Abstract
Most bioplastics are based on polysaccharides, which are either synthesized from a variously sourced monomer or extracted from some biomass waste. In many cases, some lignocellulosic fibers are then added to the obtained bioplastics to form biocomposites and extend their range of applications beyond packaging films and generically easily biodegradable materials. Plant-extracted tannins, which, as such, might also be building blocks for bioplastics, do nonetheless represent a useful complement in their production when added to polysaccharide-based plastics and biocomposites, since they offer other functions, such as bioadhesion, coloration, and biocidal effect. The variety of species used for tannin extraction and condensation is becoming very wide and is also connected with the local availability of amounts of bio-waste from other productions, such as from the food system. This work tries to summarize the evolution and recent developments in tannin extraction and their increasing centrality in the production of polysaccharide-based plastics, adhesives, and natural fiber composites.
1. Introduction
Tannins are plant polyphenols, typically developed as metabolites to defend their leaves against herbivorous insects, either because their odor repels them or, more directly, due to their toxicity [1]. They take their name from their traditional use in the tanning of leather materials, which involves soaking and mechanical softening, other than protection from environmental degradation [2]. Studies on tannin extraction, spanning over a few decades, have also identified their possible medical and pharmacological use, for example, as a potential substitute for antibiotics [3]. However, their very variable toxicity, depending on their origin, and their capacity to reduce nutrient bioavailability in the gut suggests that a targeted pharmaceutical use against some inflammatory diseases, together with non-food applications, is an improved way to apply them [4]. It has also been highlighted, e.g., in the case of cassava, that treatment at temperatures such as 60 °C would reduce leaf toxicity and solubilize tannins [5].
Other possibilities are related to their anti-inflammatory potential, e.g., on arthritis, using tannins obtained from Anacardium occidentale [6], due to their interaction with some therapeutic target proteins, as is the case with black myrobalan (Terminalia chebula) fruits [7], or on nonmalignant respiratory diseases, such as allergic asthma [8]. Beyond their medical use, the application of tannins in materials can be of interest. As a source of phenols, they can be applied and functionalized as raw materials to produce bio-based resins, for example, from green tea, to obtain bio-epoxies [9]. Bio-epoxies, though at least partially bio-based, are not more easily recyclable than conventional ones, also, because they are often, on the grounds of mechanical performance, used as blends with petrochemical ones, with only limited bio-content, as low as 20% or less [10]. Another possibility, which requires the complete separation of tannins from sugars, is their application in polysaccharide-based bioplastics, which is one form of obtaining bio-polyesters from renewable resources [11].
The principal families of polysaccharide-based bioplastics are the following:
- Poly(lactic acid) (PLA) is normally obtained from the saccharose dimer, as extracted from biological sources, such as corn starch [12]. In this case, for example, to obtain mechanical improvement of the polymer, tannins from Quebracho wood mixture and Kraft lignin were added in different amounts [13].
- Bacterial poly(hydroxyalkanoates) (PHA) [14]: also in this case, tannins are able to provide stabilization to the relevant bioplastic structure [15].
- Thermoplastic starches (TPSs), which represent a general category of compounds useful as a matrix for bioplastics, especially when disposing of biomass waste of different origins, are most often used for food packaging films [16]. In this case, normally glycerol is used as the main plasticizer, of which tannin-rich biomasses, such as spent coffee grounds (SCGs), can act as effect enhancers [17].
The objective of this review is to discuss the various roles of tannins in polysaccharide-based plastics. In particular, these molecules are obtained from a large number of species and do represent by-products of other sectors, such as the production of food additives, or the development of medical treatments. The actions that tannins are able to perform in this sector are diverse, from recently promoting enzymatic polymerization of biomass-derived polyphenols, e.g., obtained from biorefinery processes [18], to offering an antioxidant effect on bioplastics [19]. Ordering and classifying the various potential actions represents an essential task given that waste biomass bioplastics are being developed in competition and at the expense of monomer-based ones, such as poly(lactic acid) (PLA). This indicates the timeliness and necessity of the present review.
The structure of this work includes a section (Section 2) in which the variety of origin of various tannins from different parts of the plant is discussed. Following this, the different functions of tannins in these materials are explored (Section 3), discussing, in particular, their use as polyesters and, hence, biomatrices (Section 3.1), as bio-adhesives on a differently obtained polysaccharide matrix (Section 3.2), or as coloring agents (Section 3.3), and, finally, in combination with lignocellulosic fibers to form biocomposites (Section 3.4).
2. Origins and Modes of Extraction of the Various Tannins Used in Bioplastics
Tannins can be extracted from a number of botanical species in different parts of the plant; this is particularly relevant whenever waste biomass is used to produce bioplastics [20]. A comprehensive table of the species is provided in [21], dividing the tannins into condensable and hydrolysable ones and focusing especially on their use for active packaging. On the other hand, restricting polysaccharide-based plastics to the packaging sector does constitute a limitation, especially when aiming to progress towards a real circular economy [22].
In particular, the parts of plants from which tannins can be extracted include the bark, buds, leaves, roots, seeds, stems, and wood. Some studies on tannins extracted from various elements of the plants are reported in Table 1, especially as regards properties that can be potentially applied to the production of bioplastics. Factors that can be considered in this regard include, e.g., the facility and completeness of the extraction of tannins, the seasonality of production, possible antioxidant and antibacterial action, and the separation or grafting of tannins on sugars. All these elements are essential in fields such as edible packaging and the potential application of these materials in the biomedical sector. However, it is fair to say that the use of bio-based plastics and composites does gradually expand towards other applications. The consequence is that not all extracted tannins are equally adapted to their use in bioplastics, a fact that needs to be considered before proceeding to further action.

Table 1.
Some species for tannins extraction and specific characteristics.
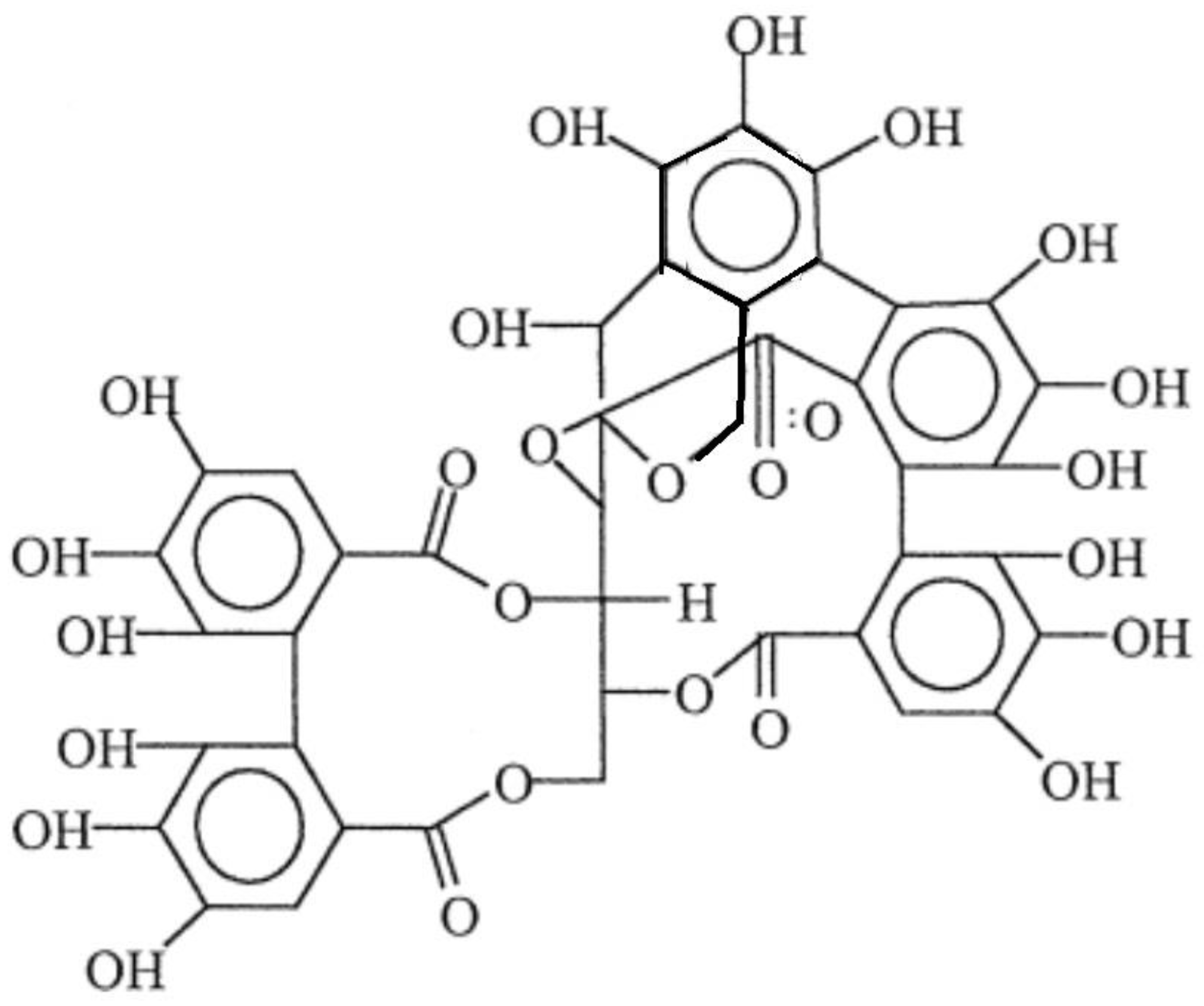
In particular, tannins from chestnut are hydrolysable, and their reactivity has therefore been considered comparable with the phenol resins’, as from evidence obtained from earlier studies. The highly complex structure of hydrolysable tannins is depicted in Figure 1 [39]. Recent considerations linked with circular economy do suggest intensifying the use of secondary raw materials from waste, which, in the particular case of tannins, often implies their availability from food residues. In some cases, such as for the production of wine, tannin extraction has been seen as being a part of a more circular approach to this system [40]. Tannins are available in a number of foodstuff items other than grapes [41]; these include various berries [42] and different types of nuts (walnuts, cashew nuts, hazelnuts, etc.) [43]. In general terms, their principal value is given by them representing a source of antioxidants. A relation has been demonstrated between antioxidant action and mechanical performance in some cases, e.g., when used in the production of bioplastics microwave-extracted tannins from walnut shells; more specifically, this demonstrated to be effective in poly(lactic acid) (PLA)/poly(butylene adipate-co-terephthalate (PBAT) blends [44].
