1. Introduction
Drinking water requirement is expanding in Egypt because of the rapidly growing population rate and development practices. The River Nile and Nile aquifers, which provide approximately 60% and 40% collectively, are the two crucial forms of water supply [
1]. In Egypt, people in rural areas rely entirely on groundwater as the source of their water supply. The aquifer is constantly recharged in the Nile Valley, primarily by infiltrating water from the irrigated fields and irrigation water from the irrigation waterways. Agrochemicals have progressively been used to supply crops with vital nutrients for improved productivity. Potassium (K), sulfur (S), nitrogen (N) and phosphorus (P) are the main nutrients in the specific sequence of absorption by plants [
1,
2,
3].
Since the development of the High Aswan Dam in the 1960s, fertilizer application has risen dramatically. The development of the High Aswan Dam, that decreased the number of suspended materials accumulated in between flooding on the surface, authorized the fertility of Egyptian soils to be preserved for millennia. For example, fertilizer application rates have gone up by 81%, 63% and 115% for P, N and K, accordingly, from 1976 to 2000 [
4,
5]. In agricultural areas, groundwater degradation happens as those nutrients leach far below the root area and also into the groundwater. Multiple instances of groundwater pollution from widespread fertilizer applications in watersheds used for farming purposes have been recorded globally over the last decades [
6,
7,
8]. In such instances, the significant ion concentrations were nitrate (
), sulfate (
),
and phosphate (
). Health issues, notably in children, have been associated with concentrations higher than their standard values in drinking water. Nitrate is the most common pollutant in groundwater globally. It can be expensive as well as complicated to clear away water that is polluted with nitrate, thus there is considerable concern in monitoring nitrate sources. Nitrate’s possible health consequences include birth implications, cancer and impairments in the nervous system, as well as methemoglobinemia, World Health Organization (WHO) [
3]. As nitrate oxidizes the iron in hemoglobin, methemoglobinemia exists, thereby lessening the ability of the blood to hold oxygen.
This research seeks to secure the source of groundwater from nitrate pollution in Upper Egypt, and moreover, to analyze the brief effect on the efficiency of pumped groundwater of the existing application of chemical nitrogen fertilizers. For this research, a municipal wellfield in Upper Egypt, the Nile valley aquifer, was identified as an examination location. Several water specimens were obtained for water safety and chemically examined. The subsequent segments include analysis and discussion of the consequences.
2. Nitrogen Fertilizers in the Research Field
Nitrate is the most effective fertilizer, based on the level of use and the medical issues of drinking water. Groundwater contamination due to nitrate is a global pandemic. Previous research has demonstrated that the other forms of fertilizer, like potassium, phosphorus and sulfate, do not leach large concentrations into the groundwater table. The primary focus of the present research is thus calculations and discussion about nitrate fertilizers.
Nitrogen is added to the soil in the research area in inorganic or organic forms. The following are the major fertilizer forms used:
Urea (NH2)2CO (46.5% N)
Ammonium nitrate NH4NO3 (33.5% N)
Ammonium sulfate NH4SO4 (20.6% N)
Calcium nitrate (NO3)2Ca (15.5% N)
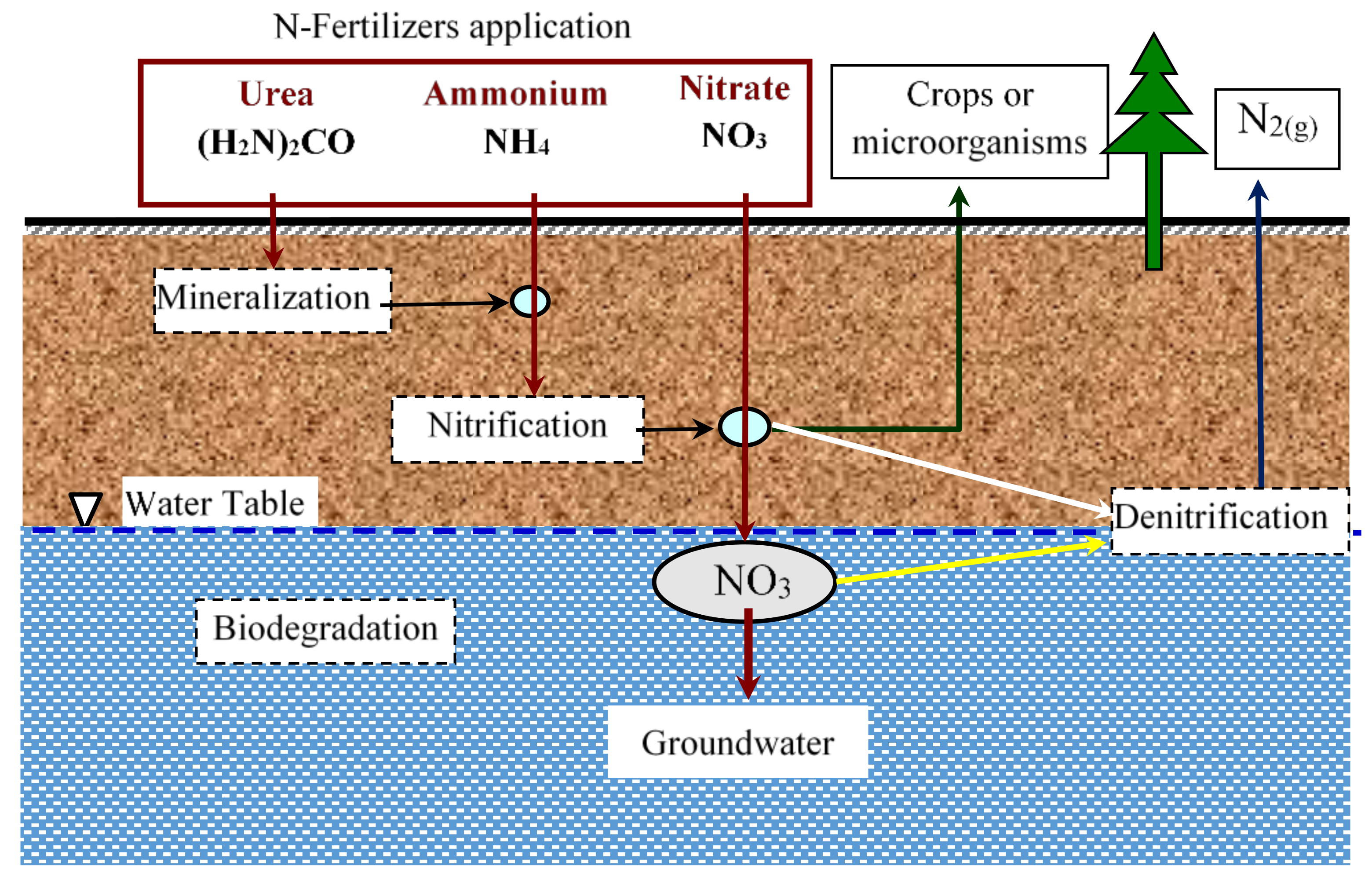
