Abstract
In the aquaculture sector, the biofunctionalization of biomaterials is discussed using materials from algae and analyzed as a possible potential strategy to overcome the challenges that hinder the future development of the application of endolysins in this field. Derived from years of analysis, endolysins have recently been considered as potential alternative therapeutic antibacterial agents, due to their attributes and ability to combat multi-resistant bacterial cells when applied externally. On the other hand, although the aquaculture sector has been characterized by its high production rates, serious infectious diseases have led to significant economic losses that persist to this day. Although there are currently interesting data from studies under in vitro conditions on the application of endolysins in this sector, there is little or no information on in vivo studies. This lack of analysis can be attributed to the relatively low stability of endolysins in marine conditions and to the complex gastrointestinal conditions of the organisms. This review provides updated information regarding the application of endolysins against multi-resistant bacteria of clinical and nutritional interest, previously addressing their important characteristics (structure, properties and stability). In addition, regarding the aquaculture sector, the biofunctionalization of biomaterials is discussed using materials from algae and analyzed as a possible potential strategy to overcome the challenges that hinder the future development of the application of endolysins in this field.
1. Introduction
Today, antibiotic resistance (AntR) represents a major threat to human health. Worldwide, by 2050, it is estimated that about 10 million deaths will be associated with AntR [1], primarily linked to longer hospital stays and increased risk of death. In addition, it is noteworthy that AntR will cause a projected global loss of around 100 billion dollars for the same year. Therefore, the widespread dissemination of AntR in both developed and developing countries results in significant costs for the public health sector [2].
Given that the development of new antibiotics is slow compared to the rapid emergence of multi-resistant bacteria (MRB), the post-antibiotic era is a fact [3]. For these reasons, there is an urgent need for the discovery and/or development of alternative antibacterial agents.
Endolysins are enzymes encoded by bacteriophages (phages) involved in bacterial lysis at the end of the lytic cycle and for the release of new viral progeny [4]. Because endolysins target the peptidoglycan (PG) layer, which is a highly conserved and unchanging component of the bacterial cell wall, the emergence of bacterial resistance against these enzymes has not been documented to date [5]. Consequently, endolysins have represented promising alternatives to conventional antibiotics to combat the AntR crisis.
Although the public health sector has paid more attention to the AntR issue and, consequently, promising results in the fight against MRBs, some productive sectors (for example, aquaculture) the scientific field in this context, are in a considerable level of delay. Currently, aquaculture has exhibited exacerbated development rates in the food production sector to meet the growing demand for fish and shellfish [6]. The intensification and the conditions of crops have meant favorable conditions for the appearance and rapid spread of new infectious diseases, affecting the production rate and bringing with it severe economic losses [7]. Usually, the control of these diseases has been carried out with the use of antibiotics [8]. However, its excessive use has represented a public health problem due to the appearance of MRB, as a result of the accumulation of antibiotics in the environment and cultivated organisms [9]. Consequently, actions have been intensified that lead to promoting the responsible use of antibacterials [8] and the search for alternative treatments [8,10,11]. Although these treatments have been effective and safe under in vitro conditions, there are few reports of their application in vivo, presenting themselves as a factor to consider in relation to their validation and approval as alternative antimicrobial agents.
The implementation of endolysins as therapeutic agents involves critical challenges. In the first instance, associated with the optimal administration strategy to reach the target of infection in appropriate concentrations without losing activity [12].
The objective of this review is, in addition to providing current information on endolysins applied against MRBs of clinical and nutritional interest, to identify the challenges that hinder the future development of their application in vivo, as well as the possible strategies to overcome these limitations in the field of aquaculture.
2. Generalities of Endolysins
Endolysins; commonly known as lysins, are phage-encoded protein products that are synthesized at the final stage of replication within the host bacterium. They are enzymes capable of hydrolyzing the host cell wall. The bacterial cell wall contains peptidoglycan (PG) as its main component. PG is a polymer consisting of repeat chains of N-acetylmuramic acid (MurNAc) and the saccharide N-acetylglucosamine (GlcNAc), linked by β(1–4) glycosidic bonds [13] (Figure 1). PG can withstand a turgor pressure of 20–50 atmospheres and disruption of the PG layer leads to osmotic shock resulting in bacterial cell death. Endolysin-mediated host lysis is regulated by proteins called holins that are also synthesized late in phage infection. Holins accumulate in the cytoplasm of the host and, at a certain concentration, are capable of oligomerizing to form channels through which the export of endolysin to the peptidoglycan layer is facilitated [14,15].
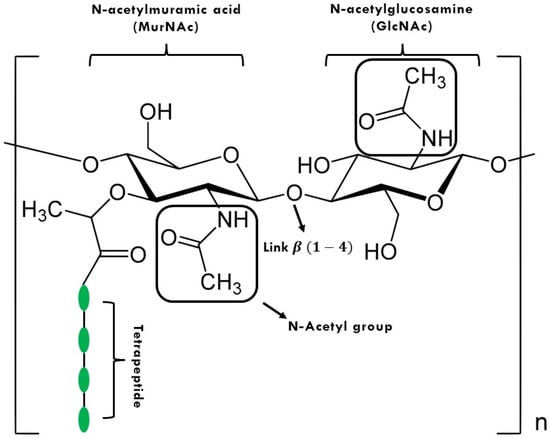
Figure 1.
Peptidoglycan (PG) structure. The letter n means the repetition of the structure “n” times.
2.1. Structure of Endolysins
Endolysins that have action against Gram-positive (G+) and Gram-negative (G−) bacteria differ in their architecture due to variations in the composition of the cell wall of both bacterial groups. In endolysins that affect G+ bacteria called globular, two distinct domains are identified called enzymatically active domain (EAD) and cell binding domain (CBD) [16]. The EAD has the necessary elements to catalyze and cleave the specific bonds of the bacterial PG, while CBD binds the enzyme to the cell wall, thus limiting its diffusion within the cell [17]. On the other hand, CBD improves the orientation of the EAD towards the insoluble PG. This targeting effect results in increased enzyme activity despite their irreversible binding [16]. In G− bacteria, the outer membrane (OM) limits CBD-based collateral damage to surrounding cells and that is why most so-called modular G− endolysins lack CBD. The G− active endolysin, designated KZ144, has been reported to have a modular structure composed of a CBD and two EADs. Interestingly, this endolysin shows a broad spectrum against various G− pathogens such as Pseudomonas aeruginosa, Bacillus subtilis, Vibrio parahaemolyticos, among others [18].
2.2. Classification of Endolysins
In addition to their wide structural variation, endolysins are also highly diverse in cleavage specificity. They can be divided into five groups (I–V), which are directed to glycosidic and amide-type bonds or peptides, present in the PG [16] (Figure 2). Glucosidases hydrolyze glycosidic bonds and include: (I) N-acetyl-β-d-glucosaminidases (EC 3.2.1.52), (II) N-acetyl-β-d-muramidases (EC 3.2.1.17, also called lysozymes or muramidases) and (III) lytic transglycosylases (EC 3.2.1.17). N-acetyl-β-d-glucosaminidases target the N-acetylglucosaminyl-β-1,4-N-acetylmuramine bond at the reducing end of GlcNAc. The other two groups break the N-acetylmuramoyl-β-1,4-N-acetylglucosamine bond; however, transglycosylases catalyze an intramolecular reaction with neighboring sugar moieties that act as electron acceptors, resulting in cleavage of the N-acetylmuramoyl-β-1,4-N-acetylglucosamine bond to form N-acetyl-1,6-anhydrous-muramoyl. Interestingly, there are no water molecules involved in this reaction, so lytic transglycosylases are not hydrolases, unlike lysozymes. (IV) N-acetylmuramoyl-l-alanine amidases (EC 3.5.1.28) hydrolyze the amide bond between MurNAc and the first amino acid of the “mother peptide” (L-alanine). Finally, the (V)-endopeptidases (EC 3.4.XX) can be classified as parent peptide-specific endopeptidases (l-alanoyl-d-glutamate endopeptidases, glutaminyl-l-lysine endopeptidases specific for interpeptidase bridges), in which they cleave peptide bonds between two amino acids in the parent peptide or the cross-link or bridge.
