Abstract
A series of previously poorly studied heterocyclic compounds, 10R-pyrido[4,3-a]phenazines, including the previously unknown parent compound, has been synthesized. The proposed synthetic approach is remarkable for its simplicity, due to the ease of the synthesis of the starting materials from readily available precursors, and is characterized by high yields of the target products, achievable under both acidic and basic catalysis. The paper discusses the synthesis conditions, optimization procedures, and X-ray crystallographic data.
1. Introduction
Phenazines are a large class of compounds found as secondary metabolites in a variety of bacteria, including Gram-negative (Pseudomonas) and Gram-positive (Streptomyces) species, as well as some archaea (Methanosarcina) [1]. The literature reports over 240 natural products featuring the phenazine core [2]. Phenazine derivatives show diverse biological activities [1,3,4,5,6,7,8,9,10], such as anticancer, antibacterial, and antiparasitic effects. Beyond their biological roles, they are also attracting attention in materials science as potential anolytes for high-voltage batteries [11,12,13] and optical sensors [14]. A significant group within the phenazine family are condensed systems like benzo- and pyridophenazines [15]. Some of these exhibit strong cytotoxic activity [8,16] and are being explored as organic anolytes for redox flow batteries (ORFBs) [11,12,13] (Figure 1).

Figure 1.
Some examples of promising condensed phenazine systems.
Several methods exist for the construction of the phenazine core [3,4,17]. One such method involves the cyclization of o-nitrosodiarylamines under acidic or basic catalysis [18,19,20,21,22,23]. Building on this approach, we previously developed an efficient method for the synthesis of 10-R-pyrido[2,3-a]phenazines based on the acid-catalyzed condensation of 7-arylamino-8-nitrosoquinolines under reflux in acetic acid [24]. The high yields and facile preparation of novel polycondensed systems motivated us to develop a synthetic approach to another heterocyclic system, pyrido[4,3-a]phenazine, for which the literature reports only a few substituted derivatives, including those with practically valuable properties [16,25,26,27].
In a recent paper [28], we demonstrated the possibility of obtaining o-nitrosodiarylamines 2a–l from readily available 5-nitroisoquinoline (1). This method is based on the reaction of direct nucleophilic substitution of hydrogen by an arylamino group (ONSH process) and allows obtaining a wide variety of o-nitrosodiarylamines of the isoquinoline series, substituted in the aniline fragment at the para position (Scheme 1).
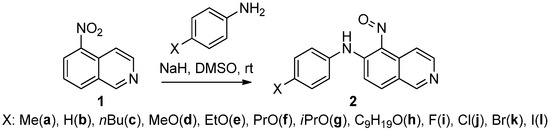
Scheme 1.
Preparation of o-nitrosodiarylamines 2a–l via direct ONSH process.
The obtained highly accessible substrates became the basis for studying the heteroannulation reaction of the quinoxaline fragment, which is a type of the Wohl–Aue reaction [29], occurring under mild conditions.
2. Materials and Methods
2.1. General Approach
1H and 13C NMR spectra were acquired on a Bruker Avance HD 400 spectrometer (Bruker BioSpin, Zurich, Switzerland). Chemical shifts were reported relative to residual solvent resonances in the appropriate deuterated solvent (DMSO) or tetramethylsilane (TMS) in the case of CDCl3. The NMR spectra of all newly synthesized compounds are available in the Supplementary Materials. HRMS data were collected on a Bruker UHR-TOF Maxis™ Impact instrument (Bruker Daltonics, Bremen, Germany) using electrospray ionization (ESI). Melting points were determined in open capillary tubes using REACH Devices RD-MP (REACH Devices, Boulder, CO, USA) and Electrothermal IA 9200 (Electrothermal, Stone, Great Britain) instruments and are uncorrected. The 5-Nitroisoquinolin was commercially available from abcr GmbH (abcr GmbH, Karlsruhe, Germany) and used without further purification. o-Nitrosodiarylamines 2a–l were obtained according to the known protocol [28]. The reaction progress and the purity of the obtained compounds were controlled by TLC on Silufol UV-254 plates. The yields of 10-R-pyrido[4,3-a]phenazines 3b–l, a description of spectral data, and images of all NMR and HRMS spectra of the new compounds are reported in the Supplementary Materials.
