Abstract
Phenytoin is an anticonvulsant drug that suffers from low aqueous solubility. The formation of phenytoin salts is a strategy employed to address this issue. A phenytoin–piperidine salt (PPD–PNT) was synthesized by solvent-assisted grinding and characterized by infrared (IR) spectroscopy, 1H and 13C Nuclear Magnetic Resonance (NMR), and powder and single crystal X-ray diffraction. The IR and NMR spectra obtained differed from those of the starting compounds, showing shifts in the N-H and C=O group signals, as well as the appearance of NH+ signals, indicating proton transfer and salt formation. Powder X-ray diffraction confirmed the formation of a new solid phase corresponding to the salt. Single crystal X-ray diffraction showed the molecular structure of the PPD–PNT salt.
1. Introduction
Most active pharmaceutical ingredients used in the manufacture of medicines are solids in the form of powders and serve as raw materials for the production of solid, liquid, semi-solid, and gaseous dosage forms. The study of the physicochemical properties of these solid ingredients is of great importance because they directly impact the stability and therapeutic effect of the drug [1].
Solubility is one of the most important physicochemical properties of an active pharmaceutical ingredient. A drug’s bioavailability depends directly on its solubility in the gastrointestinal tract and its permeation through the cell membrane. The passage of the drug through biological membranes requires that it be dissolved; therefore, low solubility of a drug will decrease its absorption [2].
Preparation of pharmaceutical salts is the most used strategy to improve the solubility of water-insoluble drugs. A salt is formed by two or more components (drug and salt coformer), which are in ionized form and interact through ionic interactions. Another advantage of pharmaceutical salts is that they can generally be crystallized, which makes them easier to handle and process [3,4]. Salt formation typically involves acid–base reactions (HA ⇌ H+ + A− and B + H+⇌BH+) and can be predicted by using the pKa rule, which states that “when the pKa difference between a cocrystallizing acid and base is greater than 2 or 3 (ΔpKa = pKa[protonated base] − pKa[acid]), salt formation is expected” [5].
Piperidine is a heterocyclic amine that has been used as a coformer of salts with pharmaceutical ingredients such as diclofenac, sulfapyrine, sulfamethazine, and fenofibric acid [6,7,8,9].
Phenytoin (5,5′-diphenylimidazolidine-2,4-dione) belongs to the hydantoin family and possesses carbonyl (C=O), amino (N-H), and benzene rings in its chemical structure (Figure 1). It is used as an anticonvulsant drug that acts by blocking calcium channels [10]. It has a low aqueous solubility (less than 0.1 g/mL), classifying it within class II of the biopharmaceutical classification system (low solubility and high permeability). The sodium salt of phenytoin also has low aqueous solubility (1 g/66 mL). Some strategies that have been developed to modify the solubility of phenytoin are: salt formation, crystal habit modification, complex formation with cyclodextrin, cosolvency with N-methylpyrrolidone, and mixtures with polymers [11,12,13,14,15,16].
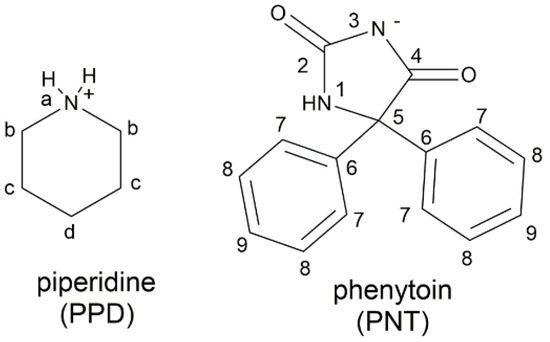
Figure 1.
Piperidine–phenytoin salt.
The formation of pharmaceutical salts generally involves the use of chemical solvents that can harm the environment and health, and incur higher economic costs. Mechanochemical synthesis (by dry or solvent-assisted grinding) is an alternative to the synthesis of salts, which is based on the application of a mechanical force to modify the solid properties of the ingredients, resulting in the formation of a new solid phase [4,17,18]. Considering the solubility limitations of phenytoin and the few options available for its administration (phenytoin and phenytoin sodium), as well as the search for alternative solid forms, the objective of this work was the synthesis of a phenytoin salt with piperidine (PPD–PNT) (Figure 1), to generate chemical and structural information about another solid form of this pharmaceutical ingredient. The obtained salt was characterized by IR spectroscopy, 1H and 13C NMR, powder X-ray diffraction, and single crystal X-ray diffraction.
