Zirconia Enrichment of Zircon from Arikya, Nasarawa State, Nigeria, by Magnetic and Gravity Separation Processes for Use as Reinforcing Agent in Composite Formulation
Abstract
1. Introduction
2. Materials and Methods
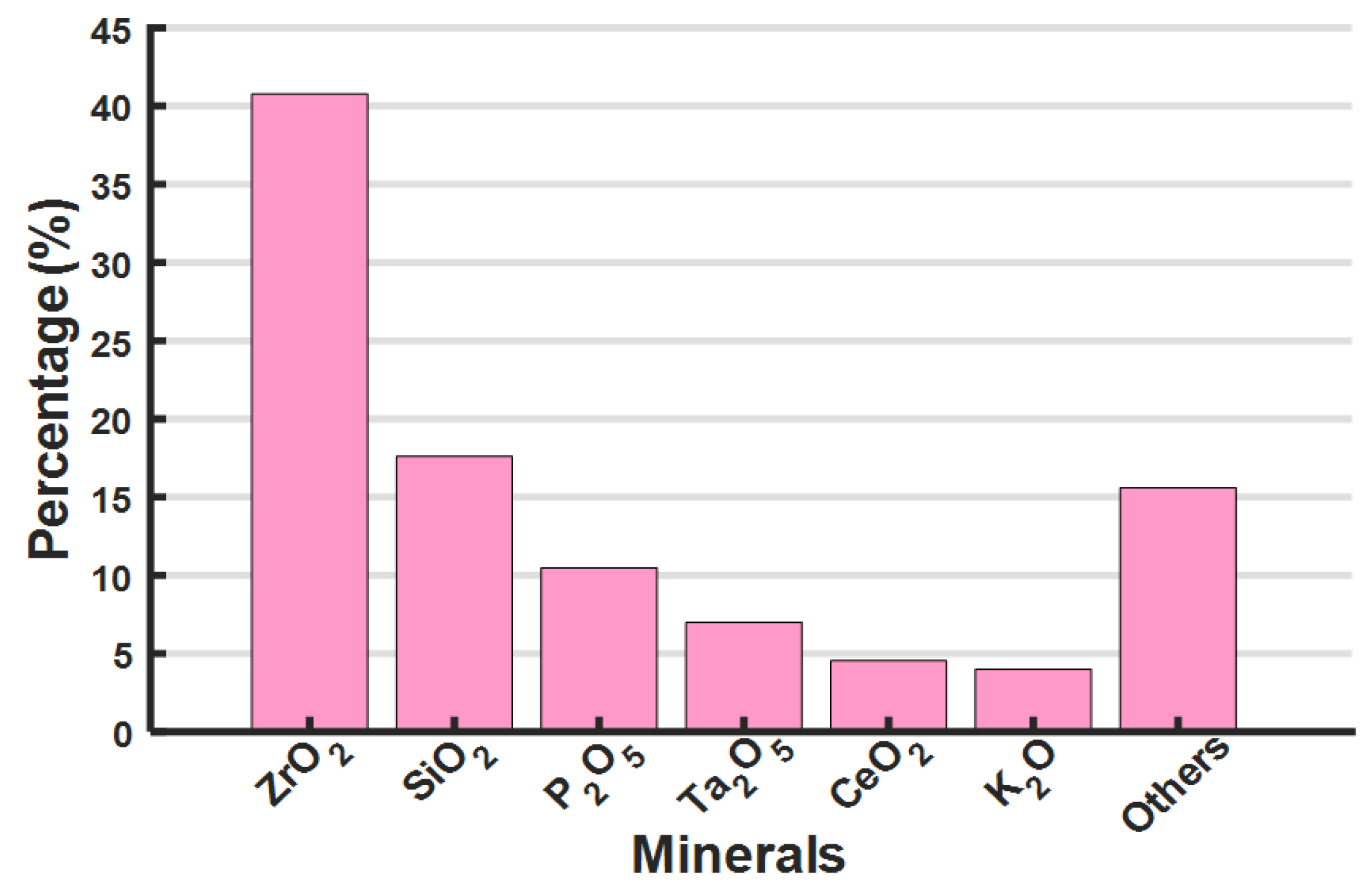
2.1. Feed Material
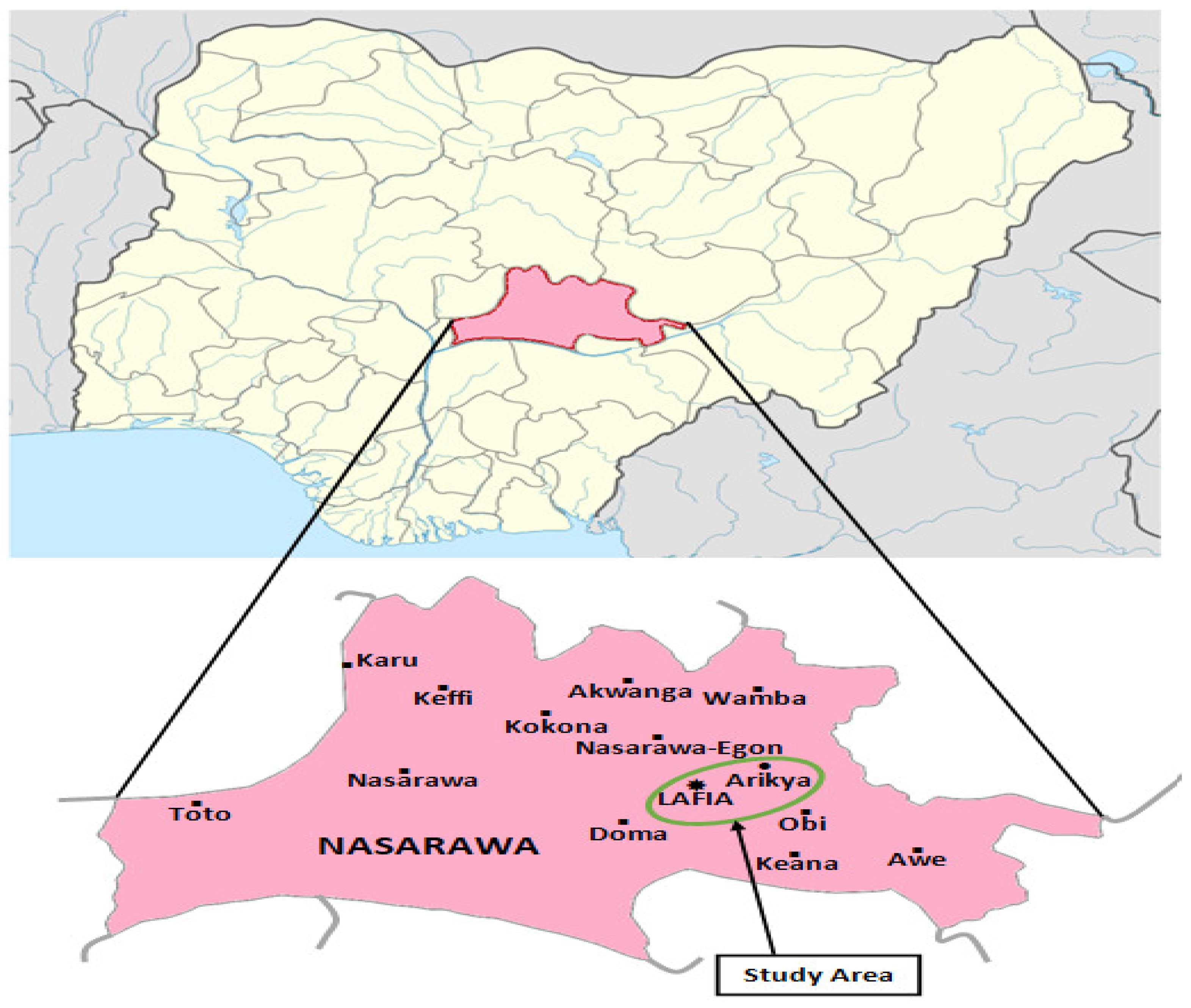
2.2. Study Area
2.3. Experimental Methods
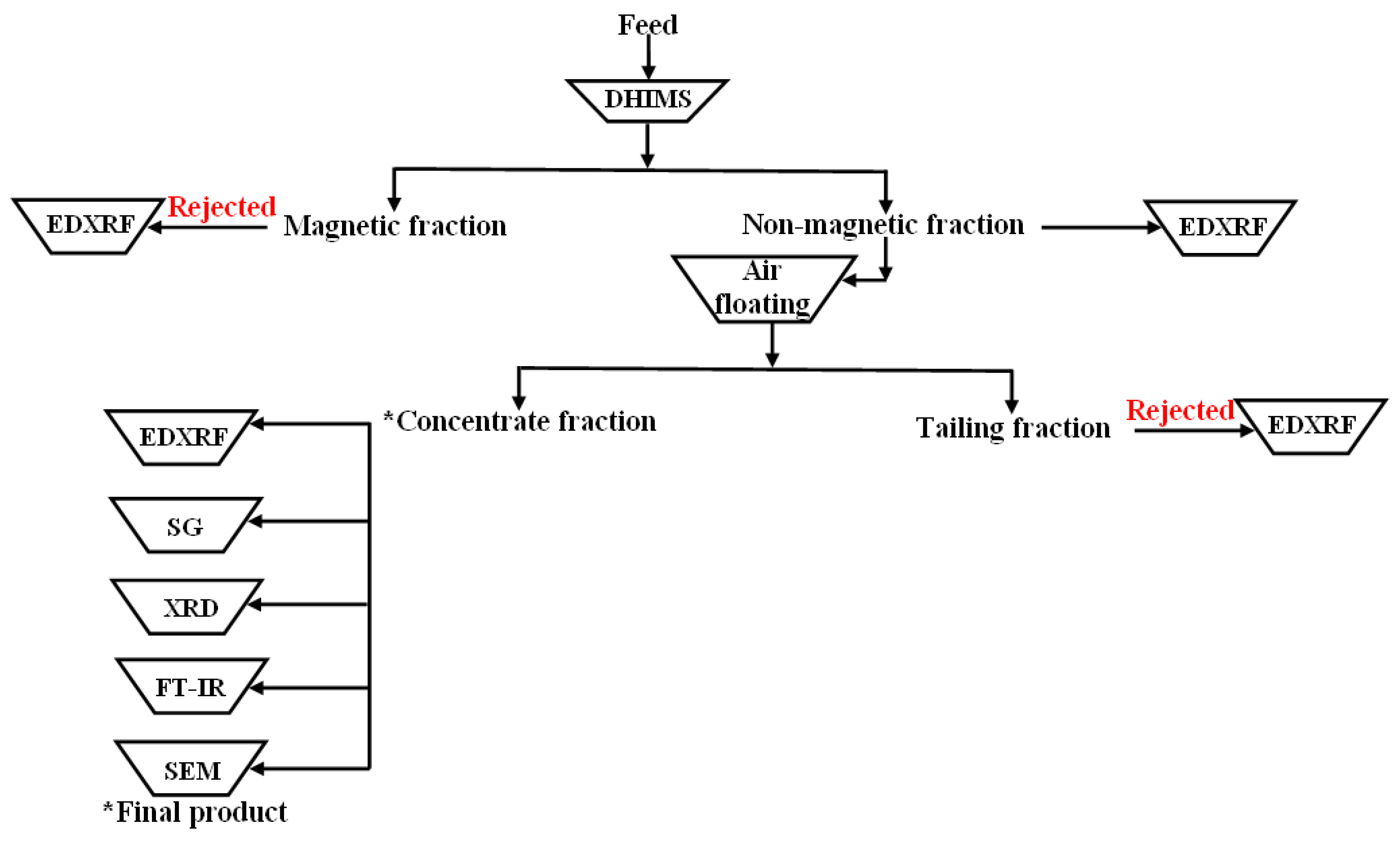
2.3.1. Beneficiation Testwork
Magnetic Separation
Gravity Separation
2.4. Characterization of Arikya Zircon Beneficiated Fractions
2.4.1. Chemical Analysis
2.4.2. Specific Gravity