2.2. Procedure for the Synthesis of 10-R-Pyrido[4,3-a]Phenazines 3a–l
Method A: A solution of the corresponding 6-arylamino-5-nitrosoisoquinoline derivatives 2a–l (0.5 mmoL) in glacial acetic acid (5 mL) was heated under reflux for 30 min. Upon completion of the reaction, the solvent was removed under reduced pressure. The resulting crude residue was dissolved in a minimal volume of dichloromethane and purified by flash column chromatography, employing ethyl acetate as the eluent for the initial fractions. The initial red-brown fraction, which contained oligomeric byproducts, was discarded. Evaporation of the solvent from the second fraction afforded the target compounds 3a–l.
Method B: To a boiling solution of potassium carbonate (2.5 mmoL) in methanol (5 mL) was added the corresponding 6-arylamino-5-nitrosoisoquinoline 2a–l (0.5 mmoL) and the mixture was refluxed for 30 min. After completion of the reaction, the mass was poured into cold water, the precipitate was filtered, washed with water, dried, and recrystallized from ethyl acetate, yielding products 3a–l.
3a 10-Methylpyrido[4,3-a]phenazine. Yellow solid; yield 119 mg (97% method A); yield 116 mg (95% method B), mp 226–227 °C, 1H NMR (400 MHz, CDCl3): δ = 9.35 (s, 1H, H-4), 9.23 (d, J = 5.6 Hz, 1H, H-1), 8.95 (d, J = 5.6 Hz, 1H, H-2), 8.21 (d, J = 8.8 Hz, 1H, H-8), 8.16 (br. s, 1H, H-11), 8.13 (d, J = 9.3 Hz, 1H, H-6), 8.09 (d, J = 9.3 Hz, 1H, H-5), 7.80 (dd, J = 8.8, 1.8 Hz, 1H, H-9), 2.72 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ = 149.4, 145.2, 143.9, 142.9, 142.6, 141.9, 140.5, 137.5, 134.7, 130.0, 129.3, 129.1, 128.5, 128.2, 118.8, 22.4. ESI-HRMS: calcd for C16H12N3 [M + H]+ 246.1026, found 246.1032 calcd for C16H11N3Na [M + Na]+ 268.0845, found 268.0849.
3b Pyrido[4,3-a]phenazine. Yellow solid; yield 95 mg (82% method A); yield 99 mg (86% method B), mp 222–223 °C 1H NMR (400 MHz, CDCl3): δ = 9.42 (br. s, 1H, H-4), 9.38 (d, J = 5.6 Hz, 1H, H-1), 8.98 (br. d, J = 4.8 Hz, 1H, H-2), 8.45-8.42 (m, 1H, H-8), 8.38-8.34 (m, 1H, H-11), 8.23 (d, J = 9.3 Hz, 1H, H-6), 8.18 (d, J = 9.3 Hz, 1H, H-5), 8.04-7.95 (m, 2H, H-9,10). 13C NMR (100 MHz, CDCl3): δ = 147.9, 144.7, 144.5, 143.3, 142.6, 140.1, 138.6 (2C), 132.3, 132.4, 130.8, 130.2, 129.7, 129.6, 128.3, 119.7. ESI-HRMS: calcd for C15H10N3 [M+H]+ 232.0869, found 232.0870; calcd for C15H10N3Na [M+Na]+ 254.0698, found 254.0689.
3c 10-Butylpyrido[4,3-a]phenazine. Yellow solid; yield 123 mg (86% method A); yield 119 mg (83% method B), mp 228–229 °C, 1H NMR (400 MHz, CDCl3): δ = 9.42 (s, 1H, H-4), 9.39 (d, J = 5.7 Hz, 1H, H-1), 8.96 (br. d, J = 5.7 Hz, 1H, H-2), 8.26 (d, J = 8.8 Hz, 1H, H-8), 8.23 (d, J = 9.3 Hz, 1H, H-6), 8.19 (d, J = 0.8 Hz, 1H, H-11), 8.15 (d, J = 9.3 Hz, 1H, H-5), 7.87 (dd, J = 8.8, 1.9 Hz, 1H, H-9), 2.99 (t, J = 7.7 Hz, 2H, CH2), 1.87-1.78 (m, 2H, CH2), 1.52-1.43 (m, 2H, CH2), 1.00 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ = 147.31, 147.27, 144.1, 143.5, 142.9, 142.2, 139.9, 139.0, 134.7, 131.2, 129.3, 128.8, 128.4, 127.8, 119.9, 36.2, 33.0, 22.5, 14.1. ESI-HRMS: calcd for C19H17N3 [M+H]+ 288.1495, found 288.1491.