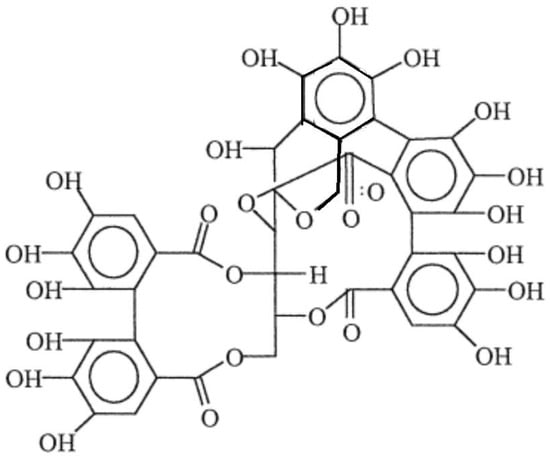
Figure 1.
Structure of the hydrolysable tannin from chestnut (Castanea spp.) (redrawn by the author).
The combination of two characteristics mainly suggests whether the use of tannin in materials is recommendable: improving mechanical performance and enhancing tolerance to weathering damage [45]. When blending with polysaccharides, tannins have the possibility to form complexes; a well-known interaction is the one between tannins and hyaluronic acid for the formation of colloidal structures [46]. In the case of polysaccharide-based bioplastics, given the compliance with a circular economy approach, substances obtained from vegetable waste are likely to be introduced in the production process [47]. This is the more general case for flavonoids, whose extraction from agro-waste to serve the production of materials has been discussed in a previous review [48]. As described above, the use of tannins, due to their antioxidant potential, can also offer other possibilities, such as their UV-blocking action [49], which has also been developed in combination with other well-renowned agents, such as titanium dioxide [50]. To extend the aims for which tannins are potentially available, their fractionation from industrial waste is necessary; this can occur in a single stage or a multi-stage procedure [51].
The agents that are commonly used for tannin extraction are ethanol or acetone, whose effectiveness can be compared, such as in [52] on grape skins, deep eutectic organic solvents [53], supercritical fluids, e.g., carbon dioxide and alcohol mixtures [54], or ultrasonic/microwave-assisted extraction [55]. The possible antifungal effects of different wood tannins, such as valonia ones, have also been discussed, which can assist in the development of bioplastics and biocomposites [56].
3. Function of Tannins in Bioplastics
3.1. Esterification of Condensed Tannins for Processing in Plastics for Various Uses
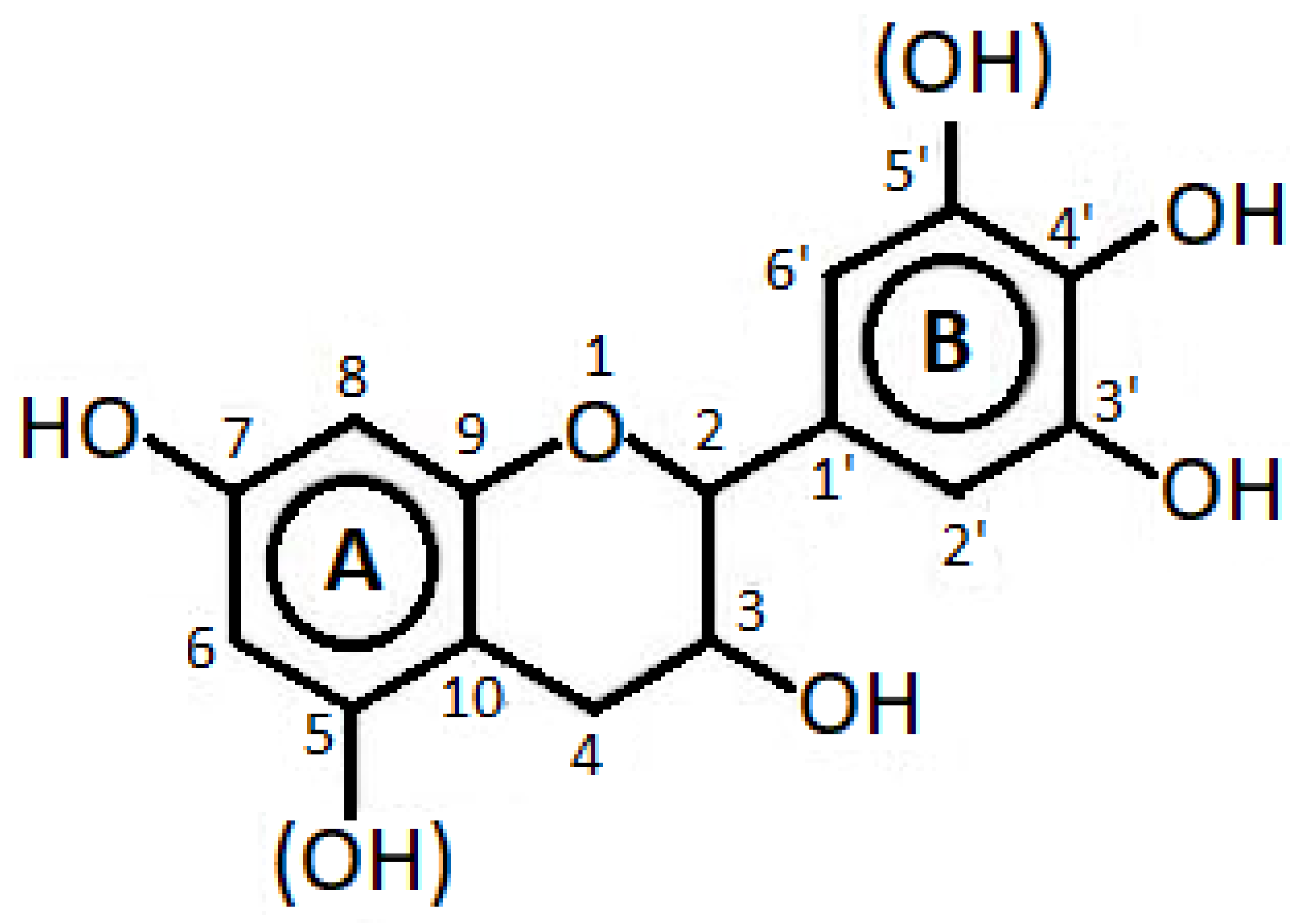
Condensed tannins (CTs), depicted in Figure 2, can be functionalized as renewable building block polymers for blending in thermoplastics [57]. In particular, they can be esterified through anhydrides with the idea of promoting their facile melt processing in plastics, which has been proved effective on poly(lactic acid) (PLA) [58]. CTs derived from Pinus radiata bark were added to biodegradable polyesters BionolleTM and BiopolTM; in this case, forming longer alkyl chain hexanoate esters promotes tannin miscibility to the biopolymers, while short-chain acetate esters acted rather as fillers in the postprocessing phase [59]. With this process, it was also possible to offer an improvement of properties to bacterial polymers, such as poly(hydroxybutyrate) (PHB), comparing the effect of tannic acid obtained from grape pomace through a hydroalcoholic extract and that of lignocellulosic biomass [60]. The valorization of grape pomace can, via a biorefinery approach, lead to the production of various chemicals, such as PHB, polyphenols, volatile fatty acids, and biogas. As discussed above, this is only one of the possibilities from the more general use of waste from wine production, such as grape stalks and residue in biopolymers as fillers for PLA, PHB, and poly(butylsuccinate) (PBS) with functionalization for the scope, yet without any real role attributed to tannins [61,62,63]. On the other side, provided a sufficient dispersion is achieved, CT can be blended with biopolymers, such as is the case for poly(hydroxybutirate-co-valerate) (PHBV) in the formation of multifunctional additives; a solvent casting process has allowed the content of tannins to be increased up to 10% [64]. Among the advantages, when introducing tannins in bioplastic packaging, is the possible colorimetric detection of ammonia vapors, which will allow detecting the possible spoilage of some food items (e.g., fish) [65]. The antioxidant properties of condensed tannins, specifically extracted from Acacia Mangium, were confirmed again, both on PLA [66] and poly(vinyl alcohol) (PVA) [67].
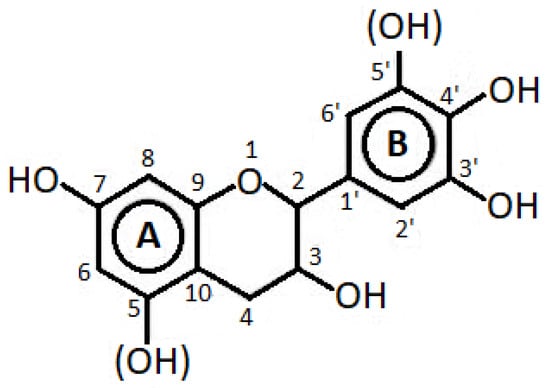
Figure 2.
Typical structure of condensed tannin (redrawn by the author).
One crucial question concerning bio-based polymers is the potential of dispersion of tannins into them; in particular, on PBS, by mapping FTIR key absorptions, acetylated tannins appeared to form discrete aggregates, able to penetrate PBS particles up to a dimension of 100 microns [68]. Aggregation capability has also been demonstrated for colloidal particles, namely using Valonia oak tannins, which improved the coagulation effect obtained by the bare use of aluminum sulfate [69]. Coagulation properties were exploited for the purpose of wastewater primary treatment for tannins extracted from Acacia Mearnsii and compared with potato starch and chitosan from crabs and prawns [70]. Tannin coagulants outperformed starch as water treatment agents in a number of aspects: for total phosphorus (70% reduction), turbidity (82% reduction), and, also, total organic carbon (TOC) (22% reduction). Their recommended concentration was measured to be equal to 5 mL/L.
3.2. Tannin-Based Bio-Adhesives
The opportunity and potential of using tannins as adhesives has been posed back in the 70s; the first application was performed in a blend with formaldehyde with the idea to modify adhesives for plywood [71,72]. The first tannin to be successfully commercialized was based on mimosa, which was more recently developed in a blend with cornstarch, aimed at being used in urea-formaldehyde resins [73]. Shear refinement enabled improving the conditions for processing mimosa tannins so that they would be processed in formaldehyde-free conditions with sufficient stability [74]. More recently, the field of mimosa tannins for bio-adhesives has also been expanded by including the M. tenuiflora species, which has been underused so far yet showed a performance to the level of commercial wood adhesives [75].