2.4.3. Mineralogical Evaluation
2.4.4. Functional Groups
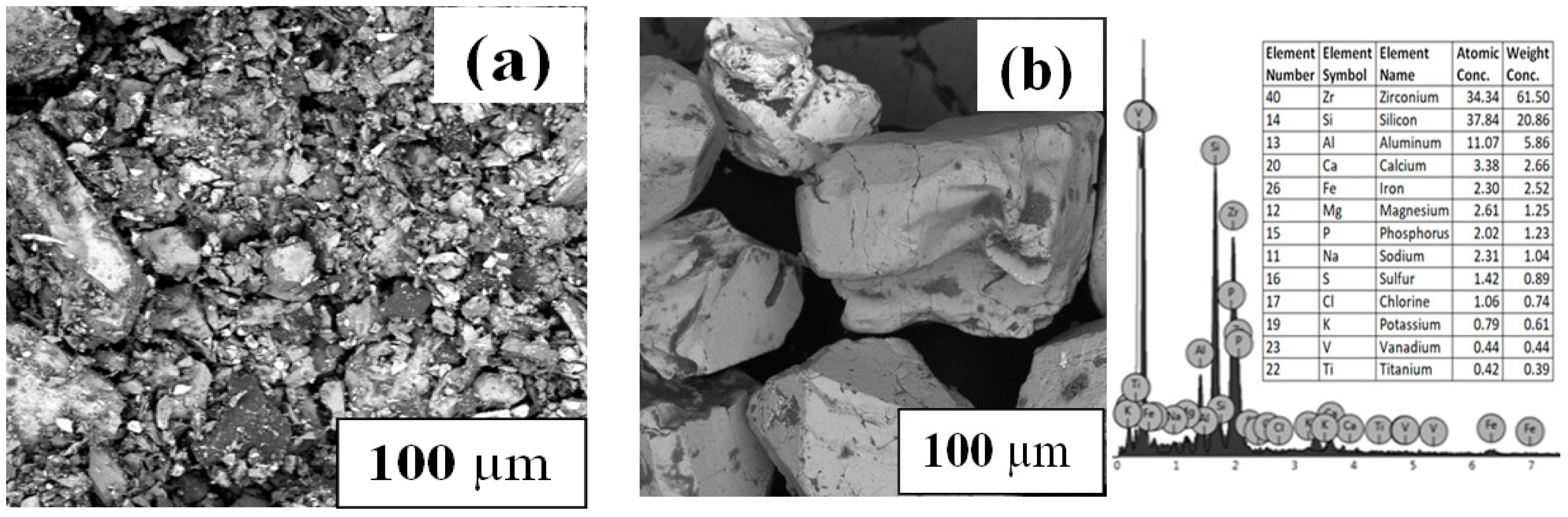
2.4.5. Morphology
3. Results and Discussion
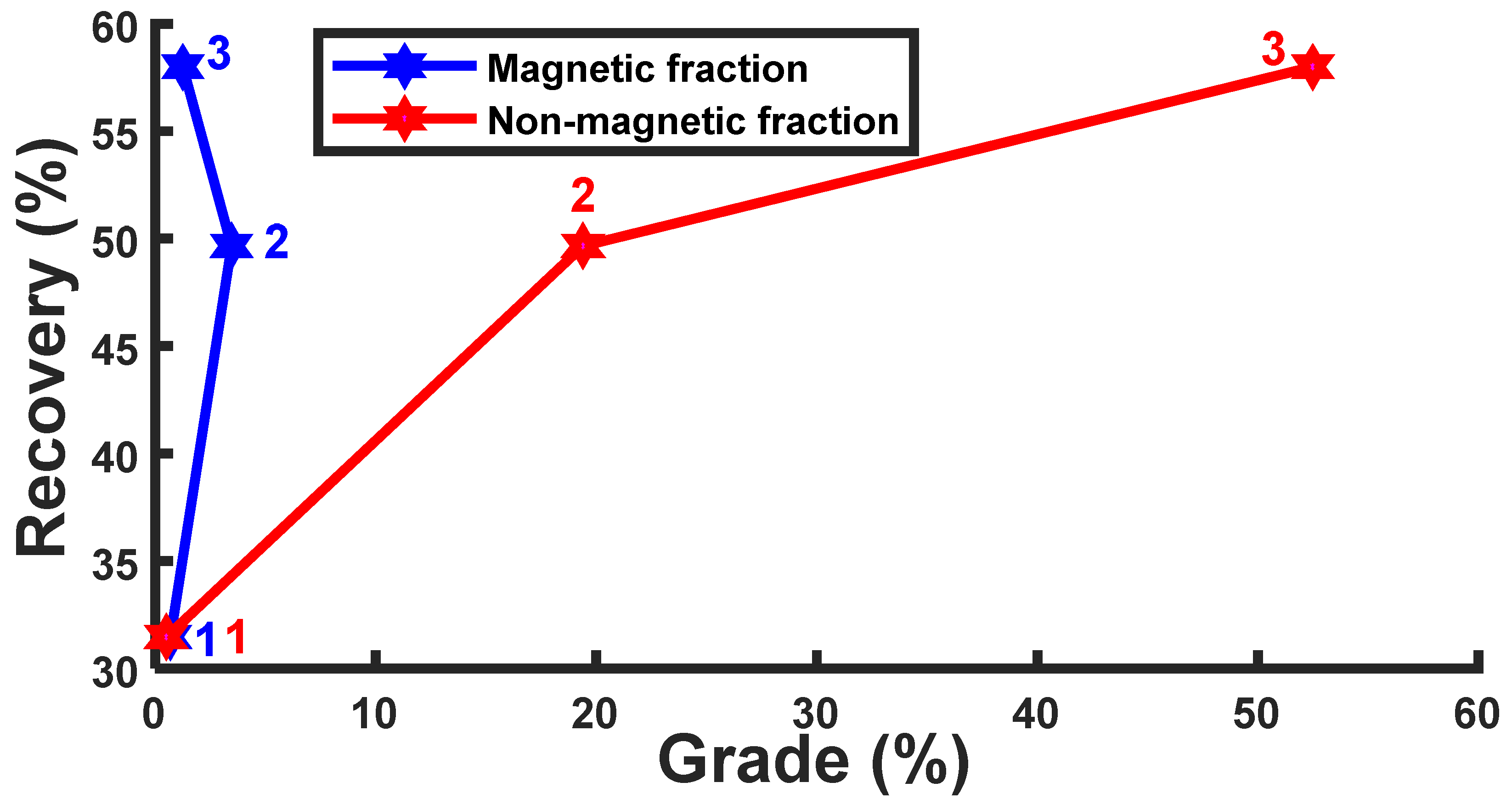
3.1. Beneficiation Studies
3.2. Dry High-Intensity Magnetic Separator
3.3. Air-Floating Separator
3.4. Characterization of Generated Products
3.4.1. Chemical Analysis of Magnetic Separation Products
3.4.2. Chemical Analysis of Gravity Separation Products
3.4.3. Specific Gravity
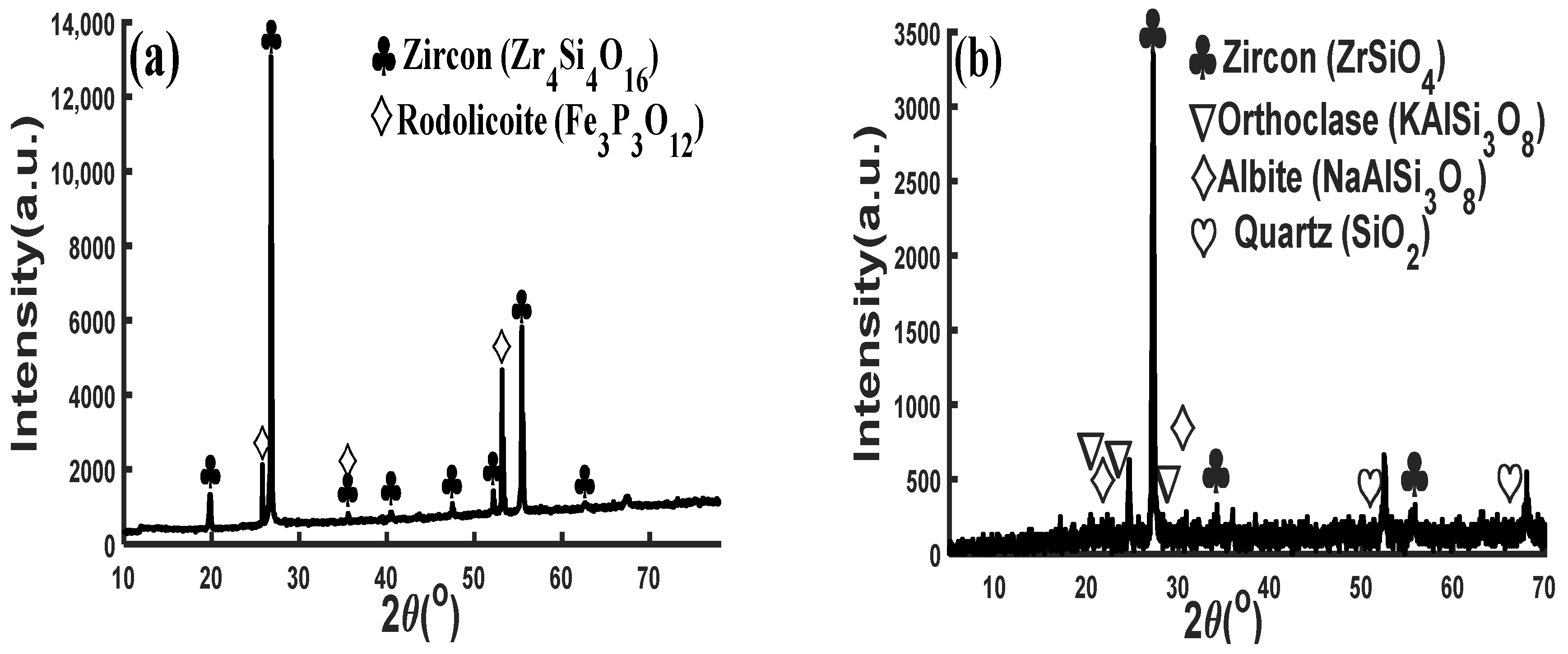
3.4.4. Mineralogical Evaluation
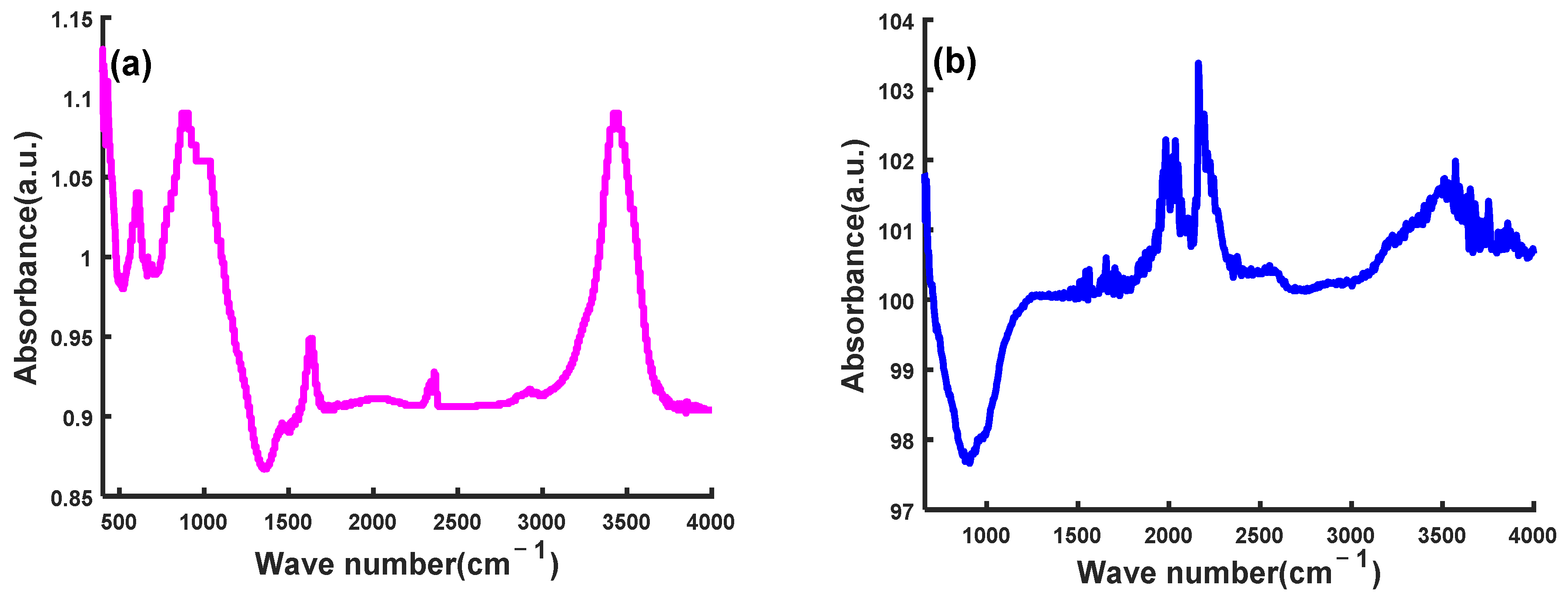