Nitrate, nevertheless, is the very predominant nitrogen species, extremely active in groundwater, and is not consumed by soil grains. The primary internal soil reactions that affect both nitrate mobility and leaching quantity are nitrification, mineralization-immobilization and denitrification, in contrast to plant uptake as well as fertilizer application, as illustrated in
Figure 1 [
3,
9,
10]. Nitrate is obtained primarily from the fertilizers in the form of nitrate or by the vadose zone’s nitrification process. Microbial oxidation of ammonium into nitrite (
) and then to nitrate is known as nitrification. The two ultimate
to
oxidation equations are specified in Equations (1) and (2):
In contrast to the depletion of nitrogen from the core areas, this is the primary reaction as it converts the comparatively unresponsive ammonium ion into nitrate that can then be denitrified or leached.
Immobilization is the integration of inorganic N into the unsaturated area by microorganisms to produce organic N. Mineralization, which is the ammonium formation throughout decomposition of microbial or organic N, is the inverse of immobilization. The major biological reaction involving nitrate species in which microorganisms turn nitrate into nitrogen gas within anaerobic environments is denitrification.
Autotrophic, as well as heterotrophic microbes, are utilized as electron acceptors and degradable organic matter or inorganic depleted compounds as terminal electron acceptors under minimal oxygen circumstances throughout the denitrification cycle. Organic matter’s heterotrophic denitrification may be interpreted via Equation (3) and also the autotrophic denitrification in the pyrite matrix of the inorganic material can be interpreted by Equation (4):
Inside the clay silt cap (Nile Valley aquifer), where organic content is accessible, heterotrophic denitrification could happen, whereas autotrophic denitrification could take place in all the graded sand-gravel surfaces as well as the clay-silt cap, within which pyrite is accessible [
11].
Bouwer [
12], as well as Goderya et al. [
13], have stated that nearly 30% to 50% of nitrogen fertilizer leaches in the
form added to the groundwater. This level of leaching has led to numerous locations globally to be polluted with nitrate.
3. Materials and Methods
3.1. Environmental Setting
A broad, parochial valley of differing widths, starting from 3 km at Aswan to around 20 km at El-Minia, and also approximately 1500 km long, has been formed by the River Nile in Egypt. The valley floor created by the River Nile floodplain is quite plain. The whole valley is primarily utilized for agriculture production, except those regions inhabited by buildings and streets. Due to comprehensive usage of nitrogen fertilizers, a municipal wellfield (26°07′29″ N, 32°04′05″ E), situated in the AbuTisht District (Qena Governorate), Upper Egypt segment of the Nile Valley, was identified as an investigation research location (
Figure 2).
Hydraulic conductivity as well as recharge are the two significant hydrological characteristics. For the aquifer of Nile Valley, the horizontal hydraulic conductivity of the silt-clay cap is 0.20 m/day, whereas the sand-gravel strata ranges from 60 to 110 m/day, as indicated by the Research Institute for Groundwater in Egypt [
14,
15]. The vertical conductivity is also 0.04 m/day and ranges from 7 to 12 m/day, respectively. The primary sand gravel aquifer is intermixed with clay lenses that decrease its hydraulic conductivity. The final hydrological factor is the recharge into the water table, which in the research region varies from 0.85 to 1.1 mm/day [
16].
