Abstract
Heterocycle cores are widely used in medicinal chemistry for developing bioactive compounds. In this scenario, using cheap and accessible starting material to build these heterocycles is desirable to obtain new drug candidates for cost-efficient processes. One easily accessible source of starting material are amino acids. Usually, these compounds are employed in peptide synthesis, but their use for building heterocycle frameworks presents another appealing opportunity. Therefore, this review highlights the application of histidine and tryptophan, two heteroaromatic amino acids, in fused heterocyclic scaffold synthesis and their use in bioactive compounds.
1. Introduction
Histidine and tryptophan are heteroaromatic amino acids with imidazole and indole rings in their structure, respectively. These two rings are among the most common rings in drugs and lead-like drugs [1,2,3], highlighting the potential application of these amino acids in the development of bioactive compounds.
The use of amino acids to make bioactive peptides and peptidomimetic compounds is very common [4,5,6,7,8,9], but they are also present in the biosynthetic pathways of some important naturally occurring alkaloids. Representative examples of alkaloids derived from histidine and tryptophan are shown in Figure 1.
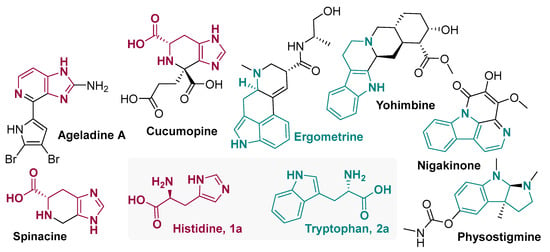
Figure 1.
Representative examples of alkaloids from histidine and tryptophan.
Spinacine, present in Panax ginseng [10], and Cucumopine, found in Agrobacterium rhizogenes [11,12], are examples of histidine derivatives formed by cyclization reaction in imidazole C-4 through Pictet–Spengler reaction. Also, this amino acid can be converted into its respective biogenic amine, histamine, and then undergo Pictet–Spengler cyclization to form, for example, Ageladine A, an important anti-angiogenic alkaloid [13].
Similar aspects can be observed for tryptophan-derivative alkaloids. Its biogenic amine, tryptamine, undergoes heterocyclization at indole C-4 to form physostigmine [14] and Pictet–Spengler-type reaction with secologanin to form strictosidine, a key intermediate in the biosynthesis of Yohimbine [15], an antagonist of α2-adrenergic receptors from the bark of the Pausinystalia yohimbe tree [16]. The antifungal [17] canthin-6-one derivative Nigakinone also comes from a Pictet–Spengler-type reaction and some oxidations to obtain the tetracyclic framework with cyclization into indole nitrogen [18]. Tryptophan can also undergo prenylation at indole C-4 to later be cyclized into the tetracyclic framework [19] present in ergometrine, a drug used in obstetrics to control postpartum bleeding [20].
Historically, natural products inspired medicinal chemistry and organic synthesis in developing several drugs [21,22]. And it was no different for these two amino acids, which are employed in the synthesis of a diverse set of bioactive heterocyclic frameworks. Therefore, considering the importance of histidine and tryptophan beyond peptides and peptidomimetics, this review brings the leading findings in the synthesis of fused heterocycles prepared from these two heteroaromatic amino acids and their application in bioactive compounds.
2. Fused Heterocyclic Scaffolds from Histidine
The most common fused heterocycle framework prepared from histidine is the tetrahydro-imidazo [4,5-c]pyridine core (Figure 2), which is present in the natural product Spinacine, an alpha-amino acid of ginseng (Panax ginseng) and spinach (Spinacia oleracea) [23]. Normally, this structural pattern is prepared using aldehyde or aldehyde-related compounds in acidic conditions, which is known as the Pictet–Spengler reaction [24,25,26]. However, basic conditions can also be used (Figure 2) [27,28,29,30]. This reaction involves heterocyclization at imidazole C-4, and the yield varies according to the conditions and the aldehyde used (Figure 2). One interesting fact regarding this tetrahydro-imidazo [4,5-c]pyridine core is that the C-6 carboxyl group of the Spinacine prevents the ring opening necessary for the racemization [31].
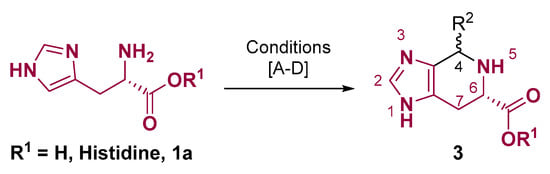
Figure 2.
