Modification of Orange Bagasse with Reactive Extrusion to Obtain Cellulose-Based Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
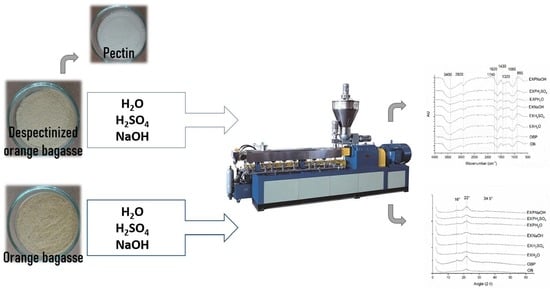
2.2.1. Extraction of Cellulose-Based Materials Using Reactive Extrusion
2.2.2. Cellulose, Hemicellulose, Lignin, and Pectin Contents
2.2.3. Fourier Transform-Infrared Spectroscopy (FTIR)
2.2.4. X-ray Diffraction (XRD)
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. Statistical Analysis
3. Results
3.1. Extraction of Cellulose-Based Materials with Extrusion
3.2. Extruded Cellulose-Based Materials Characterization
3.2.1. Fourier Transform-Infrared Spectroscopy (FTIR)
3.2.2. X-ray Diffraction (XRD)
3.2.3. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantovan, J.; Gil-Giraldo, G.A.G.; Marim, B.M.; Garcia, P.S.; Baron, A.M.; Mali, S. Cellulose-based materials from orange bagasse employing environmentally friendly approaches. Biomass Conv. Bioref. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Mantovan, J.; Giraldo, G.A.G.; Marim, B.M.; Kishima, J.O.G.; Mali, S. Valorization of orange bagasse through one-step physical and chemical combined processes to obtain a cellulose-rich material. J. Sci. Food Agric. 2021, 101, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processes based on reactive extrusion. Cellulose 2020, 27, 1975–1988. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A Green approach based on reactive extrusion to produce nanofibrillated cellulose from oat hull. Waste Biomass Valori. 2021, 12, 1051–1070. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Int. Food Res. J. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Pérez-Carrillo, E.; Serna-Saldívar, S.O.; Campanella, O.H.; Welti-Chanes, J. Functional and compositional changes of orange peel fiber thermally-treated in a twin extruder. LWT 2019, 111, 673–681. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S.; Tsai, Y.H.; Chang, S.K. In vitro hypoglycemic, cholesterol-lowering and fermentation capacities of fiber-rich orange pomace as affected by extrusion. Int. J. Biol. Macromol. 2019, 124, 796–801. [Google Scholar] [CrossRef]
- Cypriano, D.Z.; da Silva, L.L.; Tasic, L. High value-added products from the orange juice industry waste. Waste Manag. 2018, 79, 71–78. [Google Scholar] [CrossRef]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange peels: From by-product to resource through lactic acid fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef]
- USDA (U.S. Department of Agriculture). Citrus: Worldmarkets and Trade. Available online: https://www.fas.usda.gov/data/citrus-worldmarkets-and-trade (accessed on 8 March 2019).
