Enhancing Electrical Conductivity of Commercially Pure Aluminium via Deep Cryogenic Treatment and Subsequent Annealing
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Deep Cryogenic Treatment (DCT)
2.3. Deep Cryogenic Treatment + Annealing Treatment (DCT+A)
2.4. Metallographic Analysis
2.5. Quantitative Assessment of Crystallite Size, Dislocation Density and Microstrain
- D—crystallite size (nm);
- k—Scherrer constant (0.94);
- λ—X-ray wavelength (1.5406 Å);
- β—full width at half maximum (FWHM) of the peak in radians;
- θ—Bragg angle in degrees.
2.6. Grain Boundary Characterisation via EBSD: LAGB and HAGB Quantification
2.7. TEM Imaging and Selected Area Electron Diffraction (SAED) Analysis
2.8. Raman Spectroscopy for Vibrational and Structural Analysis
2.9. Electrical Conductivity
- μ—electron mobility (cm2/V·s);
- n—charge carrier concentration for aluminium (1.8 × 1029 m−3);
- q—electron charge (1.6 × 10−19 C);
- σ—electrical conductivity (S/m).
3. Results
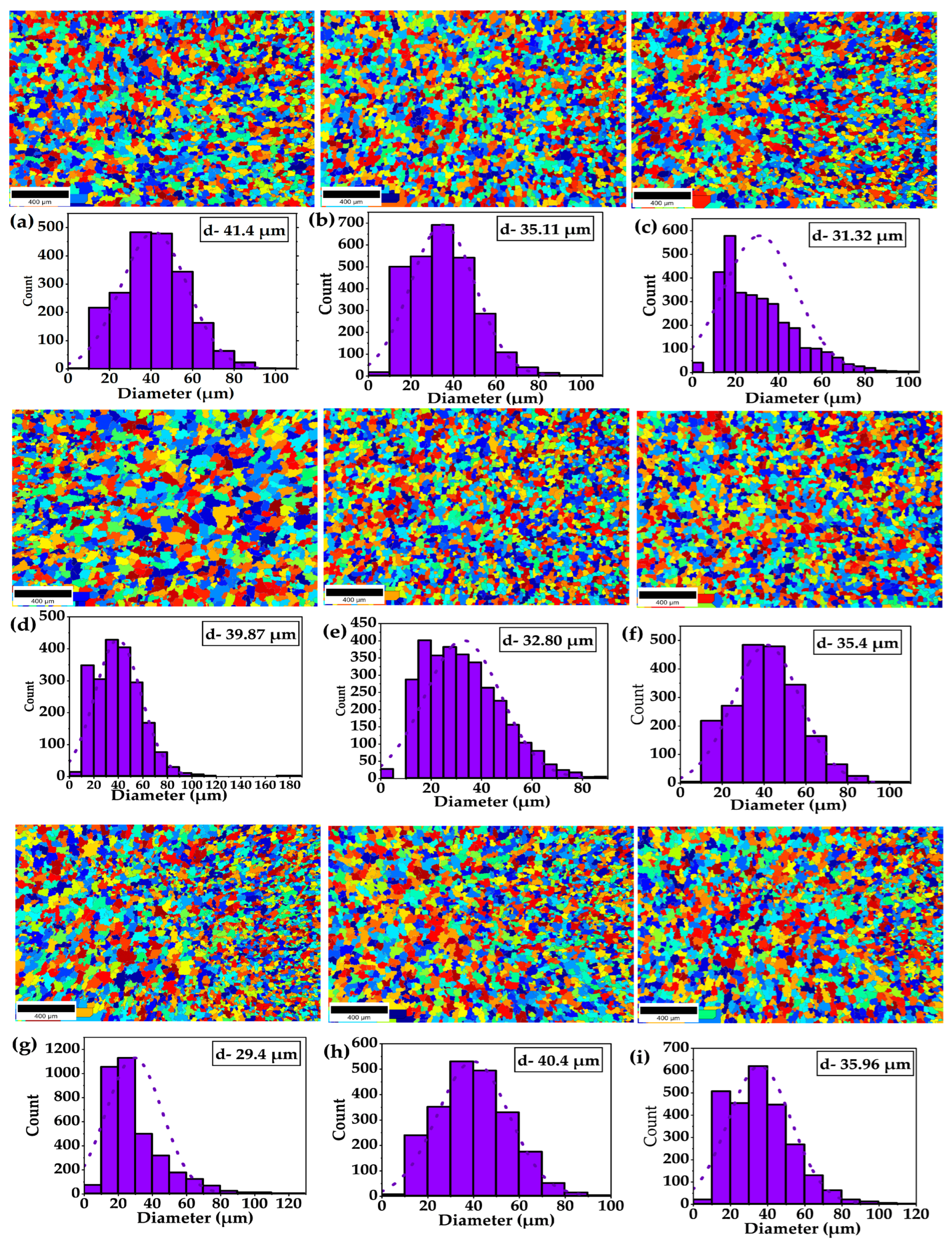
3.1. Effect of Soaking Hours on Microstructural Evolution
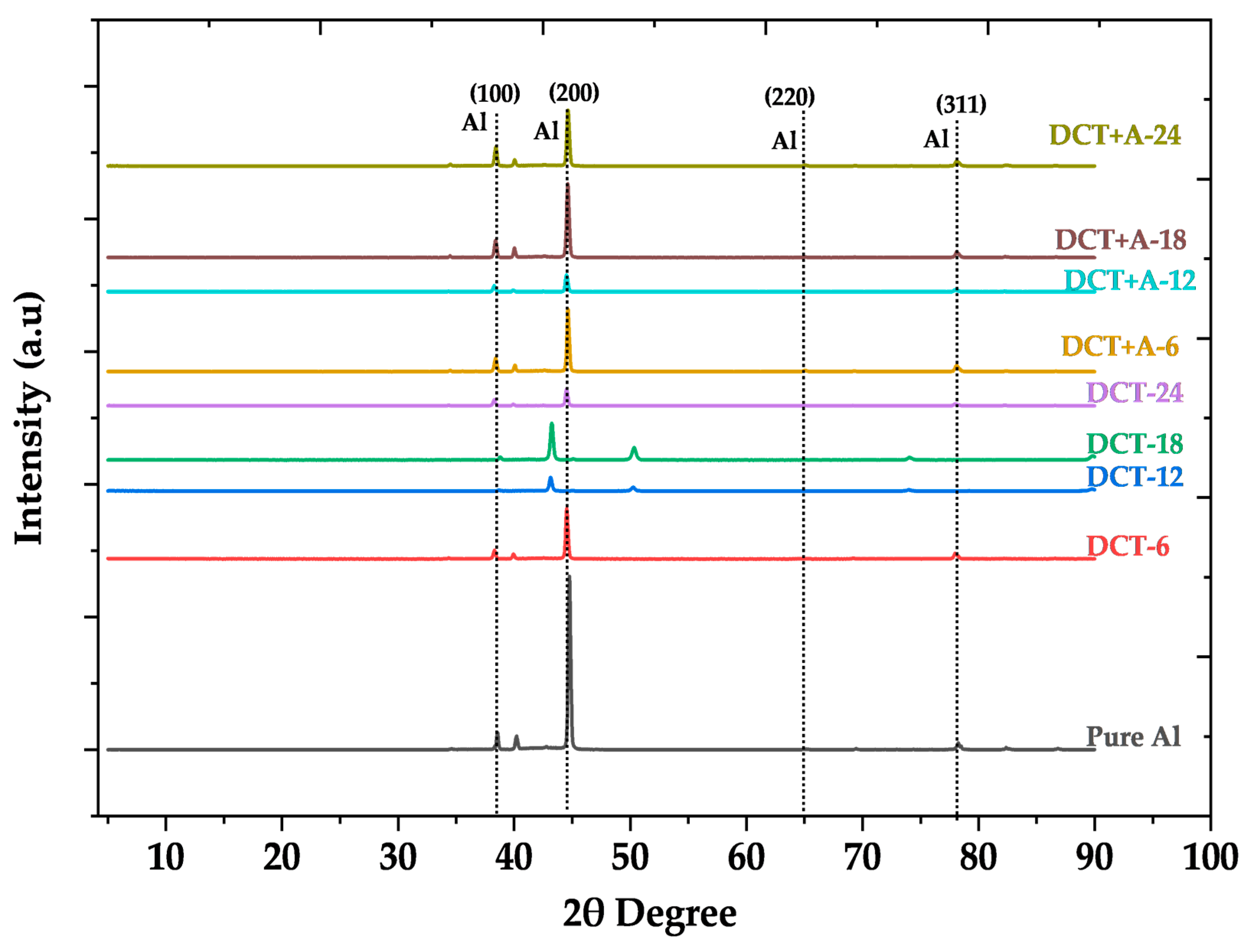
3.2. XRD-Based Crystallographic Structure and Analysis
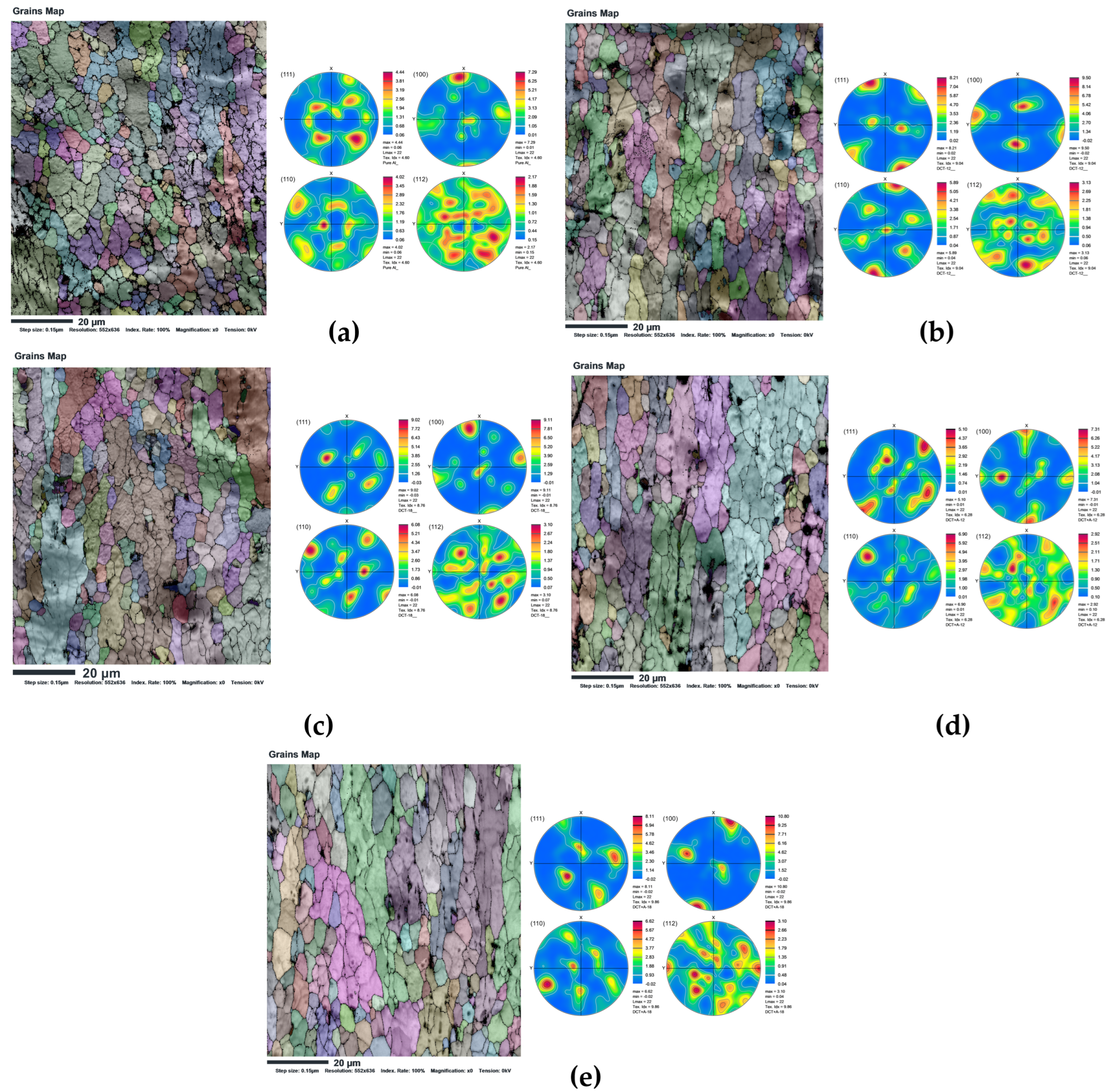
3.3. Grain Boundary Characteristics: LAGB and HAGB Distribution
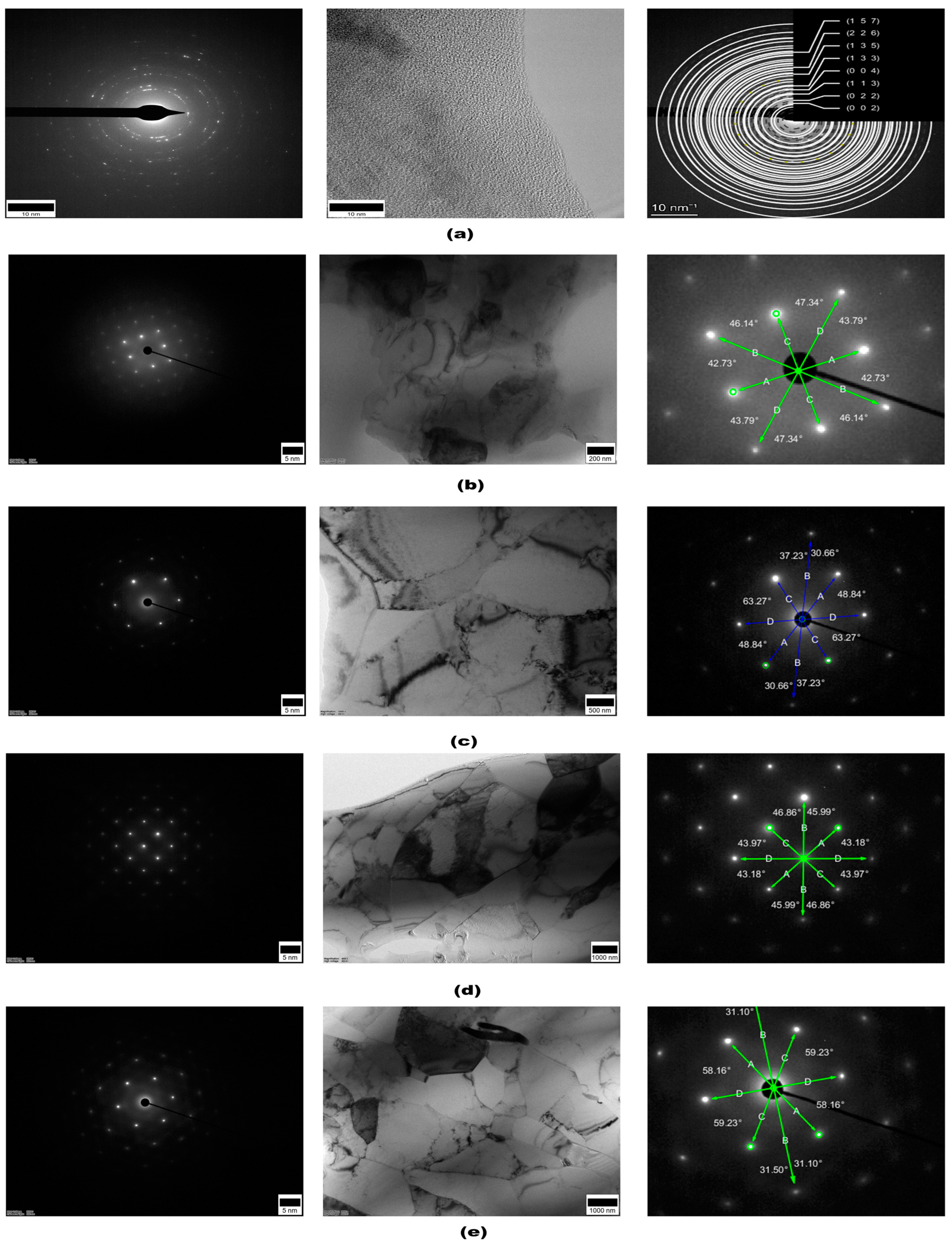
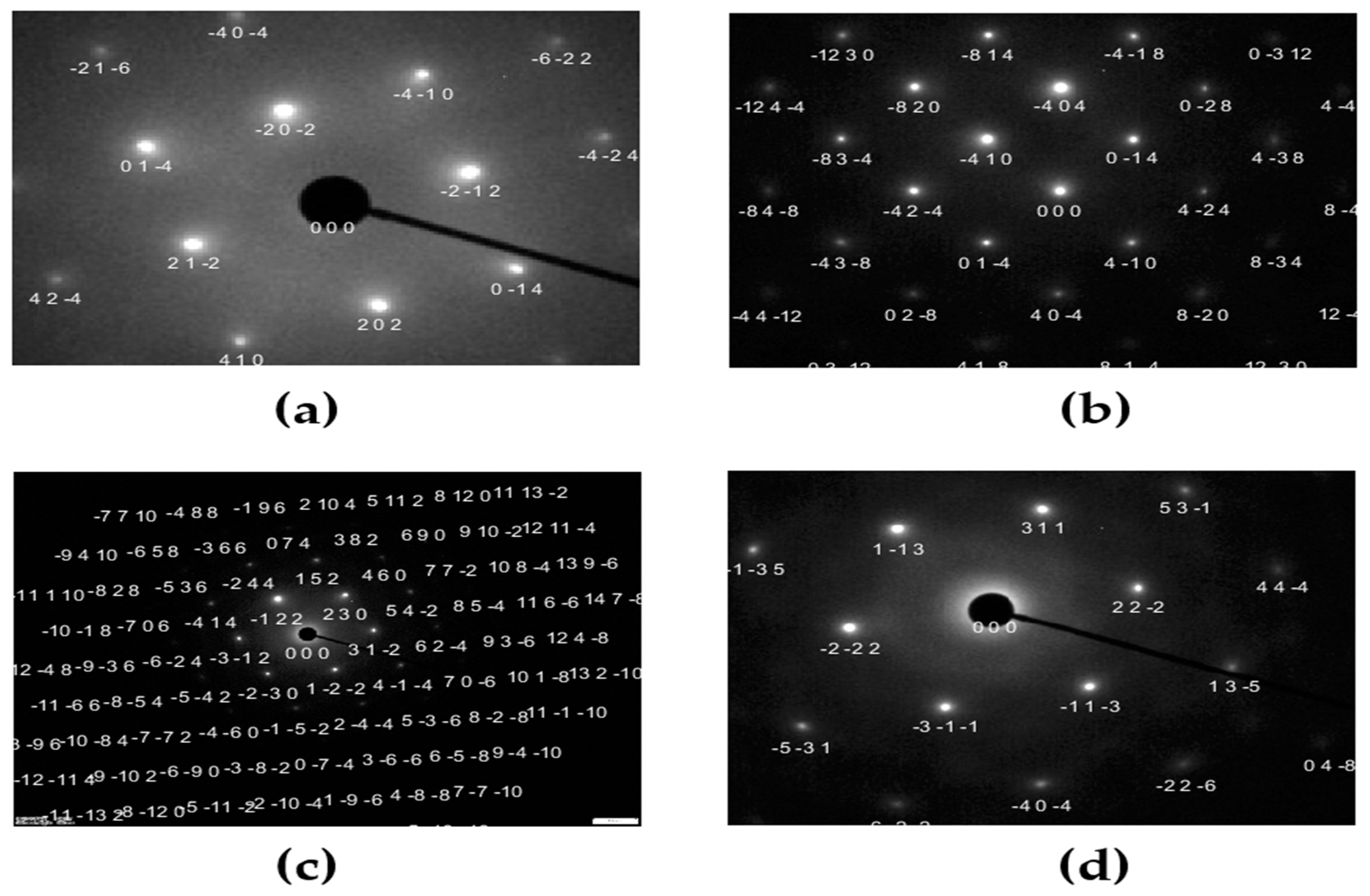
3.4. Microstructural Analysis via TEM and SAED Patterns
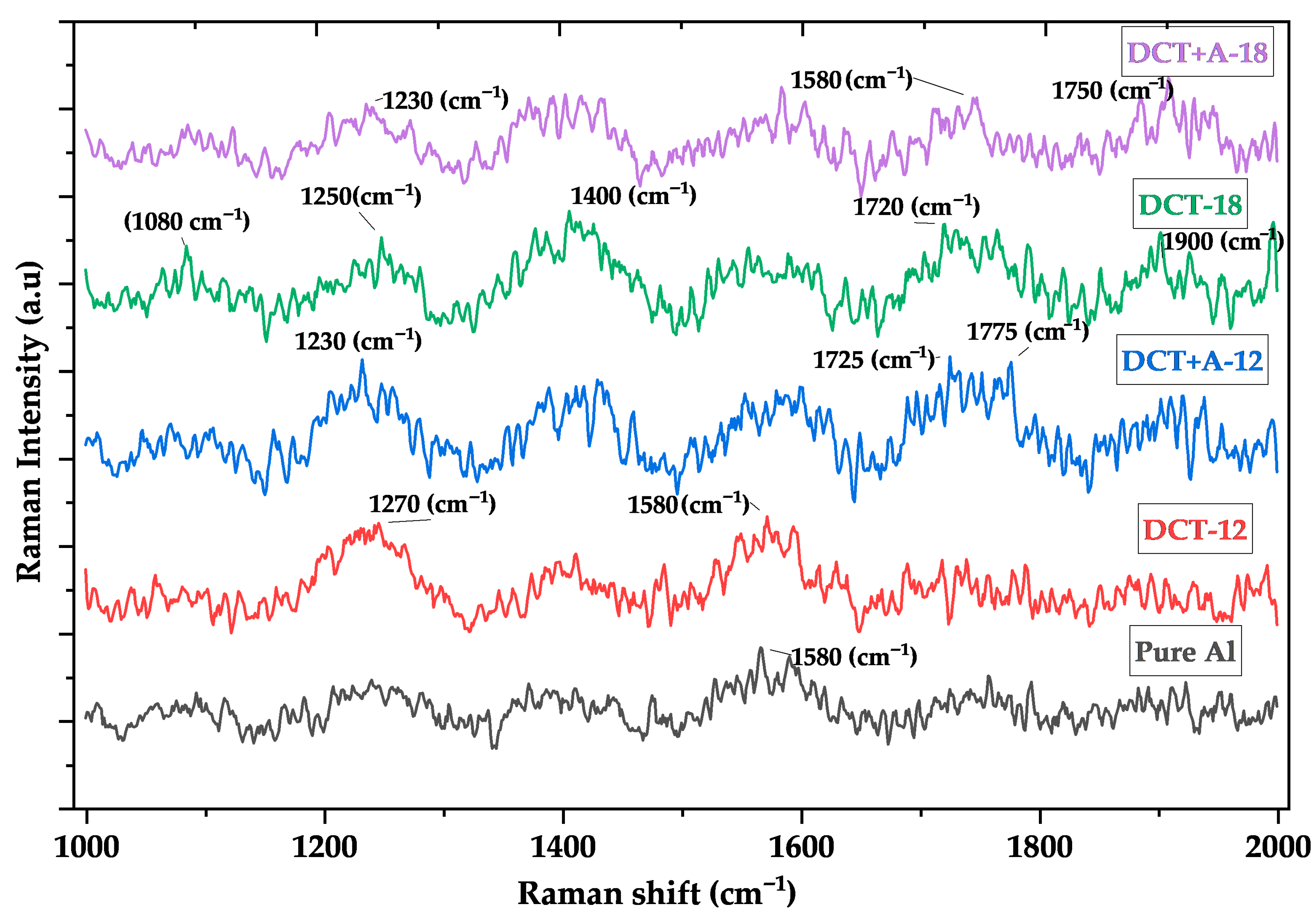
3.5. Raman Spectroscopy
3.6. Electrical Conductivity Analysis Using Four-Point Probe Technique
4. Discussion
4.1. Influence of Soaking Hours on Microstructural Evolution
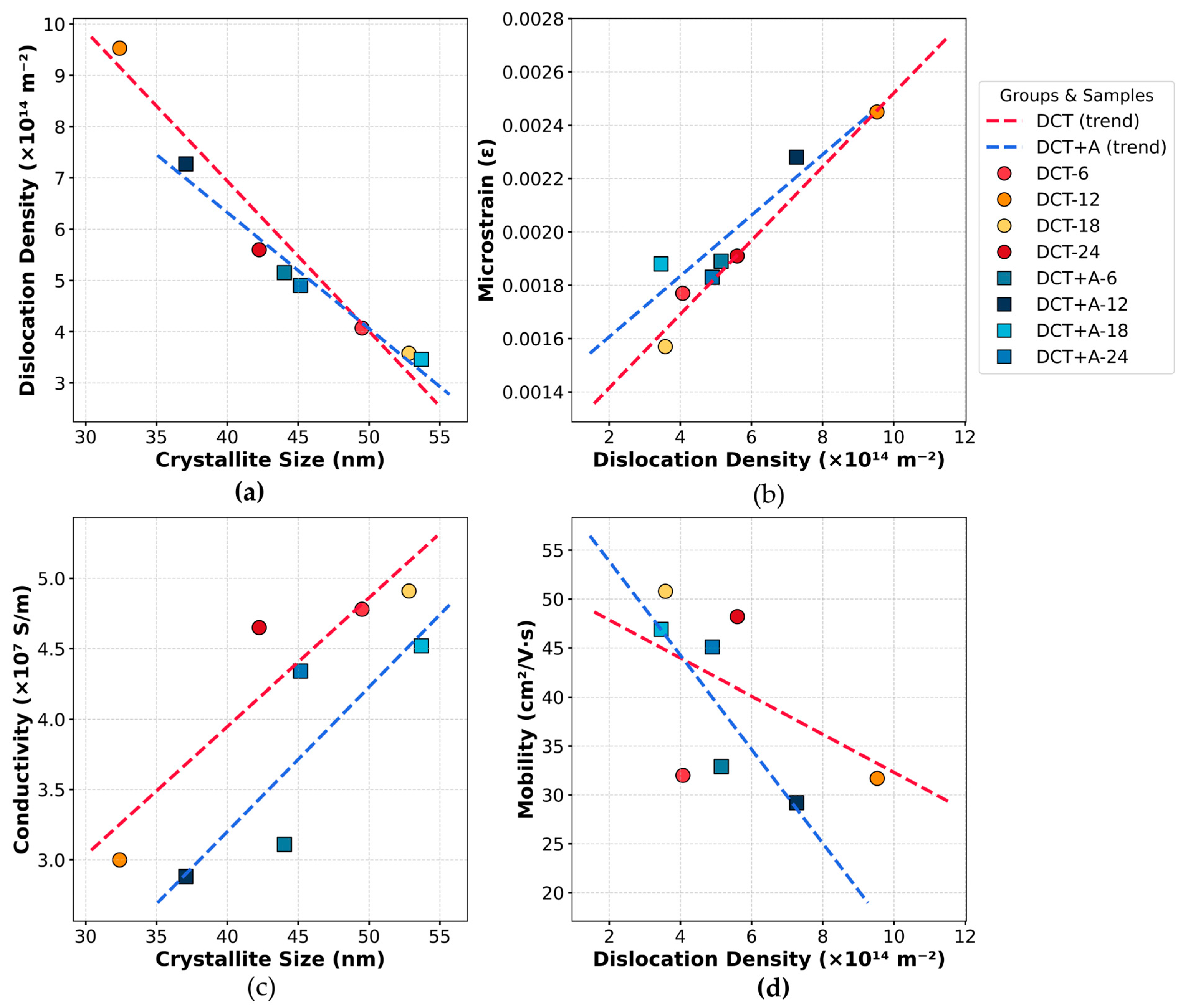
4.2. Effect of Cryogenic Treatment on Crystallite Size, Dislocation Density, and Microstrain
