Prediction of Key Parameters in the Design of CO2 Miscible Injection via the Application of Machine Learning Algorithms
Abstract
1. Introduction
2. Literature Review
3. Data Collection
3.1. Solubility (Rs)
3.2. Interfacial Tension (IFT)
3.3. Minimum Miscibility Pressure (MMP)
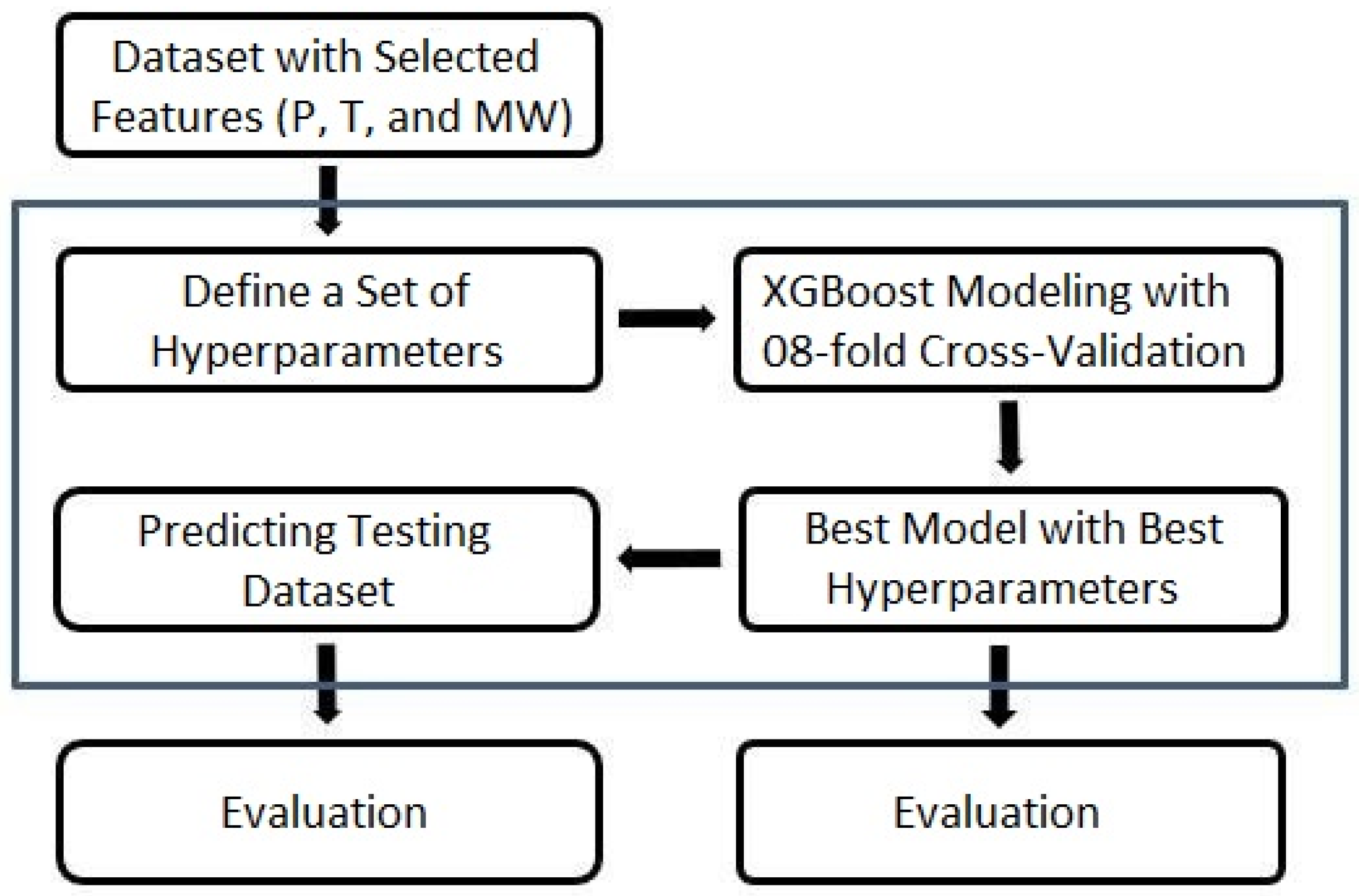
4. Model Implementation
- Dead oil solubility model: the training and validation set comprised 85% of the dataset (90 samples), and a test set formed 15% of the dataset (15 samples).
- Live oil solubility model: the training set contained 80% of the dataset (60 samples), and a test set held 20% of the dataset (14 samples).
- Interfacial tension model: the training set included 80% of the dataset (856 samples), a cross-validation set made up 1/8 of the training set (107 samples), and a test set represented 20% of the dataset (215 samples).