3.4.5. Functional Groups and Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- RMRDC. Non-metallic Mineral Endowments in Nigeria; Raw Materials Research and Development Council: Abuja, Nigeria, 2010. [Google Scholar]
- MMSD. Nigeria’s Mining and Metal Sector, Investment Promotion Brochure; Ministry of Mines and Steel Development: Abuja, Nigeria, 2017.
- FGN. Federal Republic of Nigeria. Nigeria Extractive Industries Transparency Initiative (NEITI) NEITI Secretariat; Federal Government of Nigeria: Abuja, Nigeria, 2011. [Google Scholar]
- MMSD. Roadmap for the Growth and Development of the Nigerian Mining Industry; Ministry of Mines and Steel Development: Abuja, Nigeria, 2016.
- USGS. 2012 Minerals Yearbook: Nigeria [Advance Release]; U.S. Geological Survey: Reston, VA, USA, 2014.
- Okoli, B.I.; Agboola, O.A.; Onwualu, A.P.; Bello, A.; Sholiyi, O.S.; Anye, V.C.; Yusuf, O.T. Characterization of Nigerian Zircon Sand and Its Suitability for Different Industrial Applications. Minerals 2023, 13, 711. [Google Scholar] [CrossRef]
- USGS. International Strategic Minerals Inventory Summary Report-Zirconium; US Geological Survey Circular: Denver, CO, USA, 1992.
