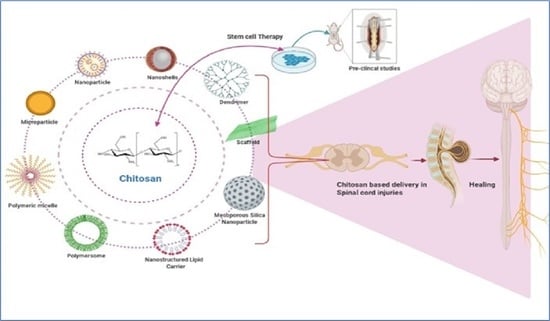
Trends of Chitosan Based Delivery Systems in Neuroregeneration and Functional Recovery in Spinal Cord Injuries
Abstract
1. Introduction
2. Stem Cell-Based Interventions of Spinal Cord Injury
2.1. MSCs: Immunomodulation and Trophic Support for SCI
2.2. Bone Marrow Stem Cells (BM-MSCs)
2.3. Embryonic Stem Cells (ESCs)
2.4. Neural Stem Cells (NSCs)
2.5. Induced Pluripotent Stem Cells (iPSCs)
2.6. Olfactory Ensheathing Cells (OECs)
2.7. Schwann Cells
2.8. Adult Endogenous Stem Cells (AESCs)
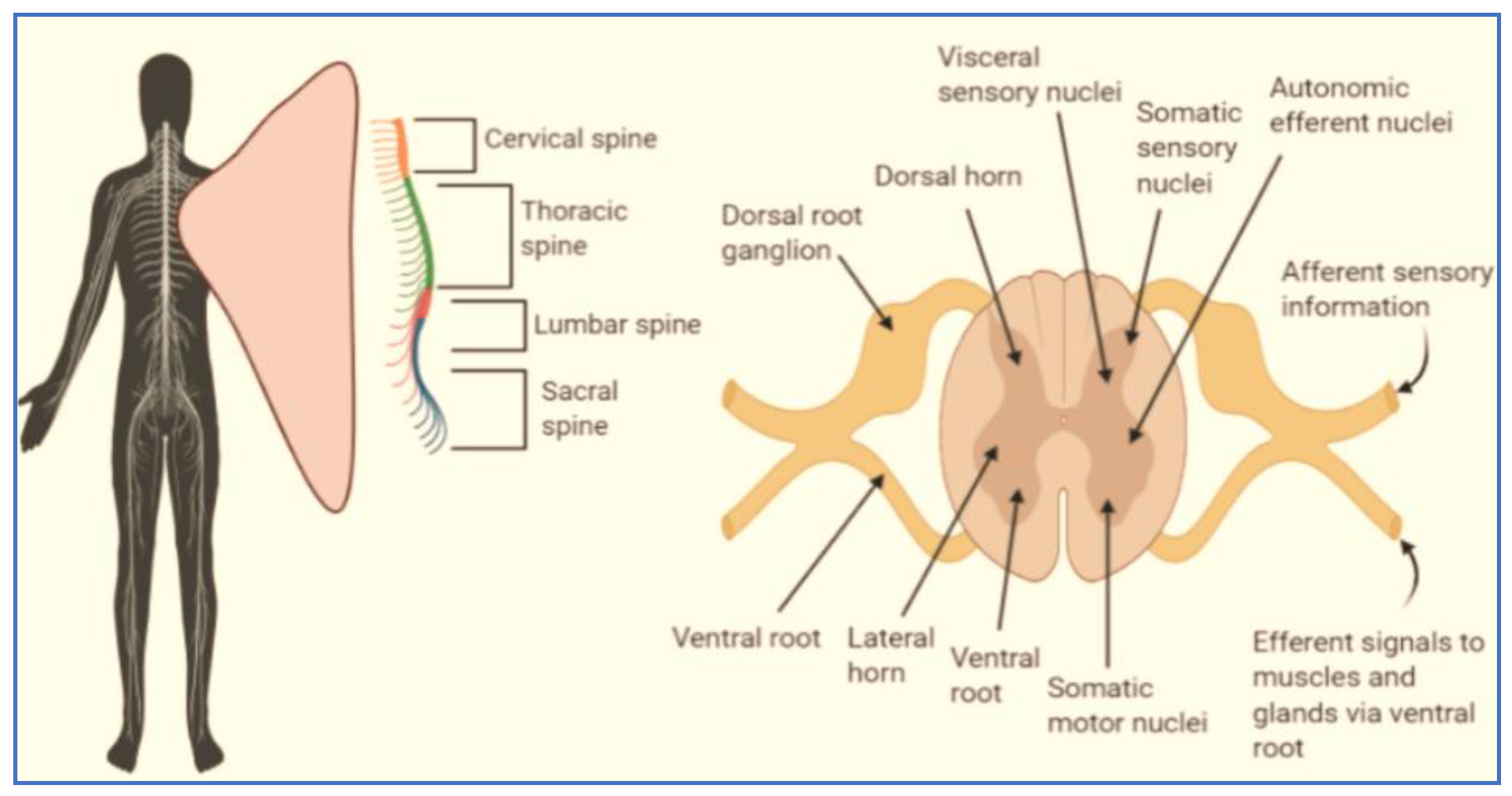
3. Factors for the Regeneration of Cells in Spinal Cord Injury
3.1. Bolus Injection
3.2. Continuous Infusion Using a Catheter or a Minipump System
4. Role of Chitosan in Neuroregeneration
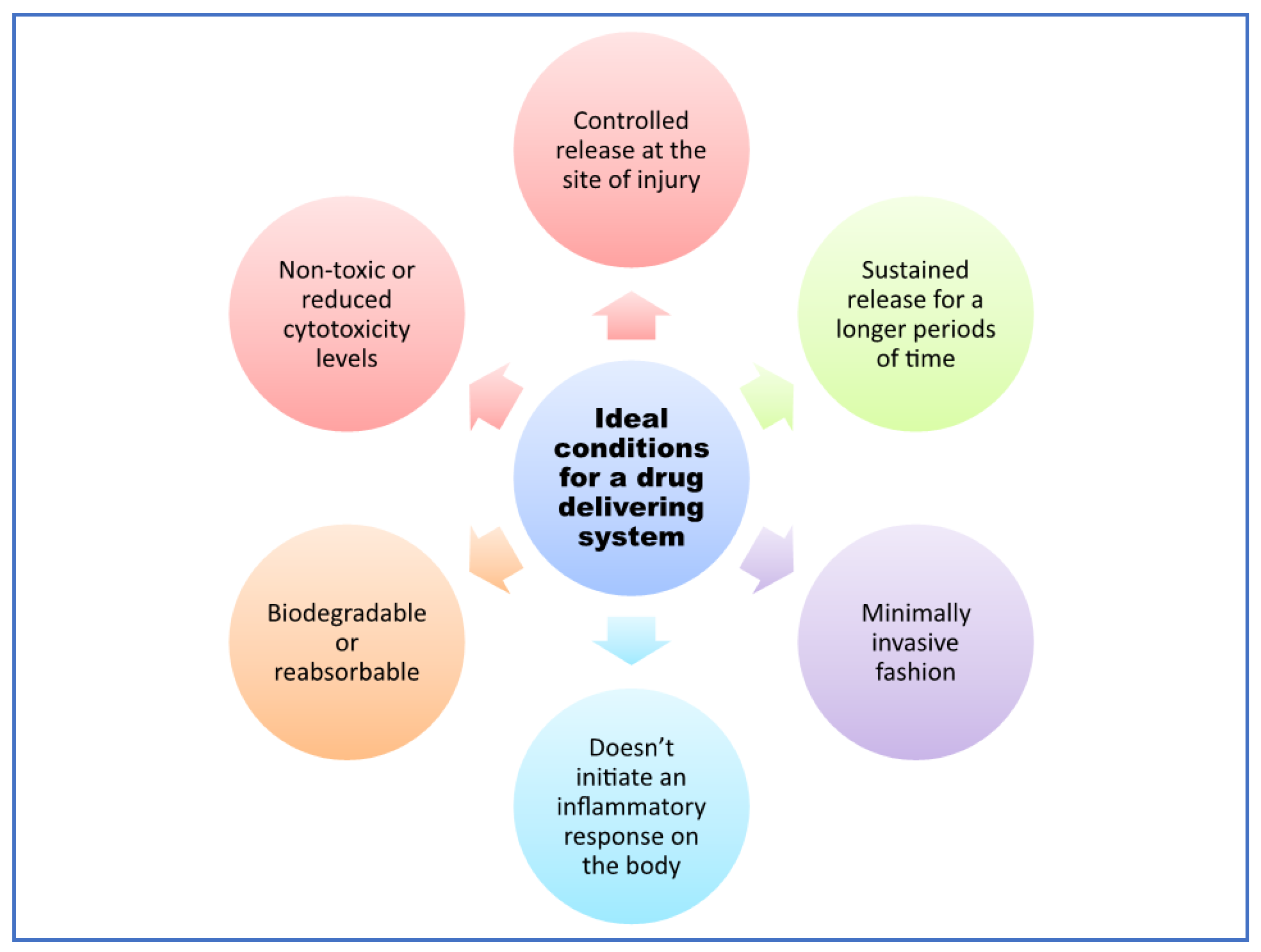
4.1. Delivery Systems for Neuroregeneration
- I.
- They actively interact with the lipid bilayer.
- II.
- The rearrangement of the membrane is spontaneous.
- III.
- They can reduce membrane defects.
4.2. Chitosan-Based Delivery in CNS Therapeutics
5. Role of Chitosan-Based Formulations in SCI
5.1. Micro/Nanoparticles (NP)
5.1.1. Iron Oxide, Gadolinium, or Cobalt Platinum Nanoparticles
5.1.2. Polymer Nanoparticles
5.2. Nanoscaffolds
5.3. Nanoemulsions
5.4. Hydro/Nano/In-Situ Gelling Systems
5.4.1. Natural Polymers
5.4.2. Synthetic Polymers
6. Recent Advancements in Chitosan-Based Stem Cell Therapy
7. Future Developments
8. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Pearce, J.M.S. The Development of Spinal Cord Anatomy. ENE 2008, 59, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, A.; Mushahwar, V.K. Spinal cord function and rehabilitation—An overview. J. Physiol. 2001, 533, 3–4. [Google Scholar] [CrossRef]
- Branco, F.; Cardenas, D.D.; Svircev, J.N. Spinal cord injury: A comprehensive review. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Polinder, S.; Meerding, W.J.; Mulder, S.; Petridou, E.; van Beeck, E.; EUROCOST Reference Group. Assessing the burden of injury in six European countries. Bull. World Health Organ. 2007, 85, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Barnabé-Heider, F.; Frisén, J. Stem cells for spinal cord repair. Cell Stem Cell 2008, 3, 16–24. [Google Scholar] [CrossRef]
- Guest, J.D.; Hiester, E.D.; Bunge, R.P. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 2005, 192, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.K.; Das, A.; Wallace, G.; Barry, J.; Vertegel, A.A.; Ray, S.K.; Banik, N.L. Spinal cord injury: A review of current therapy, future treatments, and basic science frontiers. Neurochem. Res. 2013, 38, 895–905. [Google Scholar] [CrossRef]
- Yousefifard, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Baikpour, M.; Safari, S.; Saadat, S.; Jafari, A.M.; Asady, H.; Tousi, S.M.T.R.; Hosseini, M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience 2016, 322, 377–397. [Google Scholar] [CrossRef]
- Ramesh, R.; Jeyaraman, M.; Chaudhari, K.; Dhamsania, H.J.; Prajwal, G.S. Mesenchymal Stem Cells—A Boon to Orthopedics. Open J. Regen. Med. 2018, 7, 19–27. [Google Scholar] [CrossRef][Green Version]