2. Materials and Methods
2.1. Reagents
Piperidine and phenytoin were purchased from Aldrich; dichloromethane, methanol, and acetonitrile were purchased from Química Meyer. All the reagents and solvents were used as received.
2.2. Mechanochemical Synthesis of the PPD–PNT Salt
The compounds (PPD and PNT in a 1:1 molar ratio) were placed in a mortar, 0.5 mL of dichloromethane was added before starting the grinding, and the mixture was ground with a pestle for 3 min. Upon completion of grinding, the resulting powder was placed in the center of the mortar, 0.5 mL of dichloromethane was added again, and the mixture was ground for an additional 3 min. The addition of 0.5 mL of dichloromethane and grinding for 3 min was repeated two more times to complete 12 min of grinding. The ground powder was stored in a glass vial.
2.3. IR Spectroscopy
Infrared spectra were collected on a Bruker Tensor 27 spectrophotometer equipped with a diamond ATR (Attenuated Total Reflection) accessory in a spectral range from 4000 to 600 cm−1 with a resolution of 4 cm−1 (16 scans).
2.4. Nuclear Magnetic Resonance
1H (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Ultrashield plus 400 MHz instrument using DMSO-d6 as solvent and TMS as the internal reference. The NMR spectra were processed with the MestReNova software version 14.2.0 [19].
2.5. X-Ray Diffraction
Powder diffraction patterns were recorded by a PANalytical X’pert Pro diffractometer with Cu Kα radiation (λ = 1.5405 Å, 45 kV, 40 mA) in a 2θ scanning range from 2° to 50°.
Single crystals suitable for monocrystal diffraction were obtained by dissolving 0.3 g of PPD–PNTground in 4 mL of a 1:1 methanol–acetonitrile mixture and leaving the solvent to evaporate.
Single crystal diffraction data of PPD–PNT were collected at 130 K with an Oxford Diffraction Gemini diffractometer (λMoKα = 0.71073 Å, monochromator: graphite) with a CCD-atlas area detector. Data collection and integration were performed with CrysAlisPro and CrysAlis RED software version 1.171.36.32 [20,21]. SHELXS and SHELXL software version 2018 were used for structure solution and refinement [22,23], and WinGX version 2023.1 [24] software was used to prepare material for publication. Full-matrix least-squares refinement was carried out by minimizing (Fo2 − Fc2)2. All non-hydrogen atoms were refined anisotropically. H atoms of the N-H group were located in a difference map and refined isotropically with Uiso(H) 1.2 Ueq for N-H. H atoms attached to C atoms were placed in geometrically idealized positions and refined as riding on their parent atoms, with C–H = 0.95 − 0.99 Å with Uiso(H) = 1.2Ueq(C) for aromatic and methylene groups. Graphic material was prepared using Mercury Software version 2020.3.0 [25]. Crystal data, data collection, and structure refinement details are summarized in Table 1.

Table 1.
Crystal data of PPD–PNTcrystal.
3. Results
3.1. Spectroscopic Characterization
The ΔpKa value between PPD (pKa = 11.1) [26] and PNT (pKa = 8.3) [27] is 2.8; therefore, the expected new solid phase is a salt. IR-ATR spectroscopy is a rapid method to determine the formation of new solid forms by the shift of the characteristic bands of the functional groups of a solid phase compared with the starting compounds (IR assignments of the characteristic frequencies of PNT, PPD, and the PPD–PNT mixtures are listed in Table 2).

Table 2.
IR frequencies (cm−1) of the NH, NH+, and C=O stretching frequencies of the starting compounds, PNTNa and the PPD–PNT salt.