- Elsner, H. Assessment Manual: Heavy Minerals of Economic Importance; BGR: Hanover, Germany, 2007. [Google Scholar]
- Volp, K.M.; Russill, J.; Donovan, G.O. Exploration for Offshore Heavy Mineral Sands by Grupo Minero Esmeralda Colombiana, Colombia. In Proceedings of the 7th International Heavy Minerals Conference ‘What Next’, Drakensberg, South Africa, 20–23 September 2009; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2009; pp. 163–176. [Google Scholar]
- USDOI. Minerals Yearbook; United States Department of Interior (USDOI), Bureau of Mines: Washington, DC, USA, 1992.
- Zircon Industry Association (ZIA). Technical Handbook on Zirconium and Zirconium Compounds. 2019. Available online: https://www.zircon-association.org (accessed on 13 April 2021).
- USGS. Zirconium and Hafnium: Critical Mineral Resources of the United States-Economic and Environmental Geology and Prospects for Future Supply; U.S. Geological Survey: Reston, VA, USA, 2017.
- Mihai, L.L.; Parlatescu, I.; Gheorghe, C.; Andeescu, C.; Bechir, A.; Pacurar, M.; Cumpata, C.N. In Vitro Study of the Effectiveness to Fractures of the Aesthetic Fixed Restorations Achieved from Zirconium and Alumina. Rev. Chim. 2014, 65, 725–729. [Google Scholar]
- Jankowiak, A.; Justin, J.F. Ultra High Temperature Ceramics for Aerospace Applications; HAL: Bangalore, India, 2014. [Google Scholar]
- Binner, J.; Porter, M.; Baker, B.; Zou, J.; Venkatachalam, V.; Diaz, V.R.; Angio, A.D.; Ramanujam, P.; Zhang, T.; Murthy, S.R.C. Selection, Processing, Properties and Applications of Ultra-high Temperature Ceramic Matrix Composites, UHTCMCs—A Review. Taylor Fr. Online 2019, 65, 389–444. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Sonber, J.K.; Sairam, K.; Majumdar, S.; Kain, V. Boron-Based Ceramics and Composites for Nuclear and Space Applications: Synthesis and Consolidation in Handbook of Advanced Ceramics and Composites; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Kogel, J.E.; Trivedi, N.C.; Barker, J.M.; Krukowski, S.T. Industrial Minerals & Rocks, 7th ed.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2006. [Google Scholar]
- Pirkle, F.L.; Podmeyer, D.A. Zircon: Origin and Uses. Trans. SME 1992, 292, 1–21. [Google Scholar]
- Samin; Poernomo, H.; Rozana, K.; Suyanti. The Processing and Validation of Zircon Sand Concentrate Process into Zircon Opacifier. AIP Conf. Proc. 2020, 2296, 020073. [Google Scholar]
- Ravishankar, S.A.; Kolla, H. Chemically Enhanced Electrostatic Separation. In Proceedings of the 7th International Heavy Minerals Conference ‘What Next’, Drakensberg, South Africa, 20–23 September 2009; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2009; pp. 203–206. [Google Scholar]
- Bulatovic, S.M. Beneficiation of Zircon Containing Ores in Handbook of Flotation Reagents: Chemistry, Theory and Practice; Elsevier B.V.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Routray, S.; Laxmi, T.; Rao, R.B. Alternate Approaches to Recover Zircon Mineral Sand from Beach Alluvial Placer Deposits and Badlands Topography for Industrial Applications. Int. J. Mater. Mech. Eng. 2013, 2, 80–90. [Google Scholar]
- Lundberg, M. Environmental Analysis of Zirconium Alloy Production. Available online: https://www.diva-portal.org/smash/get/diva2:475527/FULLTEXT01.pdf (accessed on 1 September 2022).
- Gouda, M.K.; Fattah, H.A.; Fathy, W.M.; Salman, S.A. Upgrading the Zirconium Content in the Egyptian Zircon Concentrate Using Alkaline Molten Salt and Leaching Processes. Int. J. Sci. Eng. Res. 2020, 11, 1591–1596. [Google Scholar]
- Tahli, L.; Wahyudi, T. Characteristic Study of Popay Zircon Sand Used for Ceramics, Refractory and Foundry Raw Materials. Indones. Min. J. 2016, 19, 1–17. [Google Scholar] [CrossRef]
- Yuhelda; Amalia, D.; Nugraha, E.P. Processing Zirconia Through Zircon Sand Smelting with NaOH as a Flux. Indones. Min. J. 2017, 19, 39–49. [Google Scholar]
- Routray, S.; Rao, R.B. Beneficiation and Characterization of Detrital Zircons from Beach Sand and Red Sediments in India. J. Miner. Mater. Charact. Eng. 2011, 10, 1409–1428. [Google Scholar] [CrossRef]
- Abdullah, M.; Triwikantoro; Umamah, C.; Andi, H.J. The Effect of pH and Calcination Temperature on the ZrO2 Phase Formation from Natural Zircon Sand of Kereng Pangi. J. Neutrino 2021, 13, 39–48. [Google Scholar]
- NASRDA. Design of Reusable Solid Rocket Motor (SRM); National Space Research and Development Agency: Abuja, Nigeria, 2012. [Google Scholar]
- NBS. Foreign Trade in Goods Statistics (Q4 2019); National Bureau of Statistics: Abuja, Nigeria, 2020.
- NPC. Federal Republic of Nigeria 2006 Population Census, Official Gazette; Nigerian Population Commission: Abuja, Nigeria, 2006.
- Manpower Nigeria. Lafia Local Government Area. Available online: https://www.manpower.com.ng/places/lga/570/lafia (accessed on 21 July 2022).
- Akomolafe, G.F.; Rahmad, Z. Relating the Land-use Changes to the Invasion of Pneumatopteris afra in Nigeria Using Remote Sensing. Pertanika J. Sci. Technol. 2020, 28, 1345–1365. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Introduction and Classification in Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Elsevier: Waltham, MA, USA, 2016. [Google Scholar]
- Wills, B.A.; Napier-Munn, T.J. Introduction in Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2006. [Google Scholar]
- ASTM D854; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. The American Society for Testing and Materials: West Conshohocken, PA, USA, 2000; pp. 1–7.
- Prakash, K. Geotechnical Engineering Laboratory Instruction Manual. Available online: https://sjce.ac.in/wp-content/uploads/2018/01/Geotechnical-Lab-Manual.pdf (accessed on 14 August 2022).