Unmodified tannins have several limitations in their application as-received for the development of bio-adhesives. These drawbacks are mainly related to the presence of non-tannin components, e.g., sugars and gums; in particular, the latter cause hydrogen bonding and electrostatic interaction, excessive viscosity, and high molecular weight of tannin extract, which can result in limited strength and ultimately in ineffective adhesion and short pot life [76]. On the other hand, tannin adhesives can generally improve water resistance of bio-based resins, e.g., those synthesized from soy oil [77]. The above issues are dealt with in different works; in particular, coupling tannins with itaconic polyamidoamine epichlorohydrin allowed multiple interactions and a higher density of cross-linking, while offering significant potential for their application in wood replacement boards [78]. Another possibility to improve the adhesive properties of tannins is the combination with bio-inspired structures; a well-known example of these is mussel byssus. Here, the bottleneck of the process is the extraction of the protein (over 95% collagen) from the byssus. On the other hand, the recombinant production has been considered with variable degrees of success, considering the three hierarchical levels of this structure, which represent collagen’s triple helix, the byssus thread, and the foot of the mussel [79]. In contrast, blending mussel protein with a soy meal/tannin mixture, inspired by a byssal cuticle, produced an adhesive with high resistance and low mildew effect [80].
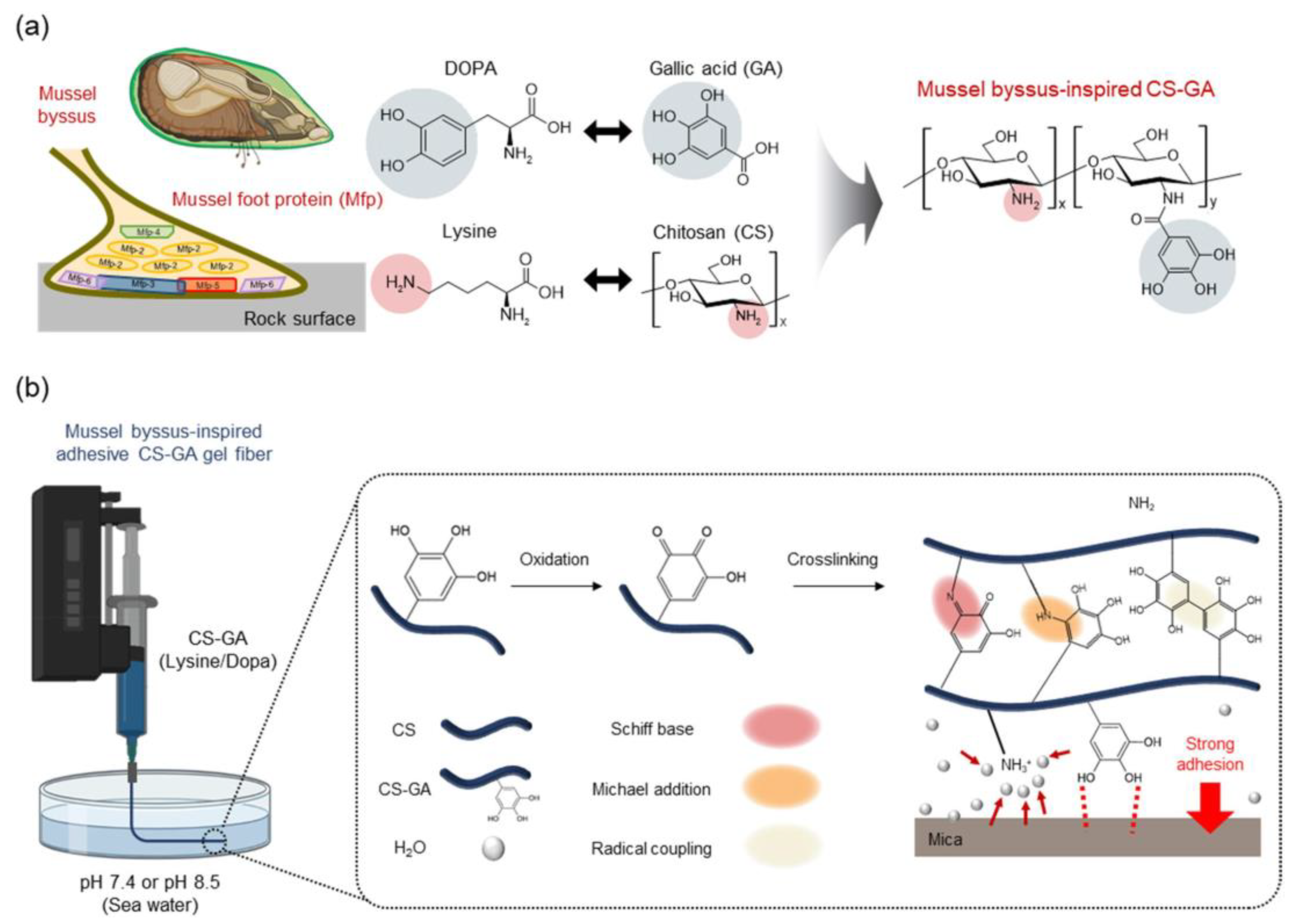
In another sense, the concept of byssus adhesion and, hence, hierarchization of structure with different functions can also be applied using tannins. In particular, a recent investigation concerned the fabrication of hierarchized blended fibers between gallol and chitosan [81] (Figure 3), developed with the idea to mimic the polysaccharide–tannin interaction [82]. On the other hand, the use of tannic acid has a recognized plasticization effect on chitosan matrices, where it can offer some crosslinking potential [83]. A branched structure was also obtained in [84] for a biomedical adhesive including ε-polylysine to form a bridge between gallic acid and collagen.
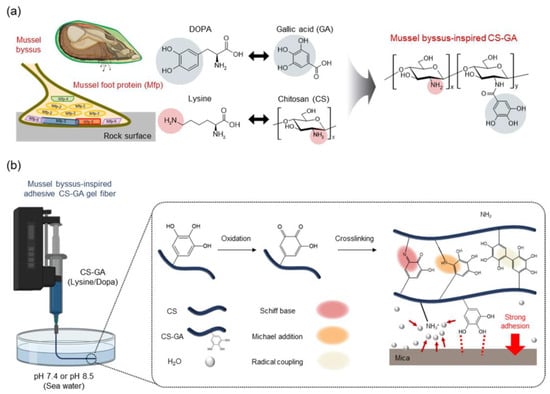
Figure 3.
Hierarchized blended fibers between gallol and chitosan, mimicking byssus function: (a) chemicals involved in adhesion; (b) biomimicry process [81].
Another question of interest is the possible application of bio-adhesives based on tannin to offer bonding to specific waste biomass, for example, that based on Triumfetta cordifolia needle-punched nonwovens, using tannins extracted from the bark of Aningeria altissima [85]. It is also worth noting that it is possible to obtain, using tannins, bio-adhesives with biocidal action by the addition of boric acid in the mixture [86].
3.3. Tannins as Coloring Agents in Bioplastics
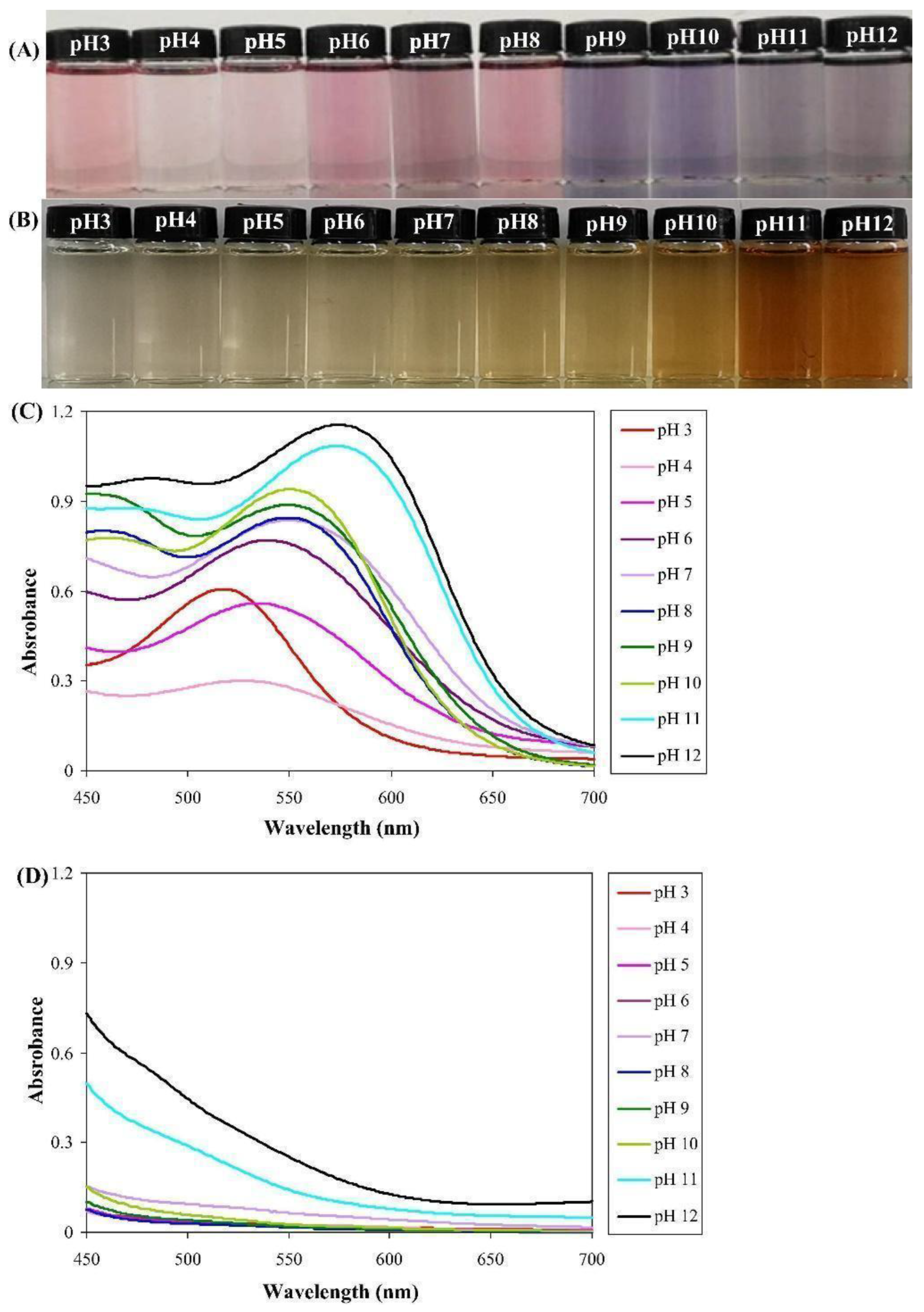
Coloration of bioplastics, such as polylactic acid (PLA), poly butyl succinate (PBS), and polycaprolactone (PCL) in various wood-like (yellowish-brown) colors, using logwood pigments, was also proposed. One important aspect to be taken into consideration was the discoloration or, more precisely, the spectral changes by irradiation using UV-A ays to simulate accelerated aging. The changes were very limited, even using an amount of pigment one order of magnitude lower than with industrial pigments, i.e., 0.5% [87]. Another possible solution is the fabrication of hybrid nanopigments based on anthocyanin in the specific case from pomegranate peel waste mixed with calcined hydrotalcite and montmorillonite (MMT) nanoclays. The possibility of varying the colors in a wide range from reddish to bluish hues has been exploited by modifying the pH of the pigment [88]. Other clays, such as bentonite, can also contribute to the process of stabilization of anthocyanins on bioplastics [89]. A full range of color variation has also been realized with κ-carrageenan films added with pomegranate peel (PPE) and flesh extracts (PFE), working in a pH interval between 3 and 12. UV-vis spectroscopy results indicated a maximum absorption peak for PFE solution, gradually shifting with pH from 520 to 580 nm, which was deemed to be due to the structural change in anthocyanins; the highest intensities were obtained in alkaline conditions. In contrast, no obvious peak was revealed when using PPE solution [90] (Figure 4).