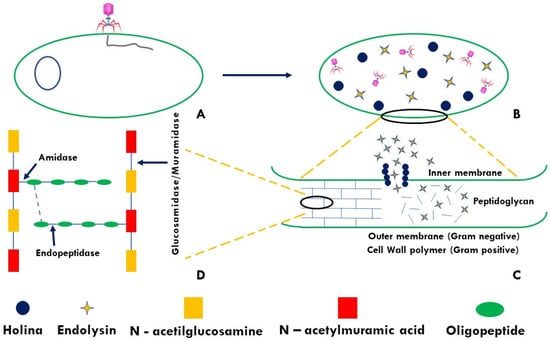
Figure 2.
Mechanism of alteration of peptidoglycan (PG) by the endolysin-holin system: (A) Infection by bacteriophage; (B) Lysis of bacterial cells; (C) Endolysin-holin mediated PG disruption; (D) Endolysin sites of action.
2.3. Unique Properties of Endolysins
In vitro and in vivo studies demonstrate the enormous potential of endolysins as antibacterial agents. Due to the proteinaceous nature of these antibacterials, doubts have been raised regarding their immunogenicity and stability. However, many of these concerns have been addressed and resolved, resulting in successful preclinical trials. Some of its strengths and weaknesses are summarized in Table 1. First of all, its specificity is noteworthy. The vast majority of antibiotics or any other antibacterial exhibit a broad spectrum of inhibition once applied, damaging the intestinal microbiota (causing dysbiosis) specifically against commensal strains, which play an important role in the health of the organism at the immunological level, as in the prevention of gastrointestinal diseases as an example. Regarding the possible development of resistance, it is known that because its site of action is the PG, which is a structure that can hardly mutate, the probability that resistant populations will appear is very low [17]. However, in endopeptidase-type endolysins, which act in the crosslinking of the PG, bacteria capable of substituting one or more amino acid residues and consequently affecting the mode of action and the effectiveness of this type of endolysins have been reported [18,19]. In this sense, the application of endolysins of types I–IV is recommended in future research, in such a way as to avoid this problem or to make use of chimeric endolysins with different sites of action [18].

Table 1.
Strengths and weaknesses of endolysins based on their antimicrobial designation and protein nature.
2.4. Endolysins with Activity against Gram-Negative Bacteria (G−)
Today, various sectors worldwide are facing unprecedented crises due to the appearance and rapid spread of microorganisms resistant to one or more antimicrobial agents. Even in the first list of global priority pathogens published by the WHO, nine of the twelve identified pathogens are G− bacteria [30]. For this reason, there is an urgent need to extend the application of endolysins directed to G-bacteria that allow them to “overcome” their OM, which represents their first defense mechanism. Although it has been reported that some of these endolysins have the innate ability to penetrate the OM, various strategies have been proposed to enhance this property. Some of them involve coupling with outer membrane permeabilizers (OMPs) and modification of endolysins by protein engineering or by functionalization into transport systems with OM-penetrating properties (Figure 3).
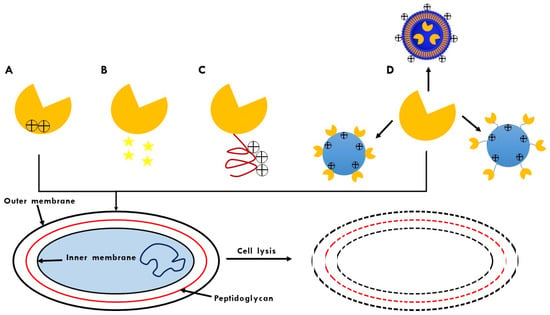
Figure 3.
OM penetration strategies for endolysins: (A) Identification of lysins with intrinsic OM permeability; (B) Use of outer membrane permeabilizers (OMP); (C) Design of endolysins with OMP-disruptive properties; (D) Formulation of endolysins in carrier systems that penetrate OM.
Table 2 summarizes some works that describe the approaches used in the last decade to enhance the effect of endolysins against G− bacteria. In general, it is noteworthy that while some endolysins have a substantial bactericidal effect against multiple G− pathogens, in other cases it is limited to a few strains of the same species. In general, differences between endolysins are visualized in terms of their effectiveness, as well as a dose-dependent effect. Based on Table 2, to achieve a bactericidal effect against G− pathogens, it can be seen that higher doses are required for the case of modular endolysins (chimeric or not) in contrast to chimeric globular endolysins, even without this particularity; however, there is no scientific evidence which affirms this observation. In short, envisioning future research projects, it would undeniably be recommended to carry out analyses and apply synergistic treatments in which endolysin G− chimerics (not endopeptidases) are included, as well as an adequate process which allows endolysin to resist different adverse conditions in which it is produced and its enzymatic activity is compromised, a functionalization process, for example [31].

Table 2.
Endolysins activity against bacteria of clinical and nutritional interest.
2.5. Stability of Endolysins
To potentially be handled as an antibacterial agent, the stability of endolysin during processing, storage, and administration is critical. Table 3 summarizes various reports of these antibacterials from preclinical studies, where various aspects of the stability of endolysins against G− such as thermostability, catalytic activity in pH ranges and storage specifications are addressed, which must be addressed for future technological development. The fact of the numerous lines of research in which these antibacterials can be applied is unquestionable. Based on this, issues related to stability should be addressed. For example, if it is linked to an industrial application, the thermostability factor and pH would be important to consider. On the other hand, if an application is glimpsed in which it is intended to exert an effect on the intestinal microbiota of an organism; whether it is terrestrial or marine, the pH parameter would be key, since it is known that there are important variations in this abiotic factor. Finally, both in the industrial sector, although more related to the field of research, the scenario of “storage for later analysis” is routine, for which storage is an important term to consider, more so because it has been documented that some endolysins may lose substantial effectiveness, as shown in Table 3. Due to the above and based on what is reported in the literature, endolysin KZ144 could be postulated as an ideal bacterial agent, since it reflects a robust enzymatic activity by maintaining its activity above 50 °C, is active in slightly acid and alkaline conditions (pH 4.5–9, optimal 6.2–6.5) and does not present a loss of activity despite storage for 4 months at 4 °C in an enzyme buffer, in addition to exhibiting a significant inhibition spectrum against important G− bacteria clinical and nutritional [18].

Table 3.
Abiotic factors that alter the stability of endolysin.
2.6. Commercial Endolysins
Finally, expressing more strengths than weaknesses, there are already formulations of endolysins aimed mainly at treating diseases and their respective symptoms resulting from the proliferation of pathogenic bacteria. Some are in the clinical phase and others are in the preclinical phase. The results of all of them are promising in relation to the combat of MRB; the companies and their respective products (endolysins) are illustrated in Table 4.

Table 4.