The aquifer of the Nile alone is not a natural supply of water, but rather serves as a reservoir. Infiltration of irrigation water as well as drainage from irrigation canals is the only significant recharge to the aquifer. Across the Nile Valley, the precipitation is exceptionally low, that is approximately 20 mm/year. In the River Nile, the surface of the water is smaller than the elevations of both the water table as well as the aquifer’s piezometic head. Thus, for the aquifer of Nile Valley, the river serves as a sewer. The aquifer in the flood zone is usually extremely fertile and the salinity of the groundwater is even less than 1000 mg/L [
2,
17]. Groundwater pollution can arise from farming, commercial or household activities in the AbuTisht region. Even so, the primary cause of contamination, since it occupies much of the inhabited area, tends to be correlated with agricultural activity (
Figure 3).
3.2. Fertilizers and Agricultural Actvities
Agricultural activities are the predominant occupation of residents in the Nile Valley, inclusive of AbuTisht County. Around 85% of the overall region of land in AbuTisht is planted. In the winter season, sugarcane, barley and berseem (Egyptian clover) are the primary crops, whereas, in the summer season, maize, corn and vegetables are the primary crops. In October–November, the winter cultivation period begins, and in May–June, the summer cycle begins. Nearly 90% of the land, nevertheless, is sugarcane-grown. Sugarcane is a seasonal plant, and it is re-grown every 5 years. Agricultural water is added by the conventional flooding process, which happens in summer and winter at a pace of 2 and 3 times a month, respectively. The irrigation scheme is composed of canals, and in open drains, the drainage water is stored. In the region, there are no essential subsurface drains.
Ammonium nitrate, urea, superphosphate, ammonium sulfate and calcium nitrate are the widely utilized commercial fertilizers in the research region. Fertilizers are distributed to farmers via the Agricultural Cooperative Society (ACS). From the field information, the application amounts of the fertilizers were calculated.
Table 1 illustrates both the times as well as the application rates of N and P in the research region. N and P fertilizer rates are classified as comprehensive to moderate. The dose of sugarcane fertilizer is split into three different applications a month apart, beginning in March of every year. The N dosage for sugarcane is greater than that of the prescribed by the Ministry of Agriculture and Land Reclamation: (MARL) dose [
18].
Sugarcanes, as well as other crops, obtain their fertilizer requirements from the irrigation water and subsurface soil, and the excessive volume will percolate. Groundwater table is 2–3 m below the ground surface. Many biological, as well as chemical transformations, happen in both the root and vadose region following fertilizer application. The volume of fertilizer nutrient leaching is similar to the total applied to the surface minus crop uptake, considering the transition of nutrients in the vadose region. As stated by several researchers [
2,
12], after the time of application, elevated concentrations at the water table were recorded. This is owing to the solute movement throughout the fertilizer application. Even so, orthophosphate (
) and nitrate (
) ions are the principal types of leaching fertilizers into the water table [
1,
13].
3.3. Drinking Wells and Water Sampling
The location under research is among the rural communities of the Qena governorate in Upper Egypt. Inhabitants of approximately 25,000 residing in 4 villages are represented by the examined wellfield. As the source of drinking water needs, the residents from these villages rely on groundwater utilizing pumping wells. The water distribution strategy comprises of one wellfield as well as a system of supply pipelines that deliver water to the villagers. The wellfield contains three deep wells with 60 m deep, 30 cm diameter and a production potential of 100 m
3/h, as illustrated in
Figure 3. Wells are constructed according to Egyptian specifications with cementing and gravel back. The collected water is pumped into a high-elevated tank, 200 m
3 volume and 35 m height. Operation plan includes one well pumping, of those three wells, every 2 h between 6:00 a.m. to 11:00 p.m. All wells are off for the rest of the night hours. It is, nevertheless, assumed that this distance inwards of approximately 60 meters of the extraction wells is protected from both chemical contaminants as well as biological pollution.
As described earlier, in this region, there is comprehensive usage of chemical fertilizers, in particular nitrogen. A feasibility analysis for this area was then conducted to track as well as preserve the source of drinking groundwater. Grab specimens were taken from the three pumping wells each month for five years between 2000 and 2018. Utilizing the total dissolved solids (TDS) meter, Hach DR 2000 spectrophotometer and Hanna portable pH meter, these water specimens were examined chemically in the region. Water analyses were carried out following the Standard Methods (APHA) [
19]. For all the years studied, 2000, 2006, 2009, 2012 and 2018, nitrate was measured each month. Other performance variables were evaluated for the entire 12 months of 2018, like pH, TDS, sulfate, phosphate and iron. This wellfield started to operate as a standby in 2018, when a centralized treatment plant operated to supply water from the Nile directly.