4.3. Effect of Cryogenic Treatment on Grain Boundary Features and Texture
4.4. TEM Microstructural Analysis and SAED Pattern Evaluation
4.5. Vibrational Analysis by Raman Spectroscopy
4.6. Influence of Cryogenic Treatment on Conductivity and Mobility
5. Conclusions
- •
- DCT-12, undergoes intense lattice deformation and refinement. XRD analysis confirmed a significant reduction in crystallite size to (32.39 nm), accompanied by the highest recorded dislocation density of (9.53 × 1014 m−2), indicating a defect-rich microstructure. In contrast, DCT-18 and DCT+A-18 facilitated notable recovery. DCT+A-18 exhibited the largest crystallite size of 53.69 nm and the lowest dislocation density 3.46 × 1014 m−2.
- •
- SEM-EBSD results revealed a shift from HAGB in untreated aluminium (74.3%) to a substantial increase in LAGB, reaching 40.1% in DCT-12, which is evidence of subgrain formation and dislocation accumulation. DCT-18 and DCT+A-18 showed a near-balanced grain boundary distribution (51.7% HAGB, 48.3% LAGB) and strong (100) texture (10.8 mrd) is achieved in both treatments, which is favourable for electron mobility in FCC metals.
- •
- TEM micrographs and SAED patterns of DCT-12 displayed distorted lattice fringes, irregular d-spacing, and angular deviations, consistent with high internal strain. The DCT+A-12 sample showed partial lattice recovery. While DCT-18 and DCT+A-18 revealed well-aligned lattice fringes and symmetric diffraction spots, indicative of minimal local strain.
- •