Histidine heterocyclization at imidazole C-4. Conditions: (A) R1 = H and R2 = H: formaldehyde, HCl, 1–12 h [25,26,30,32]. (B) R1 = Me and R2 = benzo [1,3]dioxole: aldehyde, pyridine, 100 °C, N2, 4 h [27] (C) R1 = H and R2 = alkyl or aryl: R1CHO, NaOH, KOH or K2CO3, MeOH or EtOH, reflux, 2–24 h [28,29,30].; (D) R1 = H and R2 = CF3: TFAE, H2O, Ar, 100 °C, 6 h [31].
Another reactivity aspect of tetrahydro-imidazo [4,5-c]pyridine framework is that if this core has an aryl group at the core C-4, a hydrogenolysis in methanol at reflux using ammonium formate rapidly converts it to 4(5)-benzyl-L-histidine [33]. This is an important way to prepare 4-benzyl histidine derivatives.
The functionalization of histidine imidazole ring can also be accomplished after amine protection by a ring cyclization with 1,1′-carbonyldiimidazole (CDI) to form a 5-oxo-5,6,7,8-tetrahydroimidazo [1,5-c]pyrimidine core. After that, the non-substituted imidazole nitrogen can be alkylated; then, the protecting group can be removed to form scaffold 5. (Figure 3) [34,35]. This protection can also be accomplished by phosgene use [36].
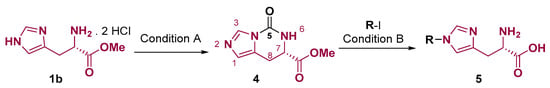
Figure 3.
Selective imidazole nitrogen alkylation via heterocyclization protection with CDI. Conditions: (A) CDI, DMF, 60 °C, 3–5 h and (B) (1) R-I, MeCN, reflux, 24 h and (2) HCl, reflux, 12–24 h [34,35].
The tetrahydro-imidazo [4,5-c]pyridine core prepared from histidine can undergo a dehydrogenation reaction to obtain the full aromatic imidazo [4,5-c]pyridine core 6 (Figure 4). Normally, in this reaction, a decarboxylative aromatization takes place using selenium dioxide [30], sulfur [37,38], 2-Iodoxybenzoic acid (IBX), or octasulfur [29]. When an ester of tetrahydro-imidazo [4,5-c]pyridine is reacted with selenium dioxide activated by trimethylsilyl polyphosphate (PPSE) in carbon tetrachloride, [39] the aromatic imidazo [4,5-c]pyridine core formed keeps the ester group [25,26].
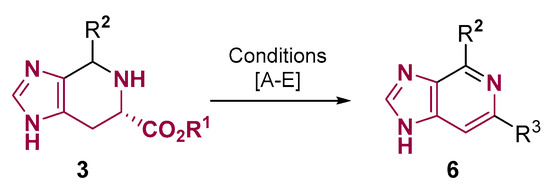
Figure 4.
Tetrahydro-imidazo [4,5-c]pyridine core dehydrogenation. Conditions: (A) R1 = Me, R2 = H or aryl and R3 = H: SeO2, AcOH, reflux, 15 min [30]; (B) R1 = H, R2 = Aryl and R3 = H: S, DMF, 120–150 °C, 2–7 h [37,38]; (C) R1 = H, R2 = Aryl and R3 = H: IBX, DMSO, 45 °C, 5–10 h [29]; (D) S8, DMF, 140 °C, 5–20 h [29]; (E) R1 = Me, R2 = H and R3 = CO2Me: SeO2, PPSE, Et3N, CCl4, reflux, 12 h [25,26].
The direct formation of aromatic imidazo [4,5-c]pyridine core from histidine was already identified from Maillard reaction conditions, in which the heating of glucose with histidine forms 2-acetyl- and 2-propionyl imidazo [4,5-c]pyridine (Figure 5) [40,41]. The reaction of histidine with another monosaccharide, D-Ribose, provided a 5–5 fused ring system 8 that showed glutathione recovery capacity (GCR) [42] and, therefore, can be used to increase the protective effects in events of prolonged and exacerbated oxidative stress [43].
