Application of Antioxidants as an Alternative Improving of Shelf Life in Foods
Abstract
:1. Introduction
2. Food Oxidation Processes
3. Naturally Occurring Antioxidants
4. Development of Lipids and Nano-Lipid Vehicles in the Conservation of Antioxidants
5. Application of Antioxidants in Edible Films
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eça, K.S.; Sartori, T.; Menegalli, F.C. Films and edible coatings containing antioxidants-a review. Braz. J. Food Technol. 2014, 17, 98–112. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimi-crobial edible films and coatings for meat and meat products preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Echávarri, J.F.; Olarte, C.; Sanz, S. Quality characteristics of minimally processed leek packaged using different films and stored in lighting conditions. Int. J. Food Sci. Technol. 2009, 44, 1333–1343. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Fontán, M.C.G.; Carballo, J. Effect of fat content on physical, microbial, lipid and protein changes during chill storage of foal liver pâté. Food Chem. 2014, 155, 57–63. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as pre-servatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, O. Haemorrhages due to defective blood coagulation do not occur in mice and guinea-pigs fed butylated hydroxy-toluene, but nephrotoxicity is found in mice. Food Chem. Toxicol. 1992, 30, 89–97. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, P.; Xue, J.; Kannan, K. Synthetic phenolic antioxidants, including butylated hydroxytoluene (BHT), in resin-based dental sealants. Environ. Res. 2016, 151, 339–343. [Google Scholar] [CrossRef]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, D.; Sarkar, S.; Mandal, A.K.; Choudhury, S.T.; Das, N. Anticarcinogenic activity of nanoencapsulated quercetin in combating diethylnitrosamine-induced hepatocarcinoma in rats. Eur. J. Cancer Prev. 2012, 21, 32–41. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control. 2020, 114, 107226. [Google Scholar] [CrossRef]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.; Santos, L. Design of microparticles containing natural antioxidants: Preparation, characterization and controlled release studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Tian, Z.; Ma, J.; Kang, M.; Ding, C.; Ming, D. Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation. Molecules 2017, 22, 2175. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, Z.; Li, F.; Rui, X.; Aisa, H.A. A comprehensive review on the research progress of vegetable edible films. Arab. J. Chem. 2021, 14, 103049. [Google Scholar] [CrossRef]