- Raman spectroscopy correlated strongly with microstructural integrity. DCT-12 and DCT+A-12, with high defect densities, exhibited sharp and intense Raman peaks (1110 a.u in DCT+A-12), signifying elevated phonon activity and lattice distortion. In contrast, DCT-18 and DCT+A-18 showed broader peaks, reflecting reduced microstrain and defect density.
- •
- DCT-12 showed a reduced conductivity of 3.0 × 107 S/m due to enhanced electron scattering from defects, and DCT-18 achieved a peak conductivity of 4.91 × 107 S/m, attributed to a crystallite size of 52.82 nm. DCT+A-18 followed with a conductivity of 4.52 × 107 S/m, benefiting from the largest crystallite size, lowest dislocation density, and optimal lattice orientation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WEDM | Wire Electrical Discharge Machining |
| Al | Aluminium |
| SPG | Severe Plastic Deformation |
| UFG | Ultrafine Grained |
| MGS | Multiple Gradient Structures |
| ECAP | Equal Channel Angular Pressing |
| CDDT | Cryogenic Dual-Direction Torsion |
| DCT | Deep Cryogenic Treatment |
| DCT+A | Deep Cryogenic Treatment followed by Heat Treatment |
| FIB-SEM-EBSD | Focused Ion Beam–Scanning Electron Microscopy-Electron Backscatter Diffraction |
| HAGB | High-Angle Grain Boundary |
| LAGB | Low-Angle Grain Boundary |
| (mrd) | Multiple of Random Distribution |
| PF | Pole Figure |
| TEM | Transmission Electron Microscopy |
| SAED | Selected Area Electron Diffraction |
| FCC | Face-Centered Cubic |
| ND | Normal Direction |
| RD | Rolling Direction |
| TD | Transverse Direction |
References
- Li, S.; Yue, X.; Li, Q.; Peng, H.; Dong, B.; Liu, T.; Yang, H.; Fan, J.; Shu, S.; Qiu, F.; et al. Development and Applications of Aluminum Alloys for Aerospace Industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Premkumar, R.; Vignesh, R.V.; Padmanaban, R.; Govindaraju, M.; Santhi, R. Investigation on the Microstructure, Microhardness, and Tribological Behavior of AA1100-hBN Surface Composite. Koroze Ochrana Materiálu 2021, 65, 1–11. [Google Scholar] [CrossRef]