Commercial endolysins and their respective manufacturing companies.
3. Strategies for the Application of Endolysins Aimed at In Vivo Studies in Aquaculture
3.1. Current Alternatives for the Treatment of Infectious Diseases
Given that infectious diseases of relevance in the aquaculture sector require continuous administration treatments, it is essential to design protein antibacterials that can be recognized by the receptors of the intestinal epithelium for their internalization and systemic administration [49,50].
Nowadays, for the local administration of protein antibacterials through food, various options have been used to distinguish the technology developed for enzymes applied in animal feed, which consists of partially purified enzyme preparations that contain additives for dry stabilization (e.g., salts with divalent ions, sugars and/or glycerol) [51,52]. However, depending on the protein antibacterial, the formulation requires a particular design, testing different stabilizers and vehicles and an evaluation of their protective effect during processing.
On the other hand, the administration of protein antibacterials in situ was carried out in the first instance for human health through genetically modified lactic acid bacteria. This improved over the years until its validation in patients with gastrointestinal problems [53,54,55]. The advantages of these as administration vehicles lie in their tolerance to gastric conditions and bile salts (which allows them to survive their passage through the digestive tract), as well as their ability to colonize the intestinal mucosa [56]. This technology is being evaluated in vivo trials for aquaculture, but focused on recombinantly-produced bacterial antigens (Streptococcus inie, Aeromonas hydrophyla, E. tarda) as an oral vaccine for fish [57,58,59]. In addition, some in vitro evaluations have expressed antibacterial proteins [60,61] and type II endolysins [62]. Therefore, lactic acid bacteria have the potential for the treatment of local bacterial diseases, as a vehicle for the administration of proteinaceous antibacterials.
A potential alternative that could be implemented for aquaculture should be considered, for example, through the administration of bioencapsulated protein antibacterials in plant cells, which is approved by the FDA as an economically viable and safe production system of protein antibacterials from their production in hydroponic systems in greenhouses [49]. The advantage lies in the folding and activity because they are retained for several years at room temperature in freeze-dried plant cells, also eliminating the maintenance of the cold chain [63].
3.2. Main Obstacles to Overcome for the Treatment of Infectious Diseases
In contrast, endolysins are just beginning to be evaluated against pathogens of aquaculture importance. To date, only the lytic capacity of some endolysins against the cell wall of some strains of interest in aquaculture, for example Vibrio parahaemolyticus, has been demonstrated. [Lysqdvp001 endolysin [64]; LysVPp1 [65]], Vibrio campbellii, Vibrio azureus [LysVPp1] [65] and Vibrio alginolyticus [endolisina cwlQ] [66]. The evaluations were carried out on pre-treated bacteria in order to expose the cell wall (dead bacteria that are used as a substrate), but their bactericidal effect was not reported, so their activity in marine conditions was not evidenced either.
In another sense, as is now known, significant differences in the intestinal environment can be found between carnivorous (salmon), herbivorous (tilapia) and omnivorous (catfish and penaeid shrimp) farmed species. In other words, carnivores have higher protease activity compared to herbivores and omnivores, while non-carnivores have higher carbohydrase activity for the digestion of plant cells. In addition, in carnivorous species part of their digestion is carried out in acidic conditions [67]. Adding to the above, the differences in enzymatic digestion are caused by other factors such as the age of the organism, as well as the degree of colonization of the intestinal microbiota in different organs. The latter represents an important point to consider from the point of view of elucidating the degree of stress that the protein antibacterial could experience in the site(s) of action, determining whether it would require additional protection. As a typical case, as one of the main species of crustaceans cultivated throughout the world, we can cite Litopenaeus vannamei, whose organs such as hepatopancreas, stomach and intestine exhibit a high rate of enzymatic activity attributed to the secretion of extracellular enzymes (amylases, chitinases, esterases and lipases), the product of microbial metabolism, highlighting the genera Pseudoaltheromones (sp.) and Vibrio (parahaemolyticus, harveyi, communis, campbelli, rotiferianus, alginolyticus), the latter being the most abundant [68]. However, despite being relevant in contributing to the digestive components of the diet, species of the Vibrio genus such as V. parahaemolyticus, V. harveyi and V. campbelli are reported as causal agents in numerous cases of mass mortalities in this cultivar; especially V. parahaemolyticus, which can induce vibriosis by certain strains [69,70,71], or induce Acute Hepatopancreatic Necrosis Disease (AHPND), and that can cause up to 100% mortality in the first 30 days of culture [72,73]. Furthermore, as we know so far, V. parahaemolyticus is known as an opportunistic pathogen, since exposure to high concentrations of nitrites, ammonia and some pesticides inhibits the immune responses of L. vannamei and increases its susceptibility to V. parahaemolyticus, which eventually leads to an increase in mortality [69,74]. Given that the immune systems of crustaceans are not as complex as those of higher organisms, strategies for the prevention and treatment of pathogens are required to sustain white shrimp cultures, for which alternatives such as the addition of zinc have been proposed [73] and β-glucans [74] in the diet and, consequently, favor the upregulated expression of TLR, a gene that reflects the immune response of white shrimp after bacterial combat. In conclusion, there is no doubt that there is an urgent need to improve, or, failing that, preserve the biological activity of endolysins by providing protection both for the marine environment, where the cultivar is developed, and throughout the entire complex gastrointestinal tract. Taking into account that said protection, as it is degraded, the released components manage to exert an immunomodulatory effect in the organism once absorbed in the intestinal epithelium.
3.3. Biofunctionalization of Endolysins as a Promising Strategy for Application in Aquaculture
At the beginning of the 21st century, venturing into the identification, extraction and isolation of bioactive compounds or nutraceuticals was a challenge. However, it is currently a highly exploited field of research. However, from the point of view of bioavailability, these compounds have a particularity when they are immersed in the food matrix, mainly due to their limited release profile in the digestive process, among other biotic and abiotic factors, which is why the incorporation of these molecules into various biomaterials has been implemented in recent years. Therefore, the generation of Biofunctionalized Polymeric Materials (BPM) has been a promising alternative in preserving the functionality of these compounds due to the fact that a prolonged supply has been achieved and, consequently, they have exerted their biological effect on the organism. Cases of success can be mentioned, including the protection of antioxidants (curcumin [75], β-carotene and α-tocopherol [76]), anti-inflammatories (dexibuprofen) [77], bioactive compounds (mangiferin) [78], enzymes [lactase [79], papain [80]] to name a few. Under this perspective, and in addition to the fact that, as far as we know, there is no experimental evidence of the therapeutic effectiveness of endolysins in aquaculture, only their preventive effect in a few evaluations and numerous experiments are evidence of their in vitro activity. The biofunctionalization of endolysins is seen as a viable and feasible strategy; however, it is crucial to determine the polymeric material(s) to be used which, in addition to contributing to the stability of the enzyme, would be interesting if they contributed significantly to the previously discussed limitations (antibacterial activity, prebiotic effect, immunomodulatory effect).
Firstly, it is noteworthy that many polymeric materials, as well as other possible elements that will make up the treatment, need to be related to the organism in question. In other words, they must facilitate the ingestion of the treatment and its acceptance (affinity to the diet), as well to be stable in seawater for at least 2.5 h due to the slow feeding of L. vannamei. In this sense, as we know so far, polymeric materials such as cellulose, hemicellulose, alginates and alginate oligosaccharides (AOS) have been added to shrimp diets because they have generated substantial stability of the formulation in seawater [81].