- Palkopoulou, S.; Joly, C.; Feigenbaum, A.; Papaspyrides, C.D.; Dole, P. Critical review on challenge tests to demonstrate de-contamination of polyolefins intended for food contact applications. Trends Food Sci. Technol. 2016, 49, 110–120. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Walker, T.R.; McGuinty, E.; Charlebois, S.; Music, J. Single-use plastic packaging in the Canadian food industry: Consumer behavior and perceptions. Humanit. Soc. Sci. Commun. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Chaple, S.; Vishwasrao, C.; Ananthanarayan, L. Edible Composite Coating of Methyl Cellulose for Post-Harvest Extension of Shelf-Life of Finger Hot Indian Pepper (Pusa jwala). J. Food Process. Preserv. 2016, 41, e12807. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of Poly(lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT 2019, 100, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2019, 55, 92–98. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the gap for bioplastic use in food packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Li, Z.; Soladoye, P.O.; Van-Hecke, T. Health Risks of Food Oxidation. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 45–81. [Google Scholar]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Jackson, V.; Penumetcha, M. Dietary oxidised lipids, health consequences and novel food technologies that thwart food lipid oxidation: An update. Int. J. Food Sci. Technol. 2019, 54, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Pereira, L.; Schweiss, M.; Barnaba, C.; Medina-Meza, I.G. The role of cholesterol oxidation products in food toxicity. Food Chem. Toxicol. 2018, 118, 908–939. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shimizu, N.; Hanzawa, Y.; Otoki, Y.; Ito, J.; Kimura, F.; Takekoshi, S.; Sakaino, M.; Sano, T.; Eitsuka, T. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography–tandem mass spectrometry. NPJ Sci. Food 2018, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Waraho, T.; McClements, D.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Estévez, M.; Luna, C. Dietary protein oxidation: A silent threat to human health? Crit. Rev. Food Sci. Nutr. 2017, 57, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 16, 96–123. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Mercola, J. Fat for Fuel: A Revolutionary Diet to Combat Cancer, Boost Brain Power, and Increase Your Energy; Hay House: Carlsbad, CA, USA, 2017. [Google Scholar]