3d 10-Methoxypyrido[4,3-a]phenazine. Yellow solid; yield 119 mg (91% method A); yield 125 mg (96% method B), mp 203–204 °C 1H NMR (400 MHz, CDCl3): δ = 9.32 (s, 1H, H-4), 9.13 (d, J = 5.4 Hz, 1H, H-1), 8.94 (d, J = 5.4 Hz, 1H, H-2), 8.17 (d, J = 9.1 Hz, 1H, H-8), 8.09 (d, J = 9.2 Hz, 1H, H-6), 8.03 (d, J = 9.2 Hz, 1H, H-5), 7.63-7.58 (m, 2H, H-9,11), 4.09 (s, 3H, OCH3). 13C NMR (100 MHz, CDCl3): δ = 161.7, 150.4, 146.3, 144.2, 142.6, 141.0, 140.7, 136.5, 130.6, 129.4, 128.6, 128.2, 126.7, 118.2, 106.7, 56.2. ESI-HRMS: calcd for C16H12N3O [M+H]+ 262.0975, found 262.0970.
Gram scale procedure: To a boiling solution of potash (0,5 g) in methanol (40 ml) 1g of N-(4-methoxyphenyl)-5-nitrosoisoquinolin-6-amine 2d was added and the mixture was refluxed for 30 min. Then, the reaction mass was diluted with water. Methanol was distilled off from the resulting mixture and the suspension of the substance in water was filtered, washed with water, dried and recrystallized from ethyl acetate yielding 10-methoxypyrido[4,3-a]phenazine 3d. Yellow solid, yield 898 mg (96%).
3e 10-Ethoxypyrido[4,3-a]phenazine. Yellow solid; yield 122 mg (89% method A); yield 125 mg (91% method B), mp 207–208 °C 1H NMR (400 MHz, CDCl3): δ = 9.31 (br. s, 1H, H-4), 9.10 (br. d, J = 4.8 Hz, 1H, H-1), 8.94 (br. d, J = 4.8 Hz, 1H, H-2), 8.17 (br. d, J = 9.3 Hz, 1H, H-8), 8.08 (d, J = 9.2 Hz, 1H, H-6), 8.03 (d, J = 9.2 Hz, 1H, H-5), 7.60 (dd, J = 9.3 Hz, J = 2.6 Hz, 1H, H-9), 7.57 (d, J = 2.6 Hz, 1H, H-11), 4.32 (q, J = 7.0 Hz, 2H, CH2), 1.58 (t, J = 7.0 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ = 160.9, 150.9, 147.0, 144.2, 142.5, 140.8 (2C), 136.2, 130.5, 129.2, 128.7, 128.2, 126.9, 118.0, 106.2, 64.6, 14.8. ESI-HRMS: calcd for C17H13N3O [M+H]+ 276.1131, found 276.1126.
3f 10-Propoxypyrido[4,3-a]phenazine. Light Yellow solid; yield 123 mg (85% method A); yield 134 mg (93% method B), mp 158–159 °C 1H NMR (400 MHz, CDCl3): δ = 9.32 (br. s, 1H, H-4), 9.15 (br. d, J = 5.5 Hz, 1H, H-1), 8.92 (d, J = 5.5 Hz, 1H, H-2), 8.16 (d, J = 9.4 Hz, 1H, H-8), 8.10 (d, J = 9.2 Hz, 1H, H-6), 8.03 (d, J = 9.2 Hz, 1H, H-5), 7.61 (dd, J = 9.4 Hz, 2.7 Hz, 1H, H-9), 7.55 (d, J = 2.7 Hz, 1H, H-11), 4.19 (t, J = 6.6 Hz, 2H, OCH2), 2.00-1.94 (m, 2H, CH2), 1.14 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ = 161.2, 149.8, 145.4, 144.3, 142.5, 141.0, 140.4, 136.9, 130.5, 129.7, 128.3, 128.2, 127.2, 118.5, 106.1, 70.5, 22.5, 10.7. ESI-HRMS: calcd for C18H15N3O [M+H]+ 290.1288, found 290.1290.