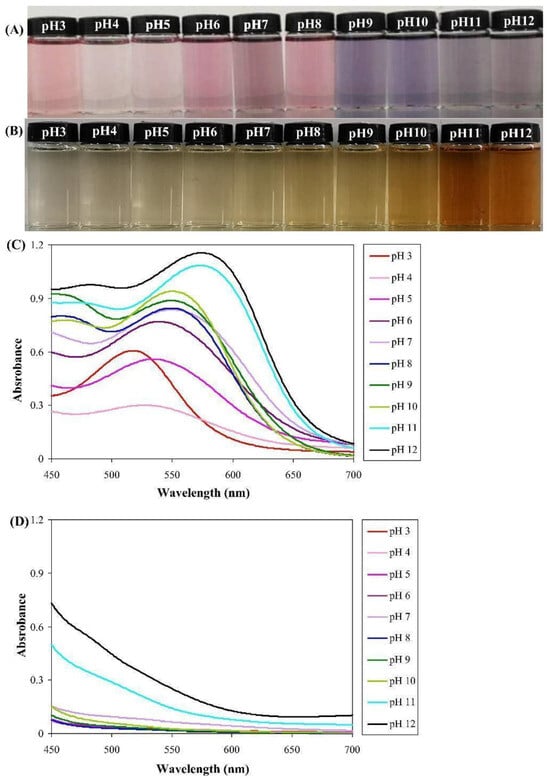
Figure 4.
Color variations of κ-carrageenan films added with (A) pomegranate flesh extracts (PFE) and (B) pomegranate peel (PPE). UV-Vis spectra of (C) PFE and (D) PPE in a pH interval between 3 and 12 [90].
The sensitivity to pH by producing changes in color can also be exploited in the case of active packaging as a way to obtain information on the conditions of preservation for the contained food [91]. In other words, as reported also in the case of mulberry tannins blended into poly(vinylalcohol) (PVA)/chitosan bioplastics, this natural tannin-based dye can provide an accurate measurement of pH, acting as an indicator, which provides, on the other hand, data on how anthocyanins do change their chemical structure [92]. Anthocyanins from purple sweet potatoes (Ipomoea batatas) have also been proposed for the production of pH indicators in a carboxymethylcellulose/starch matrix to monitor the preservation of fish, offering additional indications about light transmittance rate and possible presence of ammonia [93].
3.4. Tannins in Biocomposites Including Lignocellulosic Fibers
Further developments led to the production of natural fiber composites using tannins as their matrix. In particular, tannin/hexamine resin being reinforced with flax–hemp fibers has been studied; the tannin extracts have been obtained once again from mimosa [94], sulfited mimosa, and quebracho [95], whose properties have been separately identified. Another natural fiber that was used in a matrix synthesized from their tannin extracts to obtain biocomposites is banana stalk fiber; in this case, tannins extracted from the bark of various African trees were also proposed, such as African copaiba (Daniellia oliveri), sycamore (Ficus sycomorus), shea tree (Butyrospermum parkii, now Vitellaria paradoxa), and neem (Azadirachta indica) [96]. An alternative could be modifying starch–flax biocomposites with tannic acid to confer biocidal properties to these materials [97].
Another fiber that was experimented on to be used as a filler for filling a 50/50 tannin–phenol matrix is coir, which yielded a sufficient reinforcement effect in an amount of up to 70 wt.% when using fibers between 3 and 6 mm long, as verified by Izod impact tests [98]. With a similar amount (up to 70 wt.%) of longer (30 mm) sisal fibers, a 60/40 phenol–mimosa tannin matrix was used; in this case, Izod impact strength showed to be improved up to a 50 wt.% fibers content [99].
The combined presence of different natural materials, e.g., tannin extracts as the resin and lignocellulosic fibers as the reinforcement, does reveal the likeliness that mold growth would take place on the biocomposite. This has been investigated using the aniline blue method after inoculation with Aspergillus niger, as regards flax fiber–tannin resin composites, pretreating the nonwoven fiber material by boric acid, and proving that they are highly resistant to mold [100]. Also, the interaction of tannins with biodegradable thermoplastic materials based on poly (lactic acid) (PLA), starch, sugarcane fiber, and nutrient compounds was demonstrated to delay the biodegradation process [101].
3.5. Tannins as Compared with Other Building Blocks for Bioplastics and Biocomposites: Humins
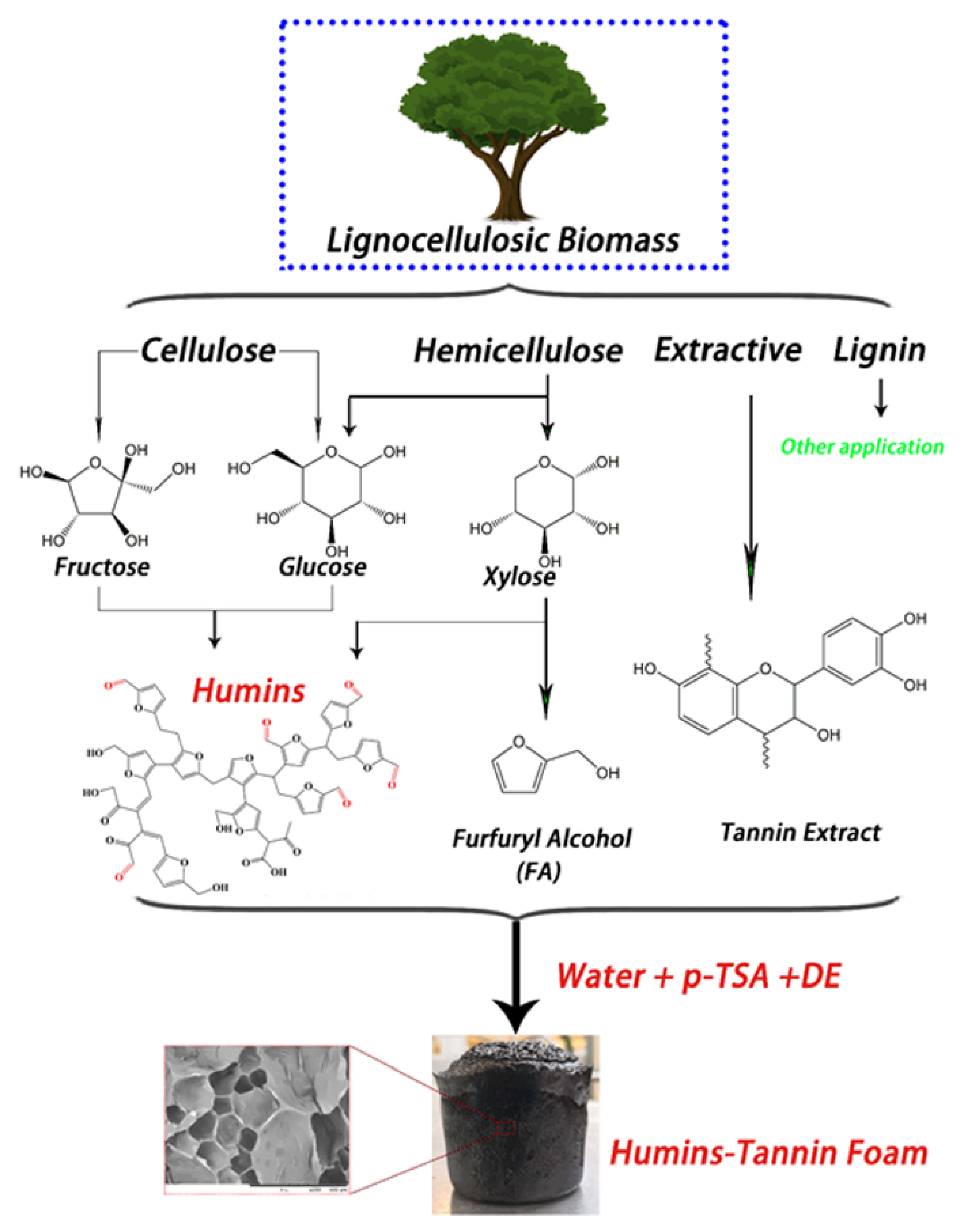
To enter the sector of constructions, structures such as tannin-based polyurethanes [102] or biofoams [103] have also been developed. This has been realized also in combination with furan to obtain tannin–furanic foams, which may equally have a quasi-exclusive bio-based content [104], with the advantage, offered by furans, of potential high temperature applications [105]. The foaming action might also involve the incorporation of polysaccharide-based products, such as humins [106]. In practice, polyfuranic humins, a biorefinery product, served to promote the self-blowing action of mimosa-extracted tannin-based foams (Figure 5). This is attributed to the fact that, under very low pH, which is a condition that seldom occurs in soil, heated humins can self-crosslink, a reaction that can be accelerated through a strong acid initiator, e.g., p-toluene sulfonic acid (pTSA) and a foaming agent (DE). Humins are the insoluble part of soil biomass, which normally are not precisely defined as for chemical composition [107] yet are precipitated in the form of solid microspheres [108], and can be obtained by an acidic treatment of polysaccharides. This insolubility in water is the main characteristic that differentiates them from tannins [109]. In general terms, humins have been considered an issue for biomass processing, yet, on the other hand, their insolubility has allowed them being mixed with tannins with a characteristic self-blowing effect at room temperature. In particular, for materials processing, humins oligomers tend to form complex crosslinked networks through physical aggregation while absorbing other molecules [110], which enable their use as building blocks for durable polysaccharide-based bioplastics [111]. Flax-fiber-reinforced composites with similar properties between humins and tannins as potential matrices have been reported [112]. Tannins can also serve in non-furanic polyurethane adhesives to the improvement of humins’ properties, where the two biomaterials appeared to form a crosslinked network gradually disentangling with temperature [113].