- Wootton-Beard, P.C.; Ryan, L. Improving public health? The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samoticha, J.; Jara-Palacios, M.J.; Hernández-Hierro, J.M.; Heredia, F.J.; Wojdyło, A. Phenolic compounds and antioxidant ac-tivity of twelve grape cultivars measured by chemical and electrochemical methods. Eur. Food Res. Technol. 2018, 244, 1933–1943. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Xu, N.; Shanbhag, A.G.; Li, B.; Angkuratipakorn, T.; Decker, E.A. Impact of phospholipid–tocopherol combinations and en-zyme-modified lecithin on the oxidative stability of bulk oil. J. Agric. Food Chem. 2019, 67, 7954–7960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C.; Zhou, X.; Zhang, M.; Chen, Y.; Nie, S.; Xie, M. Combined application of gallate ester and α-tocopherol in oil-in-water emulsion: Their distribution and antioxidant efficiency. J. Dispers. Sci. Technol. 2019, 41, 909–917. [Google Scholar] [CrossRef]
- Barzegar, T.; Fateh, M.; Razavi, F. Enhancement of postharvest sensory quality and antioxidant capacity of sweet pepper fruits by foliar applying calcium lactate and ascorbic acid. Sci. Hortic. 2018, 241, 293–303. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M. Effect of ascorbic acid postharvest treatment on enzymatic browning, phenolics and antioxidant capacity of stored mung bean sprouts. Food Chem. 2018, 239, 1160–1166. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Lipophilization of Resveratrol and Effects on Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 8617–8625. [Google Scholar] [CrossRef]
- Farhoosh, R.; Nyström, L. Antioxidant potency of gallic acid, methyl gallate and their combinations in sunflower oil triacyl-glycerols at high temperature. Food Chem. 2018, 244, 29–35. [Google Scholar] [CrossRef]
- Palma, M.; Robert, P.; Holgado, F.; Márquez-Ruiz, G.; Velasco, J. Antioxidant Activity and Kinetics Studies of Quercetin, Epicatechin and Naringenin in Bulk Methyl Linoleate. J. Am. Oil Chem. Soc. 2017, 94, 1189–1196. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Imran, M.; Imran, A.; Hussain, S. Oxidative stability and lipid oxidation flavoring vol-atiles in antioxidants treated chicken meat patties during storage. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- de Carli, C.; Moraes-Lovison, M.; Pinho, S.C. Production, physicochemical stability of quercetin-loaded nanoemulsions and evaluation of antioxidant activity in spreadable chicken pâtés. LWT 2018, 98, 154–161. [Google Scholar] [CrossRef]
- Artaud-Wild, S.M.; Connor, S.L.; Sexton, G.; Connor, W.E.; Artaud-Wild, S.M.; Connor, S.L.; Sexton, G.; Connor, W.E. Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. A paradox. Circulation 1993, 88, 2771–2779. [Google Scholar] [CrossRef] [Green Version]
- Peluso, I.; Villaño Valencia, D.; Chen, C.O.; Palmery, M. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Nutraceuticals and Functional Foods 2018. Oxid. Med. Cell. Longev. 2018, 2018, 3824509. [Google Scholar] [CrossRef] [Green Version]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [Green Version]