- Aravamudhan, S.; Premkumar, N.; Yerrapragada, S.S.; Mani, B.P.; Viswanathan, K. Separation Based on Shape Path II: Newton’s Separation Efficiency. Powder Technol. 1984, 39, 93–98. [Google Scholar] [CrossRef]
- Hiroaki, M.; Ko, H.; Hideto, Y. Classification in Powder Technology: Handling and Operations, Process Instrumentation, and Working Hazards; CRC Press Taylor and Francis Group: Broken Sound Parkway, NW, USA, 2006. [Google Scholar]
- Sarangua, N.; Watanabe, Y.; Echigo, T.; Hoshino, M. Chemical Characteristics of Zircon from Khaldzan Burgedei Peralkaline Complex, Western Mongolia. Minerals 2019, 9, 10. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Matijevic, E. Preparation and Characterization of Nanosized Zirconium (Hydrous) Oxide Particles. J. Mater. Res. 1997, 12, 3286–3292. [Google Scholar] [CrossRef]
- Guo, G.Y.; Chen, Y.L.; Ying, W.J. Thermal, Spectroscopic and X-ray Diffractional Analyses of Zirconium Hydroxides Precipitated at low pH Values. Mater. Chem. Phys. 2004, 84, 308–314. [Google Scholar] [CrossRef]
- Dwivedi, R.; Maurya, A.; Verma, A.; Prasad, R.; Bartwal, K.S. Microwave Assisted Sol-gel Synthesis of Tetragonal Zirconia Nanoparticles. J. Alloys Compd. 2011, 509, 6848–6851. [Google Scholar] [CrossRef]


| (a) Feed | |||||||
 | |||||||
| Fraction | Weight (g) | Grade (%) | Recovery (%) | ||||
| ZrO2 | SiO2 | Fe2O3 | ZrO2 | SiO2 | Fe2O3 | ||
| Feed | F | f | R | ||||
| 100 | 40.77 | 17.61 | 0.73 | - | |||
| Magnetic fraction | T | t | |||||
| 59.95 | 1.27 | 3.47 | 0.71 | 57.99 | 49.66 | 31.47 | |
| Non-magnetic fraction | C | c | |||||
| 45.05 | 52.48 | 19.41 | 0.51 | ||||
| (b) Non-Magnetic Fraction | |||||||
 | |||||||
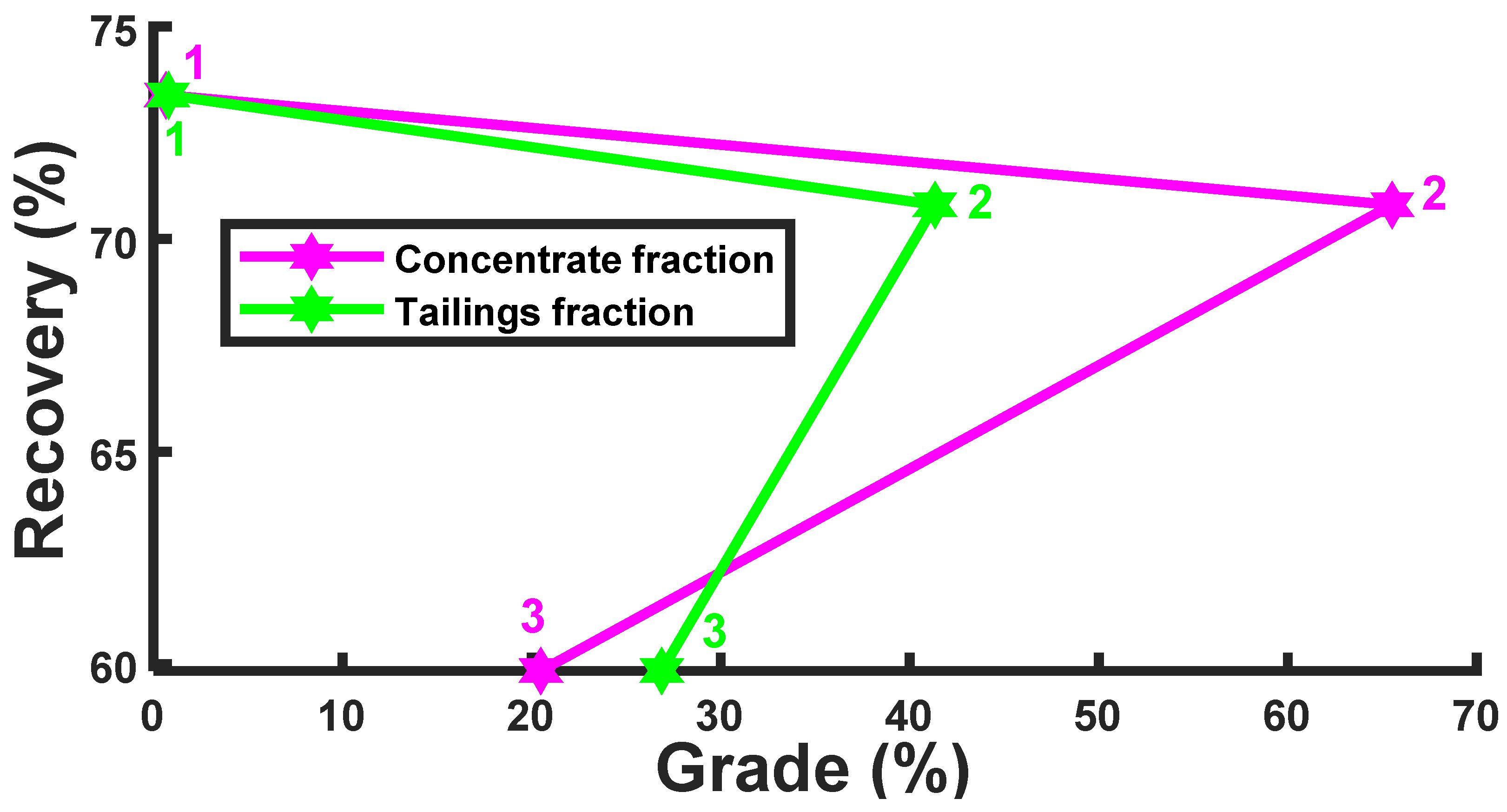
| Feed (non-magnetic fraction) | - | - | |||||
| Concentrate fraction | C 25.55 | 65.52 | c 20.48 | 0.66 | 70.81 | 59.84 | 73.39 |
| Tailings fraction | T 17.00 | 41.34 | t 26.88 | 0.78 | |||
| (a) | ||||||||
| Element/Oxide | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO |
| Content (%) | 0.71 | 3.47 | 1.44 | 0.80 | 10.08 | 0.15 | 0.91 | 0.00 |
| Element/Oxide | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | As2O3 | PbO |
| Content (%) | 0.52 | 0.00 | 0.00 | 0.01 | 0.00 | 0.14 | 0.00 | 0.13 |
| Element/Oxide | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | Br | SrO |