The IR spectra of PNT and PPD were assigned as previously reported [28,29,30]. The IR spectra of PPD–PNT (ground and single crystal) showed differences with respect to the starting compounds (Figure 2 and Table 2). To confirm the salt formation, the IR frequencies of PPD–PNTground/crystal were compared with the IR frequencies of phenytoin sodium salt (PNTNa) [28]. In PNTNa (a deprotonated form of PNT), the C=O signal shifts to a lower wavenumber appearing at 1674 cm−1 and 1687 cm−1, while in PPD–PNTground they were observed at 1703 cm−1 (ΔνC=O = −67 cm−1) and 1643 cm−1 (ΔνC=O = −72 cm−1) (in PPD–PNTcrystal they appeared at 1702 cm−1 and 1644 cm−1, ΔνC=O = −68 cm−1 and −71 cm−1 respectively), which is in agreement with the deprotonation of carboxylic acids, where the C=O band shifts to a lower wavenumber due to the electron delocalization to form the carboxylate ion [31]. The N-H signal of PNTNa is reported at 3318 cm−1, while in PPD–PNTground/crystal, it was observed at 3153 cm−1 (ΔνN-H = −47 cm−1). When theophylline is deprotonated to form salts, the NH band shifts by 30–40 cm−1 [32].
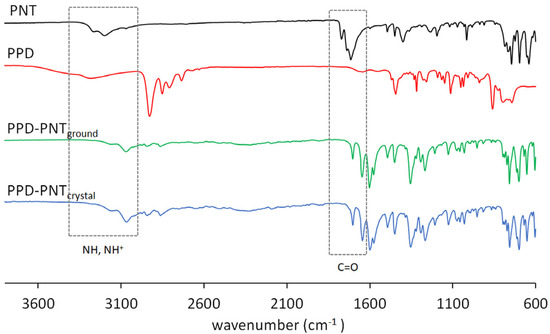
Figure 2.
IR spectra of PNT (phenytoin); PPD (piperidine); PPD–PNTground (piperidine–phenytoin ground powder), and PPD–PNTcrystal (piperidine–phenytoin single crystal).
The signal at 3070 cm−1 in the IR spectrum of PPD–PNTground (3065 cm−1 in PPD–PNTcrystal) belongs to the NH2+ group of piperidine, which is another signal indicating the proton transfer to form the salt, in a similar way to that reported for piperidine–sulfamethazine salt (3076 cm−1) [8], and piperidinium–4-carboxylic acid hydrogen squarate (3073 cm−1) [33].
The frequencies of the aromatic/alkyl and C-N groups also appeared shifted in the IR spectra of PPD–PNTground/crystal (Table 2).
The NMR spectroscopy study (the chemical shifts of which are listed in Table 3, and the atom numbering is according to Figure 1) was performed to characterize the PPD–PNT salt in solution. The 1H NMR spectrum of starting PNT (Figure 3) showed the N-H characteristic signals [34] at 11.13 ppm and 9.33 ppm, and the aromatic signals in the 7.42–7.34 ppm range. PPD showed the N-H signal at 2.86 ppm, and the rest of the alkyl signals in the 2.63–1.41 ppm range. The absence of the NH3 signal of PNT (11.13 ppm) in the 1H NMR spectra of PPD–PNTground/crystal (Figure 3) is evidence of the salt formation and proton transfer from PNT to PPD to form the PPD–PNT salt. The NH1 signal appeared as a broad signal at 9.24 ppm in PPD–PNTground and at 9.19 ppm in PPD–PNTcrystal (similar to the 1H chemical shifts reported for phenytoin derivatives [35,36]); the aromatic signals remained in the same range.

Table 3.
NMR chemical shifts (ppm in DMSO-d6) of PNT (phenytoin), PPD (piperidine), PPD–PNTpowder (piperidine–phenytoin ground powder), and PPD–PNTcrystal (piperidine–phenytoin single crystal). (N.O. = not observed).
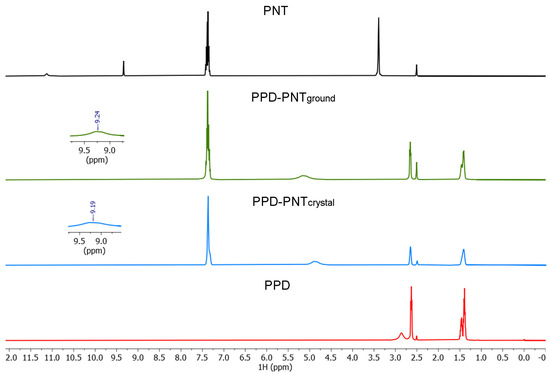
Figure 3.