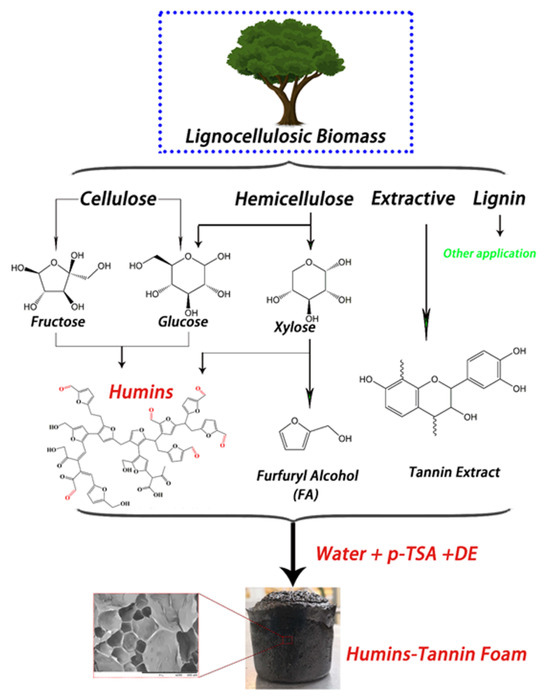
Figure 5.
Crosslinking action of humin–tannin foam through acid self-crosslinking [106].
4. Discussion
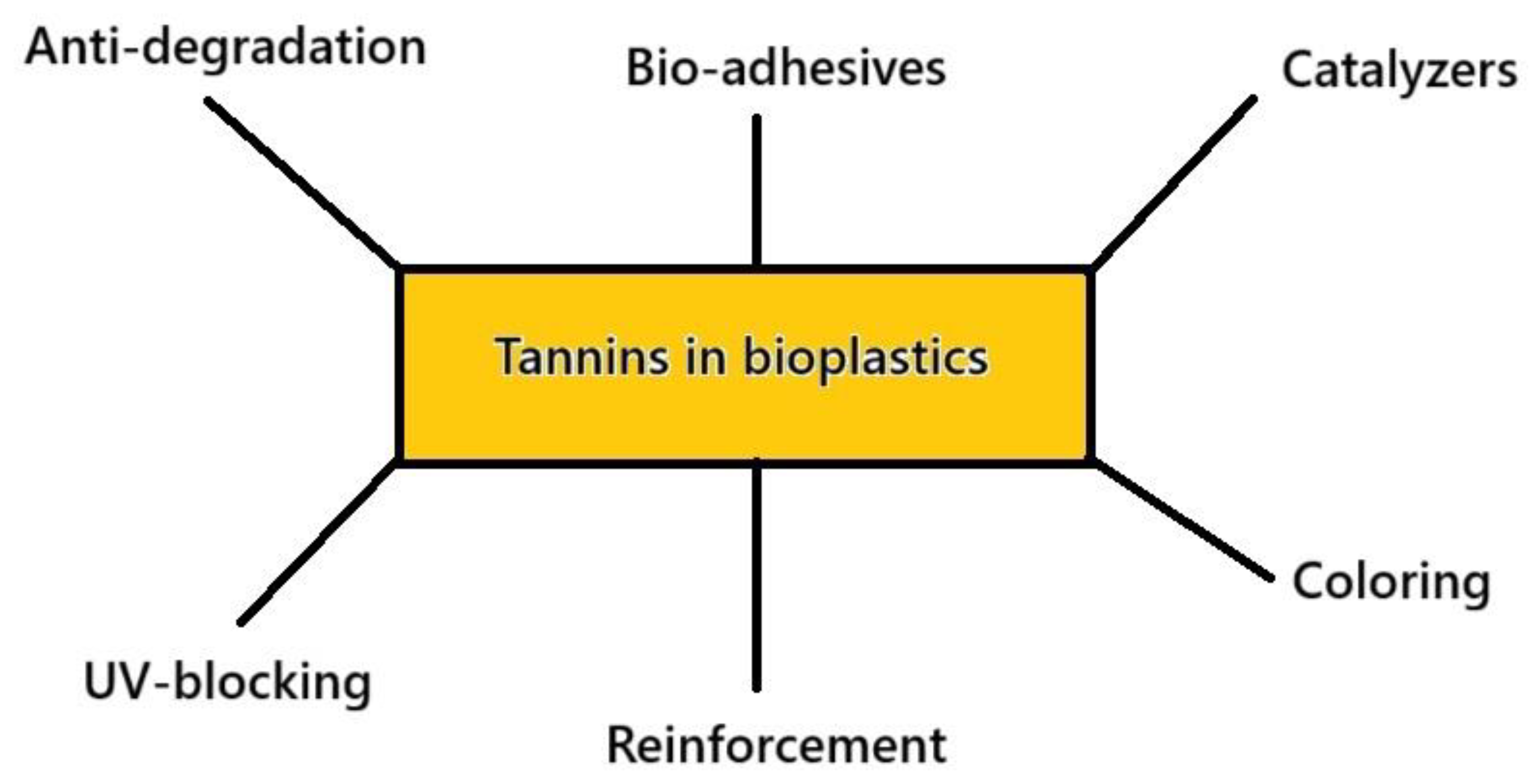
Tannins’ use in bioplastics and biocomposites serves various purposes, which allow them to act as matrices or, generically, adhesives, coloring agents, antibacterial principles, and in combination with other principles, such as humins, to self-crosslink as foams. A scheme summarizing these different applications is given in Figure 6.
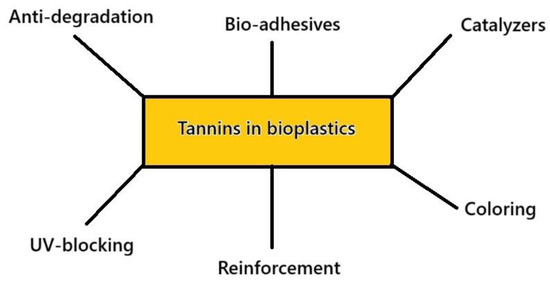
Figure 6.
Some amongst the various roles of tannins in bioplastics.
It is suggested that the perspectives of future applications would concentrate on some specific themes. These are listed below:
- (i)
- The development of effective crosslinkers to use tannins for wood-based panels; after initial attempts, early in this century, on polyethyleneimine [114], nowadays one of the most diffused is hexamethylenetetramine (HMTA) [115], which forms tannin and amino methylene bridges. This is a formaldehyde-free technology that is supposed to coexist with binderless principles, based on the modification of wood to promote adhesion [116]. Research is continuing: natural crosslinkers, such as those based on proteins, though thermoset, tend to become thermoplastic under the effect of moisture and generally contamination; this is the case for casein [117]. In practice, therefore, attention is concentrated on aldehyde (aliphatic, cyclic, or aromatic) or amine functional groups, according to the specific reactive groups on the various adhesives based on natural substances [118]. However, the drive towards achieving full-biobased adhesives is by no means exhausting; a glucose–tannin combination has in fact been also proposed for wood adhesion, which proved highly performant [119].
- (ii)
- As for other bio-based products, the principal bottleneck is the requirement for thorough characterization of these materials, especially as far as the market for thermosetting polymers and composites is concerned, where specifications need to be supplied and constantly verified for their variability over time. This occurs, e.g., in the field of bio-epoxies [120] and bio-polyurethanes [121]. In the latter case, the tannin-based resin can also be used as a modifier for lignocellulosic fibers to improve their adhesion to further matrices; this has been, for example, proposed with ramie fibers [122]. Fiber modification is generally a widely diffused practice; tannin-rich plants, such as persimmon, are important candidates for that purpose, despite limitations due to their seasonal availability, which may hinder the possible industrialization of the process [123].
- (iii)
- In the field of biocomposites and of the increasing number of lignocellulosic fibers that are employed with that purpose [124], tannin-based matrices provide a very suitable location for their effective bonding. It is no surprise that the last years have witnessed the development of tannin resin composites with various natural fibers, such as Urena lobata [125] and Grewia bicolor [126]. Together with high interfacial properties obtained, these materials are able to present also antibacterial and anti-mold properties [127], which represented a limit so far for fully bio-based biocomposites.
In more general terms, it can be suggested that, given the fact that tannins are necessary by-products of many food-based systems, their application in bioplastics could enhance their life perspective and move away from a single-use product (SUP) philosophy; in this sense, a reinforcement action is particularly adapted [128]. This also requires considering the improvement of the mechanical strength of the bioplastic obtained, which appears one of the most important challenges so far [129].
As is the case for natural materials, not different from obtaining cellulose or lignin from biomass, the extraction of tannins has very variable yield depending on the species yet also on the method followed. Further indications are given in [130], where the literature is classified according to the means of extraction (organic solvents, hot water, pressurized or not, ionic liquids, supercritical fluids or water, and assisted by microwave or ultrasound), methods which can also be combined. Some comparative studies do also exist, for example, in [131] performing different extraction methods (maceration, infusion, microwave, and microwave with water) on 10-year-old Acacia mollissima bark samples as regards polyphenols, hydrolisable, and condensed tannins. The results were suggestive of the potential of the innovative method using microwave, though, in terms of scaling-up towards industrialization, it might not always be convenient to go for it [132].
Further complications for global productions, though recommended in terms of circular economy, are the use of tannins from local cultivations, e.g., for the function of bio-adhesives to be used in wood boards; in some cases, such as for tannins from Aningeria, these proved particularly suitable for their use as bio-adhesives [133]. Other most recent tannin applications, such as the possibility to enhance enzymatic polymerization of lignin fractions through the laccase process by Pinus radiata bark extraction, appeared challenging due to the variability of properties and incomplete characterization of tannins [134]. In the same way, it is not easy to control the modification of biodegradability patterns introduced by the addition of tannins, such as in the case of chestnut bark ones [135]. It is suggested though that, also, given the variety and the potential of tannins and the possible combination with typically non-hydrolysable elements, such as humins, a sensible approach would tend to make use of them in bio-based yet durable items. In that case, controlled and limited biodegradability would be obviously preferable.