- Huang, F.; Lu, K. Effects of Impurity on Microstructure and Hardness in Pure Al Subjected to Dynamic Plastic Deformation at Cryogenic Temperature. J. Mater. Sci. Technol. 2011, 27, 628–632. [Google Scholar] [CrossRef]
- Lipińska, M.; Bazarnik, P.; Lewandowska, M. The Influence of Severe Plastic Deformation Processes on Electrical Conductivity of Commercially Pure Aluminium and 5483 Aluminium Alloy. Arch. Civ. Mech. Eng. 2016, 16, 717–723. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Niu, G.; Mao, J. Conductive Al Alloys: The Contradiction between Strength and Electrical Conductivity. Adv. Eng. Mater. 2021, 23, 2001249. [Google Scholar] [CrossRef]
- Czerwinski, F. Aluminum Alloys for Electrical Engineering: A Review. J. Mater. Sci. 2024, 59, 14847–14892. [Google Scholar] [CrossRef]
- Fu, H.; Guo, K.; Wu, Z.; Mai, R.; Chin, C.-L.; Ma, C.-K. Experimental Investigation of a Novel CFRP-Steel Composite Tube-Confined Seawater-Sea Sand Concrete Intermediate Long Column. Int. J. Integr. Eng. 2024, 16, 466–475. [Google Scholar] [CrossRef]
- Aravind, T.V.; Arul, S. Effect of Deep Cryogenic Treatment on C93700 Bearing Bush Material Used in Submersible Pumps. Mater. Today Proc. 2021, 46, 4685–4690. [Google Scholar] [CrossRef]
- Praveen Kumar, R.; Arul, S. Improving the Wear and Corrosion Resistance of Nickel Aluminium Bronze by Deep Cryogenic Treatment. Mater. Today Proc. 2021, 46, 4915–4918. [Google Scholar] [CrossRef]