- Salewski, R.P.; Mitchell, R.A.; Shen, C.; Fehlings, M.G. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015, 24, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Uchida, K.; Nakajima, H.; Matsuo, H.; Sugita, D.; Yoshida, A.; Honjoh, K.; Johnson, W.E.B.; Baba, H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells 2015, 33, 1902–1914. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Kishk, N.A.; Gabr, H.; Hamdy, S.; Afifi, L.; Abokresha, N.; Mahmoud, H.; Wafaie, A.; Bilal, D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabilit. Neural Repair 2010, 24, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; He, C.; Zhao, Y.; Wang, J.; Tang, M.; Li, J.; Wu, Y.; Ao, L.; Hu, X. Human umbilical cord blood stem cell transplantation for the treatment of chronic spinal cord injury: Electrophysiological changes and long-term efficacy. Neural Regen. Res. 2013, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, E.A.L.; de Castro Nicodemo, M.; da Luz Oliveira, C.; Chaves, D.C.; Santâ’Anna, L.B. Amniotic Membrane in the Treatment of Spinal Cord Injuries. Biomed. J. Sci. Tech. Res. 2017, 1, 1520–1522. [Google Scholar] [CrossRef][Green Version]
- Aras, Y.; Sabanci, P.A.; Kabatas, S.; Duruksu, G.; Subasi, C.; Erguven, M.; Karaoz, E. The Effects of Adipose Tissue-Derived Mesenchymal Stem Cell Transplantation during the Acute and Subacute Phases Following Spinal Cord Injury. Turk. Neurosurg. 2016, 26, 127–139. [Google Scholar] [CrossRef]
- Jin, M.C.; Medress, Z.A.; Azad, T.D.; Doulames, V.M.; Veeravagu, A. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg. Focus 2019, 46, E10. [Google Scholar] [CrossRef] [PubMed]
- Shroff, G. Human Embryonic Stem Cell Therapy in Chronic Spinal Cord Injury: A Retrospective Study. Clin. Transl. Sci. 2016, 9, 168–175. [Google Scholar] [CrossRef]
- Shao, A.; Tu, S.; Lu, J.; Zhang, J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019, 10. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Long, L.; Lu, X.; Zhu, K.; Tuo, Y.; Chen, N.; Zhao, X.; Wang, L.; Wan, Y. Transplantation of Wnt5a-modified NSCs promotes tissue repair and locomotor functional recovery after spinal cord injury. Exp. Mol. Med. 2020, 52, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yao, H.; Dong, Q.; Zhang, Y.; Yang, Y.; Zhang, Y.; Yang, Z.; Xu, M.; Xu, R. Neurotrophy and immunomodulation of induced neural stem cell grafts in a mouse model of closed head injury. Stem Cell Res. 2017, 23, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Chao, H.-M.; Chern, E.; Hsu, S.-H. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef] [PubMed]
- Goel, A. Stem cell therapy in spinal cord injury: Hollow promise or promising science? J. Craniovertebr. Junction Spine 2016, 7, 121–126. [Google Scholar] [CrossRef]
- Marquardt, L.M.; Doulames, V.M.; Wang, A.T.; Dubbin, K.; Suhar, R.A.; Kratochvil, M.J.; Medress, Z.A.; Plant, G.W.; Heilshorn, S.C. Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Sci. Adv. 2020, 6, eaaz1039. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, F.; Gates, M.; Holmberg, E.G. Role of endogenous Schwann cells in tissue repair after spinal cord injury. Neural Regen. Res. 2013, 8, 177–185. [Google Scholar] [CrossRef]
- McDonough, A.; Martínez-Cerdeño, V. Endogenous Proliferation after Spinal Cord Injury in Animal Models. Stem Cells Int. 2012, 2012, e387513. [Google Scholar] [CrossRef]
- Hao, P.; Duan, H.; Hao, F.; Chen, L.; Sun, M.; Fan, K.S.; Sun, Y.E.; Williams, D.; Yang, Z.; Li, X. Neural repair by NT3-chitosan via enhancement of endogenous neurogenesis after adult focal aspiration brain injury. Biomaterials 2017, 140, 88–102. [Google Scholar] [CrossRef]
- Rubin, L.L.; Staddon, J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef]