4. Results and Discussion
4.1. Physiochemical Water Quality of Wellfiled in Different Seasons
Different important physiochemical parameters of the pumped groundwater measured each month in 2018 are given in
Table 2. Measurements of pH, TDS, NO
3, PO
4, SO
4 and Fe ions are given in mg/L. The recent results from this study are in agreement with the previous measurements for the same region in Nile Valley presented in References [
4,
5,
20,
21,
22]. The measured values of those selected specific parameters, in general, are less than the permissible limits for drinking water according to the Egyptian Standards. Regarding TDS, the monthly values ranged between 500 and 590 mg/L, which are less than the allowable limit (1000 mg/L) for Egyptian drinking water. Winter (January–March) values of TDS are higher than summer (May–September) values. This variation might be due to less recharge of aquifer in winter from low irrigation water that has less TDS than groundwater, in general. Seasonal changes of NO
3 and long-term trends and correlation with sulfate will be presented and discussed in the next sections. There are variations in the values of pH and Fe, but they are out of our research focus.
4.2. Seasonal Vairiations of Nitrate throughout the Year
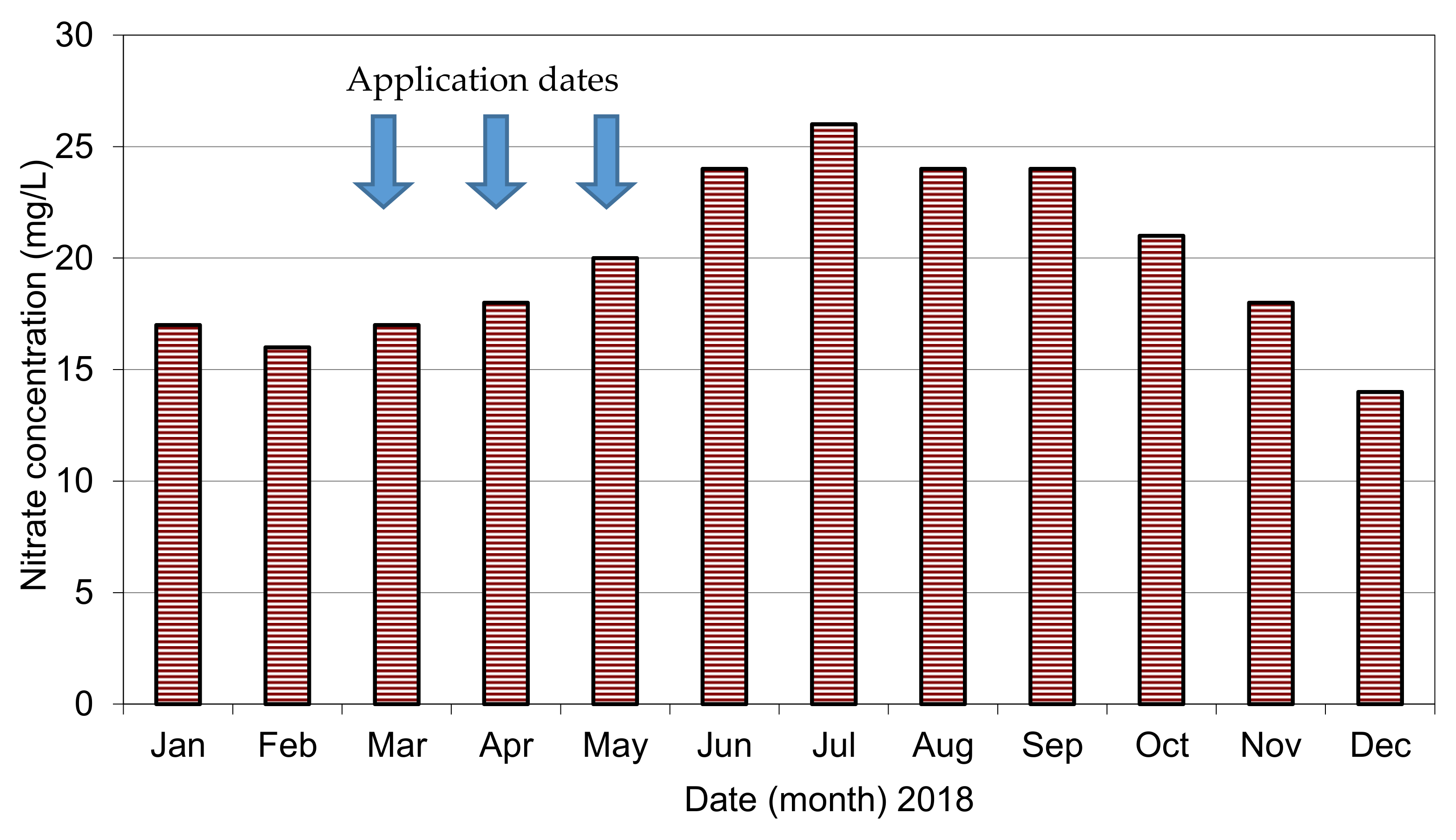
Figure 4 shows nitrate concentrations each month and for different seasons. Nitrate ranged from 14 to 26 mg/L. All nitrate results are less than the Egyptian Standard, which is 45 mg/L. Dates of N-fertilizer applications into crops are illustrated in
Figure 4. According to Bouwer [
12], nitrate (N) values more than 3.0 mg/L are above natural levels and indicate an anthropogenic (human-related) source. According to this, it is clear that all measured values of nitrate are above natural levels in the study area.