| Content (%) | 0.00 | 0.00 | 0.00 | 0.01 | 1.27 | 0.10 | 0.00 | 0.47 |
| Element/Oxide | Nb2O5 | Bi2O3 | Sb2O3 | Co3O4 | CdO | HfO2 | Ag2O | CeO2 |
| Content (%) | 0.47 | 0.09 | 0.64 | 0.00 | 0.00 | 0.00 | 0.00 | 23.47 |
| Element/Oxide | BaO | Au | WO3 | MoO3 | La2O3 | ThO2 | Sn2O | |
| Content (%) | 1.00 | 0.00 | 0.03 | 0.00 | 13.78 | 1.88 | 0.92 | |
| (b) | ||||||||
| Element/Oxide | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO |
| Content (%) | 0.51 | 19.41 | 2.86 | 0.00 | 3.60 | 0.00 | 0.67 | 0.00 |
| Element/Oxide | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | As2O3 | PbO |
| Content (%) | 0.58 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.32 | 0.09 |
| Element/Oxide | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | Br | SrO |
| Content (%) | 0.00 | 0.00 | 0.00 | 0.00 | 52.48 | 0.09 | 0.00 | 0.74 |
| Element/Oxide | Nb2O5 | Bi2O3 | Sb2O3 | Co3O4 | CdO | HfO2 | Ag2O | CeO2 |
| Content (%) | 0.39 | 0.29 | 0.62 | 0.30 | 0.15 | 0.28 | 0.00 | 1.11 |
| Element/Oxide | BaO | Au | WO3 | MoO3 | La2O3 | ThO2 | Sn2O | |
| Content (%) | 1.00 | 0.00 | 0.00 | 0.33 | 1.59 | 0.42 | 6.44 | |
| (a) | ||||||||
| Element/Oxide | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO |
| Content (%) | 0.66 | 20.48 | 2.66 | 0.40 | 3.45 | 0.00 | 0.54 | 0.00 |
| Element/Oxide | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | As2O3 | PbO |
| Content (%) | 0.47 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.37 | 0.07 |
| Element/Oxide | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | Br | SrO |
| Content (%) | 0.00 | 0.00 | 0.01 | 0.00 | 65.52 | 0.12 | 0.00 | 0.79 |
| Element/Oxide | Nb2O5 | Bi2O3 | Sb2O3 | Co3O4 | CdO | HfO2 | Ag2O | CeO2 |
| Content (%) | 0.55 | 0.27 | 0.95 | 0.30 | 0.15 | 0.29 | 0.00 | 1.36 |
| Element/Oxide | BaO | Au | WO3 | MoO3 | La2O3 | ThO2 | Sn2O | |
| Content (%) | 1.00 | 0.00 | 0.00 | 0.33 | 0.98 | 0.49 | 5.55 | |
| (b) | ||||||||
| Element/Oxide | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO |
| Content (%) | 0.78 | 26.88 | 5.98 | 0.09 | 11.19 | 0.00 | 1.79 | 0.03 |
| Element/Oxide | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | As2O3 | PbO |
| Content (%) | 0.69 | 0.00 | 0.02 | 0.00 | 0.00 | 0.02 | 0.33 | 0.08 |