In principle, the cellulose molecule (β-glucan) should be highlighted, since today it is widely used for the biofunctionalization of bioactive compounds [82,83,84]. However, it is a challenge to determine the spacer group that will act as a “bridge” between the enzyme and the cellulose, as at this time there are numerous methods to achieve it [85]. Regardless, it is worth noting the use of chlorinated compounds (acyl chlorides), since, in addition to generating a deprotonation in the hydroxyl group (characteristic of cellulose) (ideal event to form a covalent bond with a characteristic group of endolysins, for example amino) it has also been shown that the reaction conditions are not strong and it does not produce aberrant reactive species [85].
As a second aspect, it would be interesting to consider the AOS that, although their study in various fields has been exploited in the last 20 years, has not been enough to be a potential candidate for incorporation into a diet in L. vannamei or any other marine organism. However, the idea of designing a formulation containing these would be viable and attractive, since they exhibit diverse biological activities as antioxidants [86,87,88,89], prebiotic effects [90,91], immunomodulatory properties [92,93,94] and antibacterial effects on bacteria of clinical and food interest [95,96,97], as well as bacteria of aquaculture interest such as V. parahaemolyticus and V. harveyi [95], the latter representing a point to highlight attributed to the potential inhibition of vibrios related to the mortality of L. vannamei. Similar to cellulose, there are factors related to the probability of success, including that the previously mentioned effects are exhibited by OSA, which are directly related to the chemical structure, specifically the ratio or quotient (M/G) of mannuronic acid (M) and guluronic acid (G). As we know so far, each monomer is related to a particular characteristic; that is, abundance of blocks of M that G is said to exert a greater biological effect, while, in an inverse sense, it correlates with a high stability against abiotic factors [86,88,89,93,95,98,99]. Therefore, it could be asserted that if the objective of the treatment is for OSA to potentially exhibit the biological effects described, then M/G > 1. However, if the objective corresponds to presenting substantial stability against an abiotic factor (for example, water marina), then M/G ˂ 1. Finally, if a scenario will be idealized in which the two phenomena will manifest themselves as much as possible, then M/G ≈ 1, in such a way that, attending to the double problem raised previously regarding the application of endolysins, it would be recommended to opt for the last condition. However, it would be important to carry out analyses corresponding to the effect of seawater on OSA (structure and bioactivity) and, consequently, establish the ideal M/G ratio.
Thirdly, to our knowledge, zinc controls the regulatory change of the immune response; that is, against antigenic stimuli it is an element that mediates the behavior of the immune system. For this reason, a diet adequate in zinc is fundamental in the efficacy of the immune system and therefore in the health of the organism. Consequently, the addition of zinc in various diets intended for marine organisms is a fact [100,101,102,103]. However, there are significant differences between zinc of inorganic origin (for example ZnO, ZnSO4) and that of organic origin (for example amino zinc), with the latter class standing. Zinc methionine (ZnMet), stands out in particular due to its excellent immune response under normal conditions, even under events in which the health of the marine organism is compromised (for example, reduction in the mortality rate after inoculation of species of the genus Vibrio) [103]. Additionally, supplementation with zinc of inorganic origin could be viewed negatively due to the fact that this type of molecule has been reported as cytotoxic and with a broad-spectrum antibacterial effect [104,105]. As a possible result, the problem of dysbiosis in the organism could be reflected, greatly altering numerous biochemical processes such as the limited availability of digestive enzymes. Finally, although normal or standard diets aimed at marine organisms have a zinc concentration (˂10 mg), this does not represent a significant content to achieve the desired biological effect. It has been reported that an important immune response is acquired at intervals of 35 to 50 mg, For this reason, the zinc content in the treatments should oscillate in the mentioned range, thus guaranteeing a potential immunomodulatory effect.
Finally, determining which endolysins could have significant stability in the marine environment represents a key point in protein antibacterial formulation. In our opinion, those endolysins that have substantial activity in conditions similar to those of seawater should be selected as a study model [106]. Thus, the acceptance requirements would first be that they exhibit activity in the presence of high ionic strength (NaCl concentrations > 0.3 M); secondly, that the activity is present in a wide pH range, particularly in alkaline conditions (with optimal activity at pH 7–9) and as a last criterion, as already reported, with bactericidal activity against Gram-negative pathogens (considering that the vibrios fall into this classification). Based on these conditions, the endolysins KZ144 [18] and LysPA26 [34] meet these characteristics, even KZ144 exhibits potential enzymatic activity when subjected to high concentrations of NaCl (200 mM and 500 mM~170% and 120% relative activity, respectively), in addition to maintaining up to 80% of its activity when exposed to solutions, with divalent cations (Mg2+ and Ca2+) characteristic of seawater [18]. In summary, it would be recommended to select endolysin KZ144 to be biofunctionalized, due to its high activity even under conditions of extreme salinity, its stability against divalent cations and, to a lesser extent, to opt for LysPA26, which, although both are derived from phages that infect G− bacteria, differ in relation to the type and amount of EAD and CBD. Therefore, it is unquestionable to carry out various analyses to elucidate the relevance of these characteristics in this context.
In summary, the urgent need to provide protection to endolysins is unquestionable, since their enzymatic activity could be compromised by biotic and abiotic factors characteristic of seawater, as well as the complex intestinal tract of L. vannamei. Therefore, the use of polymeric materials such as cellulose and OSA would be excellent candidates to be biofunctionalized with these enzymes, which, in addition to being part of the native diet of this crustacean, chemically represent an ideal molecule to be loaded and reflect a robust bioactive profile, respectively. In addition, the immune system plays an important role in the health of the crustacean, both under normal and induced conditions, for which the addition of zinc sources of organic origin would exacerbate this characteristic, influencing its growth, as well as preventing it from contracting an infectious disease. Finally, it is required that, in order for the endolysins to be biofunctionalized, they are stable when immersed in seawater. Based on its chemical composition, KZ144 would be postulated as ideal in this context and LysPA26 as a second option (Figure 4).
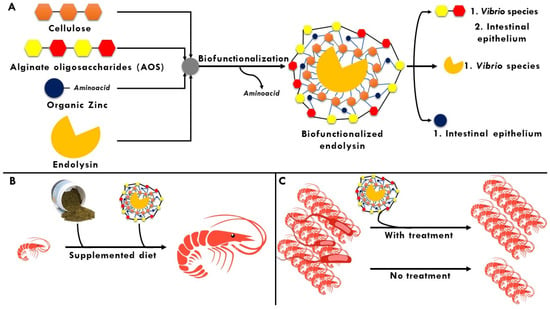
Figure 4.
Biofunctionalization as a potential strategy to combat bacteria of interest in aquaculture. (A) Main molecules to be biofunctionalized with endolysins and suitable target sites after being released from the matrix; (B) Biofunctionalized endolysin as a potential additive in standard diets for L. vannamei; (C) Biofunctionalized endolysin as a possible prophylactic/palliative treatment in cultivars of L. vannamei.
4. Current Trends of Endolysins Applied to In Vivo Studies in Aquaculture
In the search for information, a typical process is to manually explore, describe and organize the retrieved results. However, today, efficient and flexible technologies can be used, capable of combining the retrieval and subsequent organization of information.
A different approach is that of grouping the results into so-called clusters. The Carrot2 program performs the “clustering” (categorization or grouping) of the documents found based on similarities between them, without prior knowledge of their characteristics. The user raises a general topic and can then be directed to analyze the more specific topics created dynamically from the results of the query.