Figure 4 shows that there are notable variations in nitrate values, especially pre- and post-fertilizer applications in the area. These elevated values of nitrate concentrations are probably due to fertilizers, septic tanks or other anthropogenic sources. However, these concentrations are higher than the previous ones reported in References [
11,
23] but less than those reported by Shamrukh [
4]. It is clear that values of nitrate after fertilizer applications are higher than nitrate before fertilizer applications,
Figure 4.
Results indicate that there is significant correlation between nitrate elevated values and fertilizer application in the study area. In addition, elevated values of nitrate in the wellfield are two months after fertilizer applications. This indicates that the travel time of nitrate from application of irrigation water with fertilizers to sand-gravel aquifer is about two months. High nitrate values continue from July to October, as seen in
Figure 4. However, the measured values of nitrate are still not matching the high amounts (640 kg N/ha) of nitrogen fertilizers. These lower nitrate values in the study area compared to other studies worldwide [
9] might be because of (i) dilution with groundwater in the abstracted aquifer, (ii) significant denitrification by bacteria in the upper layer of the aquifer system and (iii) high N-uptake by sugarcane crops in the study area. Therefore, more observation wells located at different depths are strongly needed to understand nitrate transport and transformations in this aquifer system in Nile Valley.
4.3. Trendline of Nitrate in the Study Area
There are few works in the literature to model and predict nitrate concentrations in groundwater wells in Nile Valley. However, in the current study, measured values of nitrate will be used to assess the trendline of nitrate in the study area. It is noted in the current results that there are seasonal variations in nitrate pre- and post-fertilizer applications. However, high nitrate values post-fertilizer applications from July to September will be used for estimating the trendline of nitrate in the study area.
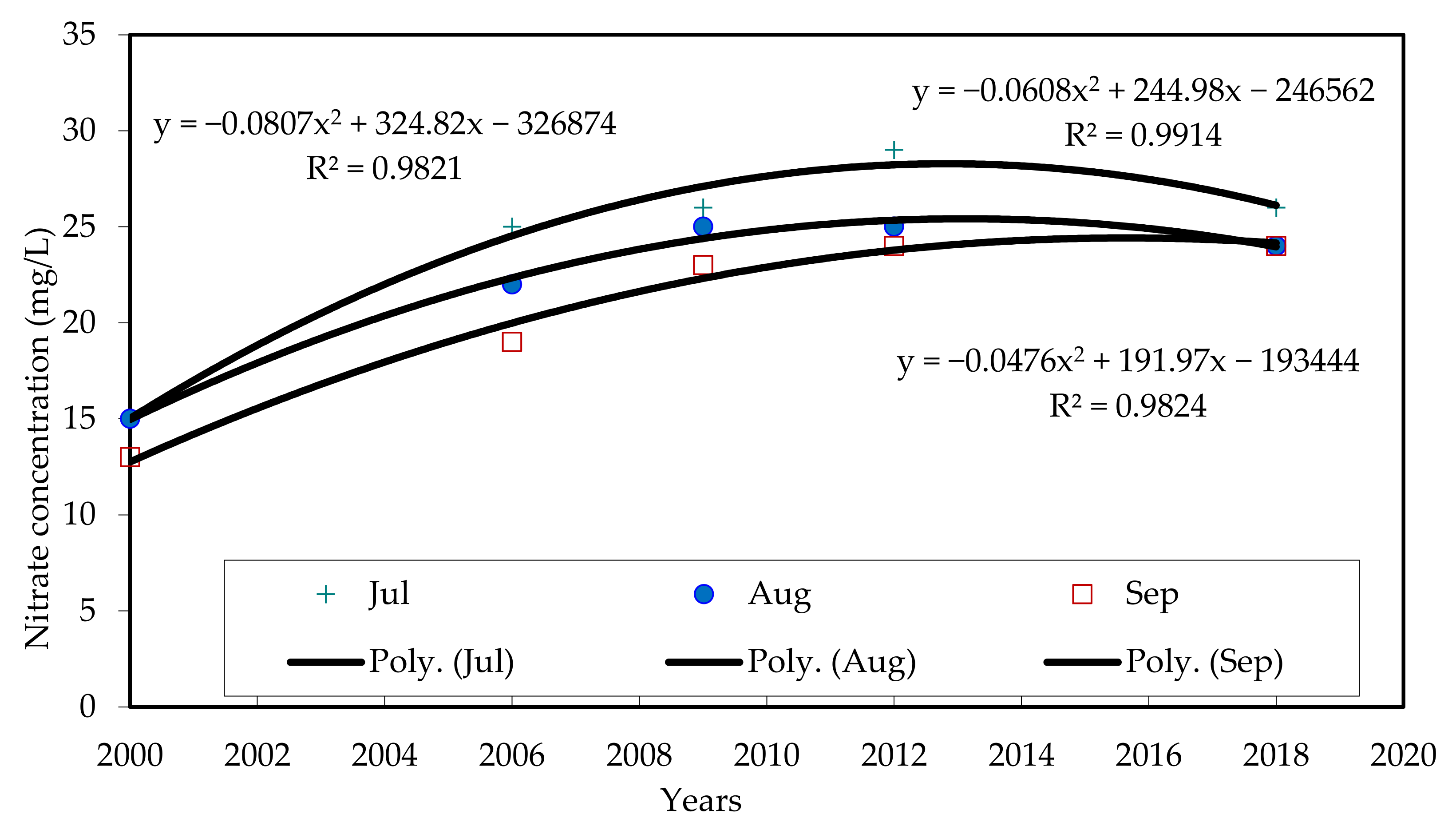
Table 3 shows the monthly measured values of nitrate for five years between 2000 and 2018. Elevated values in July, August and September post-fertilizer applications are highlighted. In general, there was an increase in nitrate values from 2000 to 2009 for all months and in 2012 for a few months. However, nitrate values did not increase after 2012 but did decrease for most of the months. This is not what was predicted by previous studies [
4,
5]. This decline in nitrate concentration needs to be confirmed in this site and other locations in coming years. However, this might be due to reductions in the fertilizers’ usage because of the high cost of fertilizers after 2016.