- Minimum miscibility pressure model: the training set consisted of 84% of the dataset (162 samples), and a test set incorporated 16% of the dataset (31 samples).
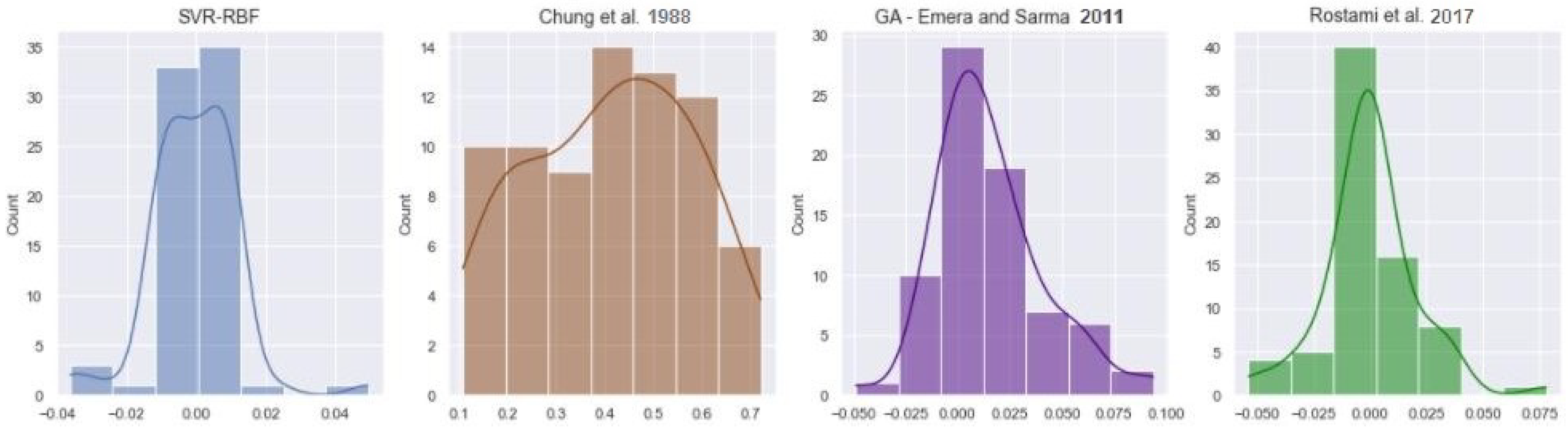
4.1. Dead Oil Solubility
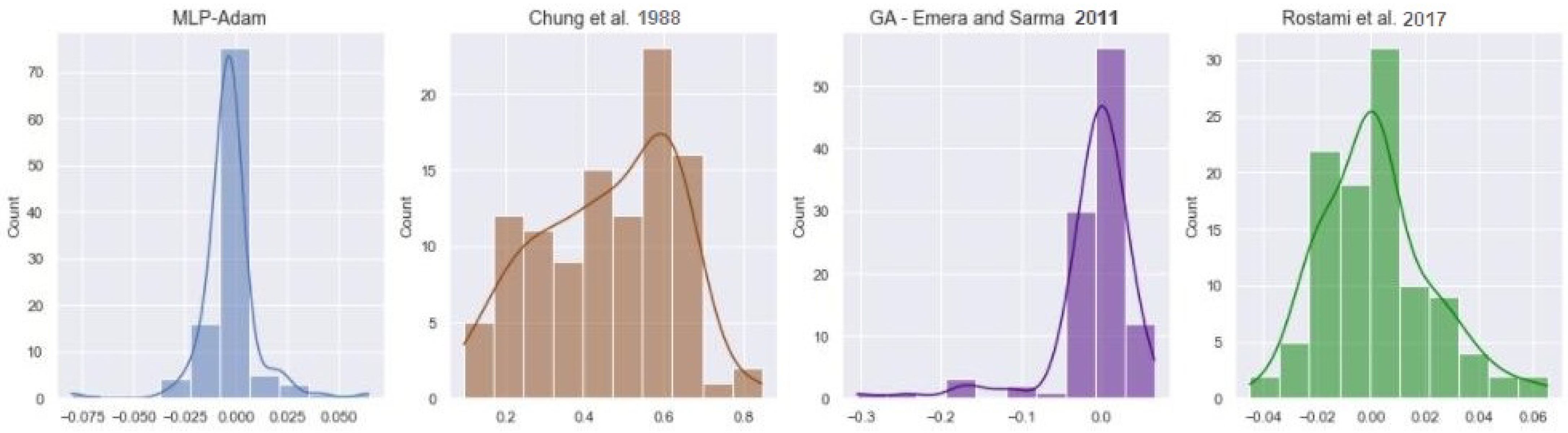
4.2. Live Oil Solubility
4.3. Interfacial Tension
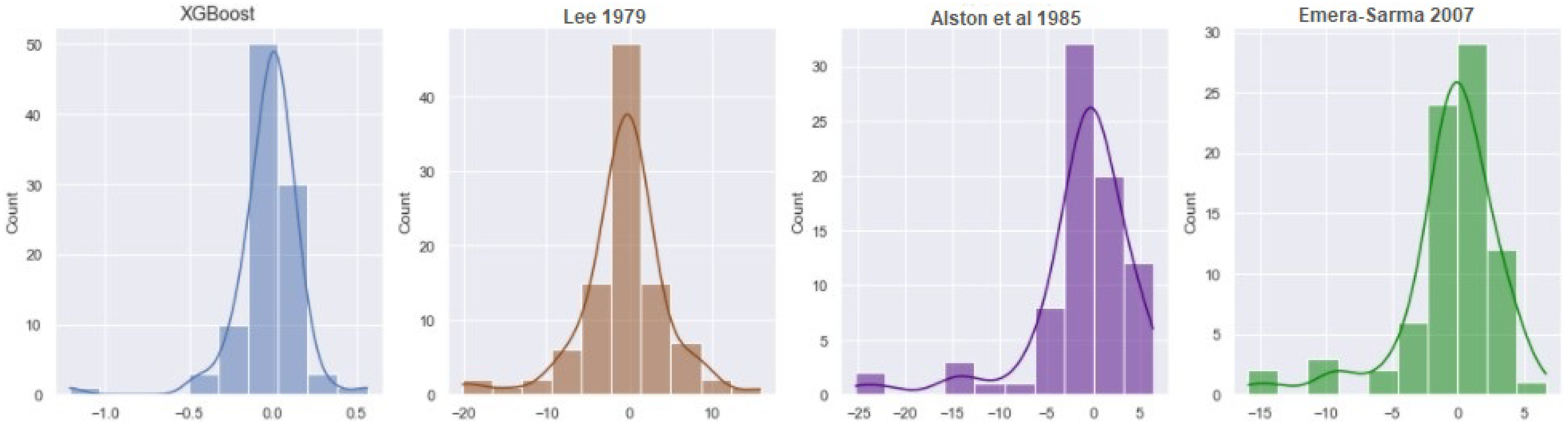
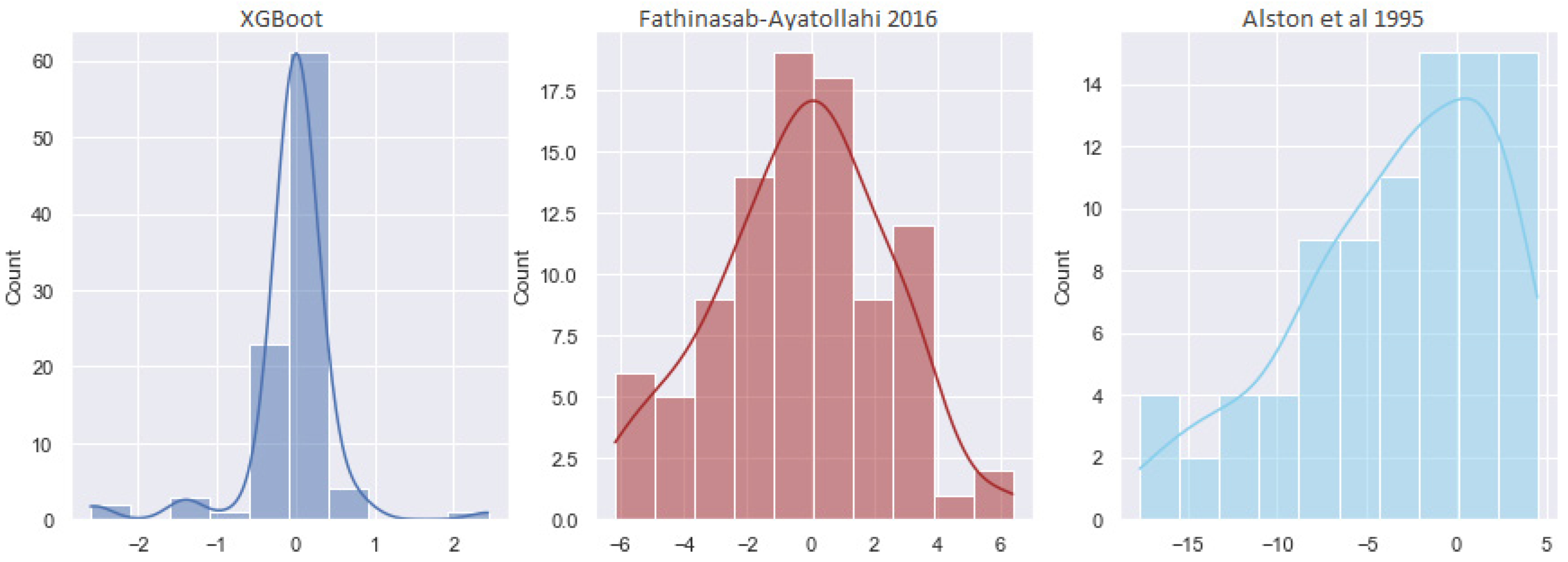
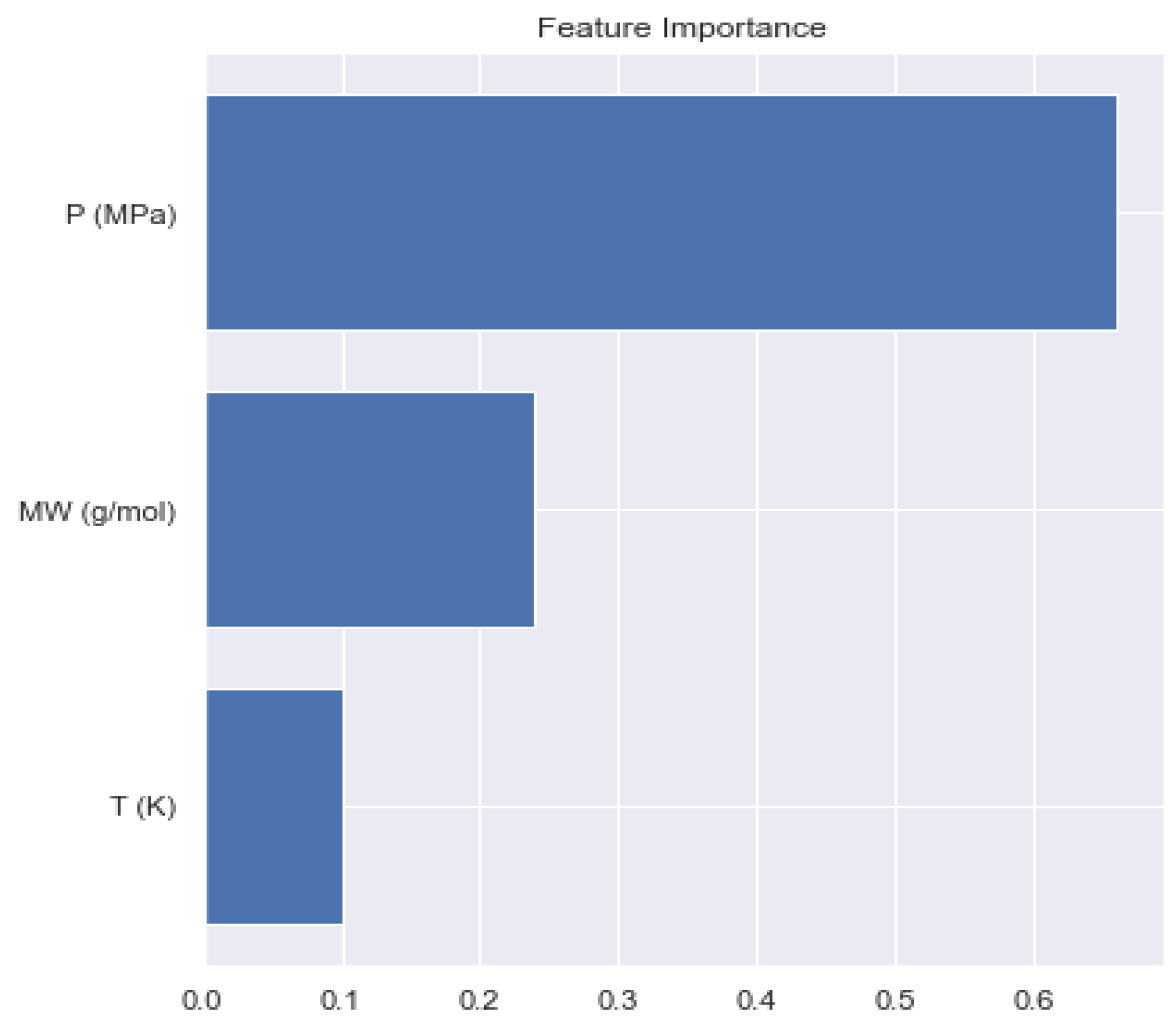
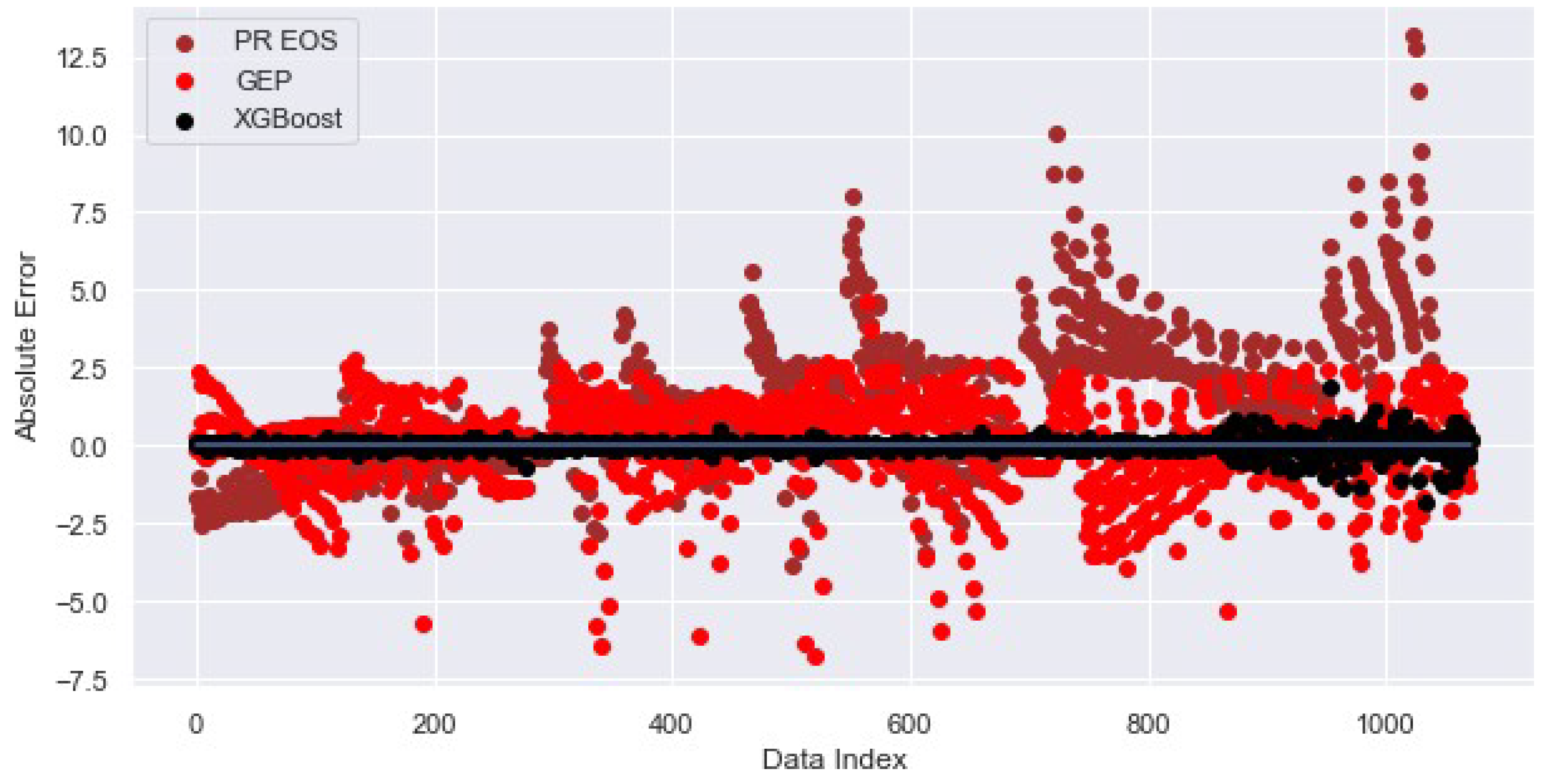
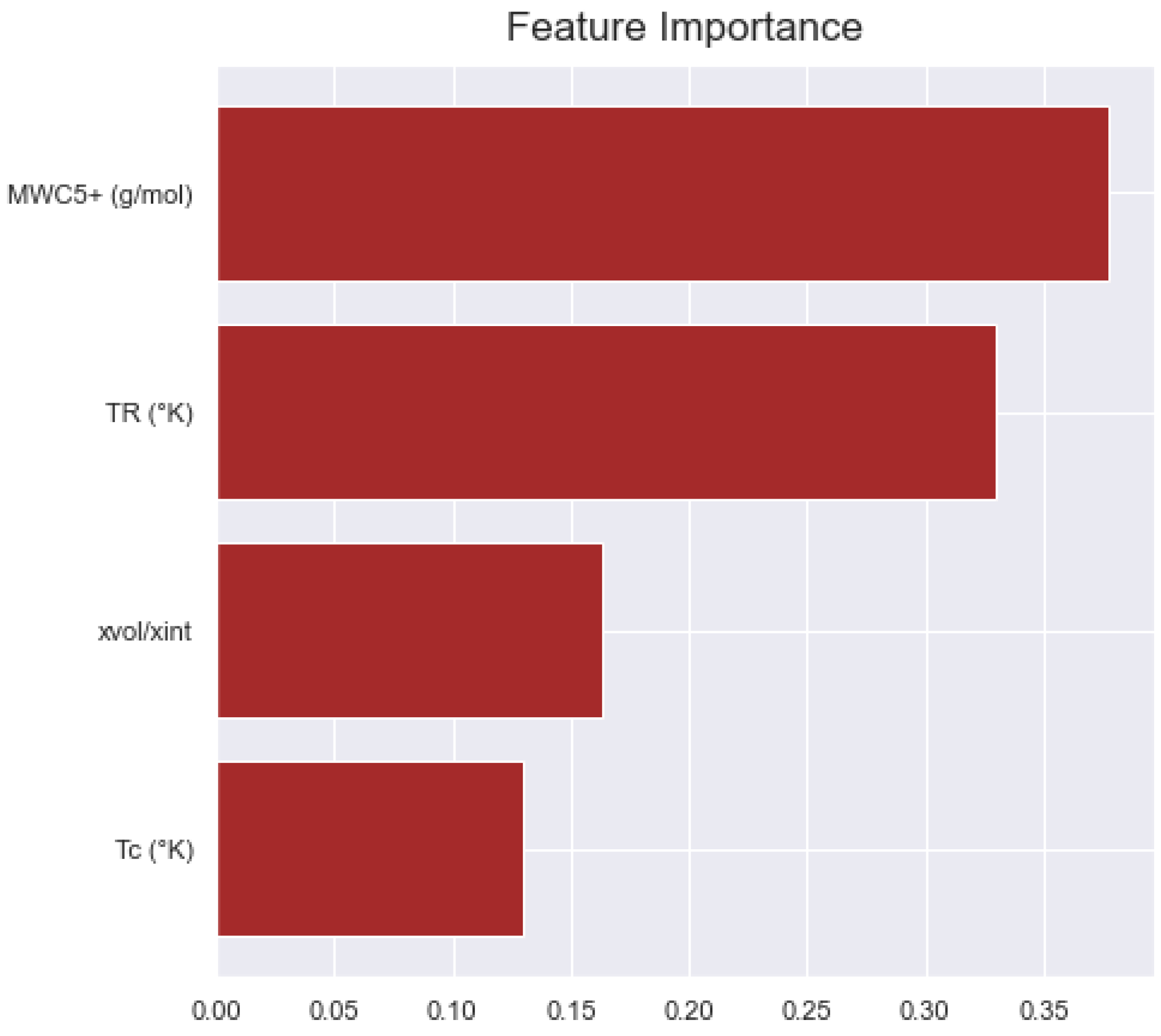
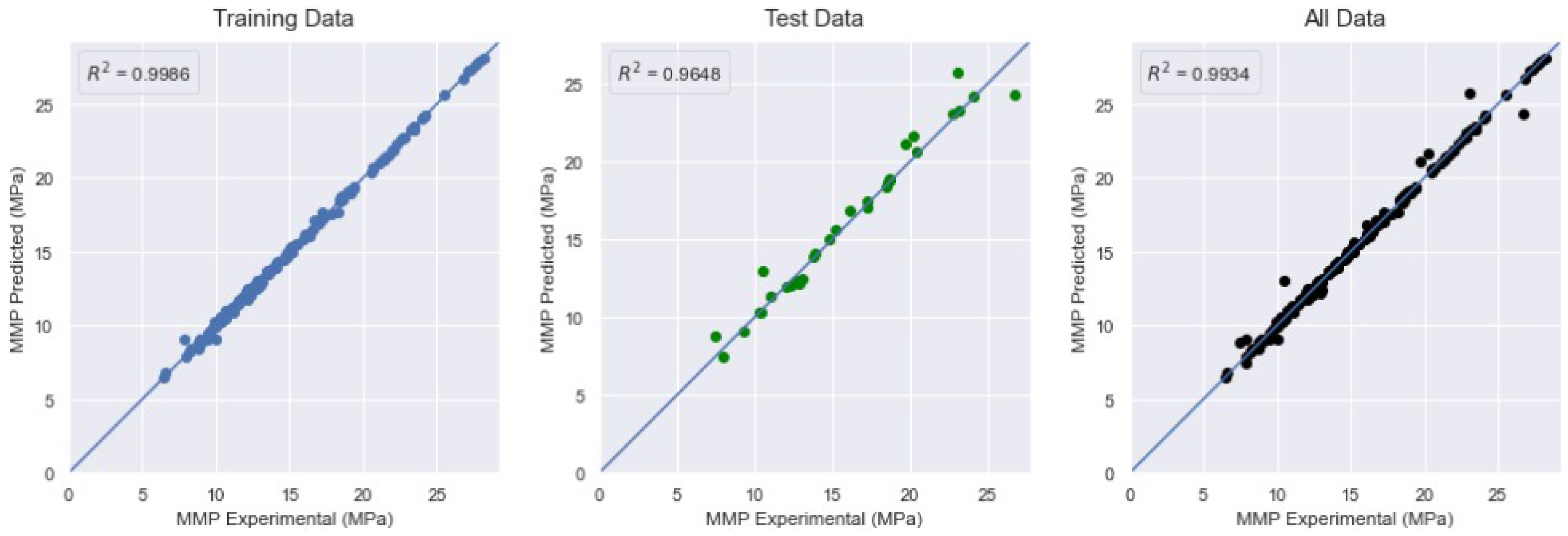
4.4. Minimum Miscibility Pressure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Average Absolute Relative Deviation (AARD (%))
- n is the total number of observations;
- Actual refers to the actual value;
- Predicted refers to the predicted value.