1H NMR spectra in DMSO-d6 of PNT (phenytoin); PPD (piperidine); PPD–PNTground (piperidine–phenytoin ground powder) and PPD–PNTcrystal (piperidine–phenytoin single crystal).
Another proof of salt formation is the shift of the NHa signal (the piperidinium signal in PPD–PNT) from 2.86 ppm in the starting PPD to 5.14 ppm in PPD–PNTground (Δδ = 2.28 ppm), and 4.89 ppm in PPD–PNTcrystal (Δδ = 2.03 ppm).
The 13C NMR signals (Figure 4 and Table 3) of C2 and C4 carbonyls were shifted from 156.44 ppm and 175.28 ppm in the starting PNT to 157.27 ppm (Δδ = 0.83 ppm) and 175.90 ppm (Δδ = 0.62 ppm) in PPD–PNTground, and 157.60 ppm (Δδ = 1.16 ppm) and 176.13 ppm (Δδ = 0.85 ppm) in PPD–PNTcrystal, due to the deprotonation causing a higher deshielding effect over C2 and C4 carbonyls, as occurs in the deprotonation of carboxylic acids [37]. PPD carbons also were sensitive to the salt formation, showing shifts from 47.29 ppm, 26.80 ppm, and 25.16 ppm in the starting PPD to 47.00 ppm, 27.14 ppm, and 25.38 ppm in PPD–PNTground (Δδ = −0.29 ppm, 0.34 ppm, and 0.22 ppm, respectively), and 46.87 ppm, 26.66 ppm, and 25.07 ppm in PPD–PNTcrystal (Δδ = −0.42 ppm, 0.14 ppm, and 0.09 ppm, respectively).
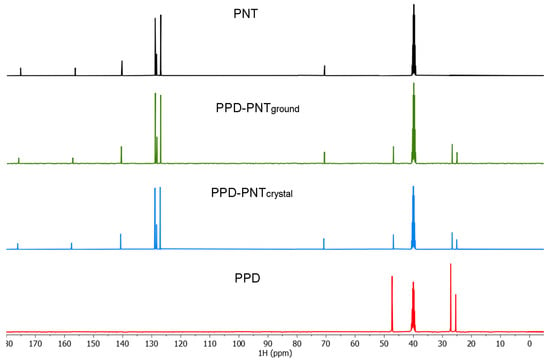
Figure 4.
13C NMR spectra in DMSO-d6 of PNT (phenytoin); PPD (piperidine); PPD–PNTground (piperidine–phenytoin ground powder) and PPD–PNTcrystal (piperidine–phenytoin single crystal).
3.2. X-Ray Diffraction
The production of the solid form of the PPD–PNT salt was confirmed because the powder X-ray diffraction pattern of PPD–PNTground (Figure 5) was different from the pattern of PNT and similar to the simulated pattern of PPD–PNTcrystal. Distinctive signals of the PPD–PNTground diffraction pattern were observed at 2θ = 9.2° (0 1 0), 13.4° (1 1 1), 15.8° (0 0 2), 22.0° (2 0 1) and 25.1° (9.3°, 13.6°, 15.9°, 22.1° and 25.1° in the simulated pattern of PPD–PNTcrystal). Signals of residual PNT appeared at 2θ = 11.4°, 20.4°, and 27.0° in the PPD–PNTground diffraction pattern.
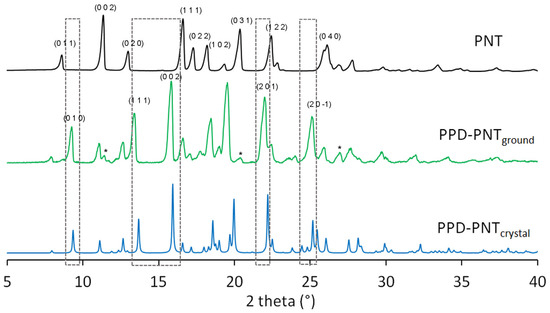
Figure 5.