Figure 5 shows nitrate measurements for five years between 2000 and 2018 (2000, 2006, 2009, 2012, 2018). Only elevated results of July, August and September are used in this trendline figure. Linear regression was not good to represent the trendline of nitrate values. To best-fit the correlations between the trendline and nitrate values for each year, nonlinear regressions were found to be the best. R
2 values for the nonlinear equations of the trendlines are higher than 95%. These nonlinear regressions (poly in our case) were used to assess the future of nitrate in this wellfield. As mentioned before, the Egyptian Standard for nitrate in drinking water is 45 mg/L. Trendlines indicate that nitrate will not reach the allowable limit of 45 mg/L in the near future, under the current conditions. This is not in agreement with the previous results from the study carried out for the same wellfield [
4]. However, the current results are in agreement with three-dimensional (3D) simulations for nitrate prediction in Nile Valley, which predicated that nitrate would take a long time (>20 years starting in 2000) to reach the allowable limits [
20].
4.4. Sulfate and Nitrate Correlations
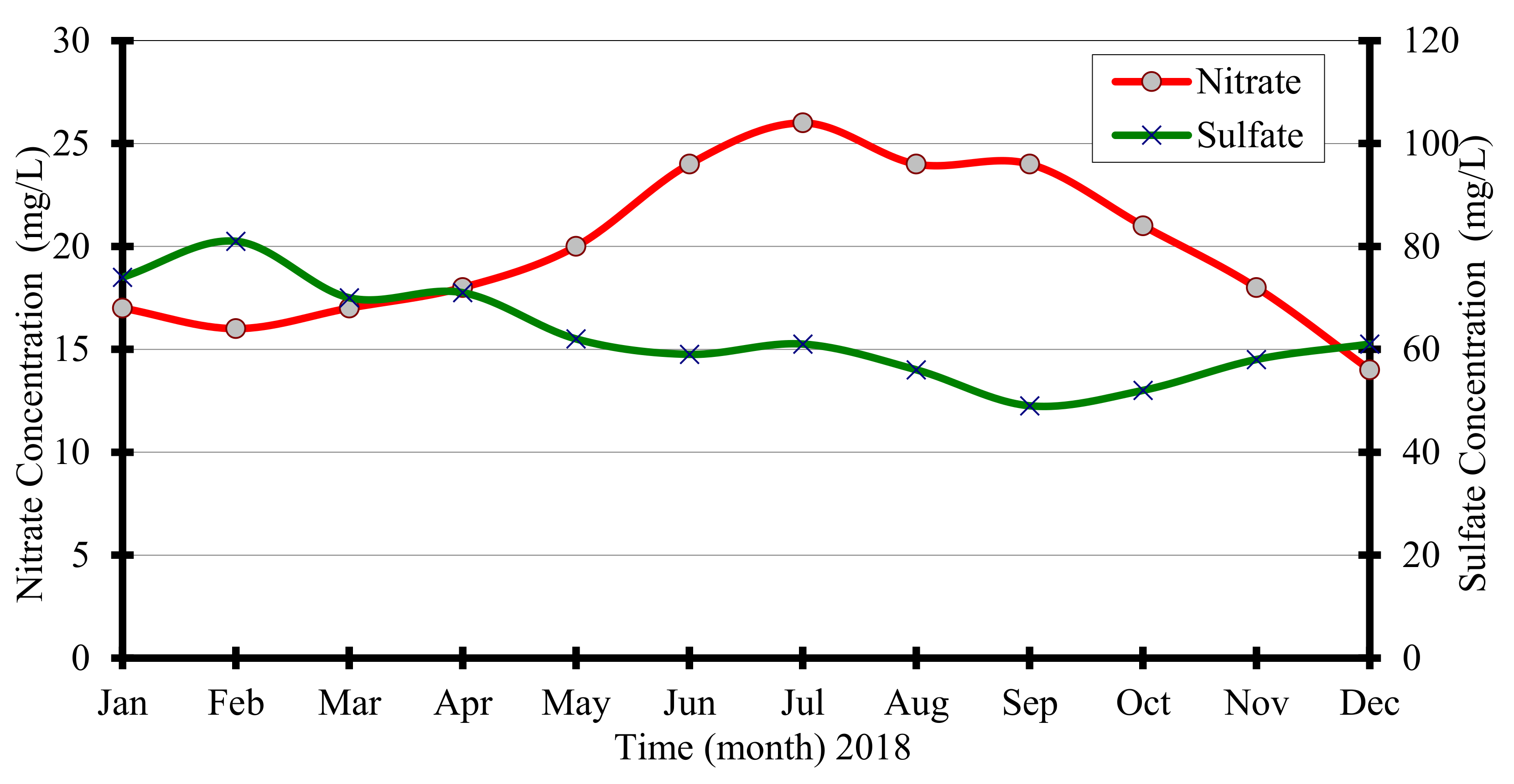
Sulfate values in the study wellfield for each month for the year 2018 were given in
Table 2. Measured values ranged from 49 to 81 mg/L. Egyptian Standard for sulfate
for drinking water is 250 mg/L. All sulfate values in this wellfield are less than the allowable limit. However, natural sulfate concentrations in the Nile aquifer are about 20 mg/L [
17]. It is worth mentioning that there is no direct application of sulfur fertilizer in the studied area. The correlation between sulfate produced from nitrate (i.e., denitrification) in the presence of pyrite was given previously in Equation (4), as reported by Dekov et al. [
11]. This type of reaction is an oxidation-reduction reaction.
Oxidation of sulfide in pyrite by O
2 depends on the availability of O
2 and it is a slow reaction. In general, pyrite contents in the sediments of the Nile Valley are about 17 kg/m
3 of S, as reported in previous studies. Thermodynamically, assimilation with the subsurface organic matter as an electron donor is more favorable than pyrite [
24]. Redox reactions of reduced S-compounds, such as pyrite, may occur in unsaturated and saturated zones. Therefore, oxidation of reduced sulfur under anaerobic conditions by nitrate (i.e., denitrification) might occur in the case of organic matter unavailability as an electron donor. Other sources of sulfate such as septic tanks might also occur.