Appendix A.2. Root Mean Square Error (RMSE)
- n is the total number of observations;
- Actual refers to the actual value;
- Predicted refers to the predicted value.
Appendix A.3. Coefficient of Determination (R2)
- SSres is the sum of squares of the residual errors.
- SStot is the total sum of squares.
Appendix B
Appendix B.1. Feed Forward Equation of our MLP-Adam Model
- Initialize the input data. Let us denote the input vector as X.
- Calculate the activations of the neurons in the first hidden layer by applying the ReLU activation function (this function computes the maximum value between 0 and the input x. If x is positive, the output is equal to x, and if x is negative, the output is set to 0) to the resulting sum to introduce non-linearity. This is carried out using the following equation:where is the interconnection weight between the input vector and the hidden layer neurons, j, is the sum of the weighted inputs and the bias, , and n is the number of neurons in the input layer. represents the resulting activation value.
- 3.
- The same process is repeated for the second hidden layer. The output of the second hidden layer is denoted as .where represents the weights connecting the first hidden layer neurons j to the second hidden layer neurons k, Represents the weighted sum of inputs for the neurons in the second hidden layer, and is the bias term. represents the activation values for this second hidden layer.
- 4.
- Finally, the output of our MLP-Adam model can be calculated by applying the purelin function to the output of the ReLU function as shown below:where is the predicted output value, represents the weights connecting the second hidden layer neurons, k, to the output layer neurons, l, is the bias term, and is the number of neurons in the hidden layer.
Appendix B.2. Example Calculations using MLP-Adam Model
| 0.423479229 | −0.518270671 | 0.088841140 | 0.164605036 | −0.182387754 |
| −0.316850155 | 0.578180193 | −0.627018213 | −0.370452255 | −0.037461437 |
| −0.260930061 | 0.121095933 | 0.302609562 | 0.177341118 | 0.035739433 |
| 0.153113961 | −0.273656278 | 0.023316100 | −0.014185284 | −0.152793422 |
| 0.326721847 | 0.163599714 | 0.017112899 | 0.437370806 | −0.370236605 |
| 0.467836350 | −0.183758318 | −0.116376496 | 0.173847764 | 0.190825283 |
| 0.207402825 | −0.402902960 | 0.277075022 | 0.077882327 | −0.256408870 |
| 0.430666834 | 0.488847017 | 0.382416307 | 0.316209614 | −0.437328159 |
| −0.378489106 | −0.191637143 | −0.586777627 | 0.073175244 | −0.207403078 |
| −0.280519455 | −0.169934719 | −0.038683220 | 0.464787781 | 0.129119664 |
| −0.012112551 | −0.279909700 | 0.314301490 | −0.553606331 | 0.127572730 |
| 0.203990727 | 0.348036944 | 0.120888933 | −0.571946859 | −0.362548828 |
| 0.144444540 | 0.227294683 | −0.281868785 | −0.386379957 | −0.244969561 | 0.250844776 | −0.042056944 | 0.090741582 |
| −1.246394872 | −0.613879323 | −0.806254267 | 0.332979083 | 0.174128487 | −0.160888448 | −0.905039012 | 0.223389938 |
| 0.158317938 | 0.136602625 | 0.250266492 | −0.048559281 | −0.043032091 | −0.009495512 | 0.364784896 | −0.316569924 |
| −0.292102873 | 0.049241617 | 0.113946393 | 0.185241475 | −0.189562544 | 0.473260581 | 0.171075671 | −0.035240747 |
| −0.311310201 | −1.128083109 | −0.132358402 | −0.147601380 | 0.150322437 | −0.051223963 | −0.059710107 | 0.302232533 |
| −0.527317762 | 0.004510418 | −0.090777598 | 0.033773034 | 0.003524607 | 0.325446367 | −0.200799241 | −1.144739747 |
| 0.641047120 | −0.064388409 | 0.391169577 | −0.684768438 | −0.434764891 | 0.371954649 | −0.063837923 | −0.090706437 |
| −0.190623462 | 0.257651656 | 0.394092589 | 0.200460493 | −0.200868785 | 0.064583137 | 0.155178993 | 0.315470844 |
| 0.193483933 | −0.301786810 | 0.255001187 | −0.513664782 | −0.427212923 | −0.234824061 | −0.042243052 | 0.111917041 |
| −1.080619454 | 0.096860095 | 0.129510939 | 0.049882758 | 0.238265812 | −1.272954463 | 0.236488863 | −0.735467910 |
| −0.364739000 | −0.515439033 | −0.178362324 | −0.179078683 | −0.595661461 | −0.054487861 | −0.096768409 | −0.003158351 |
| −0.499953687 | 0.379382699 | −0.177857115 | −0.423149019 | −0.938039004 | 0.343048214 | −0.956486344 | 0.245499372 |
| −0.792608916 | −0.343328714 | −0.205415770 | −0.539200484 | 0.158580690 | 0.079246789 | 0.300516456 | |
| 0.365020424 | −0.149115592 | −0.426100313 | 0.130489438 | 0.123922713 | 0.187488675 | ||
| 0.137588575 | 0.520926713 | −0.278029352 | −0.333180844 | −0.322128087 | −0.186551764 | ||