- Yao, E.; Zhang, H.; Ma, K.; Ai, C.; Gao, Q.; Lin, X. Effect of Deep Cryogenic Treatment on Microstructures and Performances of Aluminum Alloys: A Review. J. Mater. Res. Technol. 2023, 26, 3661–3675. [Google Scholar] [CrossRef]
- Woodcraft, A.L. Recommended Values for the Thermal Conductivity of Aluminium of Different Purities in the Cryogenic to Room Temperature Range, and a Comparison with Copper. Cryogenics 2005, 45, 626–636. [Google Scholar] [CrossRef]
- Su, L.; Deng, G.; Luzin, V.; Wang, H.; Wang, Z.; Yu, H.; Li, H.; Tieu, A.K. Effect of Cryogenic Temperature Equal Channel Angular Pressing on Microstructure, Bulk Texture and Tensile Properties of AA1050. Mater. Sci. Eng. A 2020, 780, 139190. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, J.; Xu, C.; Liu, W.; Li, D.; Wang, G.; Tang, G.; Geng, L. Deformation Behavior of Pure Aluminum at Room and Cryogenic Temperatures. J. Mater. Res. Technol. 2024, 31, 2355–2366. [Google Scholar] [CrossRef]
- Cai, S.L.; Li, D.Q.; Liu, S.C.; Si, J.J.; Gu, J.; Zhou, L.X.; Cheng, Y.F.; Koch, C.C. Cryogenic Reciprocating Torsion Induced Nanoscale Precipitation in Aluminum Wire with Exceptional Strength and Electrical Conductivity. Mater. Sci. Eng. A 2022, 860, 144276. [Google Scholar] [CrossRef]
- Cai, S.L.; Li, D.Q.; Liu, S.C.; Cheng, Y.F.; Li, J.H.; Koch, C.C. Multiple Gradient Structures Driving Higher Tensile Strength and Good Capacity to Absorbed Energy in Aluminum Wire Processed by Cryogenic Pre-Torsion. Scr. Mater. 2022, 210, 114436. [Google Scholar] [CrossRef]
- Cruz Feliciano, A.; Ghosh, A.; Kacher, J.; Graber, L.; Jin, Z. Lightweight Clad Bi-Metal Conductors for Cryogenic Applications. IOP Conf. Ser. Mater. Sci. Eng. 2024, 1302, 012036. [Google Scholar] [CrossRef]