- Adegbola, P.; Aderibigbe, I.; Hammed, W.; Omotayo, T. Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: A review. Am. J. Cardiovasc. Dis. 2017, 7, 19–32. [Google Scholar]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antioxidant and Anti-inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing Brain Injury, Cerebral Ischemia, Cognition Deficit, Anxiety, and Depression. Oxidative Med. Cell. Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; McClements, D. Nanotechnology Approaches for Increasing Nutrient Bioavailability. Adv. Food Nutr. Res. 2017, 81, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Thanh, V.M.; Nguyen, T.H.; Tran, T.V.; Ngoc, U.P.; Ho, M.N.; Nguyen, T.T.; Chau, Y.N.T.; Tran, N.Q.; Nguyen, C.K.; Nguyen, D.H. Low systemic toxicity nanocarriers fabricated from heparin-mPEG and PAMAM dendrimers for controlled drug release. Mater. Sci. Eng. C 2018, 82, 291–298. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Nanoencapsulation: Techniques and developments for food applications. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–61. [Google Scholar]
- Shende, P.; Ture, N.; Gaud, R.; Trotta, F. Lipid- and polymer-based plexes as therapeutic carriers for bioactive molecules. Int. J. Pharm. 2019, 558, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.; Quintanar-Guerrero, D.; González-Reza, R. Nanocontainers in food preservation: Techniques and uses. Smart Nanocontain. 2020, 137–155. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, M.; Liu, X.; Huang, C.; Gu, Z.; Cao, Y. Evaluation of toxicity of halloysite nanotubes and multi-walled carbon nanotubes to endothelial cells in vitro and blood vessels in vivo. Nanotoxicology 2020, 14, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Betker, J.L.; Anchordoquy, T.J. The Use of Lactose as an Alternative Coating for Nanoparticles. J. Pharm. Sci. 2020, 109, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, Z.; Jafari, S.M. Application of Lipid Nanocarriers for the Food Industry. Ref. Ser. Phytochem. 2018, 1–43. [Google Scholar] [CrossRef]
- Jose, S.; Anju, S.; Cinu, T.; Aleykutty, N.; Thomas, S.; Souto, E. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef]