| 0.191870614 | 0.492062687 | −0.308154106 | −0.205118045 | 0.259233176 | 0.440697550 | ||
| −0.230481609 | −0.726262688 | 0.058385573 | −0.124779440 | −0.023145271 | 0.217374727 | ||
| 0.379300296 | 0.162133157 | 0.567164421 | 0.756009399 | −0.201348185 | −0.275265455 | ||
| −0.633766531 | 0.062475737 | 0.018612951 | −0.710203170 | 0.197099491 | 0.088092155 | ||
| 0.158039510 | −0.123929366 | 0.011550034 | 0.471806019 | −0.221232160 | −0.171224877 | ||
| 0.196399033 | −0.388778716 | −0.568655312 | 0.230788096 | −0.103322580 | −0.453178435 | ||
| 0.274261921 | −0.640708744 | 0.155315384 | 0.250834226 | 0.017402615 | −0.166323795 | ||
| 0.512196242 | −0.019978577 | −0.330687165 | 0.177631750 | 0.079844228 | 0.371093213 | ||
| −0.256439089 | 0.436899453 | −0.405297756 | 0.383212924 | −0.086818188 | 0.109248526 |
| MW | γ | T | Ps | Rs-Pred | Rs-Exp | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1.54606763 | 0.90499909 | 2.66878506 | 0.76176893 | 0.3657942 −1.9596565 0.68460845 −0.1123078 0.6618012 0.56967878 0.49840513 1.93238358 −2.4762450 −0.2075494 0.2726109 0.15474298 | 0.3657942 0 0.68460845 0 0.6618012 0.56967878 0.49840513 1.93238358 0 0 0.2726109 0.15474298 | 0.01417779 −0.9757543 −0.6840449 0.2759657 0.39354511 −2.3092059 0.31163391 0.64580446 −0.1985719 −2.0788913 −0.7179282 −0.8703367 | 0.01417779 0 0 0.2759657 0.39354511 0 0.31163391 0.64580446 0 0 0 0 | 0.4256788 | 0.42 |
References
- Holdren, J.P. Population and the energy problem. Popul. Env. 1991, 12, 231–255. [Google Scholar] [CrossRef]
- Laherrere, J.; Hall, C.B.; Bentley, R. How much oil remains for the world to produce? Comparing assessment methods, and separating fact from fiction. Curr. Res. Environ. Sustain. 2022, 4, 100174. [Google Scholar] [CrossRef]
- Ozotta, O.; Ostadhassan, M.; Lee, H.; Pu, H.; Kolawole, O.; Malki, M.L. Time-dependent Impact of CO2-shale Interaction on CO2 Storage Potential. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference, Abu Dhabi, United Arab Emirates, 18 March 2021; pp. 15–18. [Google Scholar]
- Clonts, M.; Mazighi, M.; Touami, M. Reservoir simulation of the planned miscible gas injection project at Rhourde El Baguel, Algeria. In Proceedings of the European Petroleum Conference, Milan, Italy, 22–24 October 1996; OnePetro: Richardson, TX, USA, 1996. [Google Scholar]
- Malki, M.L.; Rasouli, V.; Saberi, M.R.; Sennaoui, B.; Ozotta, O.; Chellal, H.A. Effect of CO2 on Mineralogy, Fluid, and Elastic Properties in Middle Bakken Formation Using Rock Physics Modeling. In Proceedings of the ARMA US Rock Mechanics/Geomechanics Symposium, Santa Fe, NM, USA, 26–29 June 2022. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; First, E.L.; Boukouvala, F.; Floudas, C.A. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU. Comput. Chem. Eng. 2015, 81, 2–21. [Google Scholar] [CrossRef]
- Merzoug, A.; Mouedden, N.; Rasouli, V.; Damjanac, B. Simulation of Proppant Placement Efficiency at the Intersection of Induced and Natural Fractures. In Proceedings of the ARMA US Rock Mechanics/Geomechanics Symposium, Santa Fe, NM, USA, 26–29 June 2022. [Google Scholar] [CrossRef]
- Afari, S.; Ling, K.; Sennaoui, B.; Maxey, D.; Oguntade, T.; Porlles, J. Optimization of CO2 huff-n-puff EOR in the Bakken Formation using numerical simulation and response surface methodology. J. Pet. Sci. Eng. 2022, 215 Pt A, 110552. [Google Scholar] [CrossRef]
- Taber, J.J.; Martin, F.D.; Seright, R.S. EOR screening criteria revisited -Part 1: Introduction to screening criteria and enhanced recovery field projects. SPE Reserv. Eng. 1997, 12, 189–198. [Google Scholar] [CrossRef]
- Sennaoui, B.; Pu, H.; Afari, S.; Malki, M.L.; Kolawole, O. Pore- and Core-Scale Mechanisms Controlling Supercritical Cyclic Gas Utilization for Enhanced Recovery under Immiscible and Miscible Conditions in the Three Forks Formation. Energy Fuels 2023, 37, 459–476. [Google Scholar] [CrossRef]
- Almobarak, M.; Wu, Z.; Daiyu, Z.; Fan, K.; Liu, Y.; Xie, Q. A review of chemical-assisted minimum miscibility pressure reduction in CO2 injection for enhanced oil recovery. Petroleum 2021, 7, 245–253. [Google Scholar] [CrossRef]
- El-Hoshoudy, A.; Desouky, S. CO2 Miscible Flooding for Enhanced Oil Recovery. In Carbon Capture, Utilization and Sequestration; InTech eBooks: London, UK, 2018. [Google Scholar] [CrossRef]
- Mouedden, N.; Laalam, A.; Mahmoud, M.; Rabiei, M.; Merzoug, A.; Ouadi, H.; Boualam, A.; Djezzar, S. A Screening Methodology Using Fuzzy Logic to Improve the Well Stimulation Candidate Selection. In All Days; OnePetro: Richardson, TX, USA, 2022. [Google Scholar] [CrossRef]