Therefore, a stratified search was performed [107] to understand the current trends of the relevant topics of this review, which was divided into three main points: the use of endolysins directed towards G-antibiotic-resistant bacteria, the use of OSA as a potential antibacterial agent in marine organisms, and, finally, the use of endolysins targeting marine bacteria using keywords and boolean operators [108]. The information obtained explaining the main issues of this project is displayed in Figure 5.
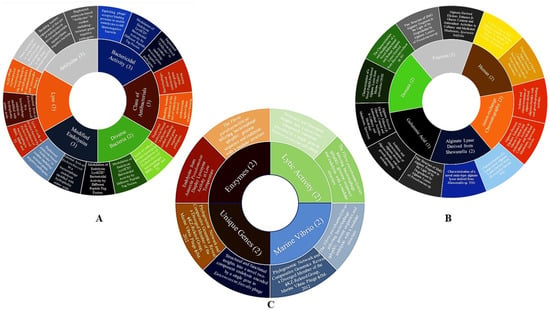
Figure 5.
Trends of central themes of this review: (A) Endolysins directed towards G-antibiotic-resistant bacteria; (B) Use of OSA as an antibacterial agent in marine bacteria; (C) Endolysins targeting marine bacteria.
4.1. Current Trends in the Use of Endolysins Targeting G-Antibiotic-Resistant Bacteria
Potential trends in the use of endolysins directed at G-antibiotic-resistant bacteria (Figure 5A) are directed at two main aspects: the discovery of endolysins from phages of various bacteria (mainly E. coli and Salmonella spp.) that have the intrinsic ability to penetrate the OM, as well as incorporate an exogenous agent to a native endolysin, providing it with certain hydrophobicity or amphiphilic characteristics. In addition, it is important to highlight that although they were isolated, purified and evaluated a few years ago, they have attracted great interest as a potential candidate for a novel antibacterial, both in the food and clinical sectors, due to the undesirable effects of the exacerbated use of antibiotics in many fields.
4.2. Current Trends in the Use of OSA as an Antimicrobial Agent in Marine Bacteria
When performing a stratified exploration for the use of OSA as an antimicrobial agent in marine bacteria (Figure 5B), limited data is presented, a trend is observed regarding the discovery and characterization of alginate lyases and their products from various marine bacteria (Vibrio splendidus, Marinimicrobium spp.,) obtaining AOS with ranges from 3 to 5 monomers. In addition, as mentioned previously, a particularity of OSA lies in its prebiotic capacity, in which a recent study stands out in relation to the effect that these have on the intestinal microbiota of marine species (Salmo solar); supplementation with this type of carbohydrates facilitates the colonization of Proteobacteria (A. parvum, A. insolitus) and spirochetes (B. andersonii), in which it has been reported that the prevalence of these bacteria is associated with the production of butyric acid. Therefore, it can be seen that supplementation with OSA to marine species is a viable and attractive alternative. However, the generated search did not show any studies related to the antibacterial effect of these oligosaccharides in marine bacteria. Consequently, the field becomes explorable and presents guidelines to carry out various analyses aimed at mitigating related problems, pointing to the eradication of one or more pathogenic bacterial genera in marine species (species of the genus Vibrio), where its prevalence in the organism eventually chains the mortality of these or, failing that, represents a source of infection after ingestion.
4.3. Current Trends in the Use of Endolysins Directed towards Marine Bacteria
In Figure 5C, the use of endolysins directed towards marine bacteria can be observed, the information found is limited and the discovery of three endolysins from phages that infect certain marine bacteria (Pseudomonas spp. and Vibrio parahaemolyticus) is rescued, although the stability aspects of these are not reported. In addition to this, there is no clear trend in the information regarding the use of these enzymes directed at marine bacteria, in such a way that it is relevant to emphasize that there are no reports that demonstrate the enzymatic or bactericidal activity of endolysins in the marine environment and, therefore, neither the generation of bioassays under these conditions.
5. Conclusions
With the rise of antibiotic resistance, scientists are paying increasing attention to the discovery of antimicrobial agents other than antibiotics. In this sense, the number of publications on endolysins and their possible applications has increased considerably during the last decade, also driven by the appearance and rapid dissemination of MRB, which makes it necessary to identify new treatment options. However, their target specificity and ability to combat both drug-sensitive and drug-resistant organisms makes them undoubtedly attractive antibacterial agents.
A major difficulty for their development is the presence of OM on the cell surface of G-bacteria which prevents the access of antibacterial enzymes to adjacent PG substrates and, thus, limits their efficacy. There is an important focus in relation to the permeabilization of endolysins through the OM to exert their lytic activity against G-bacteria. We have summarized the main approaches reported in the last few decades, in which both in vitro and in vivo data affirm that globular endolysins are promising antimicrobial agents against MRBs.
While understanding the safety and distribution profiles of endolysins after administration would require further in vivo studies, formulation sciences such as biofunctionalization are a suitable tactic to improve the efficacy and stability of globular endolysins.
Aquaculture has shown substantial development rates that have led to opportune conditions for the appearance and accelerated spread of new infectious diseases. Given that these diseases of relevance in the aquaculture sector require treatments that are stable in seawater and resist adverse conditions during and at the end of the complex gastrointestinal journey of the marine organism, it is undeniable to venture into the strategy of biofunctionalization of endolysins. In this regard, it is crucial to consider that the molecule to be loaded has a suitable chemical structure for this process, in addition to exhibiting a considerable bioactive profile and, in the same way, being able to exert an immunomodulatory effect. This is especially crucial as the latter contributes considerably to the health of the marine organism, including in normal conditions as in disease scenarios. We have addressed the main candidate molecules for this complex treatment, in which cellulose, OSA and zinc of organic origin would play relevant roles in combating MRB of aquaculture importance, as well as represent a future potential additive in standardized diets.
Author Contributions
Conceptualization, C.E.C.-G. and J.A.S.-B.; methodology, V.Z.-G.; investigation, C.E.C.-G.; writing—original draft preparation, C.E.C.-G.; writing—review and editing, A.P.-L. and C.S.C.-F.; visualization, V.Z.-G.; supervision, A.P.-L.; project administration, C.S.C.-F.; funding acquisition, J.A.S.-B. All authors have read and agreed to the published version of the manuscript.