- Oehlke, K.; Behsnilian, D.; Mayer-Miebach, E.; Weidler, P.G.; Greiner, R. Edible solid lipid nanoparticles (SLN) as carrier system for antioxidants of different lipophilicity. PLoS ONE 2017, 12, e0171662. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.; Ghoshal, G.; Katare, O.P. Beta-carotene-encapsulated solid lipid nano-particles (BC-SLNs) as promising vehicle for cancer: An investigative assessment. AAPS PharmSciTech 2019, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging ap-plications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pec-tin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3, 4-dihydroxyphenylglycol on beef meat during re-frigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control. 2020, 110, 107018. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Physical and Antioxidant Properties of Cassava Starch–Carboxymethyl Cellulose Incorporated with Quercetin and TBHQ as Active Food Packaging. Polymers 2020, 12, 366. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoen-capsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef] [PubMed]
- Giteru, S.G.; Oey, I.; Ali, M.A.; Johnson, S.K.; Fang, Z. Effect of kafirin-based films incorporating citral and quercetin on storage of fresh chicken fillets. Food Control. 2017, 80, 37–44. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Starch films loaded with donut-shaped starch-quercetin microparticles: Characterization and release kinetics. Int. J. Biol. Macromol. 2018, 118, 2201–2207. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and active furcellaran-gelatin films con-taining green or pu-erh tea extracts: Characterization, antioxidant and antimicrobial potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef]
- Olorunda, A.O.; Aworh, O.C. Effects of tal pro-long, a surface coating agent, on the shelf life and quality attributes of plantain. J. Sci. Food Agric. 1984, 35, 573–578. [Google Scholar] [CrossRef]

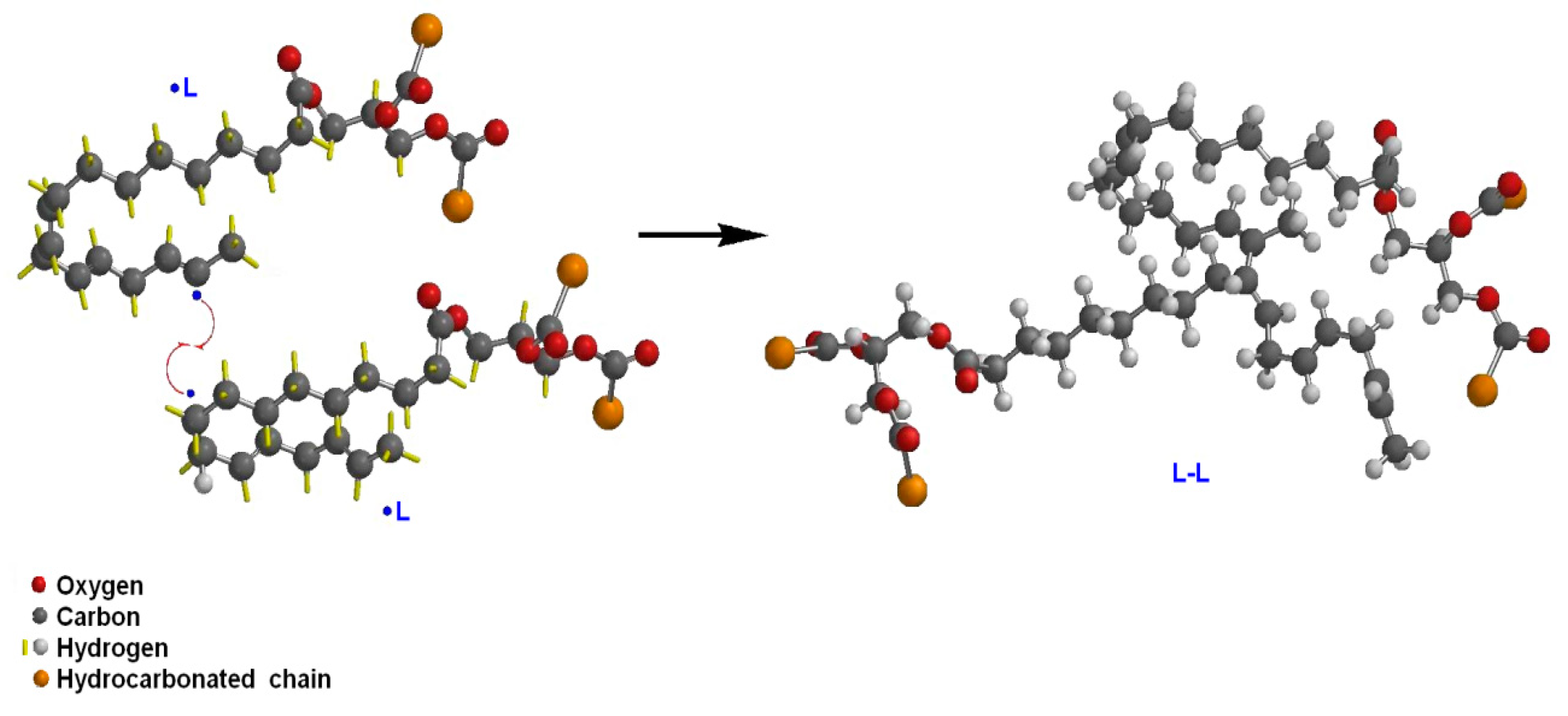

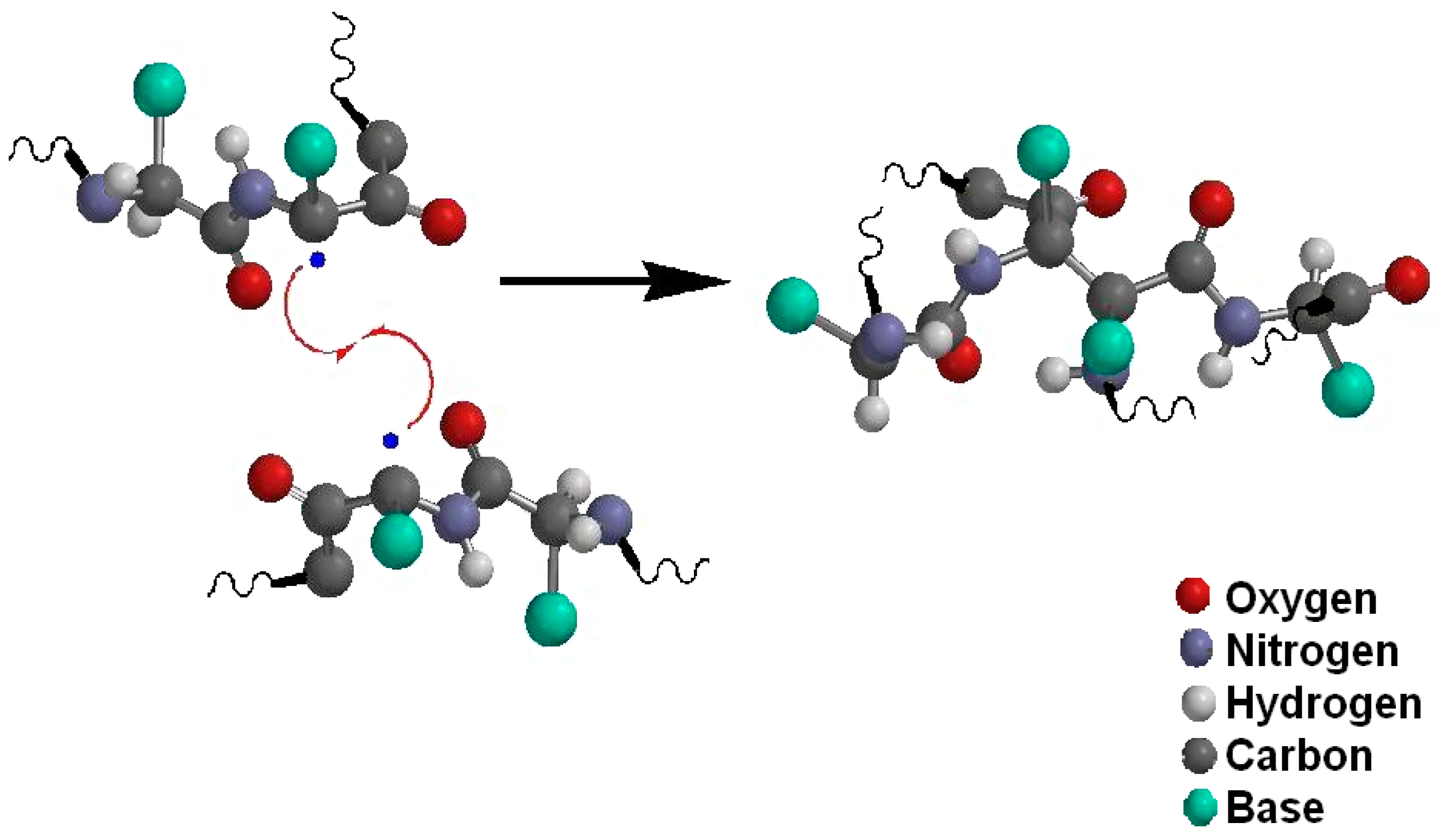
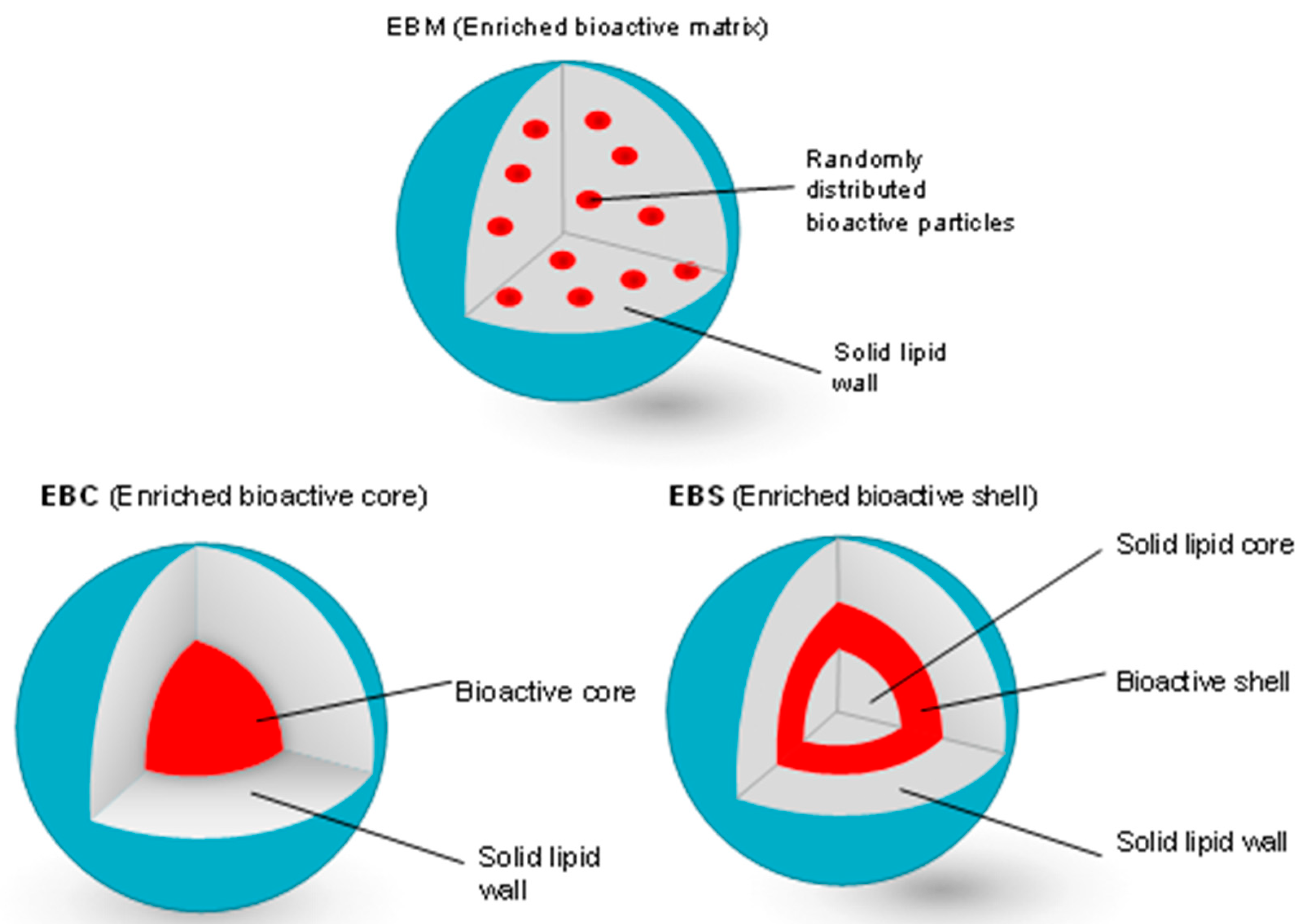
| Ref. | Major Findings | Description | Antioxidant |
|---|---|---|---|
| [45] | Aa comparative study between different tocopherols and tocotrienols for the inhibition of the oxidation of vegetable oils and animal fats was carried out. It was found that at low concentrations, α-tocopherol is more efficient in scavenging free radicals, while γ-tocopherol was better at relatively high concentrations. | Tocopherols and tocotrienols | Vitamins |
| [46] | α-Tocopherol presented a better antioxidant performance in lipids when it is in the presence of phospholipids such as phosphatidylethanolamine. | ||
| [47] | The synergistic effect between propylgalate and α-tocopherol was compared. The authors found that the antioxidant properties in oil-in-water emulsions were greater than when only the tocopherol was used. This effect was attributed to a regeneration of the vitamin by the action of propylgalate. | ||