Powder X-ray diffraction patterns of PNT (phenytoin), PPD–PNTground (piperidine–phenytoin ground powder), and PPD–PNTcrystal (piperidine–phenytoin single crystal). Numbers in brackets are the Miller indices. (*) Signals of residual PNT.
There are a few reports about crystal structures of multicomponent forms of phenytoin. Only the following crystal structures have been reported: the cocrystal of PNT-1-(4-bromophenyl)-4-dimethylamino-2,3-dimethyl-3-pyrazolin-5-one (CCDC REFCODE: DPHPZL [38]) where the PNT molecules adopt the R22(8) imide type pattern (Figure 6), as well as the cocrystal of PNT-9-ethyladenine 2,4-pentanedione solvate (CCDC REFCODE: DPHEAD10 [39]), and PNT-choline salt (REFCODE: COXYUK [11]) where, in both crystal structures, the PNT molecules form the R22(8) urea type pattern (Figure 6).
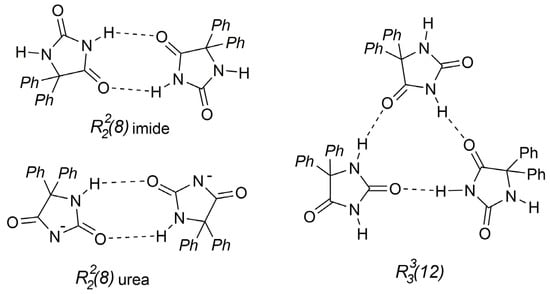
Figure 6.
PNT–PNT hydrogen bond patterns found in crystalline structures of PNT.
Single crystal X-ray diffraction showed the molecular structure (triclinic, P-1 space group) and confirmed the proton transfer from PNT to PPD to form the PPD–PNTcrystal salt because the PPD molecule showed the NH2+ group and the PNT molecule showed the O=C-N− group (Figure 7a). PPD and PNT are connected by a N-H+···N− hydrogen bond, forming the 1:1 salt (hydrogen bond details are listed in Table 4). A 1D supramolecular tape involving a R22(8) urea type PNT–PNT interaction (Figure 7b), and an R44(12) PNT2-PPD2 interaction is extended along the (16 17 16) plane. The 2D supramolecular architecture showed a supramolecular tape where one PNT molecule is interlinked by a C-H···π interaction (C1-H1CA···Cg(3) = 2.44 Å; Cg(3) = C7/C8/C9/C10/C11/C12) to one PPD molecule and extended along the a axis by the N1C-H3N···N1 hydrogen bond. Free PNT forms a supramolecular tape where three molecules of PNT are interlinked by N-H···O=C hydrogen bonds, depicting an R33(12) motif (Figure 7c). Formation of PPD–PNTcrystal involves the breaking of this pattern of hydrogen bond interactions and the molecular rearrangement to include PNT, now showing the characteristic R22(8) urea pattern between PNT molecules and the R44(12) pattern that connects PPD with PNT.
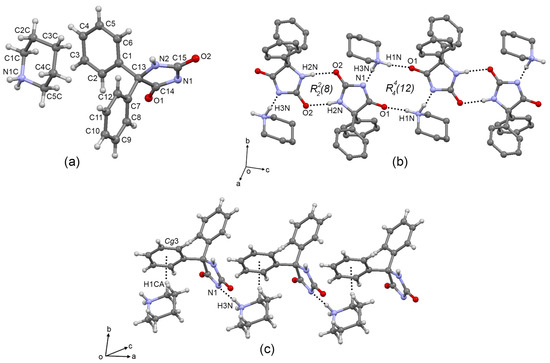
Figure 7.
(a) Molecular structure of PPD–PNTcrystal at 50% probability level. (b) 1D supramolecular tape of formed by N-H···O=C, N-H+···N, and N-H+···O=C hydrogen bonds showing the R22(8) and R44(12) motifs. (c) 2D supramolecular tapes of PPD–PNTcrystal showing C-H···π interactions and N-H+···N hydrogen bond. Some H atoms have been omitted for clarity. Dashed lines represent hydrogen bonds or non-covalent interactions.