- Boualam, A.; Rasouli, V.; Dalkhaa, C.; Djezzar, S. Stress-Dependent Permeability and Porosity in Three Forks Carbonate Reservoir, Williston Basin. In Proceedings of the 54th U.S. Rock Mechanics/Geomechanics Symposium, Physical Event Cancelled, Golden, CO, USA, 28 June–1 July 2020. [Google Scholar]
- Boualam, A.; Rasouli, V.; Dalkhaa, C.; Djezzar, S. Advanced Petrophysical Analysis and Water Saturation Prediction in Three Forks, Williston Basin. In Proceedings of the SPWLA Annual Logging Symposium, Online, 24 June–29 July 2020. [Google Scholar] [CrossRef]
- Koroteev, D.; Tekic, Z. Artificial intelligence in oil and gas upstream: Trends, challenges, and scenarios for the future. Energy AI 2021, 3, 100041. [Google Scholar] [CrossRef]
- Dargahi-Zarandi, A.; Hemmati-Sarapardeh, A.; Shateri, M.; Menad, N.A.; Ahmadi, M. Modeling minimum miscibility pressure of pure/impure CO2-crude oil systems using adaptive boosting support vector regression: Application to gas injection processes. J. Pet. Sci. Eng. 2020, 184, 106499. [Google Scholar] [CrossRef]
- Sambo, C.; Liu, N.; Shaibu, R.; Ahmed, A.A.; Hashish, R.G. A Technical Review of CO2 for Enhanced Oil Recovery in Unconventional Oil Reservoirs. Geoenergy Sci. Eng. 2022, 221, 111185. [Google Scholar] [CrossRef]
- Fath, A.H.; Pouranfard, A.-R. Evaluation of miscible and immiscible CO2 injection in one of the Iranian oil fields. Egypt. J. Pet. 2014, 23, 255–270. [Google Scholar] [CrossRef]
- Lv, Q.; Zheng, R.; Guo, X.; Larestani, A.; Hadavimoghaddam, F.; Riazi, M.; Hemmati-Sarapardeh, A.; Wang, K.; Li, J. Modelling minimum miscibility pressure of CO2-crude oil systems using deep learning, tree-based, and thermodynamic models: Application to CO2 sequestration and enhanced oil recovery. Sep. Purif. Technol. 2023, 310, 123086. [Google Scholar] [CrossRef]
- Yang, G.; Li, X. Modified Peng-Robinson equation of state for CO2/hydrocarbon systems within nanopores. J. Nat. Gas Sci. Eng. 2020, 84, 103700. [Google Scholar] [CrossRef]
- Kiani, S.; Saeedi, M.; Nikoo, M.R.; Mohammadi, A.H. New model for prediction of minimum miscibility pressure and CO2 solubility in crude oil. J. Nat. Gas Sci. Eng. 2020, 80, 103431. [Google Scholar] [CrossRef]
- Ahmed, T. Minimum Miscibility Pressure from EOS. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 4–8 June 2000. [Google Scholar] [CrossRef]
- Alshuaibi, M.; Farzaneh, S.A.; Sohrabi, M.; Mogensen, K. An Accurate and Reliable Correlation to Determine CO2/Crude Oil MMP for High-Temperature Reservoirs in Abu Dhabi. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019. [Google Scholar] [CrossRef]
- Jhalendra, R.K.; Kumar, A. Reliable estimate of minimum miscibility pressure from multiple possible EOS models for a reservoir oil under data constraint. Pet. Sci. Technol. 2022, 40, 1898–1913. [Google Scholar] [CrossRef]
- Sinha, U.; Dindoruk, B.; Soliman, M. Prediction of CO2 Minimum Miscibility Pressure MMP Using Machine Learning Techniques. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar] [CrossRef]
- Shakeel, M.; Khan, M.R.; Kalam, S.; Khan, R.A.; Patil, S.; Dar, U.A. Machine Learning for Prediction of CO2 Minimum Miscibility Pressure. In Proceedings of the Society of Petroleum Engineers—Middle East Oil, Gas and Geosciences Show, MEOS, Manama, Bahrain, 19–21 February 2023; SPE Middle East Oil and Gas Show and Conference, MEOS, Proceedings; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2023. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Zhang, Y.; Sun, L.; Yuan, S. Four Methods to Estimate Minimum Miscibility Pressure of CO2-Oil Based on Machine Learning. Chin. J. Chem. 2019, 37, 1271–1278. [Google Scholar] [CrossRef]
- Ekechukwu, G.K.; Falode, O.; Orodu, O.D. Improved Method for the Estimation of Minimum Miscibility Pressure for Pure and Impure CO2–Crude Oil Systems Using Gaussian Process Machine Learning Approach. ASME J. Energy Resour. Technol. 2020, 142, 123003. [Google Scholar] [CrossRef]
- Dong, P.; Liao, X.; Chen, Z.; Chu, H. An improved method for predicting CO2 minimum miscibility pressure based on artificial neural network. Adv. Geo-Energy Res. 2019, 3, 355–364. [Google Scholar] [CrossRef]
- Huang, C.; Tian, L.; Zhang, T.; Chen, J.; Wu, J.; Wang, H.; Wang, J.; Jiang, L.; Zhang, K. Globally optimized machine-learning framework for CO2 hydrocarbon minimum miscibility pressure calculations. Fuel 2022, 329, 125312. [Google Scholar] [CrossRef]