To assess the correlation between nitrate and sulfate values in the studied aquifer,
Figure 6 is given. Results in the figure shows that when nitrate values decrease, sulfate content increases. Plotted results in this figure indicate that Equation (4) is valid and takes place in the study area of Nile Valley. However, there are other sources in both vadose and saturated zones that may contribute to elevated sulfate in the wells, such as dissolution of gypsum sediments, redox reactions of reduced sulfur compounds and septic tanks. However, in the presence of oxygen and/or nitrate as electron acceptors and reduced sulfur as an electron donor, redox reactions may occur in the study area.
5. Conclusions
A wellfield in Upper Egypt used as a potable water supply was investigated for long-term groundwater monitoring in the Nile Valley aquifer. Nitrate and other physiochemical quality parameters were conducted to assess anthropogenic impact to protect potable water from contamination. In general, all the measured quality parameters were less than the Egyptian Standards for potable water. However, elevated values of nitrate and sulfate above naturally occurring levels were detected in the well’s groundwater. These results indicate that anthropogenic activities, especially nitrogen fertilizers, have an influence on the quality of groundwater in Nile Valley. However, recent nitrate values are less than the previous reported values ten years ago. One reason for this decreasing trend is possibly due to the reduction of fertilizers’ usage due to its increasing cost after 2016. There were high nitrate values in well water post-fertilizer application for sugarcane crops in the study area. Trendline of nitrate (nonlinear regression, poly equation) indicates that nitrate in well water will not cross the allowable limits for potable water in the near future under the current conditions in the study area. In addition, results indicate that there is an inverse correlation between sulfate and nitrate. It is noted that sulfate is increasing while nitrate is decreasing, probably due to denitrification converting it to sulfate due to redox reactions in the subsurface. Results indicate that there is a strong need to establish a groundwater quality monitoring system in the Nile Valley located at different depths. A monitoring program should include periodical sampling of both observation and water supply wells for nitrate, sulfate, chloride and other physiochemical parameters. Isotopes’ measurements are useful to confirm the sources of nitrate and sulfate in the Nile Valley aquifer.