| Element/Oxide | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | Br | SrO |
| Content (%) | 0.00 | 0.00 | 0.01 | 0.00 | 41.34 | 0.09 | 0.00 | 0.69 |
| Element/Oxide | Nb2O5 | Bi2O3 | Sb2O3 | Co3O4 | CdO | HfO2 | Ag2O | CeO2 |
| Content (%) | 0.67 | 0.24 | 0.79 | 0.30 | 0.14 | 0.29 | 0.00 | 0.43 |
| Element/Oxide | BaO | Au | WO3 | MoO3 | La2O3 | ThO2 | Sn2O | |
| Content (%) | 1.00 | 0.00 | 0.01 | 0.20 | 0.00 | 0.29 | 3.55 | |
| Composition | Percentage (%) | ||
|---|---|---|---|
| USGS and USDOI Specifications [7,17] | Arikya Zircon | ||
| Unprocessed [6] | Processed | ||
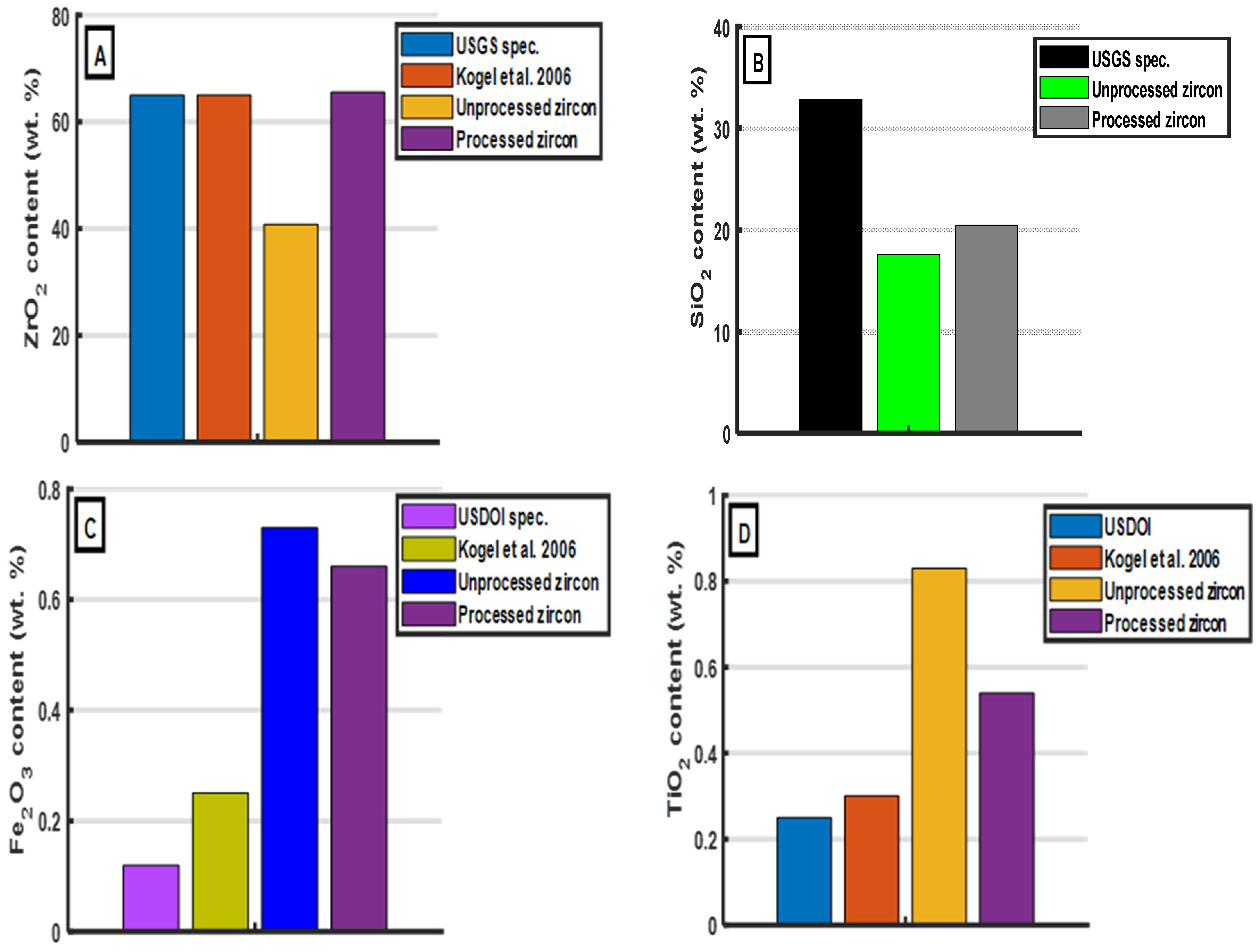
| ZrO2 | Minimum 65.00 | 40.77 | 65.52 |
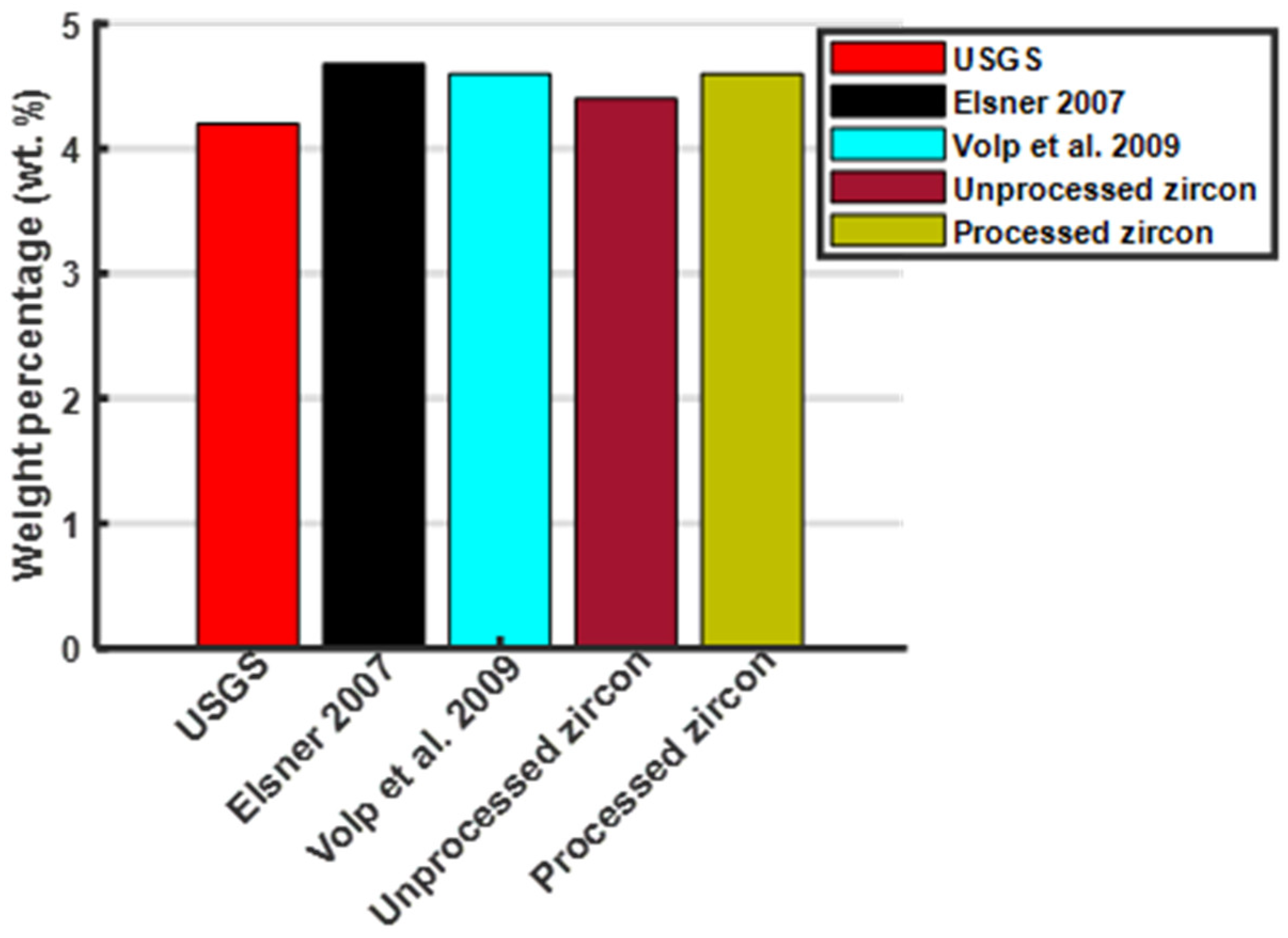
| HfO2 | 0.40 to 2.00 | n.d or bdl * | 0.29 |
| SiO2 | 32.80 | 17.61 | 20.48 |
| Fe2O3 | 0.12 | 0.73 | 0.66 |
| TiO2 | 0.25 | 0.83 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoli, B.I.; Agboola, O.A.; Onwualu, A.P.; Bello, A.; Sholiyi, O.S.; Anye, V.C.; Yusuf, O.T. Zirconia Enrichment of Zircon from Arikya, Nasarawa State, Nigeria, by Magnetic and Gravity Separation Processes for Use as Reinforcing Agent in Composite Formulation. Eng 2024, 5, 180-197. https://doi.org/10.3390/eng5010010
Okoli BI, Agboola OA, Onwualu AP, Bello A, Sholiyi OS, Anye VC, Yusuf OT. Zirconia Enrichment of Zircon from Arikya, Nasarawa State, Nigeria, by Magnetic and Gravity Separation Processes for Use as Reinforcing Agent in Composite Formulation. Eng. 2024; 5(1):180-197. https://doi.org/10.3390/eng5010010
Chicago/Turabian StyleOkoli, Benneth Ifenna, Olufemi A. Agboola, Azikiwe Peter Onwualu, Abdulhakeem Bello, Olusegun Samuel Sholiyi, Vitalis C. Anye, and Olatunbosun T. Yusuf. 2024. "Zirconia Enrichment of Zircon from Arikya, Nasarawa State, Nigeria, by Magnetic and Gravity Separation Processes for Use as Reinforcing Agent in Composite Formulation" Eng 5, no. 1: 180-197. https://doi.org/10.3390/eng5010010
APA StyleOkoli, B. I., Agboola, O. A., Onwualu, A. P., Bello, A., Sholiyi, O. S., Anye, V. C., & Yusuf, O. T. (2024). Zirconia Enrichment of Zircon from Arikya, Nasarawa State, Nigeria, by Magnetic and Gravity Separation Processes for Use as Reinforcing Agent in Composite Formulation. Eng, 5(1), 180-197. https://doi.org/10.3390/eng5010010