Table 4.
Hydrogen bond geometry (Å, °).
Deprotonation causes changes in the bond distances of the imidazolidinedione ring of PNT. An analysis of the bond distances of PPD–PNTcrystal (Figure 8) compared with the phenytoin sodium methanol hydrate (PNTNa-MeOH-H2O, REFCODE: COMREE [40]) (deprotonated form) and the starting PNT (protonated form) revealed that the imidazolidinedione fragment in PPD–PNTcrystal is similar to PNTNa-MeOH-H2O (Figure 8). The C-N imide distances become shorter in PPD–PNTcrystal (1.338(1) Å and 1.386(1) Å) with respect to PNT (1.350(4) Å and 1.394(4) Å), and similar to what was observed in PNTNa-MeOH-H2O (1.339(3) Å and 1.371(2) Å). Also, the C-O distances in PPD–PNTcrystal became longer (1.238(1) Å and 1.237(1) Å) compared with PNT (1.224(4) Å and 1.204(4) Å), similar also to PNTNa-MeOH-H2O (1.239(2) Å and 1.247(3) Å). The rest of the bond distances of the imidazolidinedione ring are shown in Figure 8.
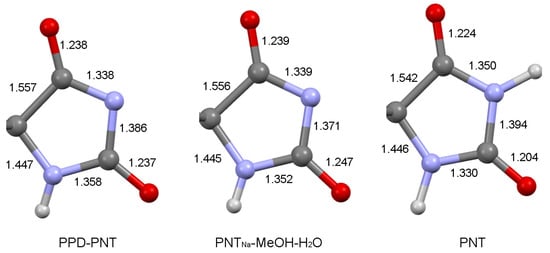
Figure 8.
Bond distances (Å) of the imidazolidinedione ring of PPD–PNTcrystal, PNTNa-MeOH-H2O, and PNT.
4. Conclusions
The PPD–PNT salt was obtained by the solvent-assisted grinding method and characterized by IR spectroscopy, 1H and 13C NMR, powder X-ray diffraction, and single crystal X-ray diffraction.
Formation of the PPD–PNT salt and the proton transfer from PNT to PPD was evidenced by: (a) the shift of the C=O band (similar to PNTNa), and the appearance of the NH2+ band in the IR spectrum of PPD–PNTground/crystal; (b) the absence of the NH3 proton, and the shift of the NHa proton in the 1H NMR spectrum of PPD–PNTground/crystal; (c) the shift of the 13C NMR signals of the carbonyls of PPD–PNground/crystal; (d) a different powder diffraction pattern of PPD–PNTground compared with the powder diffraction pattern of PNT; and (e) the appearance of the NH2+ and O=C-N− groups, and the changes in the bond distances (similar to the PNTNa-MeOH-H2O) in the molecular structure obtained by single crystal X-ray diffraction of PPD–PNTcrystal.
The crystal structure of PPD–PNTcrystal showed a 1:1 stoichiometry, forming a N-H+···N− hydrogen bond between PPD and PNT and depicting 1D and 2D supramolecular tapes.