- Ge, D.; Cheng, H.; Cai, M.; Zhang, Y.; Dong, P. A New Predictive Method for CO2-Oil Minimum Miscibility Pressure. Geofluids 2021, 2021, 8868592. [Google Scholar] [CrossRef]
- Chemmakh, A.; Merzoug, A.; Ouadi, H.; Ladmia, A.; Rasouli, V. Machine Learning Predictive Models to Estimate the Minimum Miscibility Pressure of CO2-Oil System. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 15–18 November 2021. [Google Scholar] [CrossRef]
- Ramdan, D.; Najmi, M.; Rajabzadeh, H.; Elveny, M.; Alizadeh, S.M.S.; Shahriari, R. Prediction of CO2 solubility in electrolyte solutions using the e-PHSC equation of state. J. Supercrit. Fluids 2022, 180, 105454. [Google Scholar] [CrossRef]
- Song, Z.; Shi, H.; Zhang, X.; Zhou, T. Prediction of CO2 solubility in ionic liquids using machine learning methods. Chem. Eng. Sci. 2020, 223, 115752. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Lu, Z.; Pan, Z.J.; Zeng, M.; Du, X.; Xiao, S. The effect of subcritical and supercritical CO2 on the pore structure of bituminous coals. J. Nat. Gas Sci. Eng. 2021, 94, 104132. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; Jia, C.; Li, X.; Wu, K.; He, M. Numerical simulation on natural gas migration and accumulation in sweet spots of tight reservoir. J. Nat. Gas Sci. Eng. 2020, 81, 103454. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Huang, S.S.; Dyer, S.B. Measurement and Prediction of PVT Properties of Heavy and Medium Oils with Carbon Dioxide; No. CONF-9502114-Vol. 1; UNITAR: New York, NY, USA, 1995. [Google Scholar]
- Kokal, S.L.; Sayegh, S.G. Phase behavior and physical properties of CO-saturated heavy oil and its constitutive fractions. In Proceedings of the Annual Technical Meeting, Calgary, AB, Canada, 9–12 June 1990; OnePetro: Richardson, TX, USA, 1990. [Google Scholar]
- Simon, R.; Graue, D.J. Generalized correlations for predicting solubility, swelling and viscosity behavior of CO2-crude oil systems. J. Pet. Technol. 1965, 17, 102–106. [Google Scholar] [CrossRef]
- Simon, R.; Rosman, A.; Zana, E. Phase-behavior properties of CO2-reservoir oil systems. Soc. Pet. Eng. J. 1978, 18, 20–26. [Google Scholar] [CrossRef]
- Sim, S.S.K.; Udegbuanam, E.; Haggerty, D.J.; Baroni, J.; Baroni, M. Laboratory experiments and reservoir simulation studies in support of CO2 injection project in Mattoon field, Illinois, USA. In Proceedings of the Annual Technical Meeting, New Orleans, LA, USA, 25–28 September 1994; OnePetro: Richardson, TX, USA, 1994. [Google Scholar]
- Zolghadr, A.; Escrochi, M.; Ayatollahi, S. Temperature and Composition Effect on CO2 Miscibility by Interfacial Tension Measurement. J. Chem. Eng. Data 2013, 58, 1168–1175. [Google Scholar] [CrossRef]
- Jaeger, P.T.; Alotaibi, M.B.; Nasr-El-Din, H.A. Influence of Compressed Carbon Dioxide on the Capillarity of the Gas−Crude Oil−Reservoir Water System. J. Chem. Eng. Data 2010, 55, 5246–5251. [Google Scholar] [CrossRef]
- Georgiadis, A.; Llovell, F.; Bismarck, A.; Blas, F.J.; Galindo, A.; Maitland, G.C.; Trusler, J.P.M.; Jackson, G. Interfacial tension measurements and modelling of (carbon dioxide + n-alkane) and (carbon dioxide + water) binary mixtures at elevated pressures and temperatures. J. Supercrit. Fluids 2010, 55, 743–754. [Google Scholar] [CrossRef]
- Cronquist, C. Carbon dioxide dynamic miscibility with light reservoir oils. In Proceedings of the Fourth Annual US DOE Symposium, Tulsa, OK, USA; 1978. [Google Scholar]
- Yellig, W.; Metcalfe, R. Determination and Prediction of CO2 Minimum Miscibility Pressures (includes associated paper 8876). J. Pet. Technol. 1980, 32, 160–168. [Google Scholar] [CrossRef]
- Alston, R.; Kokolis, G.; James, C. CO2 minimum miscibility pressure: A correlation for impure CO2 streams and live oil systems. Soc. Pet. Eng. J. 1985, 25, 268–274. [Google Scholar] [CrossRef]
- Yuan, H.; Johns, R.T.; Egwuenu, A.M.; Dindoruk, B. Improved MMP correlations for CO2 floods using analytical gas flooding theory. In Proceedings of the Society of Petroleum Engineers—SPE/DOE Symposium on Improved Oil Recovery, IOR, Tulsa, OK, USA, 17–21 April 2004; (Proceedings—SPE Symposium on Improved Oil Recovery; Vol. 2004-April); Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2004. [Google Scholar]
- Chen, B.L.; Huang, H.D.; Zhang, Y. An Improved Predicting Model for Minimum Miscibility Pressure (MMP) of CO2 and Crude Oil. J. Oil Gas Technol. 2013, 35, 126–130. [Google Scholar]