Funding
Carlos Eduardo Camacho González thanks CONACYT-Mexico for the financial support, grant registration number: 934865. Funding for this research was partially provided by CONACYT-Mexico Grant 247842.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kongkham, B.; Prabakaran, D.; Puttaswamy, H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 2020, 147, 104762. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.K.; Pandya, K.; Khan, I.D. Antimicrobial resistance: A public health challenge. Med. J. Armed Forces India 2015, 71, 178–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; Reed, R.H. Determination of turgor pressure in Bacillus subtilis: A possible role for K+ in turgor regulation. J. Gen. Microbiol. 1990, 136, 2521–2526. [Google Scholar] [CrossRef] [Green Version]
- Calero-Cáceres, W.; Ye, M.; Balcázar, J.L. Bacteriophages as Environmental Reservoirs of Antibiotic Resistance. Trends Microbiol. 2019, 27, 570–577. [Google Scholar] [CrossRef]
- FAO. Meeting the Sustainable Development Goal. In The State of World Fisheries and Aquaculture, 2018th ed.; FAO: Rome, Italy, 2018; p. 227. [Google Scholar]
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious Diseases Affect Marine Fisheries and Aquaculture Economics. Annu. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef] [Green Version]
- Bondad-Reantaso, M.G.; Arthur, J.R.; Subasinghe, R.P. Improving biosecurity through prudent and responsible use of veterinary medicines in aquatic food production. In FAO Aquaculture Newsletter; FAO: Rome, Italy, 2012. [Google Scholar]
- Santos, L.; Ramos, F. Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: A review. Trends Food Sci. Technol. 2016, 52, 16–30. [Google Scholar] [CrossRef]
- Dy, R.L.; Rigano, L.A.; Fineran, P.C. Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem. Soc. Trans. 2018, 46, 1605–1613. [Google Scholar] [CrossRef]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [Green Version]
- Zermeño-Cervantes, L.A.; González-Acosta, B.; Martínez-Díaz, S.F.; Cardona-Félix, C.S. Antibacterial proteins and peptides as potential treatment in aquaculture: Current status and perspectives on delivery. Rev. Aquac. 2020, 12, 1135–1156. [Google Scholar] [CrossRef]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Kramer, K.; Ebel, F.; Scherer, S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 2002, 44, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Volckaert, G.; Cornelissen, A.; Lagaert, S.; Michiels, C.W.; Hertveldt, K.; Lavigne, R. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages? KZ and EL. Mol. Microbiol. 2007, 65, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as Antimicrobials. Adv. Virus Res. 2012, 83, 299–365. [Google Scholar] [CrossRef] [Green Version]
- Jado, I. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 2003, 52, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective Removal of Staphylococcal Biofilms by the Endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef] [Green Version]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.-P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 Is a Highly Efficient Antibacterial against Multidrug-Resistant Strains and Persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Shen, Y.; Foster-Frey, J.; Powell, A.M.; Bauchan, G.; Lease, R.A.; Mohammadi, H.; Harty, W.J.; et al. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; González, S.; Campelo, A.B.; Martínez, B.; Rodríguez, A.; García, P. Downregulation of Autolysin-Encoding Genes by Phage-Derived Lytic Proteins Inhibits Biofilm Formation in Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e02724-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witzenrath, M.; Schmeck, B.; Doehn, J.M.; Tschernig, T.; Zahlten, J.; Loeffler, J.M.; Zemlin, M.; Müller, H.; Gutbier, B.; Schütte, H.; et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia*. Crit. Care Med. 2009, 37, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Li, X.; Hu, L.; Cheng, M.; Xia, F.; Gong, P.; Wang, B.; Ge, J.; Zhang, H.; et al. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci. Rep. 2016, 6, 29344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djurkovic, S.; Loeffler, J.M.; Fischetti, V.A. Synergistic Killing of Streptococcus pneumoniae with the Bacteriophage Lytic Enzyme Cpl-1 and Penicillin or Gentamicin Depends on the Level of Penicillin Resistance. Antimicrob. Agents Chemother. 2005, 49, 1225–1228. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Barros, M.; Vennemann, T.; Gallagher, D.T.; Yin, Y.; Linden, S.B.; Heselpoth, R.D.; Spencer, D.J.; Donovan, D.M.; Moult, J.; et al. A bacteriophage endolysin that eliminates intracellular streptococci. eLife 2016, 5, e13152. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Abouhmad, A.; Mamo, G.; Dishisha, T.; Amin, M.A.; Hatti-Kaul, R. T4 lysozyme fused with cellulose-binding module for antimicrobial cellulosic wound dressing materials. J. Appl. Microbiol. 2016, 121, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.-J.; Lin, N.-T.; Hu, A.; Soo, P.-C.; Chen, L.-K.; Chen, L.-H.; Chang, K.-C. Antibacterial activity of Acinetobacter baumannii phage ϕAB2 endolysin (LysAB2) against both Gram-positive and Gram-negative bacteria. Appl. Microbiol. Biotechnol. 2011, 90, 529–539. [Google Scholar] [CrossRef]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel Phage Lysin Capable of Killing the Multidrug-Resistant Gram-Negative Bacterium Acinetobacter baumannii in a Mouse Bacteremia Model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, B.; Roszniowski, B.; Espaillat, A.; Kęsik-Szeloch, A.; Majkowska-Skrobek, G.; Kropinski, A.M.; Briers, Y.; Cava, F.; Lavigne, R.; Drulis-Kawa, Z. Klebsiella phages representing a novel clade of viruses with an unknown DNA modification and biotechnologically interesting enzymes. Appl. Microbiol. Biotechnol. 2017, 101, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larpin, Y.; Oechslin, F.; Moreillon, P.; Resch, G.; Entenza, J.M.; Mancini, S. In vitro characterization of PlyE146, a novel phage lysin that targets Gram-negative bacteria. PLoS ONE 2018, 13, e0192507. [Google Scholar] [CrossRef]
- Sykilinda, N.; Nikolaeva, A.; Shneider, M.; Mishkin, D.; Patutin, A.; Popov, V.; Boyko, K.; Klyachko, N.; Miroshnikov, K. Structure of an Acinetobacter Broad-Range Prophage Endolysin Reveals a C-Terminal α-Helix with the Proposed Role in Activity against Live Bacterial Cells. Viruses 2018, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Hu, K.; Xie, Y.; Liu, Y.; Mu, D.; Guo, H.; Zhang, Z.; Zhang, Y.; Chang, D.; Shi, Y. A Novel Phage PD-6A3, and Its Endolysin Ply6A3, With Extended Lytic Activity against Acinetobacter baumannii. Front. Microbiol. 2019, 9, 3302. [Google Scholar] [CrossRef]