- Kalsi, S.S.; Storey, J.G.; Lumsden, G.; Badcock, R.A. Airplane Motors Employing Superconducting DC Field Windings and Conventional Conductor AC Windings. IOP Conf. Ser. Mater. Sci. Eng. 2024, 1302, 012017. [Google Scholar] [CrossRef]
- Kumar, U.; Swaminathan, P.; Vaghela, H. Investigation of the Emittance Properties of Multilayer Insulation Used in Cryogenic Applications. IOP Conf. Ser. Mater. Sci. Eng. 2025, 1327, 012225. [Google Scholar] [CrossRef]
- Shinozaki, K.; Mizutani, T.; Fujii, T.; Onaka, T.; Nakagawa, T.; Sugita, H. Thermal Property Measurements of Al-Alloy for Space Cryogenic Missions. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1241, 012013. [Google Scholar] [CrossRef]
- Alyani, A.; Kazeminezhad, M. Annealing Behavior of Aluminum after Low-Temperature Severe Plastic Deformation. Mater. Sci. Eng. A 2021, 824, 141810. [Google Scholar] [CrossRef]
- Achuthankutty, A.; Saravanan, R.; Nagarajan, H.; Pasunuri, V.; Gopal, N.H.; Ramesh, A.; Arangot, S.; Thekkuden, D.T. Cryo-Rolled AA5052 Alloy: Insights into Mechanical Properties, Formability, and Microstructure. J. Manuf. Mater. Process. 2024, 8, 284. [Google Scholar] [CrossRef]
- Mavlyutov, A.M.; Bondarenko, A.S.; Murashkin, M.Y.; Boltynjuk, E.V.; Valiev, R.Z.; Orlova, T.S. Effect of Annealing on Microhardness and Electrical Resistivity of Nanostructured SPD Aluminium. J. Alloys Compd. 2017, 698, 539–546. [Google Scholar] [CrossRef]
- Zeybek, Y.; Kayış, C.; Diler, E.A. Improving Electrical Conductivity of Commercially Pure Aluminium: The Synergistic Effect of AlB8 Master Alloy and Heat Treatment. Materials 2025, 18, 364. [Google Scholar] [CrossRef]
- ASTM E3; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM E1558; Guide for Electrolytic Polishing of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2021.