Author Contributions
Conceptualization, J.S.G.-G. and H.G.-O.; methodology, M.I.A.-M.; formal analysis and validation, M.F.-Á., A.P.-C. and F.J.M.-M.; investigation, M.I.A.-M.; writing—original draft preparation and editing, J.S.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de la Cañada, grant number UNCA PFI-01/23, and Facultad de Química, UNAM, grant number PAIP 5000-9112.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gokhale, M.Y.; Mantri, R.V. API solid-form screening and selection. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Zhang, G.G.Z., Mantri, R.V., Chen, Y., Yu, L., Eds.; Academic Press: Oxford, UK, 2017; pp. 85–112. [Google Scholar] [CrossRef]
- Mantri, R.V.; Sanghvi, R. Solubility of pharmaceutical solids. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Zhang, G.G.Z., Mantri, R.V., Chen, Y., Yu, L., Eds.; Academic Press: Oxford, UK, 2017; pp. 3–22. [Google Scholar] [CrossRef]
- Wiedmann, T.S.; Naqwi, A. Pharmaceutical salts: Theory, use in solid dosage forms and in situ preparation in an aerosol. Asian J. Pharm. Sci. 2016, 11, 722–734. [Google Scholar] [CrossRef]
- Mithu, S.H.; Economidou, S.; Trivedi, V.; Bhatt, S.; Douroumis, D. Advanced Methodologies for Pharmaceutical Salt Synthesis. Cryst. Growth Des. 2021, 21, 1358–1374. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Fini, A.; Cavallari, C.; Bassini, G.; Ospitali, F.; Morigi, R. Diclofenac salts, part 7: Are the pharmaceutical salts with aliphatic amines stable? J. Pharm. Sci. 2012, 101, 3157–3168. [Google Scholar] [CrossRef]
- Pratt, J.; Hutchinson, J.; Stevens, C.L.K. Sulfapyridine (polymorph III), sulfapyridine dioxane solvate, sulfapyridine tetrahydrofuran solvate and sulfapyridine piperidine solvate, all at 173 K. Acta Cryst. 2011, C67, o487–o491. [Google Scholar] [CrossRef]
- González-González, J.S.; Pérez-Espinoza, S.; Martínez-Martínez, F.J.; Pineda-Contreras, A.; Canseco-Martínez, M.Á.; Flores-Alamo, M.; García-Ortega, H. Crystal structure and characterization of the sulfamethazine–piperidine salt. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2023, 79, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Varsa S, R.B.; Ramanujan, G.M.; Prajapati, A.K.; Chernyshev, V.V.; Sanphui, P. Solid-State Diversity of Fenofibric Acid: Synthon Polymorphs and Salts with Altered Solubility and Dissolution. Cryst. Growth Des. 2025, 25, 720–733. [Google Scholar] [CrossRef]
- Bartollino, S.; Chiosi, F.; di Staso, S.; Uva, M.; Pascotto, A.; Rinaldi, M.; Hesselink, J.M.K.; Costagliola, C. The retinoprotective role of phenytoin. Drug Des. Dev. Ther. 2018, 12, 3485–3489. [Google Scholar] [CrossRef]
- Dean, P.M.; Turanjanin, J.; Yoshizawa-Fujita, M.; MacFarlane, D.R.; Scott, J.L. Exploring an Anti-Crystal Engineering Approach to the Preparation of Pharmaceutically Active Ionic Liquids. Cryst. Growth Des. 2009, 9, 1137–1145. [Google Scholar] [CrossRef]
- Chiang, P.C.; Wong, H. Incorporation of physiologically based pharmacokinetic modeling in the evaluation of solubility requirements for the salt selection process: A case study using phenytoin. AAPS J. 2013, 15, 1109–1118. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Bolourtchian, N.; Dinarvand, R. Crystal modification of phenytoin using different solvents and crystallization conditions. Int. J. Pharm. 2003, 250, 85–97. [Google Scholar] [CrossRef]
- Agrawal, S.; Gaikwad, S.; Patel, R.; Shinde, L.; Deshmukh, A. Synthesis and Formulation Development of Phenytoin by Inclusion Complexation. Indian J. Pharm. Sci. 2021, 83, 955–962. [Google Scholar] [CrossRef]
- Khajir, S.; Shayanfar, A.; Acree Jr, W.E.; Jouyban, A. Effects of N-methylpyrrolidone and temperature on phenytoin solubility. J. Mol. Liq. 2019, 285, 58–61. [Google Scholar] [CrossRef]
- Widanapathirana, L.; Tale, S.; Reineke, T.M. Dissolution and solubility enhancement of the highly lipophilic drug phenytoin via interaction with poly (N-isopropylacrylamide-co-vinylpyrrolidone) excipients. Mol. Pharm. 2015, 12, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Hasa, D.; Perissutti, B.; Cepek, C.; Bhardwaj, S.; Carlino, E.; Grassi, M.; Invernizzi, S.; Voinovich, D. Drug salt formation via mechanochemistry: The case study of vincamine. Mol. Pharm. 2013, 10, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; De la O-Contreras, M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Mnova Structure Elucidation, version 14.2.0-26256; Mestrelab Research: Santiago de Compostela, Spain, 2021.