- Chung, F.T.H.; Jones, R.A.; Burchfield, T.E. Recovery of Viscous Oil Under High Pressure by CO2 Displacement: A Laboratory Study. In Proceedings of the International Meeting on Petroleum Engineering, Tianjin, China, 1–4 November 1988. [Google Scholar] [CrossRef]
- Rostami, A.; Arabloo, M.; Kamari, A.; Mohammadi, A.H. Modeling of CO2 solubility in crude oil during carbon dioxide enhanced oil recovery using gene expression programming. Fuel 2017, 210, 768–782. [Google Scholar] [CrossRef]
- Emera, M.K.; Sarma, H.K. Prediction of CO2 Solubility in Oil and the Effects on the Oil Physical Properties. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 1233–1242. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Paszczynski, S.; Wilamowski, B.M. Advantages of Radial Basis Function Networks for Dynamic System Design. IEEE Trans. Ind. Electron. 2011, 58, 5438–5450. [Google Scholar] [CrossRef]
- Mirzaie, M.; Tatar, A. Modeling of interfacial tension in binary mixtures of CH4, CO2, and N2 -alkanes using gene expression programming and equation of state. J. Mol. Liq. 2020, 320 Pt B, 114454. [Google Scholar] [CrossRef]
- Lee, I. Effectiveness of Carbon Dioxide Displacement under Miscible and Immiscible Conditions; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1979. [Google Scholar]
- Emera, M.K.; Javadpour, F.; Sarma, H.K. Genetic algorithm (GA)-based correlations offer more reliable prediction of minimum miscibility pressures (MMP) between reservoir oil and CO2 or flue gas. J. Can. Pet. Technol. 2007, 46, 19–25. [Google Scholar] [CrossRef]
- Fathinasab, M.; Ayatollahi, S. On the determination of CO2–crude oil minimum miscibility pressure using genetic programming combined with constrained multivariable search methods. Fuel 2016, 173, 180–188. [Google Scholar] [CrossRef]
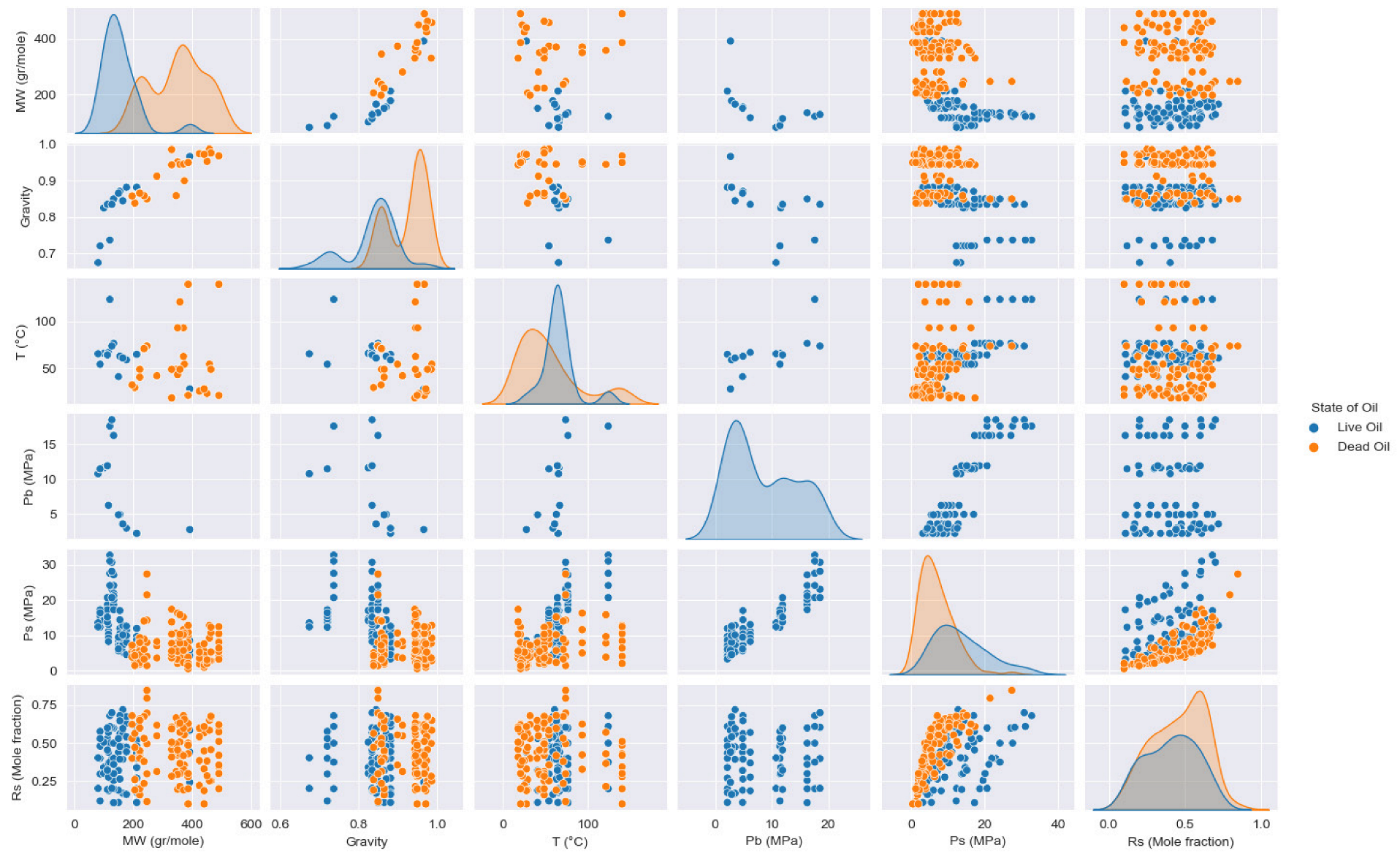
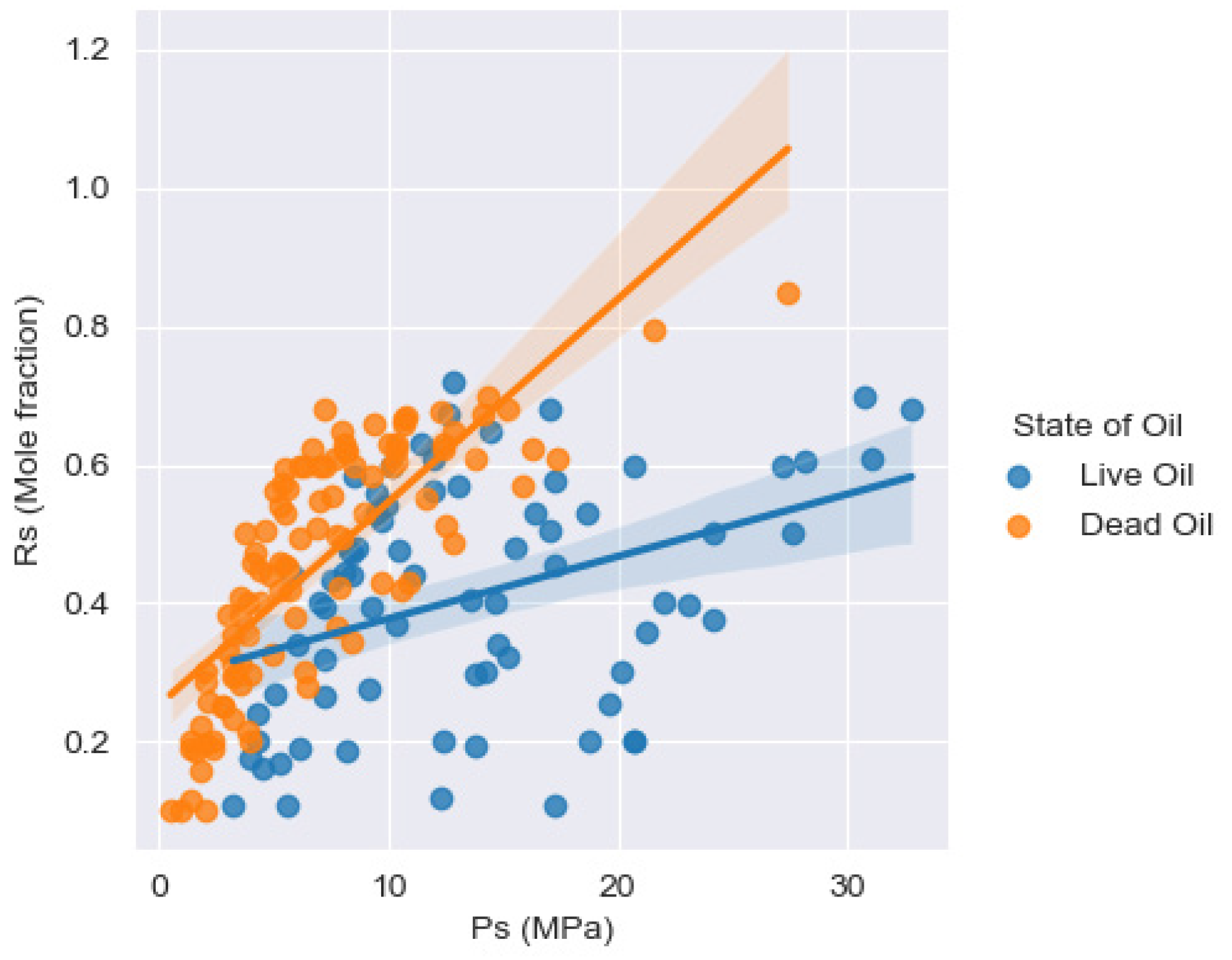
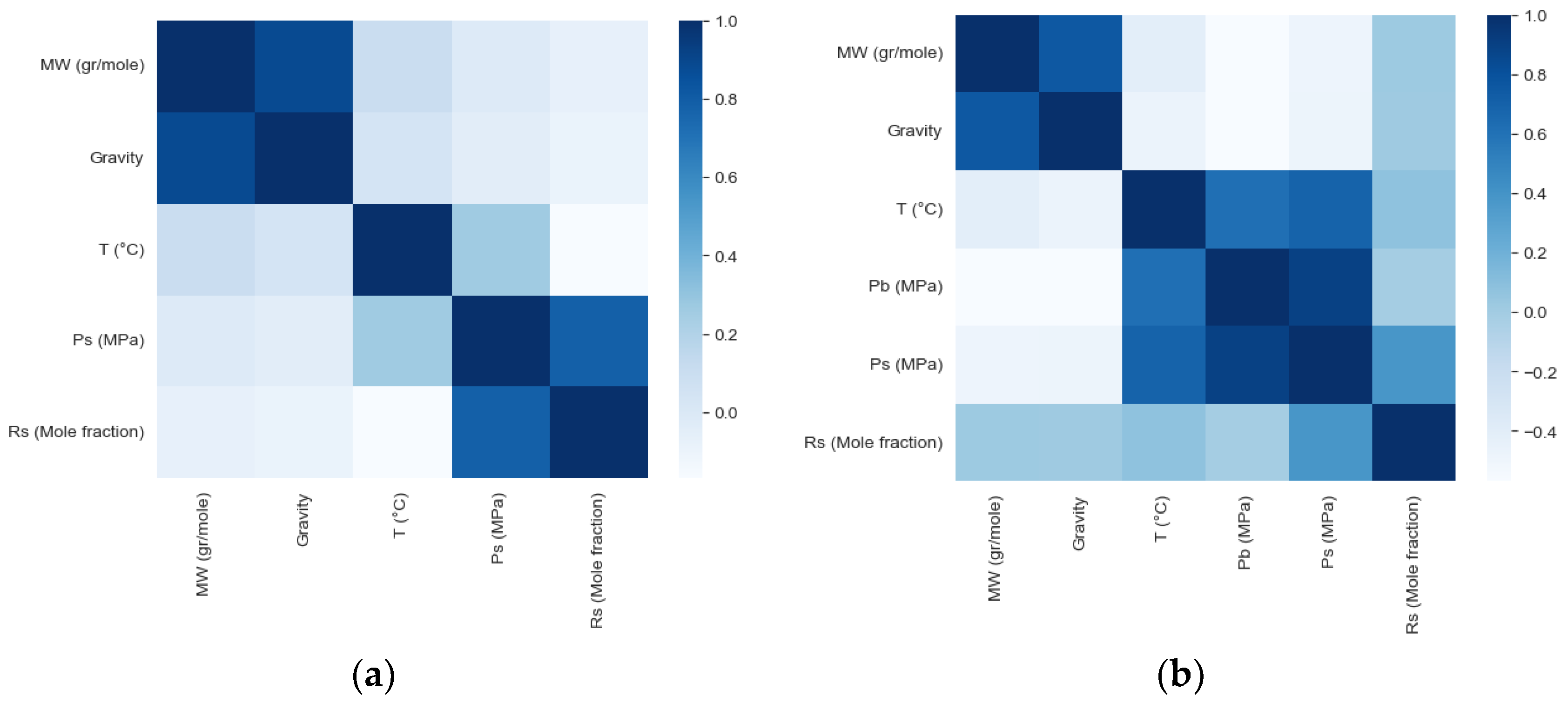
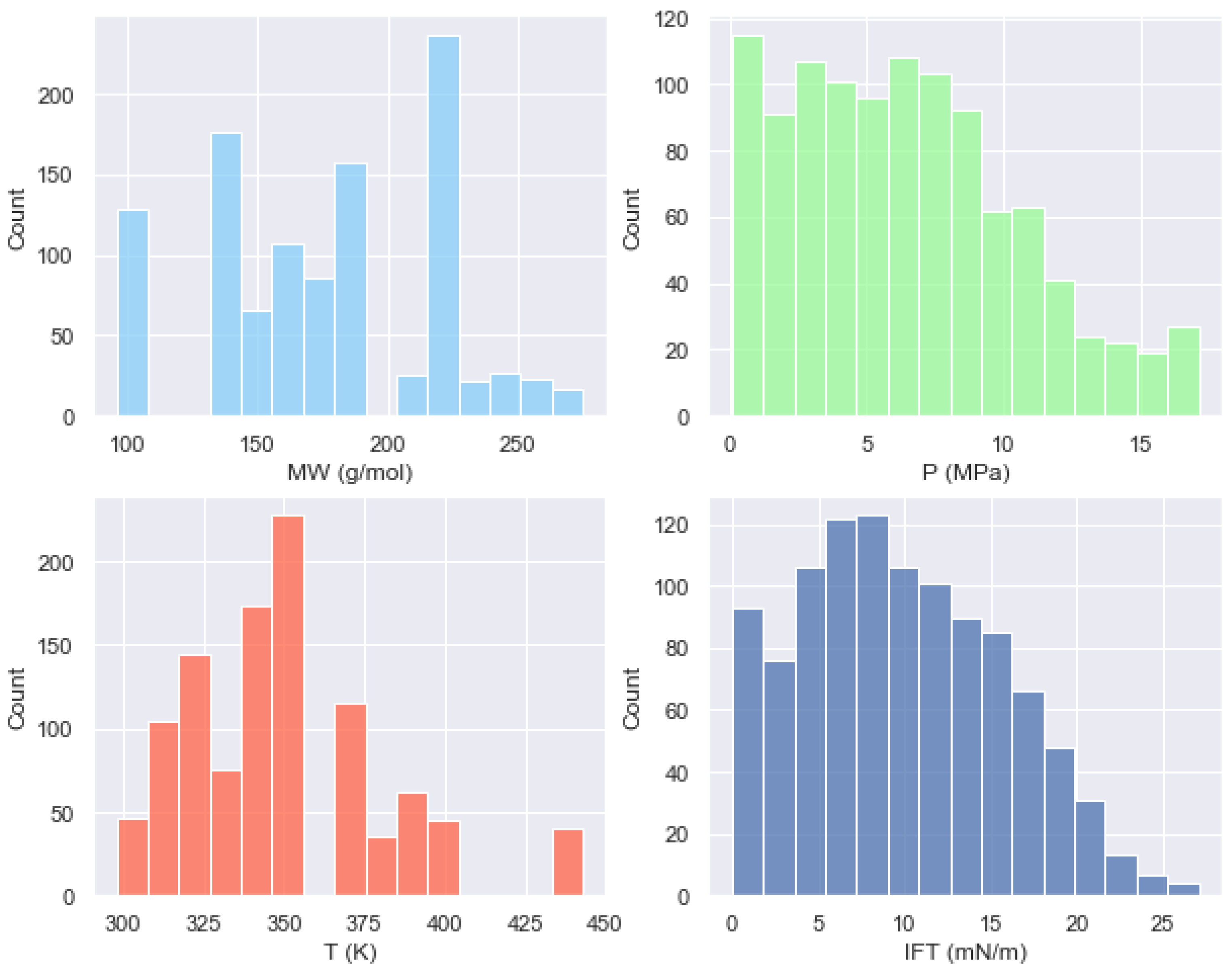
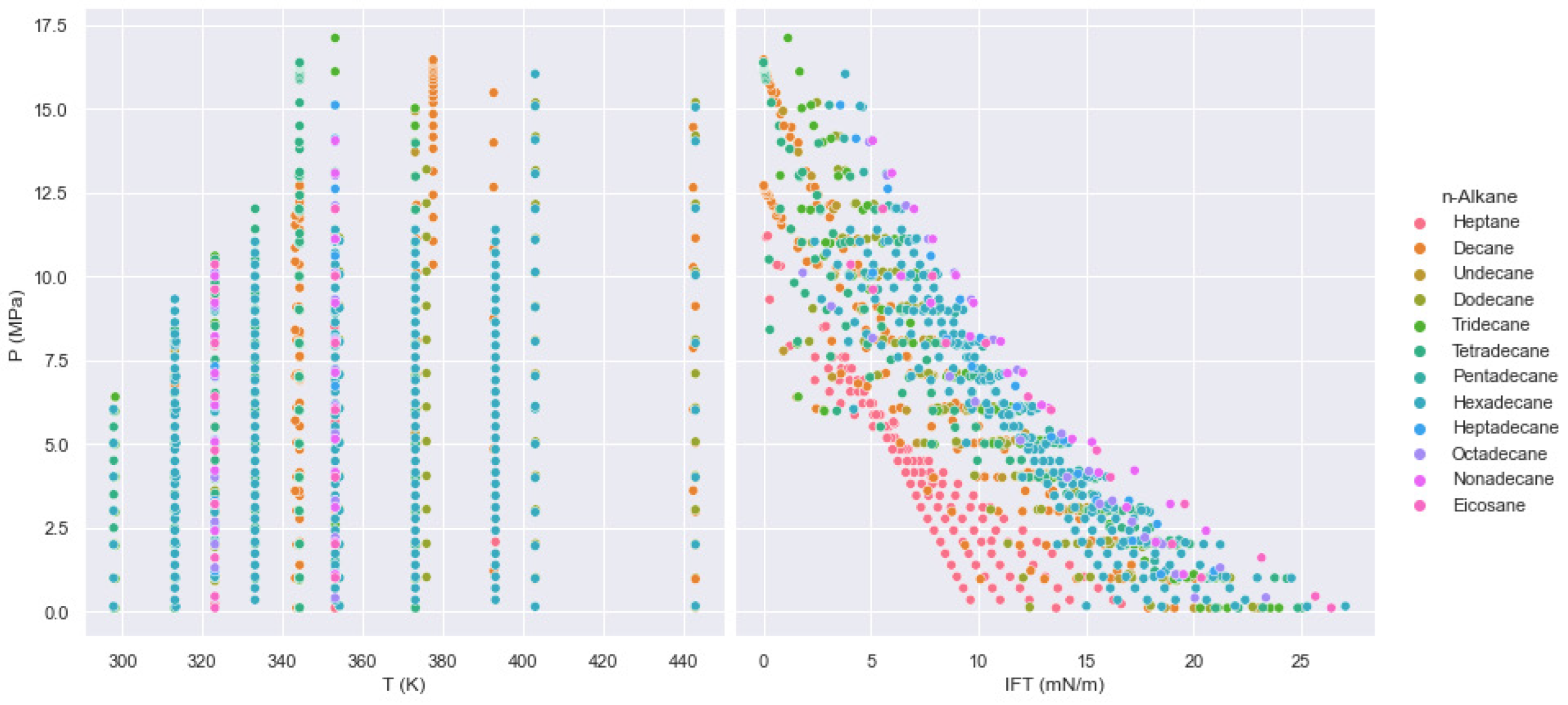
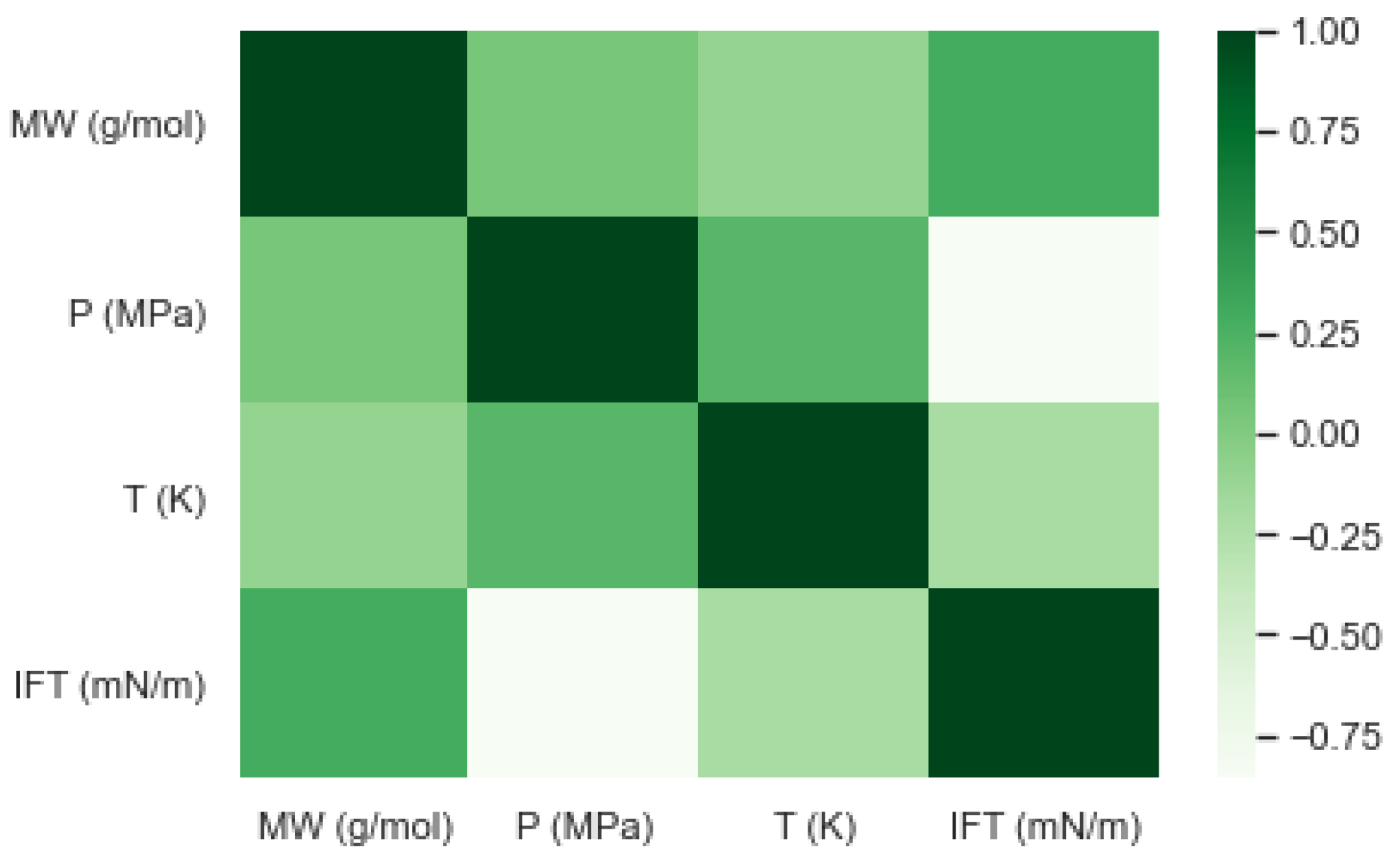
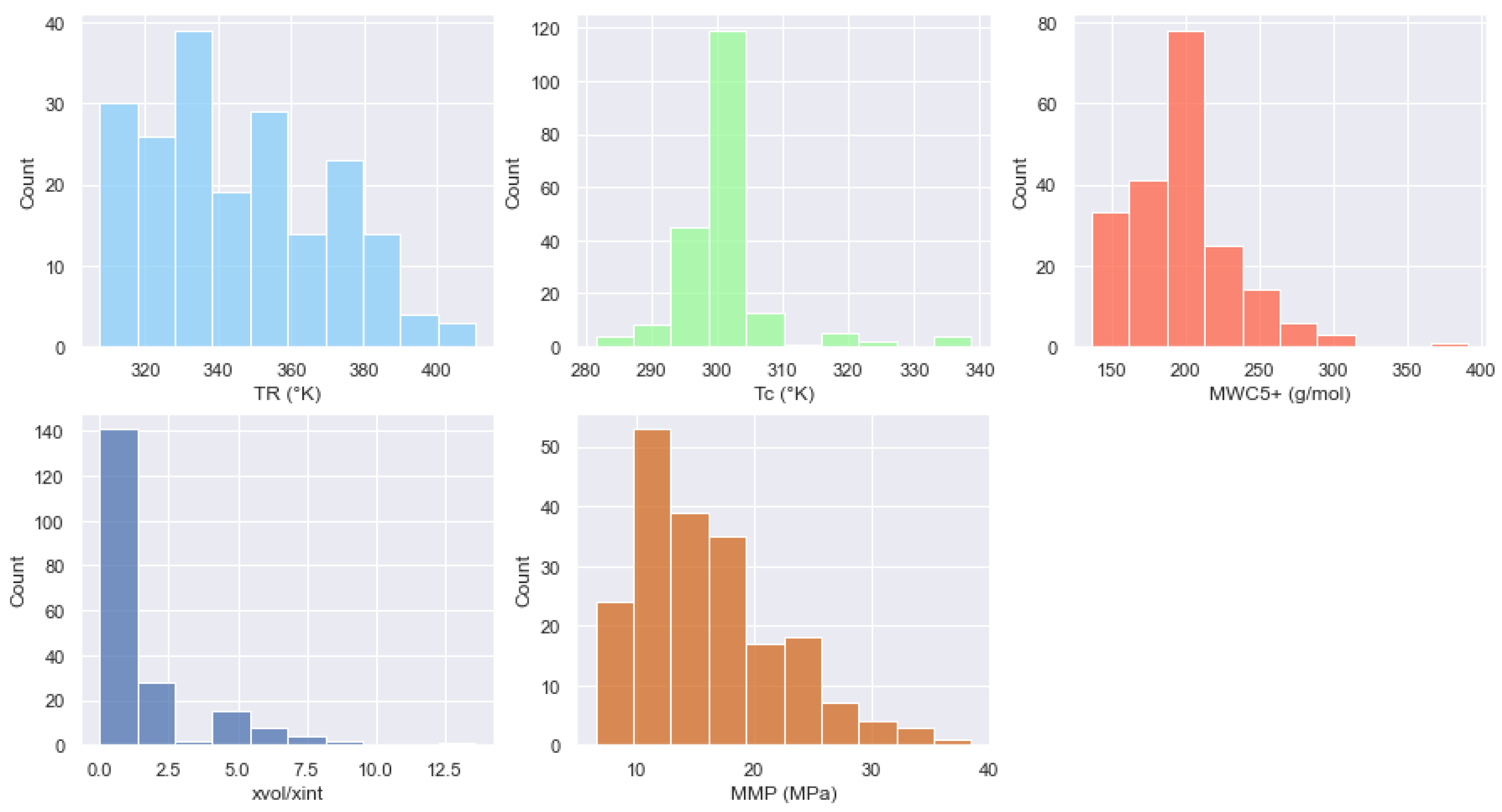
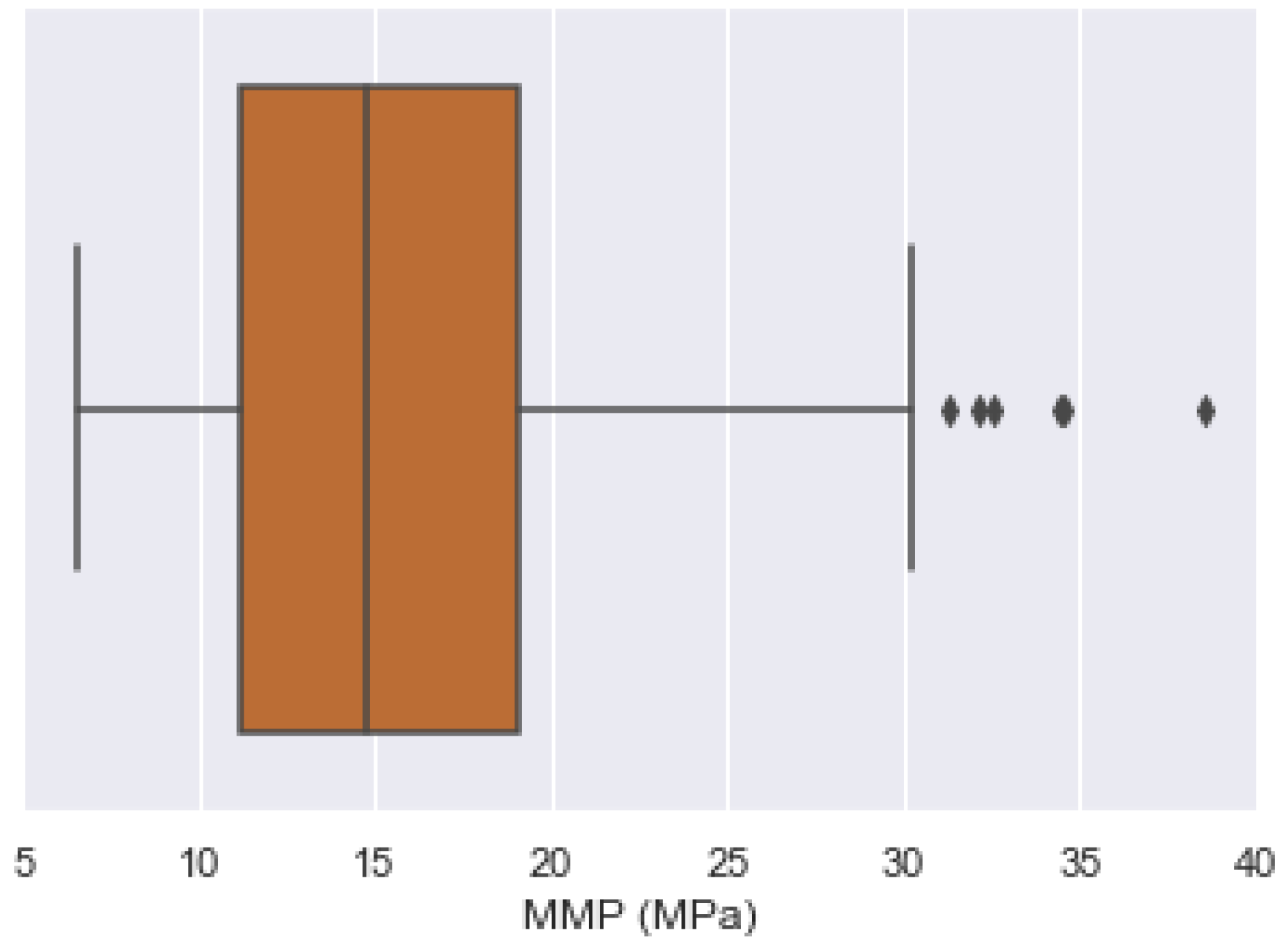
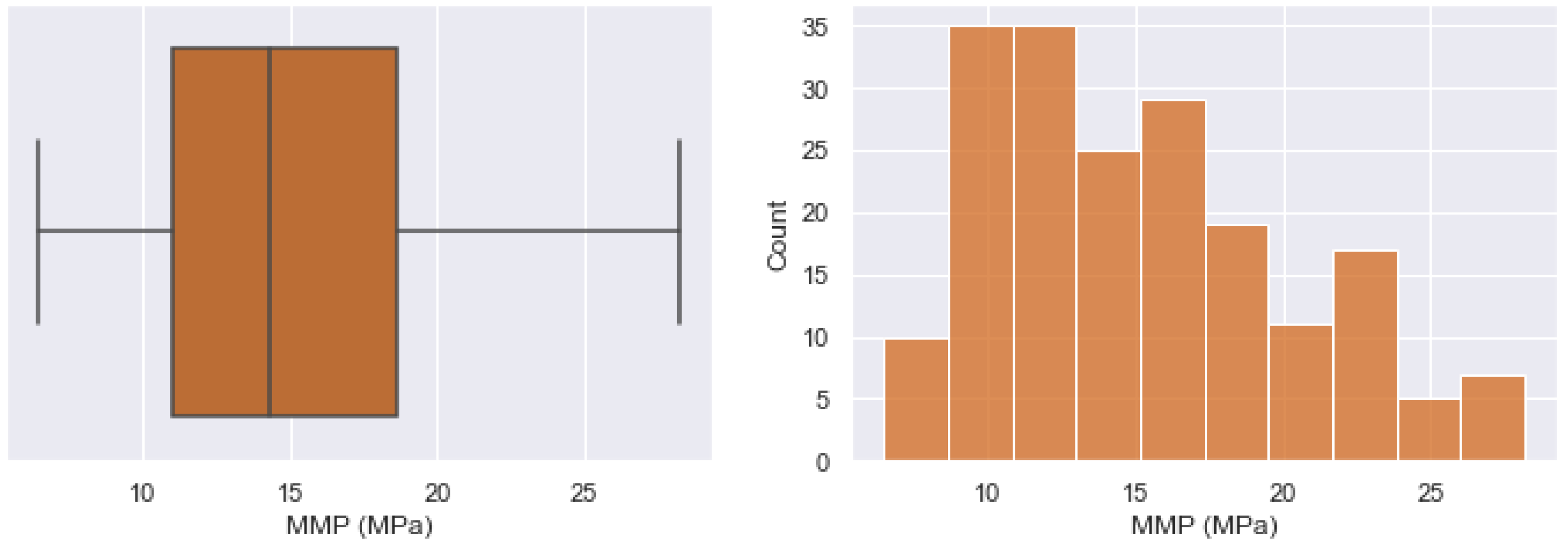
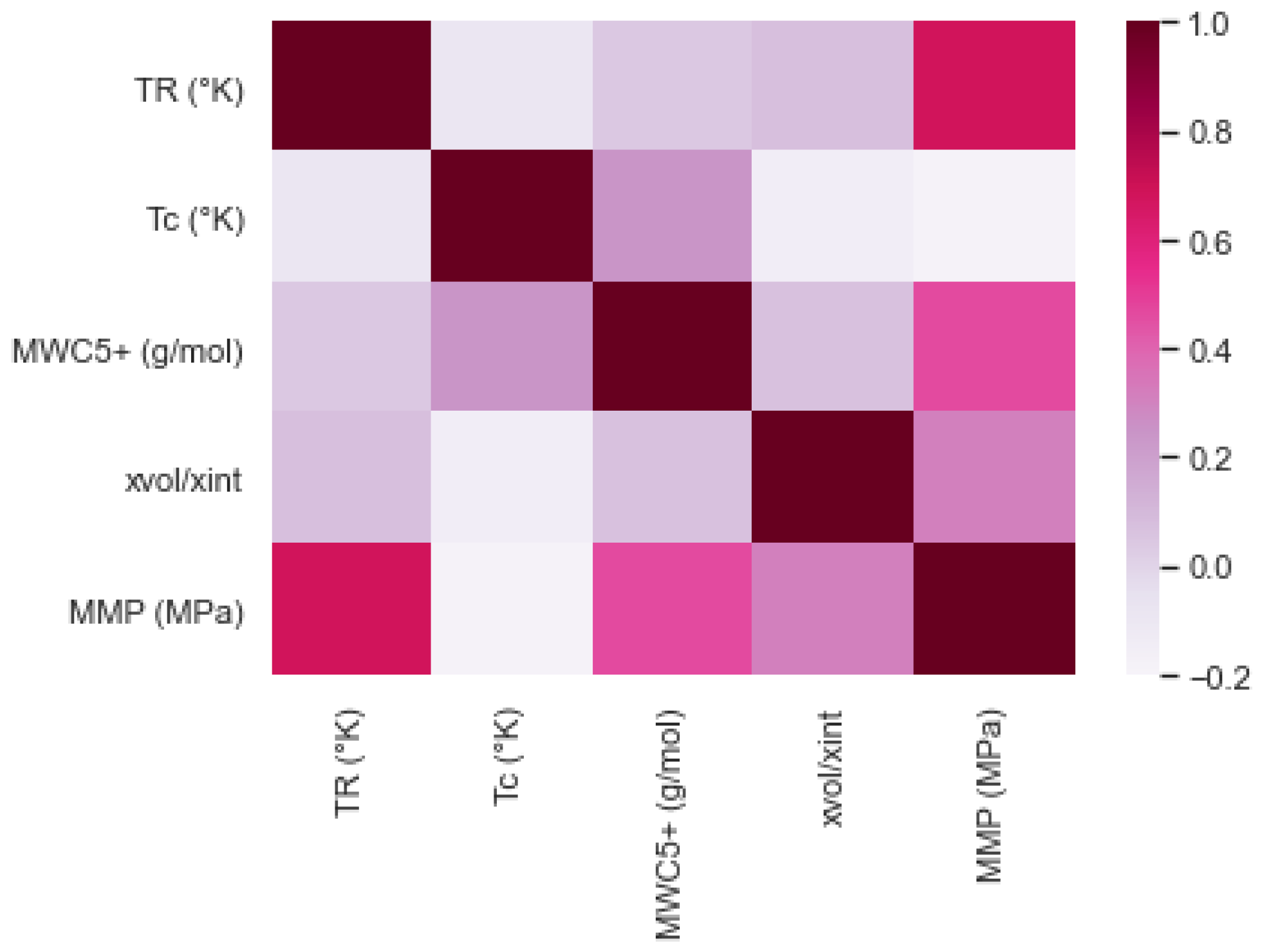



| Oil State | Experimental Data | No. of Samples | Mean | Std | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|---|
| Dead Oil | MW (gr/mole) | 105 | 350.6415 | 92.0752 | 196 | 246 | 358 | 424 | 490 |
| γ | 105 | 0.9257 | 0.0481 | 0.8382 | 0.8654 | 0.9452 | 0.9677 | 0.9867 | |
| T (°C) | 105 | 53.8450 | 35.75 | 18.33 | 26.17 | 48.89 | 69.0275 | 140 | |
| Ps (MPa) | 105 | 6.9716 | 4.5963 | 0.5 | 3.5475 | 6.02 | 9.5725 | 27.38 | |
| Rs (Mole fraction) | 105 | 0.4575 | 0.1725 | 0.1 | 0.313 | 0.4789 | 0.6048 | 0.847 | |
| Live Oil | MW (gr/mole) | 74 | 152.8364 | 61.9598 | 80.7 | 115.7 | 133.2 | 173.575 | 391.6 |
| γ | 74 | 0.8371 | 0.0617 | 0.6748 | 0.8348 | 0.8498 | 0.8789 | 0.9663 | |
| T (°C) | 74 | 65.9297 | 19.122 | 28 | 59 | 64.7 | 67 | 123.9 | |
| Pb (MPa) | 74 | 8.5052 | 5.8059 | 2.15 | 3.05 | 6.2 | 11.91 | 18.52 | |
| Ps (MPa) | 74 | 13.6241 | 7.1675 | 3.23 | 8.3075 | 12.33 | 17.24 | 32.76 | |
| Rs (Mole fraction) | 74 | 0.4103 | 0.1677 | 0.1083 | 0.2716 | 0.4182 | 0.5381 | 0.7201 |
| Oil State | Experimental Data | MW (gr/mole) | γ | T (°C) | Pb (MPa) | Ps (MPa) |
|---|---|---|---|---|---|---|
| Dead Oil | Rs (Mole fraction) | −0.0713 | −0.0934 | −0.1696 | - | 0.7813 |
| Live Oil | Rs (Mole fraction) | 0.0231 | 0.0181 | 0.0774 | −0.0132 | 0.3844 |
| Experimental Data | No. Of Samples | Mean | Std | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| MW (g/mol) | 1071 | 175.6069 | 64.6520 | 96 | 134 | 175 | 222 | 275 |
| P (MPa) | 1071 | 6.3848 | 4.1064 | 0.097 | 3.025 | 6 | 9.085 | 17.1 |
| T (K) | 1071 | 350.6999 | 31.6949 | 297.85 | 323.175 | 344.3 | 373.1 | 443.05 |
| IFT (mN/m) | 1071 | 9.8366 | 5.8556 | 0.001 | 5.225 | 9.37 | 14.15 | 27.05 |
| Experimental Data | MW (gr/mole) | P (MPa) | T (K) |
|---|---|---|---|
| IFT (mN/m) | 0.2918 | −0.8577 | −0.2042 |
| Experimental Data | No. of Samples | Mean | Std | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| TR (K) | 201 | 345.4395 | 24.3101 | 307.55 | 327.59 | 338.71 | 362.040 | 410.37 |
| Tc (K) | 201 | 302.7178 | 8.3058 | 281.45 | 295.29 | 304.19 | 304.190 | 338.77 |
| MWC5+ (g/mol) | 201 | 194.6348 | 40.1033 | 136.26 | 171.1 | 187.80 | 211.213 | 391 |
| xvol/xint | 201 | 1.5955 | 2.0928 | 0 | 0.51 | 0.74 | 1.5 | 13.6067 |
| MMP (MPa) | 201 | 16.0235 | 6.1184 | 6.50 | 11.138 | 14.80 | 19.12 | 38.52 |
| Experimental Data | TR (K) | Tc (K) | MWC5+ (g/mol) | xvol/xint |
|---|---|---|---|---|