3g 10-Isopropoxypyrido[4,3-a]phenazine. Yellow solid; yield 130 mg (90% method A); yield 133 mg (92% method B), mp 184–185 °C 1H NMR (400 MHz, CDCl3): δ = 9.35 (1H, br. s, H-4), 9.21 (1H, d, J = 5.5 Hz, H-1), 8.93 (1H, br. d, J = 5.5 Hz, H-2), 8.17 (1H, d, J = 9.4 Hz, H-8), 8.13 (1H, d, J = 9.2 Hz, H-6), 8.05 (1H, d, J = 9.2 Hz, H-5), 7.60 (1H, d, J = 2.7 Hz, H-11), 7.58-7.56 (1H, m, H-9), 4.92-4.88 (1H, m, OCH), 1.52 (6H, d, J = 6.1 Hz, (CH3)2). 13C NMR (100 MHz, CDCl3): δ = 160.1, 149.3, 144.6, 144.4, 142.5, 141.0, 140.3, 137.3, 130.6, 130.1, 128.3, 128.14, 128.12, 118.9, 106.9, 71.1, 21.9. ESI-HRMS: calcd for C18H15N3O [M+H]+ 290.1288, found 290.1289.
3h 10-(Nonyloxy)pyrido[4,3-a]phenazine. Light green solid; yield 157 mg (84% method A); yield 162 mg (86% method B), mp 118–119 °C 1H NMR (400 MHz, CDCl3): δ = 9.36 (br. s, 1H, H-4), 9.24 (br. d, J = 5.5 Hz, 1H, H-1), 8.93 (d, J = 5.5 Hz, 1H, H-2), 8.18 (d, J = 9.1 Hz, 1H, H-8), 8.15 (d, J = 9.4 Hz, 1H, H-6), 8.07 (d, J = 9.4 Hz, 1H, H-5), 7.63 (dd, J = 9.1 Hz, 2.1 Hz, 1H, H-9), 7.58 (br. s, 1H, H-11), 4.00 (t, J = 7.0 Hz, 2H, CH2-CH2O), 1.97-1.92 (m, 2H, CH2-CH2O), 1.57-1.53 (m, 2H, CH2-CH3), 1.42-1.28 (m, 10H, (CH2)5), 0.89 (t, J = 6.7 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ = 161.4, 148.9, 144.5, 144.0, 142.6, 141.2, 140.2, 137.6, 130.5, 130.3, 128.3, 128.1, 127.6, 119.0, 106.1, 69.2, 32.0, 29.7, 29.5, 29.4, 29.1, 26.2, 22.8, 14.3. ESI-HRMS: calcd for C24H27N3O [M+H]+ 374.2227, found 374.2227.
3i 10-Fluoropyrido[4,3-a]phenazine. Light yellow solid; yield 97 mg (78% method A); yield 106 mg (85% method B), mp 271–272 °C 1H NMR (400 MHz, CDCl3): δ = 9.45 (br. s, 1H, H-4), 9.38 (br. d, J = 5.7 Hz, 1H, H-2), 8.99 (d, J = 5.7 Hz, 1H, H-1), 8.39-8.37 (m, 1H, H-8), 8.23 (d, J = 9.3 Hz, 1H, H-6), 8.19 (d, J = 9.3 Hz, 1H, H-5), 8.03 (dd, J = 9.2 Hz, 2.8, 1H, H-9), 7.84-7.79 (m, 1H, H-11). 13C NMR (100 MHz, CDCl3): δ = 163.7 (d, 1JCF = 255.4 Hz), 147.4, 144.1 (d, 4JCF = 3.0 Hz), 143.3 (d, 3JCF = 13.7 Hz), 142.6, 142.0, 140.4, 138.6, 132.1 (d, 3JCF = 10.2 Hz), 131.0, 129.3, 128.6, 124.0 (d, 2JCF = 27.5 Hz), 120.1, 112.8 (d, 2JCF = 21.2 Hz). ESI-HRMS: calcd for C15H9FN3 [M+H]+ 250.0775, found 250.0766
3j 10-Chloropyrido[4,3-a]phenazine. Yellow solid; yield 106 mg (80% method A); yield 114 mg (86% method B), mp 280–281 °C 1H NMR (400 MHz, CDCl3) δ 9.33 (s, 1H), 9.11 (s, 1H), 9.00 (s, 1H), 8.42 (s, 1H), 8.27 (d, J = 9.1 Hz, 1H), 8.10 (q, J = 9.4 Hz, 2H), 7.88 (d, J = 9.1 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 150.92, 147.69, 144.51, 142.42, 142.34, 141.62, 136.75, 136.29, 132.70, 130.82, 130.76, 128.97, 128.63, 128.09, 118.28. ESI-HRMS: calcd for C15H9ClN3 [M+H]+ 266.0484, found 266.0480.