- CrysAlisPro, version 1.171.36.32; Oxford Difraction Ltd.: Abingdon, UK, 2013.
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP forWindows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Luna, O.F.; Gomez, J.; Cárdenas, C.; Albericio, F.; Marshall, S.H.; Guzmán, F. Deprotection reagents in Fmoc solid phase peptide synthesis: Moving away from piperidine? Molecules 2016, 21, 1542. [Google Scholar] [CrossRef]
- Nation, R.L.; Evans, A.M.; Milne, R.W. Pharmacokinetic drug interactions with phenytoin (Part I). Clin. Pharmacokinet. 1990, 18, 37–60. [Google Scholar] [CrossRef]
- Dharani, S.; Rahman, Z.; Ali, S.F.B.; Afrooz, H.; Khan, M.A. Quantitative estimation of phenytoin sodium disproportionation in the formulations using vibration spectroscopies and multivariate methodologies. Int. J. Pharm. 2018, 539, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, S.; Jin, R. NMR and FT-IR analysis of new molecular complex 1-piperidine-carboxylate-piperidinium-H2O. Wuhan Univ. J. Nat. Sci. 2008, 13, 93–97. [Google Scholar] [CrossRef]
- Kadam, A.; Jangam, S.; Oswal, R. Application of Green Chemistry Principle in Synthesis of Phenytoin and Its Biogical Evaluation as Anticonvulsant Agents. J. Chem. 2011, 8, S47–S52. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharmaceutics 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Bartoszak-Adamska, E.; Dega-Szafran, Z.; Komasa, A.; Szafran, M. Structural and spectroscopic properties of piperidinium-4-carboxylic acid hydrogen squarate. Vib. Spectrosc. 2015, 81, 13–21. [Google Scholar] [CrossRef]
- Luchian, R.; Vinţeler, E.; Chiş, C.; Vasilescu, M.; Leopold, N.; Chiş, V. Molecular structure of phenytoin: NMR, UV-Vis and quantum chemical calculations. Croat. Chem. Acta 2015, 88, 511–522. [Google Scholar] [CrossRef]
- Botros, S.; Khalil, N.A.; Naguib, B.H.; El-Dash, Y. Synthesis and anticonvulsant activity of new phenytoin derivatives. Eur. J. Med. Chem. 2013, 60, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Guerrab, W.; Jemli, M.E.; Akachar, J.; Demirtaş, G.; Mague, J.T.; Taoufik, J.; Ibrahimi, A.; Ansar, M.H.; Alaoui, K.; Ramli, Y. Design, synthesis, structural and molecular characterization, toxicity, psychotropic activity and molecular docking evaluation of a novel phenytoin derivative: 3-decyl-5, 5-diphenylimidazolidine-2, 4-dione. J. Biomol. Struct. Dyn. 2022, 40, 8765–8782. [Google Scholar] [CrossRef] [PubMed]
- Akkurt, N.; Al-Jumaili, M.H.A.; Ocak, H.; Cakar, F.; Torun, L. Synthesis and liquid crystalline properties of new triazine-based π-conjugated macromolecules with chiral side groups. Turk. J. Chem. 2020, 44, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Uno, T.; Shimizu, N. Structure of 5,5-diphenylhydantoin–1-(4-bromophenyl)-4-dimethylamino-2,3-dimethylpyrazolin-5-one (1:1). Acta Cryst. 1980, B36, 2794–2796. [Google Scholar] [CrossRef]
- Camerman, A.; Mastropaolo, D.; Camerman, N. Molecular structure of acetylacetone. A crystallographic determination. J. Am. Chem. Soc. 1983, 105, 1584–1586. [Google Scholar] [CrossRef]
- Shah, H.S.; Chaturvedi, K.; Zeller, M.; Bates, S.; Morris, K. A threefold superstructure of the anti-epileptic drug phenytoin sodium as a mixed methanol solvate hydrate. Acta Crystallogr. Sect. C 2019, C75, 1213–1219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).