Finally, further uses of tannins in bioplastics can be envisaged, which have received very limited attention so far and cannot be considered already available at the present state-of-the-art. An example is the application of tannins as bio-based flame retardants for bioplastics; their ability to form stable char has already been clarified [136], though many competitors are present in that respect, for which reason it is not possible to propose their diffuse use. Tannins appear nonetheless an essential and versatile component of bioplastics, whose potential is still to be fully exposed, while the research in this field appears to gradually expand and diversify.
Author Contributions
Conceptualization, G.R. and S.G.; literature review, C.S. and S.G.; writing (original draft), C.S.; writing (final version), G.R. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were generated.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochem 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Covington, A.D. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Padmaja, G. Evaluation of techniques to reduce assayable tannin and cyanide in cassava leaves. J. Agric. Food Chem. 1989, 37, 712–716. [Google Scholar] [CrossRef]
- Thomas, M.M.G.; Barbosa Filho, J.M. Anti-inflammatory actions of tannins isolated from the bark of Anacardium occidentale L. J. Ethnopharmacol. 1985, 13, 289–300. [Google Scholar] [CrossRef]
- Sanmuga Priya, E.; Senthamil Selvan, P.; Ajay, B. Tannin rich fraction from Terminalia chebula fruits as Anti-inflammatory agent. J. Herbs Spices Med. Plants 2018, 24, 74–86. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Rajasekar, N.; Sivanantham, A. Therapeutic potential of plant-derived tannins in non-malignant respiratory diseases. J. Nutr. Biochem. 2021, 94, 108632. [Google Scholar] [CrossRef]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind. Crops Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- Capretti, M.; Giammaria, V.; Santulli, C.; Boria, S.; Del Bianco, G. Use of Bio-Epoxies and Their Effect on the Performance of Polymer Composites: A Critical Review. Polymers 2023, 15, 4733. [Google Scholar] [CrossRef]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Anwer, M.A.; Naguib, H.E.; Celzard, A.; Fierro, V. Comparison of the thermal, dynamic mechanical and morphological properties of PLA-Lignin & PLA-Tannin particulate green composites. Compos. Part B Eng. 2015, 82, 92–99. [Google Scholar]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process. Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Gontard, N.; Aouf, C. Extending biopolyesters circularity by using natural stabilizers: A review on the potential of polyphenols to enhance poly (hydroxyalkanoates) thermal stability while preserving its biodegradability. Polym. Test. 2022, 110, 107561. [Google Scholar] [CrossRef]
- Rahardiyan, D.; Moko, E.M.; Tan, J.S.; Lee, C.K. Thermoplastic starch (TPS) bioplastic, the green solution for single-use petroleum plastic food packaging–A review. Enzym. Microb. Technol. 2023, 168, 110260. [Google Scholar] [CrossRef]
- Masssijaya, S.Y.; Lubis, M.A.R.; Nissa, R.C.; Nurhamiyah, Y.; Nugroho, P.; Antov, P.; Lee, S.-H.; Papadopoulos, A.N.; Kusumah, S.S.; Karlinasari, L. Utilization of spent coffee grounds as a sustainable resource for the synthesis of bioplastic composites with polylactic acid, starch, and sucrose. J. Compos. Sci. 2023, 7, 512. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An integrated green biorefinery approach towards simultaneous recovery of pectin and polyphenols coupled with bioethanol production from waste pomegranate peels. Bioresource Technol. 2018, 266, 322–334. [Google Scholar]
- Mallegni, N.; Cicogna, F.; Passaglia, E.; Gigante, V.; Coltelli, M.B.; Coiai, S. Natural Antioxidants: Advancing Stability and Performance in Sustainable Biobased and Biodegradable Plastics. Compounds 2025, 5, 4. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Agrippina, F.D.; Ismayati, M.; Hidayati, S.; Pratama, B.P. Utilization of Tannins with Various Polymers for Green-Based Active Packaging: A Review. J. Sylva Lestari 2024, 12, 648–683. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Fedorov, V.S.; Ryazanova, T.V. Bark of Siberian conifers: Composition, use, and processing to extract tannin. Forests 2021, 12, 1043. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Kluge, R.A.; Appezzato-da-Glória, B. The accumulation of tannins during the development of ‘Giombo’ and ‘Fuyu’ persimmon fruits. Sci. Hortic. 2014, 172, 292–299. [Google Scholar] [CrossRef]
- Daskalakis, I.; Stavrakaki, M.; Sotirakoglou, K.; Biniari, K. Variations in the levels of individual phenolic compounds in grapevine latent buds during eco-dormancy, following chemically-induced stress conditions. Agronomy 2021, 11, 1798. [Google Scholar] [CrossRef]
- Feeny, P.P.; Bostock, H. Seasonal changes in the tannin content of oak leaves. Phytochem 1968, 7, 871–880. [Google Scholar] [CrossRef]
- Hernes, P.J.; Benner, R.; Cowie, G.L.; Goni, M.A.; Bergamaschi, B.A.; Hedges, J.I. Tannin diagenesis in mangrove leaves from a tropical estuary: A novel molecular approach. Geochim. Cosmochim. Acta 2001, 65, 3109–3122. [Google Scholar] [CrossRef]
- Patra, A.; Prasath, V.A. Isolation of detoxified cassava (Manihot esculenta L.) leaf protein by alkaline extraction-isoelectric precipitation: Optimization and its characterization. Food Chem. 2024, 437, 137845. [Google Scholar] [CrossRef]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Antimicrobial activities of tannins extract from guava leaves (Psidium guajava L.) on pathogens microbial. Int. J. Sci. Technol. Res. 2014, 3, 236–241. [Google Scholar]
- Oszmianski, J.; Wojdylo, A.; Lamer-Zarawska, E.; Swiader, K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007, 100, 579–583. [Google Scholar] [CrossRef]
- Bakkali, A.T.; Jaziri, M.; Ishimaru, K.; Tanaka, N.; Shimomura, K.; Yoshimatsu, K.; Homes, J.; Vanhaelen, M. Tannin production in hairy root cultures of Lawsonia inermis. J. Plant Physiol. 1997, 151, 505–508. [Google Scholar] [CrossRef]
- Kantar, F.; Pilbeam, C.J.; Hebblethwaite, P.D. Effect of tannin content of faba bean (Vicia faba) seed on seed vigour, germination and field emergence. Ann. Appl. Biol. 1996, 128, 85–93. [Google Scholar] [CrossRef]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and anti-inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J. Food Biochem. 2014, 38, 259–270. [Google Scholar] [CrossRef]
- Zhang, S.J.; Lin, Y.M.; Zhou, H.C.; Wei, S.D.; Lin, G.H.; Ye, G.F. Antioxidant tannins from stem bark and fine root of Casuarina equisetifolia. Molecules 2010, 15, 5658–5670. [Google Scholar] [CrossRef]
- Wei, S.D.; Zhou, H.C.; Lin, Y.M.; Liao, M.M.; Chai, W.M. MALDI-TOF MS analysis of condensed tannins with potent antioxidant activity from the leaf, stem bark and root bark of Acacia confusa. Molecules 2010, 15, 4369–4381. [Google Scholar] [CrossRef]
- Giovando, S.; Koch, G.; Romagnoli, M.; Paul, D.; Vinciguerra, V.; Tamantini, S.; Marini, F.; Zikeli, F.; Mugnozza, G.S. Spectro-topochemical investigation of the location of polyphenolic extractives (tannins) in chestnut wood structure and ultrastructure. Ind. Crops Prod. 2019, 141, 111767. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A. Considerations on the macromolecular structure of chestnut ellagitannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2002, 85, 429–437. [Google Scholar] [CrossRef]
- Singh, N.B.; De, A.; Shukla, S.K.; Guin, M. Bioplastic from renewable biomass. In Handbook of Bioplastics and Biocomposites Engineering Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 49–79. [Google Scholar]
- Tang, G.Y.; Zhao, C.N.; Liu, Q.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.B. Potential of grape wastes as a natural source of bioactive compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef]
- Jiang, Y.; Subbiah, V.; Wu, H.; Bk, A.; Sharifi-Rad, J.; Suleria, H.A. Phenolic profiling of berries waste and determination of their antioxidant potential. J. Food Qual. 2022, 2022, 5605739. [Google Scholar] [CrossRef]
- Karamać, M. In-vitro study on the efficacy of tannin fractions of edible nuts as antioxidants. Eur. J. Lipid Sci. Technol. 2009, 111, 1063–1071. [Google Scholar] [CrossRef]
- Muccilli, V.; Maccarronello, A.E.; Rasoanandrasana, C.; Cardullo, N.; de Luna, M.S.; Pittala, M.G.; Riccobene, P.M.; Carroccio, S.C.; Scamporrino, A.A. Green3: A green extraction of green additives for green plastics. Heliyon 2024, 10, e24469. [Google Scholar] [CrossRef]
- Koopmann, A.K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-based hybrid materials and their applications: A review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef] [PubMed]
- Carn, F.; Guyot, S.; Baron, A.; Pérez, J.; Buhler, E.; Zanchi, D. Structural properties of colloidal complexes between condensed tannins and polysaccharide hyaluronan. Biomacromolecules 2012, 13, 751–759. [Google Scholar] [CrossRef]
- Merino, D.; Quilez-Molina, A.I.; Perotto, G.; Bassani, A.; Spigno, G.; Athanassiou, A. A second life for fruit and vegetable waste: A review on bioplastic films and coatings for potential food protection applications. Green Chem. 2022, 24, 4703–4727. [Google Scholar] [CrossRef]