- Gaillard, P.J.; Visser, C.C.; de Boer, A.G. Targeted delivery across the blood-brain barrier. Expert Opin. Drug Deliv. 2005, 2, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug delivery to the brain. J. Cereb. Blood Flow Metab. 1997, 17, 713–731. [Google Scholar] [CrossRef] [PubMed]
- Lavik, E.; Kuehn, M.H.; Kwon, Y.H. Novel drug delivery systems for glaucoma. Eye 2011, 25, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Gierdalski, M.; Kublik, A.; Skangiel-Kramska, J.; Kossut, M. Effects of implantation of Alzet 1007D osmotic minipumps upon 2-deoxyglucose uptake in the cerebral cortex of mice. Acta Neurobiol. Exp. (Wars) 1993, 53, 577–580. [Google Scholar]
- Follett, K.A.; Boortz-Marx, R.L.; Drake, J.M.; DuPen, S.; Schneider, S.J.; Turner, M.S.; Coffey, R.J. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology 2004, 100, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.D.; York, M.M.; Paice, J.A. Catheter systems for intrathecal drug delivery. J. Neurosurg. 1995, 83, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Tumor physiology and antibody delivery. Front. Radiat. Ther. Oncol. 1990, 24, 32–46. [Google Scholar]
- Brem, R.L.H. Polymer-based Drug Delivery to the Brain. 1996. Available online: www.sciandmed.com/sm/journalviewer.aspx?issue=1061&article=745 (accessed on 9 January 2021).
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Borgens, R.B.; Shi, R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000, 14, 27–35. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Costantino, L.; Gandolfi, F.; Tosi, G.; Rivasi, F.; Vandelli, M.A.; Forni, F. Peptide-derivatized biodegradable nanoparticles able to cross the blood-brain barrier. J. Control. Release 2005, 108, 84–96. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neurosci. 2008, 9, S4. [Google Scholar] [CrossRef]
- Xu, G.; Yong, K.-T.; Roy, I.; Mahajan, S.D.; Ding, H.; Schwartz, S.A.; Prasad, P.N. Bioconjugated quantum rods as targeted probes for efficient transmigration across an in vitro blood-brain barrier. Bioconjug. Chem. 2008, 19, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Borgens, R.B. The preparation of polypyrrole surfaces in the presence of mesoporous silica nanoparticles and their biomedical applications. Nanotechnology 2010, 21, 205102. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Borgens, R.B. The effect of an electrically conductive carbon nanotube/collagen composite on neurite outgrowth of PC12 cells. J. Biomed. Mater. Res. A 2010, 95, 510–517. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Borgens, R.; Ivanisevic, A. Repairing the Damaged Spinal Cord and Brain with Nanomedicine. Small 2008, 4, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shi, R.; Borgens, R.B.; Ivanisevic, A. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine 2008, 3, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, J.; Borgens, R.B. Neuroprotection by chitosan nanoparticles in oxidative stress-mediated injury. BMC Res. Notes 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010, 213, 1513–1520. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Ivanisevic, A.; Borgens, R.B. A mesoporous silica nanosphere-based drug delivery system using an electrically conducting polymer. Nanotechnology 2009, 20, 275102. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Alolabi, H.; Shafiei, A.; Kang, N.; Policova, Z.; Cox, P.N.; Acosta, E.; Hair, M.L.; Neumann, A.W. Chitosan Enhances the In Vitro Surface Activity of Dilute Lung Surfactant Preparations and Resists Albumin-Induced Inactivation. Pediatric Res. 2006, 60, 125–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, Y.; Shi, R.; Ivanisevic, A.; Borgens, R.B. Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury. J. Neurosci. Res. 2010, 88, 1433–1444. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan nanoparticle-based neuronal membrane sealing and neuroprotection following acrolein-induced cell injury. J. Biol. Eng. 2010, 4, 2. [Google Scholar] [CrossRef]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Osgood, C.J.; Xu, X.-H.N. Silver Nanoparticles Induce Developmental Stage-Specific Embryonic Phenotypes in Zebrafish. Nanoscale 2013, 5, 11625–11636. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Jakovcevski, I.; Poulia, N.; Djogo, N.; Schulz, F.; Martinovic, T.; Ciric, D.; Loers, G.; Vossmeyer, T.; Weller, H.; et al. Intraspinal Delivery of Polyethylene Glycol-coated Gold Nanoparticles Promotes Functional Recovery After Spinal Cord Injury. Mol. Ther. 2015, 23, 993–1002. [Google Scholar] [CrossRef]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; de Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Kim, Y.-T.; Bratt-Leal, A.M.; Lee, H.; Bellamkonda, R.V. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials 2008, 29, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.-Y.; Kong, X.-H.; Wang, H.-J.; Chang, J.; Zhang, D.-P.; Ban, D.-X.; Feng, S.-Q. Novel multifunctional polyethylene glycol-transactivating-transduction protein-modified liposomes cross the blood-spinal cord barrier after spinal cord injury. J. Drug Target. 2010, 18, 420–429. [Google Scholar] [CrossRef]
- Bulte, J.W.; Zhang, S.; van Gelderen, P.; Herynek, V.; Jordan, E.K.; Duncan, I.D.; Frank, J.A. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: Magnetic resonance tracking of cell migration and myelination. Proc. Natl. Acad. Sci. USA 1999, 96, 15256–15261. [Google Scholar] [CrossRef]
- Callera, F.; de Melo, C.M.T.P. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007, 16, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Amemori, T.; Romanyuk, N.; Jendelova, P.; Herynek, V.; Turnovcova, K.; Prochazka, P.; Kapcalova, M.; Cocks, G.; Price, J.; Sykova, E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res. Ther. 2013, 4, 68. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Millward, J.M.; Schnorr, J.; Taupitz, M.; Wagner, S.; Wuerfel, J.T.; Infante-Duarte, C. Iron oxide magnetic nanoparticles highlight early involvement of the choroid plexus in central nervous system inflammation. ASN Neuro 2013, 5, e00110. [Google Scholar] [CrossRef] [PubMed]
- Floris, S.; Blezer, E.L.A.; Schreibelt, G.; Döpp, E.; van der Pol, S.M.A.; Schadee-Eestermans, I.L.; Nicolay, K.; Dijkstra, C.D.; de Vries, H.E. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: A quantitative MRI study. Brain 2004, 127, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; McBain, S.C.; Dobson, J.; Chari, D.M. Uptake of systemically administered magnetic nanoparticles (MNPs) in areas of experimental spinal cord injury (SCI). J. Tissue Eng. Regen. Med. 2009, 3, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.I.; Pickard, M.R.; Granger, N.; Chari, D.M. Magnetic nanoparticle-mediated gene transfer to oligodendrocyte precursor cell transplant populations is enhanced by magnetofection strategies. ACS Nano 2011, 5, 6527–6538. [Google Scholar] [CrossRef]