3k 10-Bromopyrido[4,3-a]phenazine. Yellow solid; yield 149 mg (96% method A); yield 149 mg (96% method B), mp 277–278 °C 1H NMR (400 MHz, CDCl3): δ = 9.36 (br. s, 1H, H-4), 9.19 (d, J = 5.6 Hz, 1H, H-2), 8.99 (d, J = 5.6 Hz, 1H, H-1), 8.61 (d, J = 2.1 Hz, 1H, H-11), 8.20 (d, J = 9.2 Hz, 1H, H-8), 8.15 (d, J = 9.4 Hz, 1H, H-6), 8.12 (d, J = 9.4 Hz, 1H, H-5), 8.01 (dd, J = 9.2, 2.1 Hz, 1H, H-9). 13C NMR (100 MHz, CDCl3): δ = 150.0, 146.5, 144.6, 142.7, 142.6, 141.3, 136.9, 135.3, 132.2, 130.8, 130.6, 129.5, 128.2, 125.3, 118.7. ESI-HRMS: calcd for C15H9BrN3 [M+H]+ 309.9974, found 309.9970.
3l 10-Iodopyrido[4,3-a]phenazine. Yellow solid; yield 141 mg (79% method A); yield 170 mg (95% method B); mp 267–268 °C 1H NMR (400 MHz, CDCl3): δ = 9.34 (1H, br. s, H-4), 9.12 (1H, br. d, J = 5.4 Hz, H-2), 8.98 (1H, d, J = 5.4 Hz, H-1), 8.84 (1H, d, J = 1.6 Hz, H-11), 8.15 (1H, dd, J = 9.1, 1.6 Hz, H-9), 8.12 (1H, d, J = 9.3 Hz, H-6), 8.07 (1H, d, J = 9.3 Hz, H-5), 8.01 (1H, d, J = 9.1 Hz, H-8). 13C NMR (100 MHz, CDCl3): δ = 150.0, 146.5, 144.7, 143.0, 142.8, 141.0, 140.3, 139.0, 136.9, 136.4, 130.60, 129.4, 128.2, 118.7, 97.4. ESI-HRMS: calcd for C15H9IN3 [M+H]+ 357.9836, found 357.9820.
3. Results and Discussion
Based on our previously reported acid-catalyzed approach [24], we investigated the heterocyclization of 6-p-tolylamino-5-nitroisoquinoline (2a), finding that it proceeds with water elimination to afford exclusively 10-methylpyrido[4,3-a]phenazine (3a). We found that boiling diarylamine (2a) in acetic acid provided the optimal reaction conditions (Table 1, Entry 1, Method A). It should be noted that the reaction can also proceed at room temperature, however, it is not complete after 24 h, with a conversion of less than 10%. The use of pTsOH as an acid catalyst for cyclization leads to the target product, but with lower yield (Entry 2,3).

Table 1.
Optimization of the reaction conditions for the preparation of 10-methylpyrido[4,3-a]phenazine (3a) from 6-p-tolylamino-5-nitrosoisoquinoline (2a) (Method A,B).
Employing various 6-arylamino-5-nitroisoquinolines, we successfully synthesized a series of previously unknown 10R-pyrido[4,3-a]phenazines, including the parent compound, in good to excellent yields (Scheme 2, Method A). Complete spectral data can be found in the Supplementary Materials file accompanying this article.
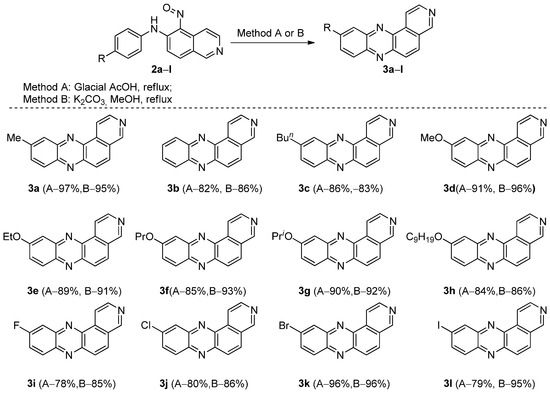
Scheme 2.
10R-pyrido[4,3-a]phenazines 3 prepared by cyclization of 6-arylamino-5-nitrosoisoquinolines 2.
Considering various approaches to phenazine synthesis from nitrosoarylamines [18,19,20,21,22,23], we investigated the feasibility of base catalysis for the preparation of our target compounds. Unlike pyrido[2,3-a]phenazines, for which this approach proved ineffective, 6-arylamino-5-nitroisoquinolines readily cyclize under these conditions. Optimization studies using 6-p-tolylamino-5-nitroisoquinoline showed that the reaction was dependent on the base used. Ultimately, we selected refluxing in commercial methanol with potassium carbonate as the base for the synthesis of our target compound library (Table 1, entry 4, Method B). As can be seen (Scheme 2), the reaction tolerates the nature of the substituent on the aryl fragment, since all yields remain in the range from 78 to 97%. In summary, both approaches afford the target products with relatively high yields; however, the base-catalyzed process proved slightly superior in some instances.