- Santulli, C. Extraction of flavonoids from agrowaste. In Extraction of Natural Products from Agro-Industrial Wastes, A Green and Sustainable Approach; Bhawani, S.A., Khan, A., Ahmad, F.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 7; pp. 111–130. ISBN 978–0-12–823349–8. [Google Scholar]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Son, H.Y.; Koo, B.I.; Lee, J.B.; Kim, K.R.; Kim, W.; Jang, J.; Yoon, M.S.; Cho, J.-W.; Nam, Y.S. Tannin–titanium oxide multilayer as a photochemically suppressed ultraviolet filter. ACS Appl. Mater. Interfaces 2018, 10, 27344–27354. [Google Scholar] [CrossRef]
- Missio, A.L.; Gatto, D.A.; Tondi, G. Exploring tannin extracts: Introduction to new bio-based materials. Braz. J. Wood Sci. 2019, 10, 88–102. [Google Scholar] [CrossRef]
- Downey, M.O.; Hanlin, R.L. Comparison of ethanol and acetone mixtures for extraction of condensed tannin from grape skin. S. Afr. J. Enol. Vitic. 2010, 31, 154–159. [Google Scholar] [CrossRef][Green Version]
- Kovač, M.J.; Jokić, S.; Jerković, I.; Molnar, M. Optimization of deep eutectic solvent extraction of phenolic acids and tannins from Alchemilla vulgaris L. Plants 2022, 11, 474. [Google Scholar] [CrossRef]
- Murga, R.; Ruiz, R.; Beltran, S.; Cabezas, J.L. Extraction of natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol. J. Agric. Food Chem. 2000, 48, 3408–3412. [Google Scholar] [CrossRef] [PubMed]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Tomak, E.D.; Gonultas, O. The wood preservative potentials of valonia, chestnut, tara and sulphited oak tannins. J. Wood Chem. Technol. 2018, 38, 183–197. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Paczkowski, S.P.; Laborie, M.P. Modification of condensed tannins: From polyphenol chemistry to materials engineering. New J. Chem. 2016, 40, 36–49. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Elliot, J.A. Esterification of condensed tannins and their impact on the properties of poly (lactic acid). Polymers 2013, 5, 344–360. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Schrade, C. Modifying biodegradable plastics with additives based on condensed tannin esters. J. Appl. Polym. Sci. 2015, 132, 41626. [Google Scholar] [CrossRef]
- Martinez, G.A.; Rebecchi, S.; Decorti, D.; Domingos, J.M.; Natolino, A.; Del Rio, D.; Bertin, L.; Da Porto, C.; Fava, F. Towards multi-purpose biorefinery platforms for the valorisation of red grape pomace: Production of polyphenols, volatile fatty acids, polyhydroxyalkanoates and biogas. Green Chem. 2016, 18, 261–270. [Google Scholar] [CrossRef]
- Nanni, A.; Parisi, M.; Colonna, M. Wine by-products as raw materials for the production of biopolymers and of natural reinforcing fillers: A critical review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef]
- Nanni, A.; Cancelli, U.; Montevecchi, G.; Masino, F.; Messori, M.; Antonelli, A. Functionalization and use of grape stalks as poly (butylene succinate)(PBS) reinforcing fillers. Waste Manag. 2021, 126, 538–548. [Google Scholar] [CrossRef]
- Jin, A.; Del Valle, L.J.; Puiggalí, J. Copolymers and Blends Based on 3-Hydroxybutyrate and 3-Hydroxyvalerate Units. Int. J. Mol. Sci. 2023, 24, 17250. [Google Scholar] [CrossRef]
- Angelini, S.; Cerruti, P.; Immirzi, B.; Poskovic, M.; Santagata, G.; Scarinzi, G.; Malinconico, M. From microbial biopolymers to bioplastics: Sustainable additives for PHB processing and stabilization. In Microbial Factories: Biodiversity, Biopolymers, Bioactive Molecules: Volume 2; Kalia, V.C., Ed.; Springer: New Delhi, India, 2015; pp. 139–160. [Google Scholar]
- Ferri, M.; Papchenko, K.; Degli Esposti, M.; Tondi, G.; De Angelis, M.G.; Morselli, D.; Fabbri, P. Fully biobased polyhydroxyalkanoate/tannin films as multifunctional materials for smart food packaging applications. ACS Appl. Mater. Interface 2023, 15, 28594–28605. [Google Scholar] [CrossRef] [PubMed]
- Ismayati, M.; Hastuti, N.; Fatriasari, W.; Lubis, M.A.R.; Suryanegara, L.; Sari, F.P.; Burhani, D.; Amalia, B.; Agrippina, F.D.; Hidayati, S.; et al. Optimization of tannin isolation from bark of Acacia mangium and application in PLA-tannin based biofilm with antioxidant properties. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2024; Volume 2973. [Google Scholar]
- Ismayati, M.; Fatah, N.A.N.; Ernawati, E.E.; Juliandri; Kusumaningrum, W. B.; Lubis, M.A.R.; Fatriasari, W.; Solihat, N.N.; Sari, F.P.; Halim, A.; et al. Antioxidant and UV-blocking activity of PVA/tannin-based bioplastics in food packaging application. Int. J. Biol. Macromol. 2024, 257, 128332. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, M.; Grigsby, W.; Harper, D.; Rials, T. Chemical imaging of the spatial distribution and interactions of tannin dispersal in bioplastic systems. Adv. Mater. Res. 2007, 29, 173–176. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, İ.A. Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles. Coll. Surf. A 2003, 229, 85–96. [Google Scholar] [CrossRef]
- Turunen, J.; Karppinen, A.; Ihme, R. Effectiveness of biopolymer coagulants in agricultural wastewater treatment at two contrasting levels of pollution. SN Appl. Sci. 2019, 1, 210. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-based adhesives. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1980, 18, 247–315. [Google Scholar] [CrossRef]
- Pizzi, A.; Scharfetter, H.O. The chemistry and development of tannin-based adhesives for exterior plywood. J. Appl. Polym. Sci. 1978, 22, 1745–1761. [Google Scholar] [CrossRef]
- Moubarik, A.; Pizzi, A.; Allal, A.; Charrier, F.; Khoukh, A.; Charrier, B. Cornstarch–mimosa tannin–urea formaldehyde resins as adhesives in the particleboard production. Starch-Stärke 2010, 62, 131–138. [Google Scholar] [CrossRef]
- Moubarik, A.; Causse, N.; Poumadere, T.; Allal, A.; Pizzi, A.; Charrier, F.; Charrier, B. Shear refinement of formaldehyde-free corn starch and mimosa tannin (Acacia mearnsii) wood adhesives. J. Adhes. Sci. Technol. 2011, 25, 1701–1713. [Google Scholar] [CrossRef]
- Lopes, P.J.G.; Calegari, L.; de Medeiros Silva, W.A.; Gatto, D.A.; de Medeiros Neto, P.N.; De Melo, R.R.; Bakke, I.A.; Delucis, R.d.A.; Missio, A.L. Tannin-based extracts of Mimosa tenuiflora bark: Features and prospecting as wood adhesives. Appl. Adhes. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Dhawale, P.V.; Vineeth, S.K.; Gadhave, R.V.; Jabeen Fatima, M.J.; Supekar, M.V.; Thakur, V.K.; Raghavan, P. Tannin as a renewable raw material for adhesive applications: A review. Mater. Adv. 2022, 3, 3365–3388. [Google Scholar] [CrossRef]
- Ghahri, S.; Mohebby, B.; Pizzi, A.; Mirshokraie, A.; Mansouri, H.R. Improving water resistance of soy-based adhesive by vegetable tannin. J. Polym. Environ. 2018, 26, 1881–1890. [Google Scholar] [CrossRef]
- Sultan, M.; Nassar, M.; Abdel Hakim, A.A.; Refaat, Y. Eco-friendly lignocellulosic particleboards bonded with free-formaldehyde itaconic polyamidoamine epichlorohydrin/soy protein adhesive. Egypt. J. Chem. 2020, 63, 961–972. [Google Scholar] [CrossRef]
- Wang, J.; Scheibel, T. Recombinant production of mussel byssus inspired proteins. Biotechnol. J. 2018, 13, 1800146. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, K.; Wu, Q.; Zhang, X.; Qian, C.; Fan, D. Mussel byssus cuticle-inspired low-carbon footprint soybean meal adhesives with high-strength and anti-mildew performance. Ind. Crops Prod. 2024, 222, 119433. [Google Scholar] [CrossRef]
- Gwak, M.A.; Choi, Y.H.; Kim, M.H.; Park, W.H. Mussel byssus-inspired gallol-enriched chitosan hydrogel fibers with strong adhesive properties. Eur. Polym. J. 2024, 204, 112673. [Google Scholar] [CrossRef]
- Argenziano, R.; Viggiano, S.; Esposito, R.; Schibeci, M.; Gaglione, R.; Castaldo, R.; Fusaro, L.; Boccafoschi, F.; Arciello, A.; Della Greca, M.; et al. All natural mussel-inspired bioadhesives from soy proteins and plant derived polyphenols with marked water-resistance and favourable antibacterial profile for wound treatment applications. J. Colloid Interface Sci. 2023, 652, 1308–1324. [Google Scholar] [CrossRef]
- Rivero, S.; Garcia, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, L.; Guo, C.; Deng, F.; Wu, H.; Chen, L.; Huang, L.; Lu, P.; Ding, C.; Ni, Y.; et al. A bioinspired gallol-functionalized collagen as wet-tissue adhesive for biomedical applications. Chem. Eng. J. 2021, 417, 127962. [Google Scholar] [CrossRef]
- Mewoli, A.E.; Segovia, C.; Njom, A.E.; Ebanda, F.B.; Biwôlé, J.J.E.; Xinyi, C.; Ateba, A.; Girods, P.; Pizzi, A.; Brosse, N. Characterization of tannin extracted from Aningeria altissima bark and formulation of bioresins for the manufacture of Triumfetta cordifolia needle-punched nonwovens fiberboards: Novel green composite panels for sustainability. Ind. Crops Prod. 2023, 206, 117734. [Google Scholar] [CrossRef]
- Efhamisisi, D.; Thevenon, M.F.; Hamzeh, Y.; Karimi, A.N.; Pizzi, A.; Pourtahmasi, K. Induced tannin adhesive by boric acid addition and its effect on bonding quality and biological performance of poplar plywood. ACS Sustain. Chem. Eng. 2016, 4, 2734–2740. [Google Scholar] [CrossRef]
- Clementi, C.; Dominici, F.; Luzi, F.; Puglia, D.; Latterini, L. Historically Inspired Strategy to Achieve Sustainable and Effective Coloration of Bioplastics. ACS Sustain. Chem. Eng. 2023, 11, 9643–9653. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Ramos, M.; Viqueira, V.; Luzi, F.; Dominici, F.; Terenzi, A.; Maron, E.; Hamzaoui, M.; Kohnen, S.; Torre, L.; et al. Anthocyanin hybrid nanopigments from pomegranate waste: Colour, thermomechanical stability and environmental impact of polyester-based bionanocomposites. Polymers 2021, 13, 1966. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.V.; Morais, A.I.; Trigueiro, P.; de Souza, J.S.N.; Damacena, D.H.; Brandão-Lima, L.C.; Bezerra, R.D.S.; Fonseca, M.G.; Silva-Filho, E.C.; Osajima, J.A. Organic–inorganic hybrid pigments based on bentonite: Strategies to stabilize the quinoidal base form of anthocyanin. Int. J. Mol. Sci. 2023, 24, 2417. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Hernandez, C.; Cadenillas, L.; Maghubi, A.E.; Caceres, I.; Durrieu, V.; Mathieu, C.; Bailly, J.D. Mimosa tenuiflora aqueous extract: Role of condensed tannins in anti-aflatoxin B1 activity in Aspergillus flavus. Toxins 2021, 13, 391. [Google Scholar] [CrossRef]