- Pickard, M.R.; Barraud, P.; Chari, D.M. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials 2011, 32, 2274–2284. [Google Scholar] [CrossRef]
- Song, H.P.; Yang, J.Y.; Lo, S.L.; Wang, Y.; Fan, W.M.; Tang, X.S.; Xue, J.M.; Wang, S. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials 2009, 31, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Hamasaki, T.; Yanada, S.; Mochizuki, Y.; Ochi, M. Magnetic targeting of bone marrow stromal cells into spinal cord: Through cerebrospinal fluid. Neuroreport 2006, 17, 1269–1272. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Cardoso, E.; Rezin, G.T.; Zanoni, E.T.; Notoya, F.d.; Leffa, D.D.; Damiani, A.P.; Daumann, F.; Rodriguez, J.C.O.; Benavides, R.; da Silva, L.; et al. Acute and chronic administration of gold nanoparticles cause DNA damage in the cerebral cortex of adult rats. Mutat. Res. 2014, 766–767, 25–30. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef]
- Donaghue, I.E.; Tator, C.H.; Shoichet, M.S. Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater. Sci. 2015, 3, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Ferrari, R.; de Paola, M.; Rossi, F.; Mariani, A.; Caron, I.; Sammali, E.; Peviani, M.; Dell’Oro, V.; Colombo, C.; et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J. Control. Release 2014, 174, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Gouritin, B.; Villarroya, H.; Eclancher, F.; Giannavola, C.; Klein, C.; Andreux, J.P.; Couvreur, P. Quantification and localization of PEGylated polycyanoacrylate nanoparticles in brain and spinal cord during experimental allergic encephalomyelitis in the rat. Eur. J. Neurosci. 2002, 15, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bohnert, D.; Borgens, R.B.; Cho, Y. Pushing the science forward: Chitosan nanoparticles and functional repair of CNS tissue after spinal cord injury. J. Biol. Eng. 2013, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.; McCarthy, D.P.; Yap, W.T.; Harp, C.T.; Getts, D.R.; Shea, L.D.; Miller, S.D. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 2014, 8, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
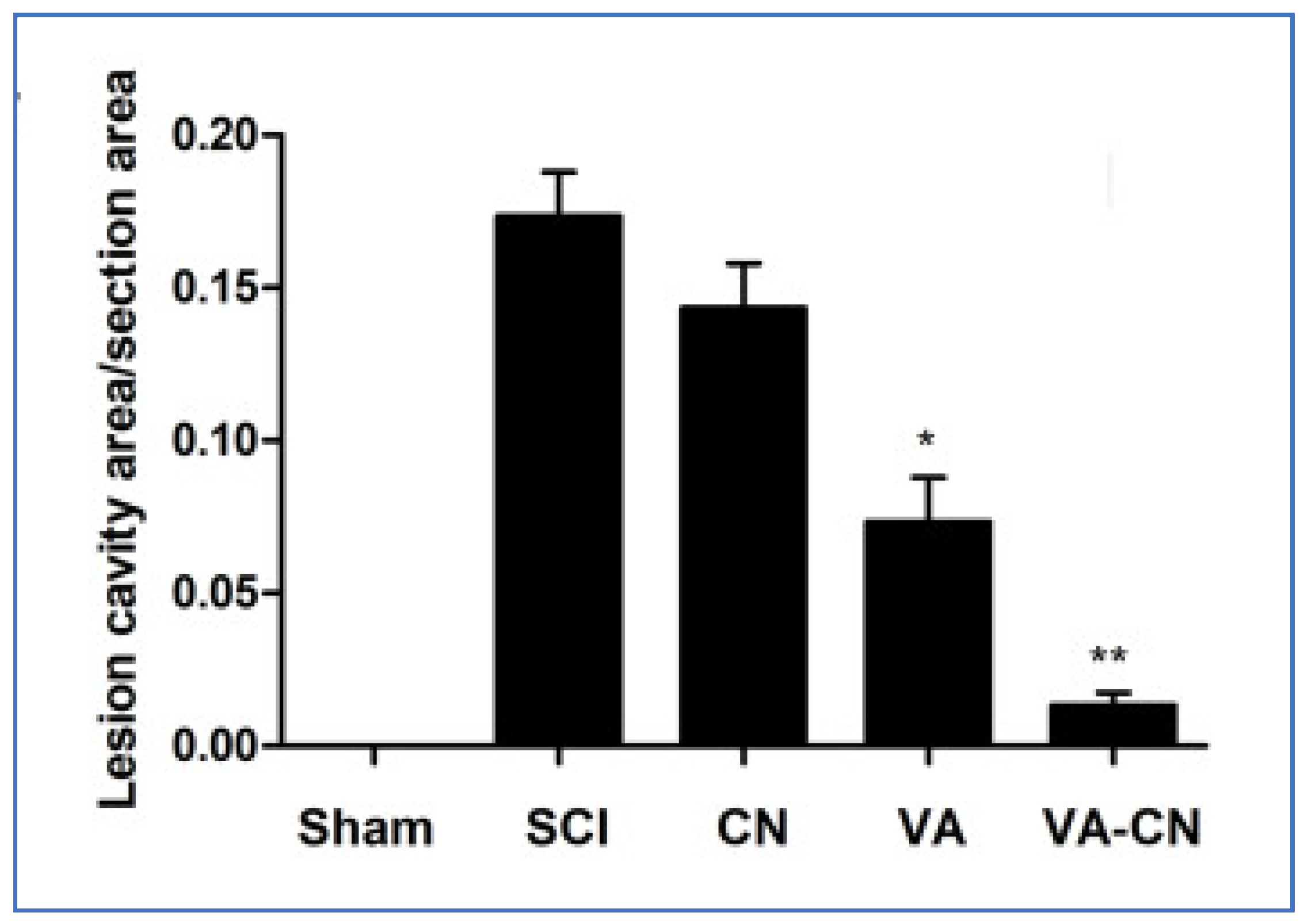
- Wang, D.; Wang, K.; Liu, Z.; Wang, Z.; Wu, H. Valproic acid-labeled chitosan nanoparticles promote recovery of neuronal injury after spinal cord injury. Aging 2020, 12, 8953–8967. [Google Scholar] [CrossRef] [PubMed]
- Millesi, H.; Zöch, G.; Reihsner, R. Mechanical properties of peripheral nerves. Clin. Orthop. Relat. Res. 1995, 314, 76–83. [Google Scholar] [CrossRef]
- Dalto, P.D.; Shoichet, M.S. Creating porous tubes by centrifugal forces for soft tissue application. Biomaterials 2001, 22, 2661–2669. [Google Scholar] [CrossRef]
- Dalton, P.D.; Flynn, L.; Shoichet, M.S. Manufacture of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes for use as nerve guidance channels. Biomaterials 2002, 23, 3843–3851. [Google Scholar] [CrossRef]
- Oudega, M.; Gautier, S.E.; Chapon, P.; Fragoso, M.; Bates, M.L.; Parel, J.M.; Bunge, M.B. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials 2001, 22, 1125–1136. [Google Scholar] [CrossRef]
- Novikov, L.N.; Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O. A novel biodegradable implant for neuronal rescue and regeneration after spinal cord injury. Biomaterials 2002, 23, 3369–3376. [Google Scholar] [CrossRef]