| [5] | Ascorbic acid not only allows one to maintain the quality of post-harvest vegetables, but also increases their shelf life and improves the properties of vegetables. | Vitamin C or ascorbic acid | |
| [48] | The effect of pre-harvest treatment with ascorbic acid and calcium lactate on bell pepper was studied. They found that the appearance and shelf life of the fruit increased with the treatment, also the amount of flavonoids in the fruit, thereby improving its antioxidant capacity. | ||
| [49] | A decrease in post-harvest enzymatic browning of mango beans of up to 50% when using an ascorbic acid treatment against a control without treatment was reported. They also noted that bean sprouts increased the polyphenol content and antioxidant capacity. | ||
| [50] | The antioxidant properties of resveratrol from the point of view of its chemical structure were studied. It was found that resveratrol inhibited lipid peroxidation by 89% compared to BHT and propylgalate, which had values of 68 and 83%, respectively. | Resveratrol | Stilbenes |
| [51] | Several resveratrol esters with long chain (C14, C16 and C18) and short chain (C3, C4, and C6) fatty acids were prepared and their antioxidant properties with different free radicals compared. It was that the antioxidant properties of long-chain resveratrol esters was better for the 2,2-diphenyl-1-picrylhydrazil (DPPH) radical. On the other hand, short chain esters showed a better antioxidant properties against 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). | ||
| [5] | Recently this antioxidant has received special attention for food preservation, because it imparts an astringent flavor. It is used mainly in acidic juice drinks such as blueberry, and grape juices. Gallic acid esters, such as propylgalate, are used to prevent lipid oxidation. | Gallic acid | Polyphenols |
| [52] | The performance of the synthetic antioxidant tertbutylhydroquinone (TBHQ) was evaluated against gallic acid, and a mixture of methyl gallate with gallic acid, in the thermal oxidation of lipids. During the thermal oxidation, so-called second-stage oxidized species are generated, that is, oxidized products of lipid peroxides. It ws found that at low temperatures TBHQ performs better, but at 120 °C, the gallic acid, and its mixture with methyl gallate, showed a better performance. | ||
| [53] | The antioxidant effect of quercetin, epicatechin and naringenin on methyl linoleate was studied. They found that naringenin had a poor antioxidant effect compared to quercetin and epicatechin. | Quercetin | |