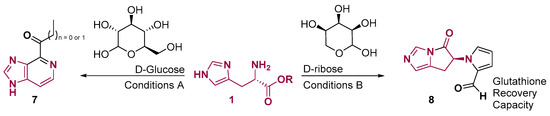
Figure 5.
Histidine reactivity with monosaccharides. Conditions: (A) R = H, 1 M phosphate buffer (pH 5.8), 150 °C, 1 h; (B) R = Me, Oxalic acid DMSO, 60 °C for 30 min, 90 °C for 30 min and 120 °C for 30 min [40,41,42].
The tetrahydro-imidazo [4,5-c]pyridine and imidazo [4,5-c]pyridine cores obtained from histidine were already employed in a diverse set of bioactive compounds (Figure 6). The most common application is in anticancer agents, such as kinase inhibitors (11 and 12, Figure 6) [26,44] and compounds 9a–b with anti-angiogenesis activity [29]. Diabetes vascular complications mediated by SSAO (semicarbazide-sensitive amine oxidase) can be treated or prevented by the use of some tetrahydro-imidazo [4,5-c]pyridine derivatives (10, Figure 6) [28]. In addition, some hydroxamate derivatives (14, Figure 6) from the imidazo [4,5-c]pyridine core showed anti-HIV activity [25] and the 4-phenyl derivative (13, Figure 6) showed good inhibition of [3H]diazepam binding to rat cerebral cortical membranes [30].
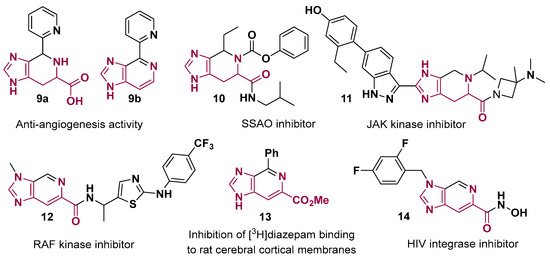
Figure 6.
Selected bioactive compounds prepared with fused heterocyclic scaffolds from histidine.
Up until now, the most common fused heterocyclic scaffolds from histidine are the 5-6 and 5-5 bicyclic cores. However, some other fused frameworks were already prepared from histidine. A hydantoin-5-6-5-fused ring system can be prepared from the tetrahydro-imidazo [4,5-c]pyridine core 3a shown above. By reacting this core with isocyanate, an imidazolidine-dione ring is formed fused to the tetrahydro-imidazo [4,5-c]pyridine. This tricyclic fused system (15, Figure 7) has potential antitumor activity by HDAC (histone deacetylase) inhibition, [45], and it was already proven not to have activity against the histamine-H3 receptor [46].

Figure 7.
5-6-5-fused ring system prepared from tetrahydro-imidazo [4,5-c]pyridine core. Condition: C2H3NCO, Et3N, DMF, 60 °C, 24 h [45].
Another tricyclic ring system can be formed by reacting tetrahydro-imidazo [4,5-c]pyridine core with chloroacetyl chloride followed by methylamine addition (Figure 8). The diketopiperazine-5-6-6 ring system at 16 showed some PDE5 (phosphodiesterase type 5) inhibition that can have utility in a variety of therapeutic areas, including the treatment of cardiovascular disorders and erectile dysfunction [27].
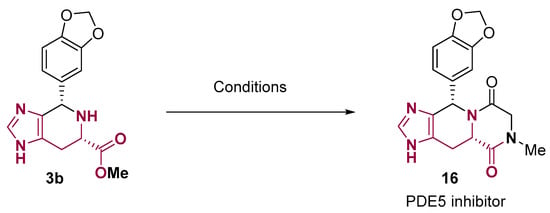
Figure 8.
5-6-6-fused ring system prepared from tetrahydro-imidazo [4,5-c]pyridine core. Conditions: (1) ClCOCH2Cl, Et3N, THF, 0 °C–r.t., 1 h, (2) Aq MeNH2, THF, 45 °C, 45 min [27].
Histidine can also be used to prepare centrocountins-related compounds through a one-pot cascade reaction [47]. Centrocountins are tetracyclic indole-quinolizine, whose synthesis was inspired by indole alkaloid natural products and has potential biological applications, such as anticancer activity [48]. The bellow quinolizine ring formation (Figure 9) involves a Pictet–Spengler step, followed by an aza-Michael addition; the N-methylated histidine reacts better than the NH counterpart [47].