- Zahir, T.; Nomura, H.; Guo, X.D.; Kim, H.; Tator, C.; Morshead, C.; Shoichet, M. Bioengineering neural stem/progenitor cell-coated tubes for spinal cord injury repair. Cell Transplant. 2008, 17, 245–254. [Google Scholar] [CrossRef]
- Nomura, H.; Zahir, T.; Kim, H.; Katayama, Y.; Kulbatski, I.; Morshead, C.M.; Shoichet, M.S.; Tator, C.H. Extramedullary chitosan channels promote survival of transplanted neural stem and progenitor cells and create a tissue bridge after complete spinal cord transection. Tissue Eng. Part A 2008, 14, 649–665. [Google Scholar] [CrossRef]
- Kim, H.; Tator, C.H.; Shoichet, M.S. Chitosan implants in the rat spinal cord: Biocompatibility and biodegradation. J. Biomed. Mater. Res. A 2011, 97, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Paino, C.L.; Bunge, M.B. Induction of axon growth into Schwann cell implants grafted into lesioned adult rat spinal cord. Exp. Neurol. 1991, 114, 254–257. [Google Scholar] [CrossRef]
- Montgomery, C.T.; Robson, J.A. New method of transplanting purified glial cells into the brain. J. Neurosci. Methods 1990, 32, 135–141. [Google Scholar] [CrossRef]
- Kim, H.; Zahir, T.; Tator, C.H.; Shoichet, M.S. Effects of Dibutyryl Cyclic-AMP on Survival and Neuronal Differentiation of Neural Stem/Progenitor Cells Transplanted into Spinal Cord Injured Rats. PLoS ONE 2011, 6, e21744. [Google Scholar] [CrossRef] [PubMed]
- Midha, R.; Shoichet, M.S.; Dalton, P.D.; Cao, X.; Munro, C.A.; Noble, J.; Wong, M.K. Tissue engineered alternatives to nerve transplantation for repair of peripheral nervous system injuries. Transplant. Proc. 2001, 33, 612–615. [Google Scholar] [CrossRef]
- Bellamkonda, R.; Ranieri, J.P.; Aebischer, P. Laminin oligopeptide derivatized agarose gels allow three-dimensional neurite extension in vitro. J. Neurosci. Res. 1995, 41, 501–509. [Google Scholar] [CrossRef]
- Woerly, S.; Doan, V.D.; Sosa, N.; de Vellis, J.; Espinosa, A. Reconstruction of the transected cat spinal cord following NeuroGel implantation: Axonal tracing, immunohistochemical and ultrastructural studies. Int. J. Dev. Neurosci. 2001, 19, 63–83. [Google Scholar] [CrossRef]
- Horn, E.M.; Beaumont, M.; Shu, X.Z.; Harvey, A.; Prestwich, G.D.; Horn, K.M.; Gibson, A.R.; Preul, M.C.; Panitch, A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J. Neurosurg. Spine 2007, 6, 133–140. [Google Scholar] [CrossRef]
- Hejcl, A.; Urdzikova, L.; Sedy, J.; Lesny, P.; Pradny, M.; Michalek, J.; Burian, M.; Hajek, M.; Zamecnik, J.; Jendelova, P.; et al. Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat. J. Neurosurg. Spine 2008, 8, 67–73. [Google Scholar] [CrossRef]
- Tomita, M.; Lavik, E.; Klassen, H.; Zahir, T.; Langer, R.; Young, M.J. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells 2005, 23, 1579–1588. [Google Scholar] [CrossRef]
- Tao, S.; Young, C.; Redenti, S.; Zhang, Y.; Klassen, H.; Desai, T.; Young, M.J. Survival migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip 2007, 7, 695–701. [Google Scholar] [CrossRef]
- Redenti, S.; Neeley, W.L.; Rompani, S.; Saigal, S.; Yang, J.; Klassen, H.; Langer, R.; Young, M.J. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials 2009, 30, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Neeley, W.L.; Redenti, S.; Klassen, H.; Tao, S.; Desai, T.; Young, M.J.; Langer, R. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials 2008, 29, 418–426. [Google Scholar] [CrossRef]