- Venter, P.B.; Senekal, N.D.; Amra-Jordaan, M.; Bonnet, S.L.; Van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 2: An electrospray mass spectrometry investigation into the chemical composition of sulfited quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 78, 156–169. [Google Scholar] [CrossRef]
- Konai, N.; Pizzi, A.; Danwe, R.; Lucien, M.A.; Lionel, K.T. Thermomechanical analysis of African tannins resins and biocomposite characterization. J. Adhes. Sci. Technol. 2021, 35, 1492–1499. [Google Scholar] [CrossRef]
- Stepczyńska, M.; Rytlewski, P.; Moraczewski, K.; Pawłowska, A.; Karasiewicz, T. Novel Biocomposite of Starch and Flax Fiber Modified with Tannic Acid with Biocidal Properties. Polymers 2024, 16, 1108. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V., Jr.; Ramires, E.C.; Razera, I.A.T.; Frollini, E. Biobased composites from tannin–phenolic polymers reinforced with coir fibers. Ind. Crops Prod. 2010, 32, 305–312. [Google Scholar] [CrossRef]
- Ramires, E.C.; Frollini, E. Tannin–phenolic resins: Synthesis, characterization, and application as matrix in biobased composites reinforced with sisal fibers. Compos. Part B Eng. 2012, 43, 2851–2860. [Google Scholar] [CrossRef]
- Segovia, C.; Sauget, A.; Besserer, A.; Kueny, R.; Pizzi, A. Evaluating mold growth in tannin-resin and flax fiber biocomposites. Ind. Crops Prod. 2016, 83, 438–443. [Google Scholar] [CrossRef]
- Moreira, A.A.; de Carvalho, F.A.; Bilck, A.P.; de Paula, M.T.; Mali, S.; Yamashita, F.; de Oliveira, A.L.M. Tannin improves the processability and delays the biodegradability of poly (lactic acid)-starch-based thermoset materials produced by injection molding made with renewable compounds. J. Appl. Polym. Sci. 2023, 140, e53815. [Google Scholar] [CrossRef]
- Zhou, X.; Du, G. Applications of tannin resin adhesives in the wood industry. In Tannins-Structural Properties, Biological Properties and Current Knowledge; Intechopen: Rijeka, Croatia, 2020; pp. 97–103. [Google Scholar]
- Pizzi, A. Tannin-based biofoams-A review. J. Renew. Mater. 2019, 7, 474–489. [Google Scholar] [CrossRef]
- Pizzi, A.; Khan, A. Furanic Rigid Foams, Furanic-Based Bioplastics and Furanic-Derived Wood Adhesives and Bioadhesives. In Furan Derivatives-Recent Advances and Applications; Khan, A., Rahman, M.M., Ramesh, M., Khan, S.A., Asiri, A.M.A., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Natali, M.; Rallini, M.; Torre, L.; Puglia, D. High temperature composites from renewable resources: A perspective on current technological challenges for the manufacturing of non-oil based high char yield matrices and carbon fibers. Front. Mater. 2022, 9, 805131. [Google Scholar] [CrossRef]
- Chen, X.; Guigo, N.; Pizzi, A.; Sbirrazzuoli, N.; Li, B.; Fredon, E.; Gerardin, C. Ambient temperature self-blowing tannin-humins biofoams. Polymers 2020, 12, 2732. [Google Scholar] [CrossRef]
- de Jong, E.; Mascal, M.; Constant, S.; Claessen, T.; Tosi, P.; Mija, A. The origin, composition, and applications of industrial humins–a review. Green Chem 2025, 27, 3136–3166. [Google Scholar] [CrossRef]
- Zavarzina, A.G.; Danchenko, N.N.; Demin, V.V.; Artemyeva, Z.S.; Kogut, B.M. Humic substances: Hypotheses and reality (a review). Eurasian Soil Sci 2021, 54, 1826–1854. [Google Scholar] [CrossRef]
- Hayes, M.H.; Mylotte, R.; Swift, R.S. Humin: Its composition and importance in soil organic matter. Adv. Agron. 2017, 143, 47–138. [Google Scholar]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in understanding the humins: Formation, prevention and application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Mija, A.; van Der Waal, J.C.; Pin, J.M.; Guigo, N.; de Jong, E. Humins as promising material for producing sustainable polysaccharide-derived building materials. Acad. J. Civ. Eng. 2015, 33, 116–121. [Google Scholar]
- Sangregorio, A.; Guigo, N.; van der Waal, J.C.; Sbirrazzuoli, N. All ‘green’composites comprising flax fibres and humins’ resins. Compos. Sci. Technol. 2019, 171, 70–77. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Essawy, H.; Fredon, E.; Gerardin, C.; Guigo, N.; Sbirrazzuoli, N. Non-Furanic Humins-Based Non-Isocyanate Polyurethane (NIPU) Thermoset Wood Adhesives. Polymers 2021, 13, 372. [Google Scholar] [CrossRef]
- Li, K.; Geng, X.; Simonsen, J.; Karchesy, J. Novel wood adhesives from condensed tannins and polyethylenimine. Int. J. Adhes. Adhes. 2004, 24, 327–333. [Google Scholar] [CrossRef]
- Oktay, S.; Pizzi, A.; Köken, N.; Bengü, B. Tannin-based wood panel adhesives. Int. J. Adhes. Adhes. 2024, 130, 103621. [Google Scholar] [CrossRef]
- Calvez, I.; Garcia, R.; Koubaa, A.; Landry, V.; Cloutier, A. Recent Advances in Bio-Based Adhesives and Formaldehyde-Free Technologies for Wood-Based Panel Manufacturing. Curr. For. Rep. 2024, 10, 386–400. [Google Scholar] [CrossRef]
- Santulli, C. Beyond buttons: Repurposing of casein-based materials in education and industry—A review. Acad. Mater. Sci. 2024, 1, 3. [Google Scholar] [CrossRef]
- Dunky, M. Applications and Industrial Implementations of Naturally-Based Adhesives. In Biobased Adhesives: Sources, Characteristics and Applications; Scrivener Publishing LL: Beverly, MA, USA, 2023; pp. 659–704. [Google Scholar]
- Zhou, Y.; Li, C.; Xu, D.; Wang, Z.; Chen, Z.; Lei, H.; Du, G. A tannin–oxidized glucose wood adhesive with high performance. Ind. Crops Prod. 2024, 222, 119603. [Google Scholar] [CrossRef]
- Borah, N.; Karak, N. Tannic acid based bio-based epoxy thermosets: Evaluation of thermal, mechanical, and biodegradable behaviors. J. Appl. Polym. Sci. 2022, 139, 51792. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Fatriasari, W.; Sari, R.K.; Antov, P.; Gajtanska, M.; Papadopoulos, A.N.; Pizzi, A. Bio-based polyurethane resins derived from tannin: Source, synthesis, characterisation, and application. Forests 2021, 12, 1516. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Laksana, R.P.B.; Sari, R.K.; Iswanto, A.H.; Kristak, L.; Antov, P.; Pizzi, A. Thermal and mechanical performance of ramie fibers modified with polyurethane resins derived from acacia mangium bark tannin. J. Mater. Res. Technol. 2022, 18, 2413–2427. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, S. Adsorption behavior of Au (III) and Pd (II) on persimmon tannin functionalized viscose fiber and the mechanism. Int. J. Biol. Macromol. 2020, 152, 1242–1251. [Google Scholar] [CrossRef]
- Nicollin, A.; Kueny, R.; Toniazzo, L.; Pizzi, A. High density biocomposite from natural fibers and tannin resin. J. Adhes. Sci. Technol. 2012, 26, 1537–1545. [Google Scholar] [CrossRef]
- Wedaïna, A.G.; Pizzi, A.; Nzie, W.; Danwe, R.; Konai, N.; Amirou, S.; Segovia, C.; Kueny, R. Performance of unidirectional biocomposite developed with Piptadeniastrum Africanum tannin resin and Urena Lobata fibers as reinforcement. J. Renew. Mater. 2021, 9, 477–493. [Google Scholar]
- Taoga, M.M.; Ebanda, F.B.; Pizzi, A.; Ndiwe, B.; Noah, P.M.A.; Essome Mbang, J.P.; Kimbi, K.K.; Akwo, E.T.; Segovia, C. Influence of the Sampling Area on the Chemical, Physical, and Mechanical Properties of Grewia bicolor (GB) Fiber for Potential Use in the Reinforcement of Tannin Matrix Composites. J. Nat. Fib. 2024, 21, 2346121. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef]
- Tabassum, Z.; Girdhar, M.; Anand, A.; Kumari, N.; Sood, B.; Malik, T.; Kumar, A.; Mohan, A. Trash to treasure: Advancing resource efficiency using waste-derived fillers as sustainable reinforcing agents in bioplastics. Mater. Adv. 2025, 6, 527–546. [Google Scholar] [CrossRef]
- Akash, P. Bioplastics from Waste Biomass: Paving the Way for a Sustainable Future. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 10–22214. [Google Scholar] [CrossRef]
- de Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier–El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef]
- Naima, R.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier–El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crops Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Pizzi, A.; Laborie, M.P.; Candan, Z. A Review on Sources, Extractions and Analysis Methods of a Sustainable Biomaterial: Tannins. J. Renew. Mater. 2024, 12, 397–425. [Google Scholar] [CrossRef]
- Konai, N.; Pizzi, A.; Raidandi, D.; Lagel, M.C.; L’Hostis, C.; Saidou, C.; Hamido, A.; Abdalla, S.; Bahabri, F.; Ganash, A. Aningre (Aningeria spp.) tannin extract characterization and performance as an adhesive resin. Ind. Crops Prod. 2015, 77, 225–231. [Google Scholar] [CrossRef]
- Romero, R.; Gonzalez, T.; Urbano, B.F.; Segura, C.; Pellis, A.; Vera, M. Exploring tannin structures to enhance enzymatic polymerization. Front. Chem. 2025, 13, 1555202. [Google Scholar] [CrossRef]
- Silva, S.B.; Freitas, O.M.; Vieira, E.F.; Delerue-Matos, C.; Domingues, V.F. A comprehensive review on valorization of chestnut processing wastes into bio-based composites and bioplastics. Polym. Compos. 2025; ahead of print. [Google Scholar] [CrossRef]
- Mhlabeni, T.L.; Chemere, E.B.; Jamiru, T.; Mhike, W. Flame retardancy of biopolymers enhanced by bio-based flame retardants: A review. J. Fire Sci. 2025, 43, 79–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).