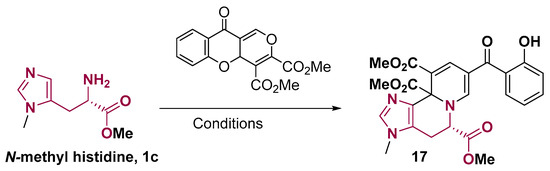
Figure 9.
Imidazo [4,5-a]quinolizine-like 5-6-6-fused ring system from histidine. Conditions: Toluene, 80 °C, 2 h, TFA [47].
A recent study described another quinolizine-like framework from histidine (Figure 10). Genihistidine A and B were described as products in the reaction of this amino acid with Genipin, a monoterpenoid extracted from Gardenia jasminoides, a herb commonly used in traditional Chinese medicine. Genipin is also involved in the formation of gardenia blue (GB) pigment, as a natural blue pigment, which is utilized as a natural food additive in numerous countries [49]. Genihistidine B also has some antiproliferative activity against colon cancer [50].

Figure 10.
Histidine reactivity with Genipin. Conditions: PBS buffer (pH = 7.35), 32 °C, 55 h [49,50].
Ultimately, a more extended ring-fused system was prepared using a tetrahydro-imidazo [4,5-c]pyridine core obtained from histidine and another tricyclic system prepared from tryptophan. This reaction formed a new hexacycle diketopiperazine framework 20 (Figure 11), which showed an anti-proliferative effect added to the inhibition in the migration and invasion of tumor cells and an in vivo anti-metastasis effect [51].
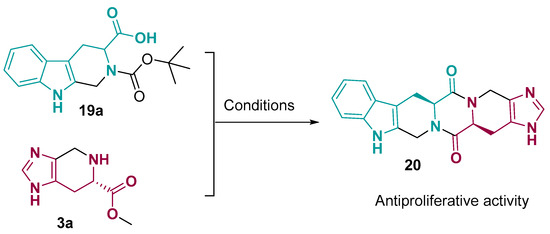
Figure 11.
Hexacyclic bearing diketopiperazine fused ring system binding tryptophan to histidine. Conditions: (1) DEPBT, THF, r.t., (2) HCl/EtOAc (4M), 0 °C, 4 h [51].
3. Fused Heterocyclic Scaffolds from Tryptophan
An intersection application of both heteroaromatic amino acids, histidine, and tryptophan, can be already observed above (Figure 11); however, in this section, data from fuse heterocyclic frameworks prepared from tryptophan are presented. For clarity, we have divided the findings according to the indole position of the cyclization and the ring system size formed. Also, the biological applications, when existing, are presented below.
3.1. Tricyclic-Fused Heterocyclic Scaffolds by Heterocyclization at Indole C-2
3.1.1. 6-5-6-Fused Ring System
Similarly to histidine, the most common heterocyclization reaction with tryptophan involves a Pictet–-Spengler-like reaction to form a tetrahydro-1H-pyrido [3,4-b]indole core (Figure 12) [52,53]. In this reaction, the tryptophan undergoes condensation with an aldehyde or ketone, followed by ring closure [54,55]. For the reaction, an acid is usually used, such as glacial acetic acid [56,57,58,59,60], sulfuric acid [61,62,63,64,65,66,67], p-toluene sulfonic acid (PTSA) [68,69], and trifluoroacetic acid (TFA) [70,71]. Same as for histidine, the basic conditions are also employed for the synthesis of tetrahydro-1H-pyrido [3,4-b]indole framework [72,73,74,75,76]. Normally, these reactions happen at high temperatures and in good yields and are broadly applied in the preparation of β-carboline alkaloids [77,78].
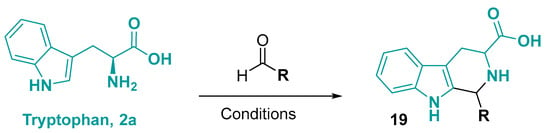
Figure 12.
Pictet–Spengler-like reaction with tryptophan. Conditions: (general acid condition): R-CHO, glacial acetic acid or sulfuric acid or PTSA/Toluene or TFA, rt–reflux 1–16 h; (general basic condition): R-CHO, NaOH/water, rt, 2–3 h [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76].