- Sodha, S.; Wall, K.; Redenti, S.; Klassen, H.; Young, M.J.; Tao, S.L. Microfabrication of a three-dimensional polycaprolactone thin-film scaffold for retinal progenitor cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011, 22, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.A.; Redenti, S.M.; Jiang, C.; Swift, J.S.; Klassen, H.J.; Smith, M.E.; Wnek, G.E.; Young, M.J. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials 2010, 31, 9–19. [Google Scholar] [CrossRef] [PubMed]
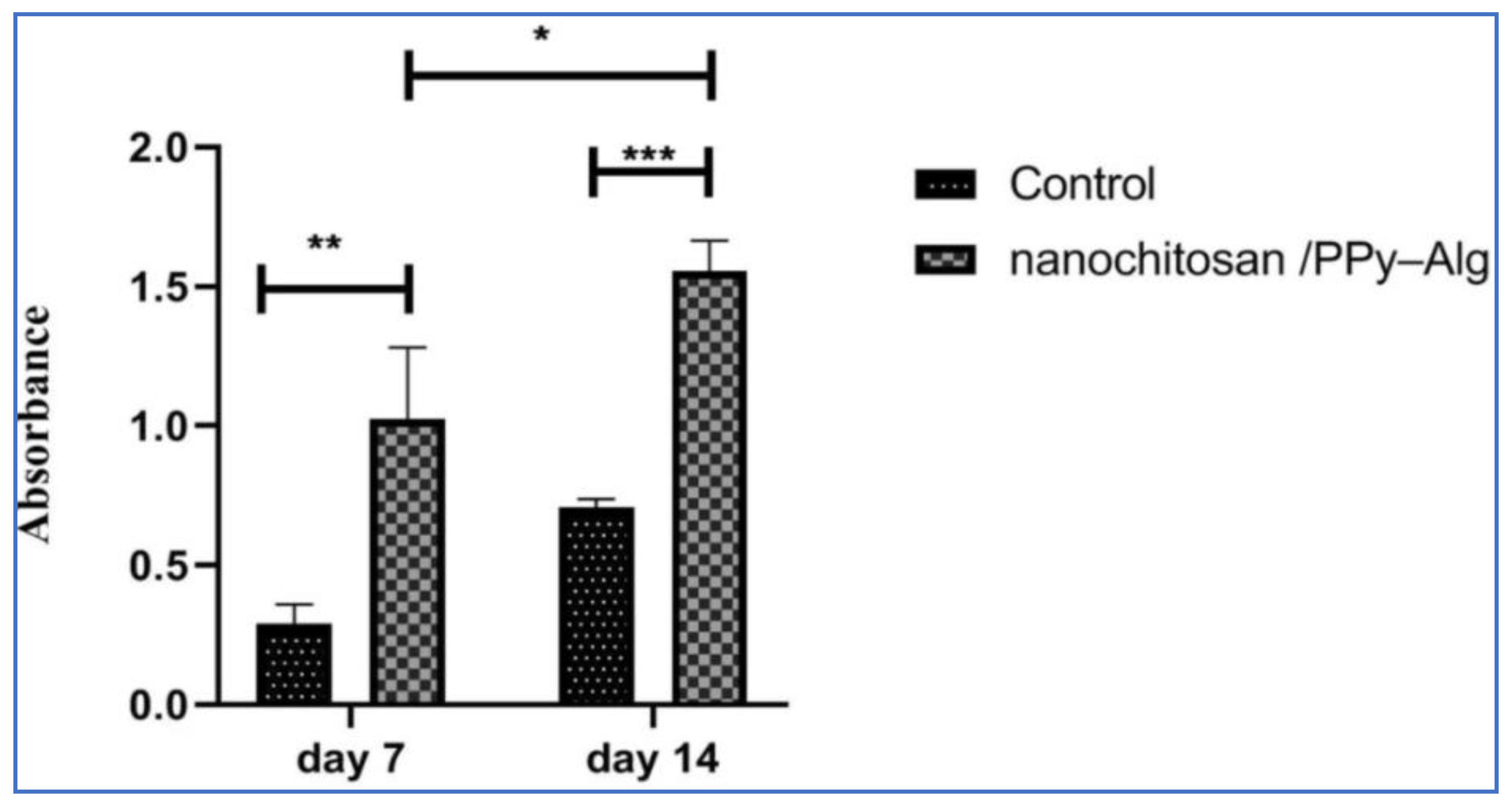
- Manzari-Tavakoli, A.; Tarasi, R.; Sedghi, R.; Moghimi, A.; Niknejad, H. Fabrication of nanochitosan incorporated polypyrrole/alginate conducting scaffold for neural tissue engineering. Sci. Rep. 2020, 10, 22012. [Google Scholar] [CrossRef]
- Chen, X.-G.; Hua, F.; Wang, S.-G.; Tang, H.-H. Albumin-Conjugated Lipid-Based Multilayered Nanoemulsion Improves Drug Specificity and Anti-Inflammatory Potential at the Spinal Cord Injury gSite after Intravenous Administration. AAPS PharmSciTech 2018, 19, 590–598. [Google Scholar] [CrossRef]
- Geng, T.-Y.; Xu, C.; Xu, G.-H. Albumin conjugated lipid nanoemulsion for site specific delivery of rapamycin at inflammatory site of spinal cord injury. Int. J. Clin. Exp. Med. 2016, 9, 21028–21037. [Google Scholar]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. A 2009, 90, 720–729. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. J. Biomed. Mater. Res. A 2006, 76, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, R.G.; van de Wetering, P.; Elbert, D.L.; Hubbell, J.A. The effect of the linker on the hydrolysis rate of drug-linked ester bonds. J. Control. Release 2004, 95, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. Available online: https://www.eurekaselect.com/73978/article (accessed on 9 January 2021). [CrossRef]
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 3340–3345. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Lampe, K.J.; Kern, D.S.; Mahoney, M.J.; Bjugstad, K.B. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: Protein distribution and the glial response. J. Biomed. Mater. Res. A 2011, 96, 595–607. [Google Scholar] [CrossRef]
- Baumann, M.D.; Kang, C.E.; Tator, C.H.; Shoichet, M.S. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials 2010, 31, 7631–7639. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.-C. Degradable Polymer Microspheres for Controlled Drug Delivery. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(.alpha.-hydroxy acid) diacrylate macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Chedly, J.; Soares, S.; Montembault, A.; von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Ghane, N.; Beigi, M.-H.; Labbaf, S.; Nasr-Esfahani, M.-H.; Kiani, A. Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J. Mater. Chem. B 2020, 8, 10712–10738. [Google Scholar] [CrossRef] [PubMed]
- Johl, S.S.; Burgett, R.A. Dermal filler agents: A practical review. Curr. Opin. Ophthalmol. 2006, 17, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lubomír Lapcík, L., Jr.; Lapcík, L.; de Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Jain, A.; Kim, Y.-T.; McKeon, R.J.; Bellamkonda, R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 2006, 27, 497–504. [Google Scholar] [CrossRef]
- Aymard, P.; Martin, D.R.; Plucknett, K.; Foster, T.J.; Clark, A.H.; Norton, I.T. Influence of thermal history on the structural and mechanical properties of agarose gels. Biopolymers 2001, 59, 131–144. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Vernengo, J.; Fussell, G.W.; Smith, N.G.; Lowman, A.M. Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 64–69. [Google Scholar] [CrossRef]