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Siles, J.A.; Vargas, F.; Gutiérrez, M.C.; Chica, A.F.; Martín, M.A. Integral valorisation of waste orange peel using combustion, biomethanisation and co-composting technologies. Bioresour. Technol. 2016, 211, 173–182. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, I.; Ravelo, M.; Segarra, S.; Tortajada, M.; Santos, V.E.; Ladero, M. Study on the effects of several operational variables on the enzymatic batch saccharification of orange solid waste. Bioresour. Technol. 2017, 245, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lopez, M.R.; Osorio-Diaz, P.; Bello-Perez, L.A.; Tovar, J.; Bernardino-Nicanor, A. Fiber concentrate from orange (Citrus sinensis L.) bagase: Characterization and application as bakery product ingredient. Int. J. Mol. Sci. 2011, 12, 2174–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar, A.K.; Godínez, L.A.; Espejel, F.; Ramírez-Zamora, R.M.; Robles, I. Optimization of the integral valorization process for orange peel waste using a design of experiments approach: Production of high-quality pectin and activated carbon. Waste Manag. 2019, 85, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Lohrasbi, M.; Pourbafrani, M.; Niklasson, C.; Taherzadeh, M.J. Process design and economic analysis of a citrus waste biorefinery with biofuels and limonene as products. Bioresour. Technol. 2010, 101, 7382–7388. [Google Scholar] [CrossRef] [PubMed]
- Ángel Siles López, J.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef]
- Bicu, I.; Mustata, F. Optimization of isolation of cellulose from orange peel using sodium hydroxide and chelating agents. Carbohydr. Polym. 2013, 98, 341–348. [Google Scholar] [CrossRef]
- Bicu, I.; Mustata, F. Cellulose extraction from orange peel using sulfite digestion reagents. Bioresour. Technol. 2011, 102, 10013–10019. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Durán, N.; Tasic, L. Nanocellulose and bioethanol production from orange waste using isolated microorganisms. J. Braz. Chem. Soc. 2013, 24, 1537–1543. [Google Scholar] [CrossRef]
- Hideno, A.; Abe, K.; Yano, H. Preparation using pectinase and characterization of nanofibers from orange peel waste in juice factories. J. Food Sci. 2014, 79, N1218–N1224. [Google Scholar] [CrossRef]
- Mariño, M.A.; Rezende, C.A.; Tasic, L. A multistep mild process for preparation of nanocellulose from orange bagasse. Cellulose 2018, 25, 5739–5750. [Google Scholar] [CrossRef]
- Mariño, M.; Lopes da Silva, L.; Durán, N.; Tasic, L. Enhanced materials from nature: Nanocellulose from citrus waste. Molecules 2015, 20, 5908–5923. [Google Scholar] [CrossRef] [PubMed]
- Gil-Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Mali, S. Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes. Polysaccharides 2021, 2, 218–233. [Google Scholar] [CrossRef]
- Weng, R.; Huang, X.; Liao, D.; Xu, S.; Peng, L.; Liu, X. A novel cellulose/chitosan composite nanofiltration membrane prepared with piperazine and trimesoyl chloride by interfacial polymerization. RSC Adv. 2020, 10, 1309–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Liu, L.; Zhang, L.; Lu, A. Strong cellulose hydrogel as underwater superoleophobic coating for efficient oil/water separation. Carbohydr. Polym. 2020, 229, 115467. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.; Mali, S. Cellulose and nanocellulose produced from lignocellulosic residues by reactive extrusion. In Biomass Extrusion and Reaction Technologies: Principles to Practices and Future Potential; Ayoub, A., Lucia, L., Eds.; ACS Publications: Washington, DC, USA, 2018; Volume 1304, pp. 175–187. [Google Scholar]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Van Soest, P.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- TAPPI TEST METHOD T222 om-88; Acid-Insoluble Lignin in Wood and Pulp, in Tappi Test Methods. Tappi Press: Atlanta, GA, USA, 1999.