- Loganathan, C.; Narayanasamy, R.; Sathiyanarayanan, S. Effect of Annealing on the Wrinkling Behaviour of the Commercial Pure Aluminium Grades When Drawn through a Conical Die. Mater. Des. 2006, 27, 1163–1168. [Google Scholar] [CrossRef]
- Sosa, J.M.; Huber, D.E.; Welk, B.; Fraser, H.L. Development and Application of MIPARTM: A Novel Software Package for Two- and Three-Dimensional Microstructural Characterization. Integr. Mater. Manuf. Innov. 2014, 3, 123–140. [Google Scholar] [CrossRef]
- ASTM E1382-9; Standard Test Methods for Determining Average Grain Size Using Semiautomatic and Automatic Image Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 2nd ed.; Addison-Wesley Series in Metallurgy and Materials; Addison-Wesley: Glenview, IL, USA, 1978; ISBN 978-0-201-01174-6. [Google Scholar]
- Williamson, G.K.; Smallman, R.E. III. Dislocation Densities in Some Annealed and Cold-Worked Metals from Measurements on the X-Ray Debye-Scherrer Spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Parveen, B.; Mahmood-ul-Hassan; Khalid, Z.; Riaz, S.; Naseem, S. Room-Temperature Ferromagnetism in Ni-Doped TiO 2 Diluted Magnetic Semiconductor Thin Films. J. Appl. Res. Technol. 2017, 15, 132–139. [Google Scholar] [CrossRef]
- Beausir, B.; Fundenberger, J.-J. Fundenberger Analysis Tools for Electron and X-Ray Diffraction, ATEX-Software; Université de Lorraine: Metz, France, 2017; Available online: http://www.atex-software.eu/ (accessed on 6 October 2025).
- Klinger, M. More Features, More Tools, More CrysTBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- ASTM B 193-02; Standard Test Method for Resistivity of Electrical Conductor Materials. ASTM International: West Conshohocken, PA, USA, 2009.
- Kittel, C. Introduction to Solid State Physics, 8th ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 978-0-471-41526-8. [Google Scholar]
- Wu, X.; He, J.; Tian, J.; Zheng, Y.; Fu, H.; Yuan, J.; Lu, S.; Huang, W. Degradation Behaviors and Damage Model of the Interface between ECC and Concrete under Sulfate Wet-Dry Cycling. Constr. Build. Mater. 2025, 476, 141283. [Google Scholar] [CrossRef]
- Alvarado, E.E.; Figueroa, I.A.; Gonzalez, G. Microstructural and Mechanical Analysis of an 1100 Aluminum Alloy Processed by Repetitive Corrugation and Straightening. Phys. Metals Metallogr. 2020, 121, 1319–1325. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Anasyida, A.S.; Zuhailawati, H.; Dhindaw, B.K.; Jabit, N.A.; Ismail, A. Characterization of Mechanical and Corrosion Properties of Cryorolled Al 1100 Alloy: Effect of Annealing and Solution Treatment. Trans. Nonferrous Met. Soc. China 2021, 31, 2949–2961. [Google Scholar] [CrossRef]
- Yu, C.; Chen, L.; Jiang, H.; Zhou, Q.; Yang, C. Effect of Deep Cryogenic-Aging Treatment on Microstructure and Mechanical Properties of 7075 Al-alloy. Chin. J. Mater. Res. 2023, 37, 120–128. [Google Scholar] [CrossRef]
- Chandra Sekhar, K.; Narayanasamy, R. Mechanical Properties and Formability of Cryorolled Commercial Pure Aluminium at Various Reductions. Mater. Today Proc. 2018, 5, 6888–6896. [Google Scholar] [CrossRef]
- Zahid, G.H.; Huang, Y.; Prangnell, P.B. Microstructure and Texture Evolution during Annealing a Cryogenic-SPD Processed Al-Alloy with a Nanoscale Lamellar HAGB Grain Structure. Acta Materialia 2009, 57, 3509–3521. [Google Scholar] [CrossRef]
- Aminah, Z.S.; Noraini, S.A.S.M.; Zuhailawati, H.; Anasyida, A.S. Effect of Solution Treatment on Al 1100 Cryorolled Sheet Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 114, 012127. [Google Scholar] [CrossRef]
- Cao, Y.; He, L.; Zhou, Y.; Wang, P.; Cui, J. Contributions to Yield Strength in an Ultrafine Grained 1050 Aluminum Alloy after DC Current Annealing. Mater. Sci. Eng. A 2016, 674, 193–202. [Google Scholar] [CrossRef]
- Izumi, M.T.; Quintero, J.J.H.; Crivoi, M.R.; Maeda, M.Y.; Namur, R.S.; De Aguiar, D.J.M.; Cintho, O.M. In Situ X-Ray Diffraction Analysis of Face-Centered Cubic Metals Deformed at Room and Cryogenic Temperatures. J. Mater. Eng. Perform. 2019, 28, 4658–4666. [Google Scholar] [CrossRef]
- Rangaraju, N.; Raghuram, T.; Krishna, B.V.; Rao, K.P.; Venugopal, P. Effect of Cryo-Rolling and Annealing on Microstructure and Properties of Commercially Pure Aluminium. Mater. Sci. Eng. A 2005, 398, 246–251. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Yao, M. Cooperative Effect of Grain Size and Cryogenic Temperature on Deformation Behavior and Mechanism of Commercial Pure Al Sheets. Mater. Sci. Eng. A 2024, 899, 146411. [Google Scholar] [CrossRef]
- George, S.; Mias, C. Effect of Rolling Temperature on Annealing of Aa1050 Aluminium Alloy. MSF 2015, 828, 200–205. [Google Scholar] [CrossRef]
- Tang, P.; Wang, H.; Cheng, X.; Zang, J.; Zhao, Y. Effects of Cryogenic Thermal Cycling Treatment on Microstructure, Mechanical Properties and Residual Stress of Selective Laser Melted Alsi10mg Alloy. Mater. Sci. Eng. A 2025, 944, 148963. [Google Scholar] [CrossRef]
- Mani, S.; Subramanian, R.K. Experimental Investigation on the Low Cycle Fatigue Performance and Fractographic Analysis of Deep Cryogenic Treated 6063 Aluminium Alloy. Structures 2023, 58, 105588. [Google Scholar] [CrossRef]