- Conova, L.; Kubinski, P.; Jin, Y.; Vernengo, J.; Neuhuber, B.; Fischer, I.; Neuhuber, B.; Lowman, A. Injectable multifunctional scaffold for spinal cord repair. In Proceedings of the 2010 IEEE 36th Annual Northeast Bioengineering Conference (NEBEC), New York, NY, USA, 26–28 March 2010; pp. 1–2. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef]
- Zhong, Y.; Bellamkonda, R.V. Biomaterials for the central nervous system. J. R. Soc. Interface 2008, 5, 957–975. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Saijilafu Wen, X.; Luo, Z.; Yang, H.; Wang, W.; Yang, L. 6-Biomaterials and scaffolds for the treatment of spinal cord injury. In Biomaterials in Translational Medicine; Yang, L., Bhaduri, S.B., Webster, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 117–139. [Google Scholar] [CrossRef]
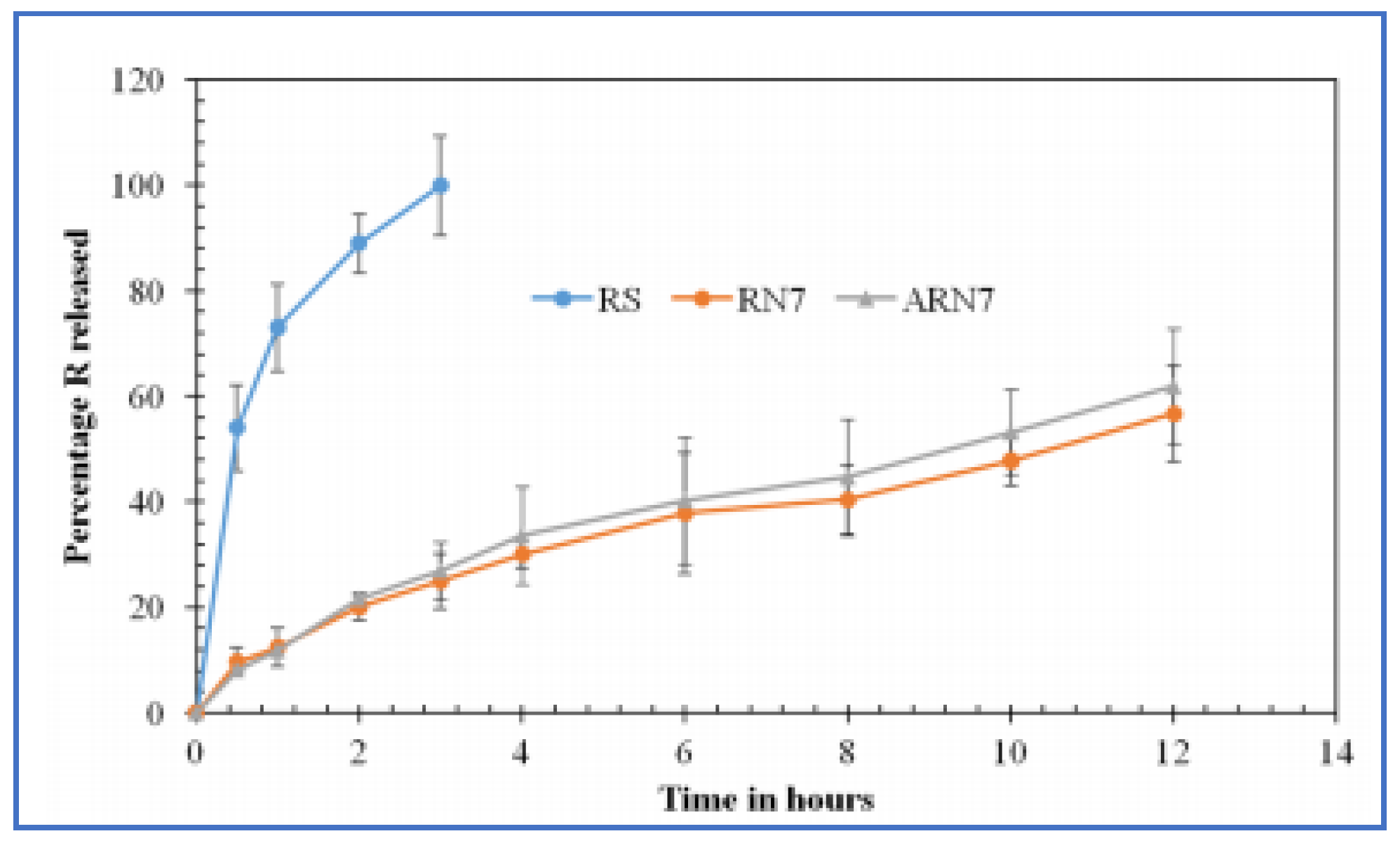
- Ni, S.; Xia, T.; Li, X.; Zhu, X.; Qi, H.; Huang, S.; Wang, J. Sustained delivery of chondroitinase ABC by poly(propylene carbonate)-chitosan micron fibers promotes axon regeneration and functional recovery after spinal cord hemisection. Brain Res. 2015, 1624, 469–478. [Google Scholar] [CrossRef]
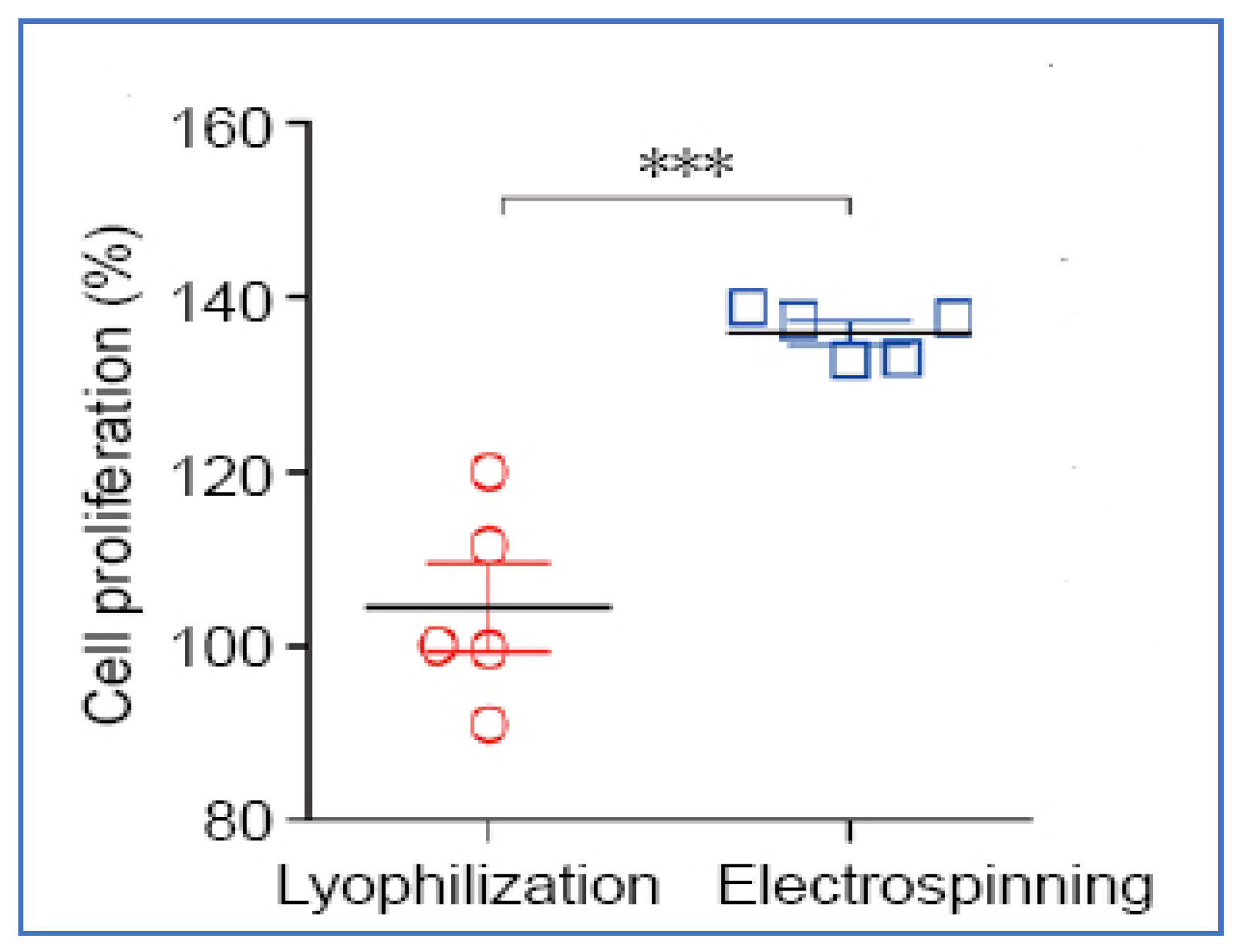
- Wu, Y.-X.; Ma, H.; Wang, J.-L.; Qu, W. Production of chitosan scaffolds by lyophilization or electrospinning: Which is better for peripheral nerve regeneration? Neural Regen. Res. 2021, 16, 1093–1098. [Google Scholar] [CrossRef]
- Qu, W.; Chen, B.; Shu, W.; Tian, H.; Ou, X.; Zhang, X.; Wang, Y.; Wu, M. Polymer-Based Scaffold Strategies for Spinal Cord Repair and Regeneration. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ham, T.R.; Neill, N.; Farrag, M.; Mohrman, A.E.; Koenig, A.M.; Leipzig, N.D. A Hydrogel Bridge Incorporating Immobilized Growth Factors and Neural Stem/Progenitor Cells to Treat Spinal Cord Injury. Adv. Healthc. Mater. 2016, 5, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Feng, G.; Gu, Z.; Sun, Y.; Bao, G.; Xu, G.; Lu, Y.; Chen, J.; Xu, L.; et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: Potential roles for spinal cord injury therapy. Cell Tissue Res. 2016, 366, 129–142. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurakula, M.; Gorityala, S.; Patel, D.B.; Basim, P.; Patel, B.; Kumar Jha, S. Trends of Chitosan Based Delivery Systems in Neuroregeneration and Functional Recovery in Spinal Cord Injuries. Polysaccharides 2021, 2, 519-537. https://doi.org/10.3390/polysaccharides2020031
Kurakula M, Gorityala S, Patel DB, Basim P, Patel B, Kumar Jha S. Trends of Chitosan Based Delivery Systems in Neuroregeneration and Functional Recovery in Spinal Cord Injuries. Polysaccharides. 2021; 2(2):519-537. https://doi.org/10.3390/polysaccharides2020031
Chicago/Turabian StyleKurakula, Mallesh, Shashank Gorityala, Devang B. Patel, Pratap Basim, Bhaumik Patel, and Saurabh Kumar Jha. 2021. "Trends of Chitosan Based Delivery Systems in Neuroregeneration and Functional Recovery in Spinal Cord Injuries" Polysaccharides 2, no. 2: 519-537. https://doi.org/10.3390/polysaccharides2020031
APA StyleKurakula, M., Gorityala, S., Patel, D. B., Basim, P., Patel, B., & Kumar Jha, S. (2021). Trends of Chitosan Based Delivery Systems in Neuroregeneration and Functional Recovery in Spinal Cord Injuries. Polysaccharides, 2(2), 519-537. https://doi.org/10.3390/polysaccharides2020031