon: Application for Electrical Energy Storage. ACS Sustain. Resour. Manag. 2024, 1, 743–756. [Google Scholar] [CrossRef]
- Bósquez-Cáceres, M.F.; Lima, L.D.; Morera Córdova, V.; Delgado, A.D.; Béjar, J.; Arjona, N.; Álvarez-Contreras, L.; Tafur, J.P. Chitosan-Carboxymethylcellulose Hydrogels as Electrolytes for Zinc–Air Batteries: An Approach to the Transition towards Renewable Energy Storage Devices. Batteries 2022, 8, 265. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, G.; Zhang, S.; Xie, C.; Li, X. A Light-Thin Chitosan Nanofiber Separator for High-Performance Lithium-Ion Batteries. Polymers 2023, 15, 3654. [Google Scholar] [CrossRef]
- Yang, X.; Wu, W.; Liu, Y.; Lin, Z.; Sun, X. Chitosan Modified Filter Paper Separators with Specific Ion Adsorption to Inhibit Side Reactions and Induce Uniform Zn Deposition for Aqueous Zn Batteries. Chem. Eng. J. 2022, 450, 137902. [Google Scholar] [CrossRef]
- Cruz-Balaz, M.I.; Bósquez-Cáceres, M.F.; Delgado, A.D.; Arjona, N.; Morera Córdova, V.; Álvarez-Contreras, L.; Tafur, J.P. Green Energy Storage: Chitosan-Avocado Starch Hydrogels for a Novel Generation of Zinc Battery Electrolytes. Polymers 2023, 15, 4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Feng, C.-N.; Li, H.-P.; Zheng, X.-C. N, O Self-Codoped Hierarchical Porous Carbon from Chitosan for Supercapacitor Electrode Active Materials. Cellulose 2021, 28, 437–451. [Google Scholar] [CrossRef]
- Quan, L.H.; Thuy, U.T.D.; Nam, P.V.; Van Chi, N.; Duong, T.X.; Hoa, N. Van Chitosan-Derived Carbon Aerogel Nanocomposite as an Active Electrode Material for High-Performance Supercapacitors. J. Sci. Adv. Mater. Devices 2023, 8, 100586. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef]
- Xue, C.-F.; Wang, L.-F.; Zhao, W.; Du, J.-Q.; Li, X.-Q.; Yan, W.-J.; Li, X.-H.; Hao, X.-G. N/O Co-Doping Biochar with Matched Pores Prepared by Co-Pyrolysis and Tailoring Activation and Its Balanced Electrochemical Supercapacitor Performance. J. Energy Storage 2023, 71, 108214. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, W.; Du, X.; Yang, X.; Wang, F.; Zhou, Y.; Pan, Y.; Lu, Y. An N,P,O-Doped Porous Carbon Electrode Material Derived from a Lignin-Modified Chitosan Xerogel for a Supercapacitor. Mater. Today Sustain. 2023, 22, 100372. [Google Scholar] [CrossRef]
- Idamayanti, D.; Rochliadi, A.; Iqbal, M.; Noer, Z.; Febrian, R.; Septiani, N.L.W.; Purwasasmita, B.S.; Yuliarto, B.; Nuruddin, A. Free-Standing Hard Carbon Anode Based on Cellulose Nanocrystal-Reinforced Chitosan Substrate for Eco-Friendly Sodium-Ion Batteries. J. Energy Storage 2024, 89, 111491. [Google Scholar] [CrossRef]
- Mayrén, A.; Alcaraz-Espinoza, J.J.; Hernández-Sánchez, A.; González, I.; Ramos-Sánchez, G. Chitosan Binders for Sustainable Lithium-Sulfur Batteries: Synergistic Effects of Mechanical and Polysulfide Trapping Properties. Electrochim. Acta 2024, 480, 143917. [Google Scholar] [CrossRef]
- Zhang, T.-W.; Chen, J.-L.; Tian, T.; Shen, B.; Peng, Y.-D.; Song, Y.-H.; Jiang, B.; Lu, L.-L.; Yao, H.-B.; Yu, S.-H. Sustainable Separators for High-Performance Lithium Ion Batteries Enabled by Chemical Modifications. Adv. Funct. Mater. 2019, 29, 1902023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.M.; Gómez-Romero, P.; González-Gil, R.M. Polysaccharides: The Sustainable Foreground in Energy Storage Systems. Polysaccharides 2025, 6, 5. https://doi.org/10.3390/polysaccharides6010005
Thomas SM, Gómez-Romero P, González-Gil RM. Polysaccharides: The Sustainable Foreground in Energy Storage Systems. Polysaccharides. 2025; 6(1):5. https://doi.org/10.3390/polysaccharides6010005
Chicago/Turabian StyleThomas, Sharin Maria, Pedro Gómez-Romero, and Rosa M. González-Gil. 2025. "Polysaccharides: The Sustainable Foreground in Energy Storage Systems" Polysaccharides 6, no. 1: 5. https://doi.org/10.3390/polysaccharides6010005
APA StyleThomas, S. M., Gómez-Romero, P., & González-Gil, R. M. (2025). Polysaccharides: The Sustainable Foreground in Energy Storage Systems. Polysaccharides, 6(1), 5. https://doi.org/10.3390/polysaccharides6010005