| [54] | The antioxidant properties of quercetin and quercetin with α-tocopherol in chicken meat were studied. Greater preservation of the meat was observed under storage conditions when quercetin was used, in addition to the elimination of odors caused by carbonyl compounds. However, the appearance of a yellow color can be avoided if tocopherol is also used in addition to the quercetin. | ||
| [55] | The oxidation of lipids and proteins in chicken pate was evaluated in the presence of quercetin and butylated hydroxytoluene (BHT). Quercetin was found to be eight times more efficient in inhibiting lipid oxidative reactions than BHT. However, quercetin was not as efficient in inhibiting protein oxidation. |
| Tradename | Manufacturer or Brand | Composition | Uses and Advantages |
|---|---|---|---|
| Crystalac Z2® | Mantrose-Haeuser Co., Inc. | Zeín | Jams and glazes |
| SemperFresh® | Pace International | Short chain sucrose esters, fatty acids and sodium salts of carboxymethyl cellulose | Coating for cherries. A Selective barrier of humidity and gases, avoids weight losses due to dehydration, preservation of color |
| Nita® | Nita Casings | Collagen or alginate | Casing or forming packaging for the protection of meat sausages during and after cooking. Selective gas barrier |
| Pro-Long® | Tal Chemicals Co. | Sucrose, fatty acid polyesters ansodium salts of carboxymethyl cellulose | Coating for freshly cut fruits and vegetables |
| NatureSeal® | AgriCoat/NatureSeal | Hydroxypropylmethyl cellulose with ascorbic acid and calcium chloride | Inhibits browning and maintains the texture and flavor of freshly cut fruits and vegetables |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Porras, C.; Román-Aguirre, M.; Cruz-Alcantar, P.; Pérez-Urizar, J.T.; Saavedra-Leos, M.Z. Application of Antioxidants as an Alternative Improving of Shelf Life in Foods. Polysaccharides 2021, 2, 594-607. https://doi.org/10.3390/polysaccharides2030036
Leyva-Porras C, Román-Aguirre M, Cruz-Alcantar P, Pérez-Urizar JT, Saavedra-Leos MZ. Application of Antioxidants as an Alternative Improving of Shelf Life in Foods. Polysaccharides. 2021; 2(3):594-607. https://doi.org/10.3390/polysaccharides2030036
Chicago/Turabian StyleLeyva-Porras, César, Manuel Román-Aguirre, Pedro Cruz-Alcantar, José T. Pérez-Urizar, and María Zenaida Saavedra-Leos. 2021. "Application of Antioxidants as an Alternative Improving of Shelf Life in Foods" Polysaccharides 2, no. 3: 594-607. https://doi.org/10.3390/polysaccharides2030036
APA StyleLeyva-Porras, C., Román-Aguirre, M., Cruz-Alcantar, P., Pérez-Urizar, J. T., & Saavedra-Leos, M. Z. (2021). Application of Antioxidants as an Alternative Improving of Shelf Life in Foods. Polysaccharides, 2(3), 594-607. https://doi.org/10.3390/polysaccharides2030036