| MMP (MPa) | 0.6845 | −0.1829 | 0.4657 | 0.3133 |
| Number of hidden layers | 2 |
| Number of neurons in the hidden layers | 12 |
| Number of epochs | 1000 |
| Optimization algorithm | Adam |
| Activation function | Relu |
| Performance Indicator | MSE, MAE |
| Validation dataset | 16 Samples |
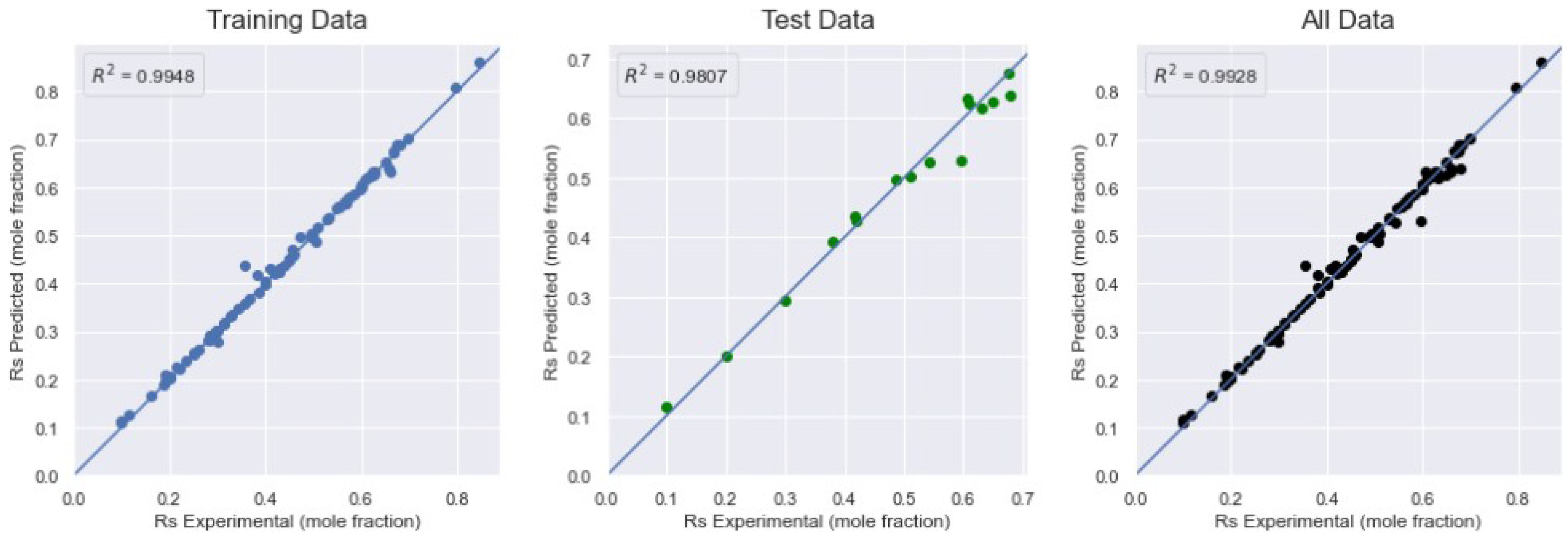
| Model | Training Data | Test Data | All Data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | |
| MLP-Adam | 2.0161 | 0.0123 | 0.9948 | 3.9629 | 0.0234 | 0.9807 | 2.3099 | 0.0145 | 0.9928 |
| Model | AARD (%) | RMSE | R2 |
|---|---|---|---|
| MLP-Adam | 2.3099 | 0.0145 | 0.9928 |
| Chung et al., 1988 [51] | 99.4213 | 0.5138 | 0.0083 |
| GA—Emera and Sarma, 2011 [53] | 6.1521 | 0.0546 | 0.8987 |
| Rostami et al., 2017 [52] | 3.8709 | 0.02045 | 0.9858 |
| Hyperparameter | C | Epsilon | Gamma |
|---|---|---|---|
| Range | 0.1–50,000 | 0.0001–0.1 | 0.001–10 |
| Optimal value | 950 | 0.039 | 0.01035 |
| Model | Training Data | Test Data | All Data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | |
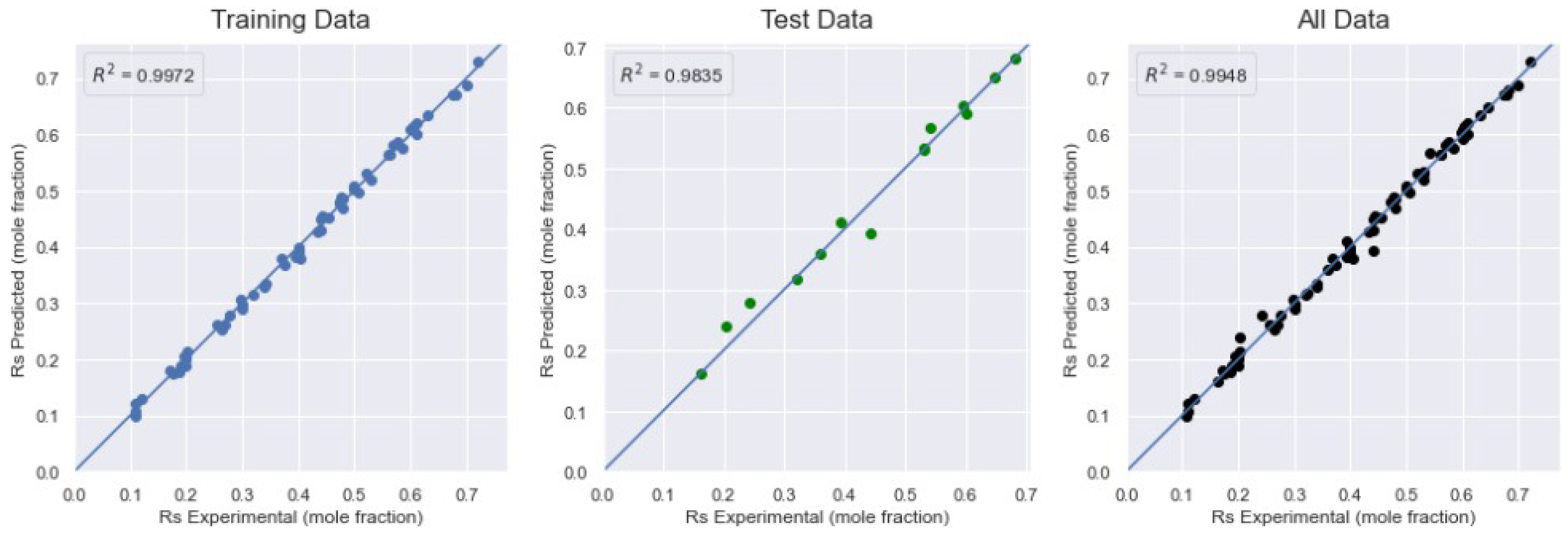
| SVR-RBF | 2.4618 | 0.0088 | 0.9972 | 4.2742 | 0.0209 | 0.9835 | 2.8047 | 0.0120 | 0.9948 |
| Model | AARD (%) | RMSE | R2 |
|---|---|---|---|
| SVR-RBF | 2.8047 | 0.0120 | 0.9948 |
| Chung et al. [51] | 99.9250 | 0.4425 | 0.0097 |
| GA—Emera and Sarma [53] | 4.9734 | 0.0295 | 0.9686 |
| Rostami et al. [52] | 3.7642 | 0.0203 | 0.9851 |
| Model | Hyperparameter | Range | Optimal Value |
|---|---|---|---|
| XGBoost | Number of trees | 100, 200, 400, 800, 1000, 2000 | 1000 |
| Regularization parameter λ | 0.0001, 0.001, 0.1, 0.3, 10, 100 | 0.001 | |
| Regularization parameter α | 0.01, 0.04, 0.09, 0.1 | 0.09 | |
| Gamma γ | 0, 0,1, 1, 10 | 0 | |
| Max. depth | 2, 4, 6, 8 | 4 | |
| Learning rate | 0.001, 0.01, 0.1 | 0.1 |
| Model | Training Data | Test Data | All Data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | |
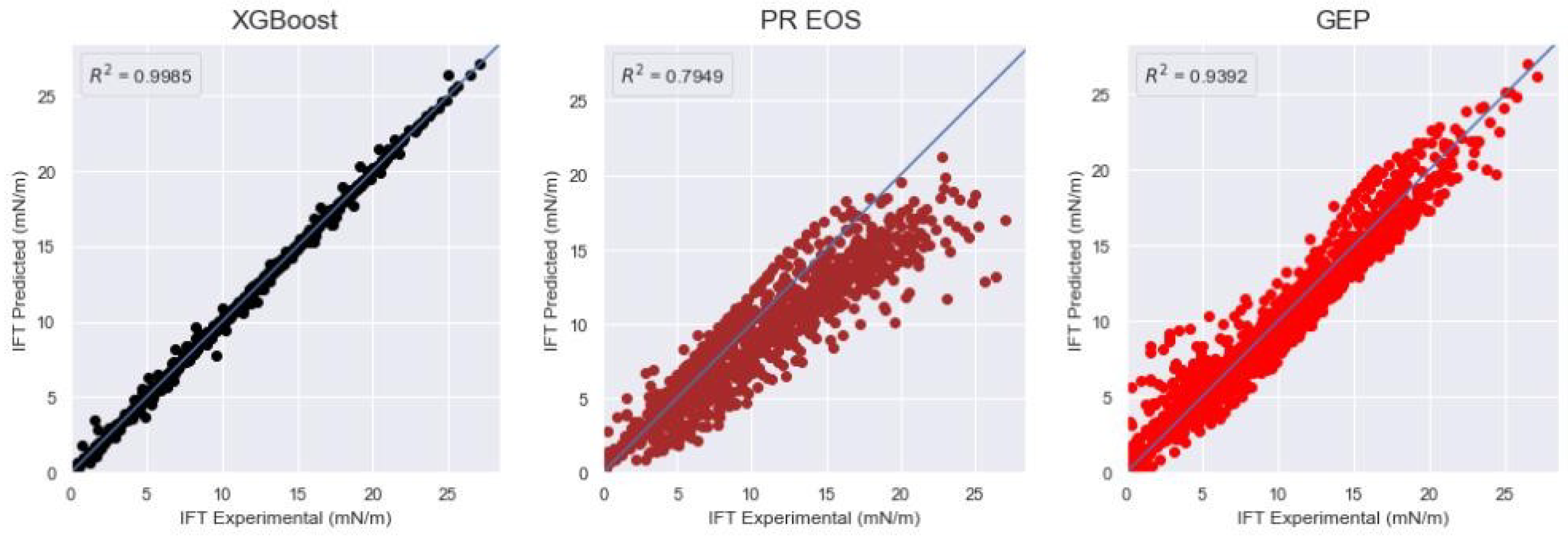
| XGBoost | 1.9386 | 0.0952 | 0.9997 | 8.6422 | 0.4698 | 0.9931 | 3.2844 | 0.2271 | 0.9985 |
| Model | AARD (%) | RMSE | R2 |
|---|---|---|---|
| XGBoost | 3.2844 | 0.2271 | 0.9984 |
| PR EOS | 60.5471 | 2.6261 | 0.7949 |
| GEP | 219.1053 | 1.4437 | 0.9391 |
| Model | Hyperparameters | Range | Optimal Value |
|---|---|---|---|
| XGBoost | Number of trees | 100, 1000, 4000, 5000, 8000 | 8000 |
| Regularization parameter λ | 0.0001, 0.001, 0.1, 0.3, 15, 100 | 15 | |
| Regularization parameter α | 0.01, 0.02, 0.09, 0.1 | 0.02 | |
| Gamma γ | 0, 0,1, 01, 10 | 0 | |
| Maximum depth | 2, 4, 6, 8 | 2 | |
| Learning rate | 0.001, 0.01, 0.1 | 0.1 |
| Model | Training Data | Test Data | All Data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | AARD (%) | RMSE | R2 | |
| XGBoost | 0.9326 | 0.1893 | 0.9986 | 4.0043 | 0.941 | 0.9648 | 1.4262 | 0.4151 | 0.9934 |
| Model | AARD (%) | RMSE | R2 | |
|---|---|---|---|---|
| Pure CO2 | XGBoost (Pure) | 0.9161 | 0.1936 | 0.9988 |
| Lee [56] | 18.781 | 5.1538 | 0.5146 | |
| Alston et al. (Pure) [48] | 18.177 | 5.5472 | 0.7063 | |
| Emera-Sarma [57] | 13.2203 | 3.7385 | 0.6161 | |
| Impure CO2 | XGBoost (Impure) | 1.9525 | 0.558 | 0.9856 |
| Alston et al. (Impure) [48] | 34.5324 | 6.4668 | 0.5967 | |
| Fathinasab-Ayatollahi [58] | 15.0134 | 2.702 | 0.7019 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamadi, M.; El Mehadji, T.; Laalam, A.; Zeraibi, N.; Tomomewo, O.S.; Ouadi, H.; Dehdouh, A. Prediction of Key Parameters in the Design of CO2 Miscible Injection via the Application of Machine Learning Algorithms. Eng 2023, 4, 1905-1932. https://doi.org/10.3390/eng4030108
Hamadi M, El Mehadji T, Laalam A, Zeraibi N, Tomomewo OS, Ouadi H, Dehdouh A. Prediction of Key Parameters in the Design of CO2 Miscible Injection via the Application of Machine Learning Algorithms. Eng. 2023; 4(3):1905-1932. https://doi.org/10.3390/eng4030108
Chicago/Turabian StyleHamadi, Mohamed, Tayeb El Mehadji, Aimen Laalam, Noureddine Zeraibi, Olusegun Stanley Tomomewo, Habib Ouadi, and Abdesselem Dehdouh. 2023. "Prediction of Key Parameters in the Design of CO2 Miscible Injection via the Application of Machine Learning Algorithms" Eng 4, no. 3: 1905-1932. https://doi.org/10.3390/eng4030108
APA StyleHamadi, M., El Mehadji, T., Laalam, A., Zeraibi, N., Tomomewo, O. S., Ouadi, H., & Dehdouh, A. (2023). Prediction of Key Parameters in the Design of CO2 Miscible Injection via the Application of Machine Learning Algorithms. Eng, 4(3), 1905-1932. https://doi.org/10.3390/eng4030108