The proposed mechanism for phenazine formation with the assistance of acid catalysis has been discussed in detail previously [19,24] and, in our view, does not require further elaboration. The base-catalyzed pathway apparently involves the promotion of intramolecular cyclization by the base through proton acceptance on the nitrogen atom and subsequent participation of the conjugate base in the formation of a good leaving group in intermediate A (Scheme 3). We note that the reaction exhibits high tolerance towards various substituents on the phenyl ring, likely because of the activating effect of the isoquinoline moiety on the electrophilic center. This ultimately allows for the synthesis of the target products in high yields under both acidic and basic conditions.
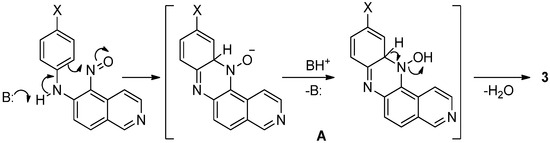
Scheme 3.
Proposed pathway for the formation of the compound 3.
The structure of 10-methylpyrido[4,3-a]phenazine 3a (CCDC 2430533) was further confirmed by X-ray crystallography (Figure 2). Suitable single crystals were obtained by slow evaporation of acetonitrile from a saturated solution of the pyridophenazine in an open vessel.
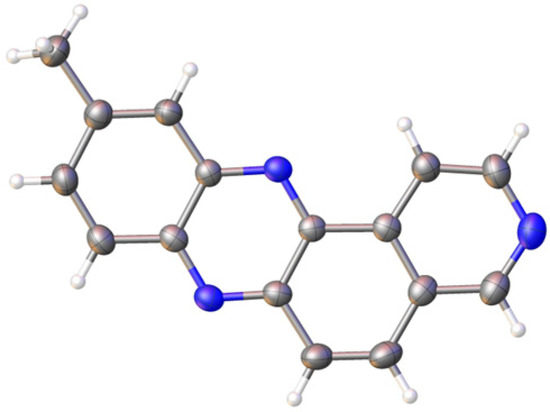
Figure 2.
ORTEP diagram of 10-methylpyrido[4,3-a]phenazine (3a). CCDC 2430533.
Molecules 3a crystallize in the orthorhombic system, space group Pca2₁, with unit cell dimensions a = 14.33537(19) Å, b = 4.48067(6) Å, c = 36.1452(5) Å, V = 2321.68(5) Å3, and Z = 8. As expected, all four aromatic rings are coplanar. Due to multiple intermolecular contacts (Figure 3a), indicating the presence of π-π interactions, the molecular packing in the crystal is formed by the formation of stacks along the crystallographic b axis (Figure. 3b). Regular layers approximately 7.89 Å wide are formed along the crystallographic c-axis, with an average interlayer distance of 0.89 Å (Figure 3c).
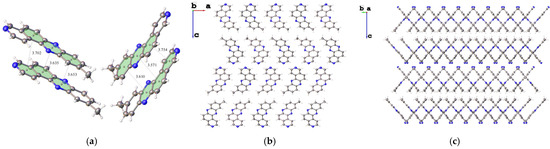
Figure 3.
Fragment of the 3a crystal packing showing intermolecular π-π interactions. (a) The main contacts between the centroids are shown (Å). (b) 2D layer of the 3a molecular packing along the crystallographic plane ac. (c) 2D layer of 3a packing on the bc plane.
4. Conclusions
An efficient protocol for the synthesis of potentially bioactive 10R-pyrido[4,3-a]phenazines has been developed. The effectiveness of both acidic and basic catalysis for the intramolecular cyclization has been demonstrated. Representatives of this poorly studied heterocyclic system, including a previously unknown parent compound, have been obtained. The structural similarity to known phenazine derivatives with valuable properties suggests the potential utility of the synthesized products for further investigation of their practical applications. Studies on the biological activity of these compounds are currently underway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6020024/s1, HRMS, NMR spectroscopy data, and X-ray analysis [28,30,31,32,33].