- Lim, J.-A.; Shin, H.; Heu, S.; Ryu, S. Exogenous Lytic Activity of SPN9CC Endolysin against Gram-Negative Bacteria. J. Microbiol. Biotechnol. 2014, 24, 803–811. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Gerstmans, H.; Thorpe, S.; Mesnage, S.; Lavigne, R.; Briers, Y. DUF3380 Domain from a Salmonella Phage Endolysin Shows Potent N-Acetylmuramidase Activity. Appl. Environ. Microbiol. 2016, 82, 4975–4981. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Le, S.; Shen, W.; Chen, Q.; Huang, Y.; Lu, S.; Tan, Y.; Li, M.; Hu, F.; Li, Y. Antibacterial Activity of a Lytic Enzyme Encoded by Pseudomonas aeruginosa Double Stranded RNA Bacteriophage phiYY. Front. Microbiol. 2018, 9, 1778. [Google Scholar] [CrossRef]
- Legotsky, S.A.; Vlasova, K.Y.; Priyma, A.D.; Shneider, M.M.; Pugachev, V.G.; Totmenina, O.D.; Kabanov, A.V.; Miroshnikov, K.A.; Klyachko, N.L. Peptidoglycan degrading activity of the broad-range Salmonella bacteriophage S-394 recombinant endolysin. Biochimie 2014, 107, 293–299. [Google Scholar] [CrossRef]
- Thummeepak, R.; Kitti, T.; Kunthalert, D.; Sitthisak, S. Enhanced Antibacterial Activity of Acinetobacter baumannii Bacteriophage ØABP-01 Endolysin (LysABP-01) in Combination with Colistin. Front. Microbiol. 2016, 7, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, H.; Vilas Boas, D.; Mesnage, S.; Kluskens, L.D.; Lavigne, R.; Sillankorva, S.; Secundo, F.; Azeredo, J. Structural and Enzymatic Characterization of ABgp46, a Novel Phage Endolysin with Broad Anti-Gram-Negative Bacterial Activity. Front. Microbiol. 2016, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Díez-Martínez, R.; de Paz, H.; Bustamante, N.; García, E.; Menéndez, M.; García, P. Improving the Lethal Effect of Cpl-7, a Pneumococcal Phage Lysozyme with Broad Bactericidal Activity, by Inverting the Net Charge of Its Cell Wall-Binding Module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P.; et al. Engineered Endolysin-Based “Artilysins” To Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, M.; Yu, J.; Wei, H. Antibacterial Activity of a Novel Peptide-Modified Lysin Against Acinetobacter baumannii and Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Yang, E.; Chang, P.-S.; Ryu, S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzym. Microb. Technol. 2019, 128, 40–48. [Google Scholar] [CrossRef]
- Kwon, K.-C.; Daniell, H. Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mol. Ther. 2016, 24, 1342–1350. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Kwon, K.-C.; Hoffman, B.E.; Kamesh, A.; Jones, N.T.; Herzog, R.W.; Daniell, H. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 2016, 80, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Haefner, S.; Knietsch, A.; Scholten, E.; Braun, J.; Lohscheidt, M.; Zelder, O. Biotechnological production and applications of phytases. Appl. Microbiol. Biotechnol. 2005, 68, 588–597. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, D.E.; Parada, J.L.; Medeiros, A.B.P.; de Carvalho, J.C.; Lacerda, L.G.; Rodríguez-León, J.A.; Soccol, C.R. Concentration by ultrafiltration and stabilization of phytase produced by solid-state fermentation. Process Biochem. 2013, 48, 374–379. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, K.; de Haard, H.; Beirnaert, E.; Dreier, T.; Lauwereys, M.; Huyck, L.; Van Huysse, J.; Demetter, P.; Steidler, L.; Remaut, E.; et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010, 3, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Roussel, Y.; Kleerebezem, M.; Pot, B. Recombinant lactic acid bacteria as mucosal biotherapeutic agents. Trends Biotechnol. 2011, 29, 499–508. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Z.; Zhang, Y.; Pan, L. Lactic acid bacteria as mucosal delivery vehicles: A realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016, 100, 5691–5701. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.R.; Bhunia, A.K. Bioengineered probiotics, a strategic approach to control enteric infections. Bioengineered 2013, 4, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuradha, K.; Foo, H.L.; Mariana, N.S.; Loh, T.C.; Yusoff, K.; Hassan, M.D.; Sasan, H.; Raha, A.R. Live recombinant Lactococcus lactis vaccine expressing aerolysin genes D1 and D4 for protection against Aeromonas hydrophila in tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2010, 109, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Beck, B.R.; Lee, S.M.; Jeon, J.; Lee, D.W.; Lee, J.I.; Song, S.K. Pellet feed adsorbed with the recombinant Lactococcus lactis BFE920 expressing SiMA antigen induced strong recall vaccine effects against Streptococcus iniae infection in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2016, 55, 374–383. [Google Scholar] [CrossRef]
- Beck, B.R.; Lee, S.H.; Kim, D.; Park, J.H.; Lee, H.K.; Kwon, S.-S.; Lee, K.H.; Lee, J.I.; Song, S.K. A Lactococcus lactis BFE920 feed vaccine expressing a fusion protein composed of the OmpA and FlgD antigens from Edwardsiella tarda was significantly better at protecting olive flounder (Paralichthys olivaceus) from edwardsiellosis than single antigen vaccines. Fish Shellfish Immunol. 2017, 68, 19–28. [Google Scholar] [CrossRef]
- Volzing, K.; Borrero, J.; Sadowsky, M.J.; Kaznessis, Y.N. Antimicrobial Peptides Targeting Gram-negative Pathogens, Produced and Delivered by Lactic Acid Bacteria. ACS Synth. Biol. 2013, 2, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Geldart, K.; Forkus, B.; McChesney, E.; McCue, M.; Kaznessis, Y. pMPES: A Modular Peptide Expression System for the Delivery of Antimicrobial Peptides to the Site of Gastrointestinal Infections Using Probiotics. Pharmaceuticals 2016, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Gervasi, T.; Horn, N.; Wegmann, U.; Dugo, G.; Narbad, A.; Mayer, M.J. Expression and delivery of an endolysin to combat Clostridium perfringens. Appl. Microbiol. Biotechnol. 2014, 98, 2495–2505. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Zhu, L.; Sherman, A.; Wang, X.; Lin, S.; Kamesh, A.; Norikane, J.H.; Streatfield, S.J.; Herzog, R.W.; Daniell, H. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials 2015, 70, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Lin, H.; Wang, J.; Mao, X. The Vibrio parahaemolyticus-infecting bacteriophage qdvp001: Genome sequence and endolysin with a modular structure. Arch. Virol. 2016, 161, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jin, Y.; Lin, H.; Wang, J.; Jiang, X. Complete Genome of a Novel Lytic Vibrio parahaemolyticus Phage VPp1 and Characterization of Its Endolysin for Antibacterial Activities. J. Food Prot. 2018, 81, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Yun, L.; Li, Y.; Tian, Y.; Liu, Q.; Huang, W.; Hu, C. Complete genomic sequence of the Vibrio alginolyticus bacteriophage Vp670 and characterization of the lysis-related genes, cwlQ and holA. BMC Genom. 2018, 19, 741. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Andreatta, E. Estado Actual y Perspectivas de la Nutrición de los Camarones Peneidos Cultivados en Iberoamérica; CYTED: Mexico, 2006. Available online: https://www.researchgate.net/profile/C-Rosas/publication/288965764_Principales_rutas_metabolicas_Utilizacion_de_la_energia/links/5984985ba6fdcc75624fbc93/Principales-rutas-metabolicas-Utilizacion-de-la-energia.pdf (accessed on 17 February 2022).