The tetrahydro-1H-pyrido [3,4-b]indole core prepared from tryptophan can be oxidized to the aromatic fused system 21 using different strategies (Figure 13 and Figure 14). The dehydrogenation keeping carboxylic group can be accomplished using trichloroisocyanuric acid (TCCA) in DMF [69], potassium permanganate in DMF [60,79,80,81,82,83,84,85,86,87,88,89,90], THF [68] or acetone [91,92], and sulfur [62,66,93,94,95,96,97,98,99,100] or octasulfur [101] in reflux of xylene. When manganese dioxide is used in toluene or benzene, reflux occurs an aromatization without decarboxylation of the ester group [75,102]. However, when free acid is on the tetrahydro-1H-pyrido [3,4-b]indole core, the use of manganese dioxide and sulfuric acid allows the decarboxylative aromatization [103,104]. A similar case happens when heating the tetrahydro-1H-pyrido [3,4-b]indole core in DMSO—the ester in the core prevents decarboxylative aromatization, but it occurs when a free acid is inside the molecule [105]. Under the same condition, when a secondary amine, e.g., tosyl, is substituted, a base addition is required for the aromatization [106].
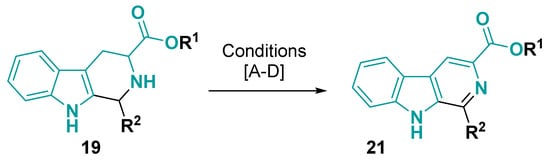
Figure 13.
Tetrahydro-1H-pyrido [3,4-b]indole core dehydrogenation. Conditions: (A) R1 = Me and R2 = aryl: TCCA, Et3N/ DMF; 0 °C-rt, 2 h [69]; (B) R1 = H or alkyl and R2 = H, alkyl or aryl: KMnO4, DMF, rt, 1–16 h [60,68,79,80,81,82,83,84,85,86,87,88,89,90,91,92]; (C) R1 = Me and R2 = H, alkyl or aryl: Sulfur or octasulfur, xylene, reflux, 8–48 h [62,66,93,94,95,96,97,98,99,100,101]; (D) R1 = Et and R2 = H: MnO2, Toluene, reflux [75,102]; (D) R1 = Me and R2 = alkyl or aryl: DMSO, 90–95 °C, 7–10 h [105,106].
Other conditions also give the aromatic pyrido [3,4-b]indole core (β-carboline core) by decarboxylative process (Figure 14). The organic base DBN (1,5-diazabicyclo [4.3.0]non-5-ene) was shown to be an efficient reagent for promoting the dehydrogenative/decarboxylative aromatization of tetrahydro-β-carbolines under air atmosphere [107]. The iodobenzene diacetate (IBD) [108] and N-chlorosuccinimide (NCS) [87,109] in DMF at room temperature are also efficient methods for decarboxylative oxidation of this core. On the other hand, iron(III) chloride needs air and higher temperature in DMF to obtain the aromatic core [110]. Potassium dichromate [111,112,113] and selenium dioxide [72,114] are the other two oxidizing agents that perform a decarboxylative aromatization in the reflux of acetic acid.
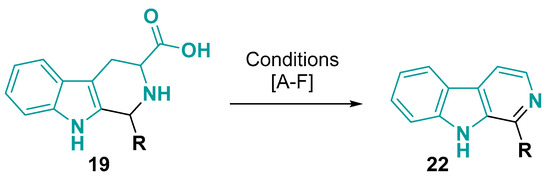
Figure 14.
Decarboxylative aromatization of tetrahydro-1H-pyrido [3,4-b]indole core: (A) R = alkyl or aryl: DBN, air, 110 °C, 12h [107]; (B) PhI(OAc)2, DMF, rt, 2h [108]; (C) R = H, alkyl or aryl: NCS, TEA, DMF, rt, 30–45 min [87,109]. (D) R = H, alkyl or aryl: K2Cr2O7(aq), AcOH, reflux, 5 min–6 h [111,112,113]; (E) R = H: SeO2, AcOH, reflux, 12h [72,114]; (F) R = H, alkyl or aryl: FeCl3, DMF, 130 °C air (O2), 1 h [110].
The β-carboline core can also be obtained directly from the reaction of tryptophan with another amino acid in the presence of iodine and trifluoracetic acid (Figure 15). For this reaction, decarboxylation, deamination, Pictet−Spengler reaction, and oxidation reactions proceeded sequentially [115].