Author Contributions
Conceptualization, A.P.E. and O.P.D.; methodology, A.P.E. and D.Y.P.; validation, O.P.D. and I.V.B., investigation, A.P.E., D.Y.P., A.A.B., A.N.L. (synthetic chemistry), D.Y.P. (mass analysis) and O.P.D. (X-ray analysis); writing—original draft preparation, A.P.E. and O.P.D.; writing—review and editing, A.P.E., A.A.B., E.K.A. and O.P.D.; visualization, E.K.A.; supervision, O.P.D.; project administration, O.P.D.; funding acquisition E.K.A. All authors have read and agreed to the published version of the manuscript.
Funding
Research was funded by a grant from the Russian Science Foundation No. 23-73-01105, https://rscf.ru/project/23-73-01105/ (accessed on 17 April 2025).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author. Crystallographic data for the structure 3a have been deposited in the Cambridge Crystallographic Data Center as a supplementary publication (CCDC 2430533, www.ccdc.cam.ac.uk/structures (accessed on 11 March 2025)). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB12 1EZ, U.K. [Fax: +44-1223 336033 or e-mail: deposit@ccdc.cam.ac.uk.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Laursen, J.B.; Nielsen, J. Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products. Available online: https://dnp.chemnetbase.com/chemical/ChemicalSearchResults.xhtml?dswid=2474 (accessed on 16 April 2025).
- Guttenberger, N.; Blankenfeldt, W.; Breinbauer, R. Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg. Med. Chem. 2017, 25, 6149–6166. [Google Scholar] [CrossRef] [PubMed]
- Miksa, B. The phenazine scaffold used as cytotoxic pharmacophore applied in bactericidal, antiparasitic and antitumor agents. Helv. Chim. Acta 2022, 105, e202200066. [Google Scholar] [CrossRef]
- Huang, W.; Wan, Y.; Zhang, S.; Wang, C.; Zhang, Z.; Su, H.; Hou, F. Recent Advances in Phenazine Natural Products: Chemical Structures and Biological Activities. Molecules 2024, 29, 4771. [Google Scholar] [CrossRef]
- Huigens, R.W.; Brummel, B.R.; Tenneti, S.; Garrison, A.T.; Xiao, T. Pyrazine and phenazine heterocycles: Platforms for total synthesis and drug discovery. Molecules 2022, 27, 1112. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Cai, J.; Wang, Y.; Li, D.; Hua, H.; Cao, H. Advances in phenazines over the past decade: Review of their pharmacological activities, mechanisms of action, biosynthetic pathways and synthetic strategies. Mar. Drugs 2021, 19, 610. [Google Scholar] [CrossRef]
- Cimmino, A.; Evidente, A.; Mathieu, V.; Andolfi, A.; Lefranc, F.; Kornienko, A.; Kiss, R. Phenazines and cancer. Nat. Prod. Rep. 2012, 29, 487–501. [Google Scholar] [CrossRef]
- Garrison, A.T.; Abouelhassan, Y.; Norwood, V.M., IV; Kallifidas, D.; Bai, F.; Nguyen, M.T.; Huigens, R.W., III. Structure–activity relationships of a diverse class of halogenated phenazines that targets persistent, antibiotic-tolerant bacterial biofilms and Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 3808–3825. [Google Scholar] [CrossRef]
- Nadtochiy, V.V.; Nikonov, I.L.; Zyryanov, G.V. Modern approaches to the synthesis of phenazine derivatives (microreview). Chem. Heterocycl. Comp. 2024, 60, 233–235. [Google Scholar] [CrossRef]
- Hollas, A.; Wei, X.; Murugesan, V.; Nie, Z.; Li, B.; Reed, D.; Wang, W. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries. Nat. Energy 2018, 3, 508–514. [Google Scholar] [CrossRef]
- Vitaku, E.; Gannett, C.N.; Carpenter, K.L.; Shen, L.; Abruña, H.D.; Dichtel, W.R. Phenazine-based covalent organic framework cathode materials with high energy and power densities. J. Am. Chem. Soc. 2019, 142, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Romadina, E.I.; Komarov, D.S.; Stevenson, K.J.; Troshin, P.A. New phenazine based anolyte material for high voltage organic redox flow batteries. Chem. Commun. 2021, 57, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Ni, Q.; Dang, L.R.; Qu, W.J.; Zhang, Y.M.; Yao, H.; Lin, Q.; Wei, T.B. Phenazine derivatives for optical sensing: A review. J. Mater. Chem. C 2020, 8, 11308–11339. [Google Scholar] [CrossRef]