Author Contributions
Conceptualization, M.S.; methodology, M.S.; resources, M.S.; writing—original draft preparation, M.S.; writing—review and editing, S.A.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors gratefully acknowledge the assistance of the manuscript reviewers that improved the original version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shamrukh, M. Effect of Chemical Fertilizers on Groundwater Quality in Upper Egypt. Ph.D. Thesis, Minia University, Minya, Egypt, 1999. [Google Scholar]
- El-Fouly, M.M.; Fawzi, A.F. Higher and Better Yields with Less Environmental Pollution in Egypt through Balanced Fertilizers Use. Fertilizers and Environment. In Proceedings of the International Symposium on Fertilizers and Environment, Salamanca, Spain, 26–29 September 1994; pp. 19–22. [Google Scholar]
- Abdel-Dayem, S.; Abdel-Ghani, M. Concentration of Agricultural Chemicals. In Proceedings of the 6th International Symposium on Drainage and Water Table Control, Nashville, TN, USA, 13–15 December 1992; pp. 353–360. [Google Scholar]
- Shamrukh, M. Seasonal and Long-term Changes in Nitrate Content of Water Supply Wells in Upper Egypt. In Proceedings of the 1st IWAYWP, Kuala Lumpur, Malaysia, 1–4 March 2010. [Google Scholar]
- Abdel-Lah, A.K. Quality Tracing of Well Water in Irrigated Agricultural Lands, Nile Valley of Egypt. In Proceedings of the International Conference for Development and the Environment in the Arab World, Assiut, Egypt, 26–28 March 2002; pp. 313–325. [Google Scholar]
- Ahmed, M.A.; Aly, A.I.M.; Hussien, R.A. Assessment of Anthropogenic Nitrate Pollution in Groundwater in Northeast Cairo using Nitrogen-15 Technique. Arab J. Nucl. Sci. Appl. 2007, 40, 55–70. [Google Scholar]
- Podlasek, A.; Bujakowski, F.; Koda, E. The spread of nitrogen compounds in an active groundwater exchange zone within a valuable natural ecosystem. Ecol. Eng. 2020, 146, 1–15. [Google Scholar]
- Cassell, E.A.; Clausen, J.C. Dynamic Simulation Modeling for Evaluating Water Quality Response to Agricultural BMP Implementation. Water Sci. Technol. 1993, 28, 635–648. [Google Scholar] [CrossRef]
- Korom, S.F. Natural Denitrification in the Saturated Zone: A Review. Water Resour. Res. 1992, 28, 1657–1668. [Google Scholar] [CrossRef]
- Spalding, R.; Exner, M. Occurrence of Nitrate in groundwater: A review. J. Environ. Qual. 1993, 22, 392–402. [Google Scholar] [CrossRef]
- Dekov, V.M.; Komy, Z.; Araujo, F.; Van Put, A.; Van Grieken, R. Chemical Composition of Sediments, Suspended Matter, River Water and Ground Water of Nile (Aswan-Sohag Traverse). Sci. Total Environ. 1997, 20, 195–210. [Google Scholar] [CrossRef]
- Bouwer, H. Effect of Irrigated Agriculture on Groundwater. J. Irrig. Drain. Eng. (ASCE) 1987, 113, 4–15. [Google Scholar] [CrossRef]
- Goderya, F.S.; Dahab, M.F.; Woldt, W.E.; Bogardi, I. Incorporation of Spatial Variability in Modeling Non-Point Source Groundwater Nitrate Pollution. Water Sci. Technol. 1996, 33, 233–240. [Google Scholar] [CrossRef]
- Research Institute for Groundwater. Hydrogeological Map of Egypt: Nag-Hammady Region (1:100,000); Ministry of Water Resources and Irrigation: Shoubra El-Kheima, Egypt, 1990. [Google Scholar]
- Bowen, R. Aspects of Egyptian Hydrogeology. Water Int. 1996, 11, 64–70. [Google Scholar] [CrossRef]
- Warner, J.W.; Gates, T.K.; Attia, F.A.; Mankarious, W.F. Vertical Leakage in Egypt’s Nile Valley: Estimation and Implication. J. Irrig. Drain. Eng. (ASCE) 1991, 117, 515–533. [Google Scholar] [CrossRef]
- Attia, F.A. Groundwater Quality in the Nile Aquifer System and Desert Fringes in Egypt. Groundw. Qual. 1989, 17, 123–129. [Google Scholar]
- MALR. Bulletin of Agriculture Economics; Central Administration of Agriculture Economics, Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2003. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1995. [Google Scholar]
- Shamrukh, M.; Corapcioglu, M.Y.; Hassona, F. Modeling the Effect of Chemical Fertilizers on Ground Water Quality in the Nile Valley Aquifer, Egypt. Ground Water 2001, 39, 59–68. [Google Scholar] [CrossRef]
- Abdalla, F.; Shamrukh, M. Quantification of River Nile/Quaternary aquifer exchanges via riverbank filtration by hydrochemical and biological indicators, Assiut, Egypt. J. Earth Syst. Sci. 2016, 125, 1697–1711. [Google Scholar] [CrossRef]
- Redwan, M.; Abdel Moneim, A.A.; Mohammed, N.E.; Masoud, A.M. Sources and health risk assessments of nitrate in groundwater, West of Tahta area, Sohag, Egypt. Epis. J. Int. Geosci. 2020, 43, 751–760. [Google Scholar] [CrossRef]
- Soltan, M.E. Characterization, Classification, and Evaluation of Some Ground Water Samples in Upper Egypt. Chemosphere 1998, 37, 735–745. [Google Scholar] [CrossRef]
- Frind, E.O.; Duynisveld, W.H.M.; Strebel, O.; Boettcher, J. Modeling of Multicomponent Transport with Microbial Transformations in Groundwater: Fuhrberg Case. Water Resour. Res. 1990, 26, 1707–1719. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).