- Tzuc, J.; Escalante, D.; Rojas Herrera, R.; Gaxiola Cortés, G.; Ortiz, M. Microbiota from Litopenaeus vannamei: Digestive tract microbial community of Pacific white shrimp (Litopenaeus vannamei). SpringerPlus 2014, 3, 280. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.P.; Xu, D.; Zhuo, Y.; Liu, H.Q.; Lu, L.Q. Identification and pathogenicity of Vibrio parahaemolyticus isolates and immune responses of Penaeus (Litopenaeus) vannamei (Boone). J. Fish Dis. 2016, 39, 1085–1097. [Google Scholar] [CrossRef]
- Kumar, B.K.; Deekshit, V.K.; Raj, J.R.M.; Rai, P.; Shivanagowda, B.M.; Karunasagar, I.; Karunasagar, I. Diversity of Vibrio parahaemolyticus associated with disease outbreak among cultured Litopenaeus vannamei (Pacific white shrimp) in India. Aquaculture 2014, 433, 247–251. [Google Scholar] [CrossRef]
- Ananda Raja, R.; Sridhar, R.; Balachandran, C.; Palanisammi, A.; Ramesh, S.; Nagarajan, K. Pathogenicity profile of Vibrio parahaemolyticus in farmed Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2017, 67, 368–381. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chen, I.-T.; Yang, Y.-T.; Ko, T.-P.; Huang, Y.-T.; Huang, J.-Y.; Huang, M.-F.; Lin, S.-J.; Chen, C.-Y.; Lin, S.-S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [Green Version]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and Experimental Evidence of Vibrio parahaemolyticus as the Causative Agent of Acute Hepatopancreatic Necrosis Disease of Cultured Shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Dechamma, M.M.; Rajeish, M.; Maiti, B.; Mani, M.K.; Karunasagar, I. Expression of Toll-like receptors (TLR), in lymphoid organ of black tiger shrimp (Penaeus monodon) in response to Vibrio harveyi infection. Aquac. Rep. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Khalil, N.M.; do Nascimento, T.C.F.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.; Palazzo, I.; Della Porta, G.; Scognamiglio, M.; Gimenez-Rota, C.; Reverchon, E. β-Carotene, α-Tocoferol and Rosmarinic Acid encapsulated within PLA/PLGA microcarriers by Supercritical Emulsion Extraction: Encapsulation efficiency, drugs shelf-life and antioxidant activity. J. Supercrit. Fluids 2019, 146, 199–207. [Google Scholar] [CrossRef]
- Ettcheto, M.; Silva, A.M.; Sánchez-López, E.; Calpena, A.C.; Egea, M.A.; Cano, A.; García, M.L.; Camins, A.; Souto, E.B.; Espina, M. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen—in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Razura-Carmona, F.F.; Pérez-Larios, A.; González-Silva, N.; Herrera-Martínez, M.; Medina-Torres, L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A. Mangiferin-Loaded Polymeric Nanoparticles: Optical Characterization, Effect of Anti-topoisomerase I, and Cytotoxicity. Cancers 2019, 11, 1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, C.J.F.; Comunian, T.A.; Kasemodel, M.G.C.; Favaro-Trindade, C.S. Microencapsulation of lactase by W/O/W emulsion followed by complex coacervation: Effects of enzyme source, addition of potassium and core to shell ratio on encapsulation efficiency, stability and kinetics of release. Food Res. Int. 2019, 121, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Boi, S.; Dellacasa, E.; Bianchini, P.; Petrini, P.; Pastorino, L.; Monticelli, O. Encapsulated functionalized stereocomplex PLA particles: An effective system to support mucolytic enzymes. Colloids Surf. B Biointerfaces 2019, 179, 190–198. [Google Scholar] [CrossRef]
- Tacon, A.G.J. 1. The essential nutrients. In The Nutrition and Feeding of Farmed Fish and Shrimp: A Training Manual, 2nd ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1987; p. 117. [Google Scholar]
- Ma, K.; Jin, X.; Zheng, M.; Gao, H. Dissolution and functionalization of celluloses using 1,2,3-triazolium ionic liquid. Carbohydr. Polym. Technol. Appl. 2021, 2, 100109. [Google Scholar] [CrossRef]
- Aguado, R.; Murtinho, D.; Valente, A.J.M. Association of antioxidant monophenolic compounds with β-cyclodextrin-functionalized cellulose and starch substrates. Carbohydr. Polym. 2021, 267, 118189. [Google Scholar] [CrossRef]
- Dacrory, S.; Hashem, A.H.; Hasanin, M. Synthesis of cellulose based amino acid functionalized nano-biocomplex: Characterization, antifungal activity, molecular docking and hemocompatibility. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100453. [Google Scholar] [CrossRef]
- Faria-Tischer, P.C.S.; Ribeiro-Viana, R.M.; Tischer, C.A. Bio-based nanocomposites. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.-Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Şen, M. Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Hiroki, T.; Takeshita, S.; Jiang, Z.; Kim, D.; Yamaguchi, K.; Oda, T. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohydr. Res. 2012, 352, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, H.; Endo, T.; Nakakita, R.; Murata, K.; Yonemoto, Y.; Okayama, K. Effect of Depolymerized Alginates on the Growth of Bifidobacteria. Biosci. Biotechnol. Biochem. 1992, 56, 355–356. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Hu, B.; Li, J.; Yu, W. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate. Nutr. Res. 2006, 26, 597–603. [Google Scholar] [CrossRef]
- Fang, W.; Bi, D.; Zheng, R.; Cai, N.; Xu, H.; Zhou, R.; Lu, J.; Wan, M.; Xu, X. Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci. Rep. 2017, 7, 1663. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wu, X.; Wang, Q.; Cai, N.; Zhang, H.; Jiang, Z.; Wan, M.; Oda, T. Immunomodulatory Effects of Alginate Oligosaccharides on Murine Macrophage RAW264.7 Cells and Their Structure–Activity Relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of Multiple Cytokine Secretion from RAW264.7 Cells by Alginate Oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Jiang, X.; Gong, J.; Hwang, H.; Liu, Y.; Guan, H. Antibacterial activity of lyase-depolymerized products of alginate. J. Appl. Phycol. 2005, 17, 57–60. [Google Scholar] [CrossRef]
- Powell, L.C.; Sowedan, A.; Khan, S.; Wright, C.J.; Hawkins, K.; Onsøyen, E.; Myrvold, R.; Hill, K.E.; Thomas, D.W. The effect of alginate oligosaccharides on the mechanical properties of Gram-negative biofilms. Biofouling 2013, 29, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate Oligosaccharides Inhibit Fungal Cell Growth and Potentiate the Activity of Antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 9, e112518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aida, T.M.; Yamagata, T.; Watanabe, M.; Smith, R.L. Depolymerization of sodium alginate under hydrothermal conditions. Carbohydr. Polym. 2010, 80, 296–302. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, X.; Jiang, Y.; Hu, X.; Mou, H.; Li, M.; Guan, H. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 2007, 21, 646–654. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Roushdy, E.M.; Hassan, F.A.M.; Mohammed, H.A.; Abdelhakim, T.M.N. Comparing the effect of diet supplementation with different zinc sources and levels on growth performance, immune response and antioxidant activity of tilapia, Oreochromis niloticus. Aquac. Nutr. 2020, 26, 1926–1942. [Google Scholar] [CrossRef]
- Kou, H.; Hu, J.; Vijayaraman, S.B.; Wang, A.-L.; Zheng, Y.; Chen, J.; He, G.; Miao, Y.; Lin, L. Evaluation of dietary zinc on antioxidant-related gene expression, antioxidant capability and immunity of soft-shelled turtles Pelodiscus sinensis. Fish Shellfish Immunol. 2021, 118, 303–312. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Jiang, L.-C. Dietary zinc requirements of grass shrimp, Penaeus monodon, and effects on immune responses. Aquaculture 2006, 254, 476–482. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Yang, Y.; Li, F.; Luo, L. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp (Litopenaeus vannamei). Aquaculture 2013, 406–407, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Razura-Carmona, F.F.; Herrera-Martínez, M.; Sáyago-Ayerdi, S.G.; Pérez-Larios, A.; Montalvo-González, E.; Ramírez-Mares, M.V.; Sánchez-Burgos, J.A. Nanoparticles of two ZnO Precursors as an Encapsulating Matrix of Mangiferin: Associated Studies to Cytotoxic Effects on Liver Cancer Cells Hep-G2 and Healthy Lung Cell Beas-2B. J. Clust. Sci. 2021, 33, 163–171. [Google Scholar] [CrossRef]
- Gao, Y.; Arokia Vijaya Anand, M.; Ramachandran, V.; Karthikkumar, V.; Shalini, V.; Vijayalakshmi, S.; Ernest, D. Biofabrication of Zinc Oxide Nanoparticles from Aspergillus niger, Their Antioxidant, Antimicrobial and Anticancer Activity. J. Clust. Sci. 2019, 30, 937–946. [Google Scholar] [CrossRef]
- Pilson, M.E.Q. An Introduction to the Chemistry of the Sea; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Stefanowski, J.; Weiss, D. Carrot2 and Language Properties in Web Search Results Clustering. In Advances in Web Intelligence; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).