- Moorthy, N.S.H.N.; Pratheepa, V.; Ramos, M.J.; Vasconcelos, V. Fused aryl-phenazines: Scaffold for the development of bioactive molecules. Curr. Drug Targets 2014, 15, 681–688. [Google Scholar] [CrossRef]
- Gamage, S.A.; Spicer, J.A.; Rewcastle, G.W.; Milton, J.; Sohal, S.; Dangerfield, W.; Denny, W.A. Structure− activity relationships for pyrido-, imidazo-, pyrazolo-, pyrazino-, and pyrrolophenazinecarboxamides as topoisomerase-targeted anticancer agents. J. Med. Chem. 2002, 45, 740–743. [Google Scholar] [CrossRef]
- Che, Y.X.; Qi, X.N.; Qu, W.J.; Shi, B.B.; Lin, Q.; Yao, H.; Wei, T.B. Synthetic strategies of phenazine derivatives: A review. J. Heterocycl. Chem. 2022, 59, 969–996. [Google Scholar] [CrossRef]
- Wrobel, Z.; Kwast, A. 2-Nitroso-N-arylanilines: Products of acid-promoted transformation of σH adducts of arylamines and nitroarenes. Synlett 2007, 10, 1525–1528. [Google Scholar] [CrossRef]
- Wrobel, Z.; Kwast, A. Simple synthesis of N-aryl-2-nitrosoanilines in the reaction of nitroarenes with aniline anion derivatives. Synthesis 2010, 22, 3865–3872. [Google Scholar] [CrossRef]
- Kwast, A.; Stachowska, K.; Wróbel, Z.; Trawczyński, A. N-Aryl-2-nitrosoanilines as intermediates in the synthesis of substituted phenazines from nitroarenes. Tetrahedron Lett. 2011, 52, 6484–6488. [Google Scholar] [CrossRef]
- Wróbel, Z.; Plichta, K.; Kwast, A. Reactivity and substituent effects in the cyclization of N-aryl-2-nitrosoanilines to phenazines. Tetrahedron 2017, 73, 3147–3152. [Google Scholar] [CrossRef]
- Demidov, O.P.; Pobedinskaya, D.Y.; Avakyan, E.K.; Amangasieva, G.A.; Borovlev, I.V. SNH Arylamination of Nitroquinolines: Access to Nitro and Nitroso Derivatives of Arylaminoquinolines. Chem. Heterocycl. Compd. 2018, 54, 875–886. [Google Scholar] [CrossRef]
- Wróbel, Z.; Więcław, M.; Bujok, R.; Wojciechowski, K. Synthesis of pyrrolo[3,2-a]phenazines from 5-nitroindoles and anilines. Monatsh. Chem. 2013, 144, 1847–1853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pobedinskaya, D.Y.; Demidov, O.P.; Borovlev, I.V.; Avakyan, E.K.; Amangasieva, G.A. Synthesis of pyrido[2,3-a]phenazines by intramolecular cyclization of 7-arylamino-8-nitrosoquinolines. Chem. Heterocycl. Compd. 2019, 55, 684–687. [Google Scholar] [CrossRef]
- Kitahara, Y.; Nakai, T.; Nakahara, S.; Akazawa, M.; Shimizu, M.; Kubo, A. Synthesis of 5, 6-, 5, 8-and 7, 8-Isoquinolinediones from the Corresponding Isoquinolinols and Dimethoxyisoquinolines. Chem. Pharm. Bull. 1991, 39, 2256–2263. [Google Scholar] [CrossRef][Green Version]
- Swoboda, D. Synthesis and Spectroscopic Characterization of Selected Phenothiazines and Phenazines Rationalized Based on DFT Calculation. Molecules 2022, 27, 7519. [Google Scholar] [CrossRef]
- Gamage, M. Pharmaceutical Compounds. U.S. Patent 2001/0034346 A1, 22 April 2003. [Google Scholar]
- Pobedinskaya, D.Y.; Demidov, O.P.; Avakyan, E.K.; Borovleva, A.A.; Larin, A.N.; Ermolenko, A.P.; Borovlev, I.V. A simple method for the synthesis of diarylamines containing a nitroso group in the ortho position based on the SNH arylamination of 5-nitroisoquinoline. Chem. Heterocycl. Compd. 2024, 60, 161–168. [Google Scholar] [CrossRef]
- Wohl, A.; Aue, W. Ueber die einwirkung von nitrobenzol auf anilin bei gegenwart von alkali. Chem. Ber. 1901, 34, 2442–2450. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.38.41; Rigaku Oxford Diffraction. 2015. Available online: https://rigaku.com/products/crystallography/x-ray-diffraction/crysalispro (accessed on 16 April 2025).
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