- Xu, B.; Lu, Q.; Liu, C.; Peng, Y.; Miao, K.; Wu, H.; Li, R.; Li, X.; Fan, G. Anisotropic Behavior and Temperature-Dependent Mechanical Properties of AA1060 Aluminum Alloy: A Comprehensive Microstructural Study. Mater. Sci. Eng. A 2024, 901, 146550. [Google Scholar] [CrossRef]
- Liu, W.; Wang, R.; Zhou, H.; Yao, M.; Sun, W.; Zhu, Y.; Li, Y. Modeling of Cryo-Deformation Based on Grain Size-Dependent Dislocation Evolution. Int. J. Mech. Sci. 2025, 285, 109813. [Google Scholar] [CrossRef]
- Zhou, F.; Witkin, D.; Nutt, S.R.; Lavernia, E.J. Formation of Nanostructure in Al Produced by a Low-Energy Ball Milling at Cryogenic Temperature. Mater. Sci. Eng. A 2004, 375, 917–921. [Google Scholar] [CrossRef]
- Karar, N. Usefulness of X-Ray Diffraction and Raman Spectra for Understanding Metal Alloy Microstructure Repeatability. IJEMS 2024, 31, 327–334. [Google Scholar] [CrossRef]
- Wu, W.; Gui, J.; Zhu, T.; Xie, Z. The Effect of Residual Stress on Whisker Reinforcements in SiCw-Al2O3 Composites during Cooling. J. Alloy. Compd. 2017, 725, 639–643. [Google Scholar] [CrossRef]
- Solonenko, D.; Žukauskaitė, A.; Pilz, J.; Moridi, M.; Risquez, S. Raman Spectroscopy and Spectral Signatures of AlScN/Al2O3. Micromachines 2022, 13, 1961. [Google Scholar] [CrossRef]
- Woo, D.J.; Hooper, J.P.; Osswald, S.; Bottolfson, B.A.; Brewer, L.N. Low Temperature Synthesis of Carbon Nanotube-Reinforced Aluminum Metal Composite Powders Using Cryogenic Milling. J. Mater. Res. 2014, 29, 2644–2656. [Google Scholar] [CrossRef]
- Revo, S.; Hamamda, S.; Ivanenko, K.; Boshko, O.; Djarri, A.; Boubertakh, A. Thermal Analysis of Al + 0.1% CNT Ribbon. Nanoscale Res. Lett. 2015, 10, 170. [Google Scholar] [CrossRef]
- González-Hernández, J.E.; Cubero-Sesin, J.M. Electrical Conductivity of Ultrafine-Grained Cu and Al Alloys: Attaining the Best Compromise with Mechanical Properties. Mater. Trans. 2023, 64, 1754–1768. [Google Scholar] [CrossRef]
- Wang, M.; Knezevic, M.; Chen, M.; Li, J.; Liu, T.; Wang, G.; Zhao, Y.; Wang, M.; Liu, Q.; Huang, Z.; et al. Microstructure Design to Achieve Optimal Strength, Thermal Stability, and Electrical Conductivity of Al-7.5wt.%Y Alloy. Mater. Sci. Eng. A 2022, 852, 143700. [Google Scholar] [CrossRef]
- Orlova, T.S.; Mavlyutov, A.M.; Bondarenko, A.S.; Kasatkin, I.A.; Murashkin, M.Y.; Valiev, R.Z. Influence of Grain Boundary State on Electrical Resistivity of Ultrafine Grained Aluminium. Philos. Mag. 2016, 96, 2429–2444. [Google Scholar] [CrossRef]
- Atanacio-Sánchez, X.; Garay-Reyes, C.G.; Martínez-García, A.; Estrada-Guel, I.; Mendoza-Duarte, J.M.; Guerrero-Seañez, P.; González-Sánchez, S.; Rocha-Rangel, E.; De Jesús Cruz-Rivera, J.; Gutiérrez-Castañeda, E.J.; et al. Enhancement of the Electrical Conductivity and Mechanical Properties of Al-Mg-Si and Al-Mg-Zn Ternary Systems After a T8 Heat Treatment. Metals 2024, 14, 1286. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, J.; Lai, Y.X.; Zhu, D.H.; Gu, Y.; Chen, J.H. Enhancing Electrical Conductivity and Strength in Al Alloys by Modification of Conventional Thermo-Mechanical Process. Mater. Des. 2015, 87, 1–5. [Google Scholar] [CrossRef]
- Du, Z.; Ye, J.; Yang, L.; Zhang, J. Enhancing Strength and Electrical Conductivity of Pure Aluminum by Microalloying with Telluride. Mater. Res. Express 2021, 8, 086511. [Google Scholar] [CrossRef]

| Specimen Classification | Treatment Condition | Soaking Hours |
|---|---|---|
| Pure Al | Untreated | - |
| DCT-n | Deep cryogenic treatment | n = 6, 12, 18, 24 |
| DCT+A-n | Deep cryogenic treatment + Annealing | n = 6, 12, 18, 24 |
| Samples | Pole Figure Intensity (mrd) | Grain Boundaries | ||||
|---|---|---|---|---|---|---|
| (111) | (100) | (110) | (112) | HAGB > 15° (%) | LAGB < 15° (%) | |
| Pure Aluminium | 4.44 | 7.29 | 4.02 | 2.17 | 74.3 | 25.7 |
| DCT-12 | 8.21 | 9.5 | 5.83 | 3.13 | 59.9 | 40.1 |
| DCT+A-12 | 5.1 | 7.31 | 6.9 | 2.92 | 54.7 | 45.3 |
| DCT-18 | 9.02 | 9.11 | 6.08 | 3.1 | 51.7 | 48.3 |
| DCT+A-18 | 8.11 | 10.8 | 6.82 | 3.1 | 54.8 | 45.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narashimhan, D.C.; Sanjivi, A. Enhancing Electrical Conductivity of Commercially Pure Aluminium via Deep Cryogenic Treatment and Subsequent Annealing. Eng 2025, 6, 328. https://doi.org/10.3390/eng6110328
Narashimhan DC, Sanjivi A. Enhancing Electrical Conductivity of Commercially Pure Aluminium via Deep Cryogenic Treatment and Subsequent Annealing. Eng. 2025; 6(11):328. https://doi.org/10.3390/eng6110328
Chicago/Turabian StyleNarashimhan, Dhandapani Chirenjeevi, and Arul Sanjivi. 2025. "Enhancing Electrical Conductivity of Commercially Pure Aluminium via Deep Cryogenic Treatment and Subsequent Annealing" Eng 6, no. 11: 328. https://doi.org/10.3390/eng6110328
APA StyleNarashimhan, D. C., & Sanjivi, A. (2025). Enhancing Electrical Conductivity of Commercially Pure Aluminium via Deep Cryogenic Treatment and Subsequent Annealing. Eng, 6(11), 328. https://doi.org/10.3390/eng6110328