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of nature cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Abobi, K.E. Optimization of oil and pectin extraction from orange (Citrus sinensis) peels: A response surface approach. J. Anal. Sci. Technol. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Duwee, Y.S.; Kiew, P.L.; Yeoh, W.M. Multi-objective optimization of pectin extraction from orange peel via response surface methodology: Yield and degree of esterification. J. Food Meas. Charact. 2022, 16, 1710–1724. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, antioxidant and antibacterial properties of fish gelatin-based edible films enriched with orange peel pectin: Wrapping application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Liu, C.; van der Heide, E.; Wang, H.; Li, B.; Yu, G.; Mu, X. Alkaline twin-screw extrusion pretreatment for fermentable sugar production. Biotechnol. Biofuels 2013, 6, 1–11. Available online: http://www.biotechnologyforbiofuels.com/content/6/1/97 (accessed on 1 July 2021). [CrossRef] [Green Version]
- Marim, B.M.; Mantovan, J.; Giraldo, G.A.; Mali, S. Environmentally friendly process based on a combination of ultrasound and peracetic acid treatment to obtain cellulose from orange bagasse. J. Chem. Technol. Biotechnol. 2020, 96, 630–638. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Langrish, T.A.G. Water-based extraction of pectin from flavedo and albedo of orange peels. Chem. Eng. J. 2006, 120, 203–209. [Google Scholar] [CrossRef]
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Larrea, M.A.; Chang, Y.K.; Bustos, F.M. Effect of some operational extrusion parameters on the constituents of orange pulp. Food Chem. 2005, 89, 301–308. [Google Scholar] [CrossRef]
- Jing, Y.; Chi, Y. Effects of twin-screw extrusion on soluble dietary fibre and pysicochemical properties of soybean residue. Food Chem. 2013, 138, 884–889. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Meneses, D.B.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R.; Rojas-Álvarez, M.; Corrales-Castillo, J.; Murillo-Araya, L.C. Pretreatment methods of lignocellulosic wastes into value-added products: Recent advances and possibilities. Biomass Convers. Biorefinery 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, D.; Kaushik, A.; Singh, M. Simultaneous extraction of lignin and cellulose nanofibrils from waste jute bags using one pot pre-treatment. Int. J. Biol. Macromol. 2018, 107, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Agblevor, F.A.; El-Zawawy, W.K. Isolation and characterization of cellulose and lignin from steam-exploded lignocellulosic biomass. BioResources 2010, 5, 397–418. [Google Scholar]
- Ahmadzadeh, S.; Nasirpour, A.; Harchegani, M.B.; Hamdami, N.; Keramat, J. Effect of electrohydrodynamic technique as a complementary process for cellulose extraction from bagasse: Crystalline to amorphous transition. Carbohydr. Polym. 2018, 188, 188–196. [Google Scholar] [CrossRef]
- Ferrer, A.; Salas, C.; Rojas, O.J. Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls. Ind. Crops. Prod. 2016, 84, 337–343. [Google Scholar] [CrossRef]




| Treatment | Cellulose Content (%) | Hemicellulose Content (%) | Lignin Content (%) | Process Yield (%) * | Cellulose Yield (%) ** | CI (%) |
|---|---|---|---|---|---|---|
| OB | 12.4 ± 0.4 f | 7.5 ± 0.1 cd | 8.5 ± 0.4 e | - | - | 18 |
| OBP | 27.9 ± 0.5 c | 6.5 ± 0.9 d | 27.1 ± 0.9 a | 55.6 | 100 | 42 |
| EXH2O | 18.8 ± 1.6 e | 10.6 ± 0.6 a | 22.5 ± 1.2 b | 79.2 | 100 | 38 |
| EXH2SO4 | 19.9 ± 0.5 de | 7.4 ± 0.2 cd | 23.7 ± 0.8 b | 37.7 | 60.5 | 37 |
| EXNaOH | 21.2 ± 0.3 d | 8.8 ± 0.9 bc | 20.1 ± 0.2 cd | 43.5 | 73.4 | 45 |
| EXPH2O | 31.8 ± 0.5 b | 10.4 ± 1.1 ab | 18.6 ± 0.2 d | 68.5 | 100 | 42 |
| EXPH2SO4 | 33.3 ± 0.6 b | 8.1 ± 0.4 c | 20.8 ± 0.3 c | 30.6 | 82.3 | 43 |
| EXPNaOH | 58.4 ± 0.6 a | 3.7 ± 0.2 e | 19.4 ± 0.1 cd | 35.5 | 92.8 | 51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantovan, J.; Yamashita, F.; Mali, S. Modification of Orange Bagasse with Reactive Extrusion to Obtain Cellulose-Based Materials. Polysaccharides 2022, 3, 401-410. https://doi.org/10.3390/polysaccharides3020024
Mantovan J, Yamashita F, Mali S. Modification of Orange Bagasse with Reactive Extrusion to Obtain Cellulose-Based Materials. Polysaccharides. 2022; 3(2):401-410. https://doi.org/10.3390/polysaccharides3020024
Chicago/Turabian StyleMantovan, Janaina, Fábio Yamashita, and Suzana Mali. 2022. "Modification of Orange Bagasse with Reactive Extrusion to Obtain Cellulose-Based Materials" Polysaccharides 3, no. 2: 401-410. https://doi.org/10.3390/polysaccharides3020024
APA StyleMantovan, J., Yamashita, F., & Mali, S. (2022). Modification of Orange Bagasse with Reactive Extrusion to Obtain Cellulose-Based Materials. Polysaccharides, 3(2), 401-410. https://doi.org/10.3390/polysaccharides3020024