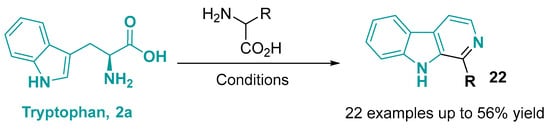
Figure 15.
Synthesis of β-carboline alkaloids from tryptophan and a second amino acid. Conditions: I2, TFA,DMSO, 120 °C, 24 h [115].
As already mentioned, the 6-5-6-fused ring system is the most explored one in the literature, including its use in biological applications. Therefore, a diverse set of these compounds was selected and presented in Figure 16.
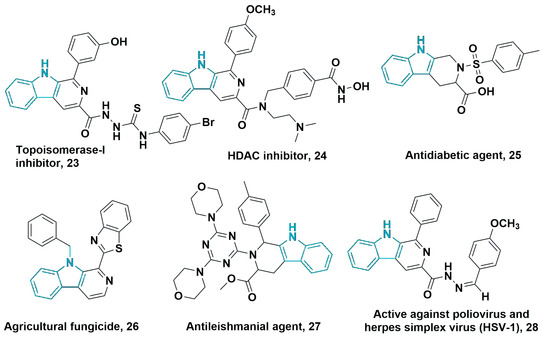
Figure 16.
Select bioactive compounds prepared using pyrido [3,4-b]indole core from tryptophan.
The tetrahydro-1H-pyrido [3,4-b]indole and pyrido [3,4-b]indole core (β-carboline) have been widely used in the development of antiproliferative agents against various cancer cell lines, including pancreatic (BxPC-3), cervical (HeLa), and prostate (PC3 and C4-2) cancers [59,81,107,115,116]. In addition, some of these compounds act as HDAC inhibitors [79,80], and 23 demonstrated anticancer activity by inhibiting topoisomerase I and kinesin spindle protein [100]. Derivatives of tetrahydro-9H-pyrido [3,4-b]indole also exhibit hypoglycemic properties comparable to or exceeding those of pioglitazone, suggesting their potential for diabetes treatment [117]. Compounds derived from β-carboline-triazine 27 have shown superior antileishmanial activity compared to sodium stibogluconate, with reduced toxicity, and have significant potential for the development of new therapeutic agents [92].
Moreover, β-carboline derivative 28 shows promising antiviral effects, with activity against HSV-1 and poliovirus [57], and has been investigated as a multifunctional acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease [94] and alcohol abuse [75]. In agriculture, compounds such as 26 stand out as potent antifungal agents against several phytopathogenic species, including Fusarium oxysporum, Rhizoctonia solani, and Botrytis cinerea [81,84,104].
3.1.2. 6-5-5-Fused Ring System
The hexahydropyrrolo [2,3-b]indole core 29 is common in bioactive natural products, such as physostigmine, a cholinesterase inhibitor used to treat glaucoma [118]. The pyrrolidinoindolines is usually obtained by the classical protocol of bromocyclization using N-bromosuccinimide without any other additives (Figure 17) [119,120]. Moreover, iodine(III)-mediated annulation provides tertiary chloride pyrrolo [2,3-b]indoline skeleton [121]. The tertiary alcohol derivative can be obtained directly by dye-sensitized photooxygenation of tryptophan [122] or unprotected tryptophan oxygenation by engineered enzyme [123].
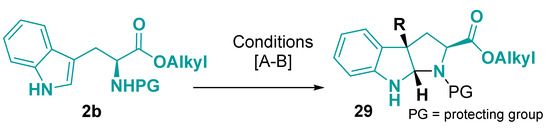
Figure 17.
Preparation of hexahydropyrrolo [2,3-b]indole core from tryptophan derivatives. (A) R = Br: NBS, CH3CN or CH2Cl2, 0 °C–rt, 30 min–4 h [119,120]; (B) R = Cl: ZnCl2, PhI(OAc)2, CH3CN, rt, 2 h [121].
3.1.3. 6-5-7-Fused Ring System
The seven-member-ring fused to indole is not the most explored framework from tryptophan, but some azepine derivatives were already prepared from this amino acid. For this reaction, an acyl-alpha-bromide in DCE and triflic acid provide the azepino [4,5-b]indole core 30 (Figure 18). The acylhydrazone derivative of this core showed some agricultural application due to their activity against the tobacco mosaic virus (TMV) [124]. The azepino [4,5-b]indole core can also be used as an intermediate in hetero-Diels–Alder cycloaddition [125].
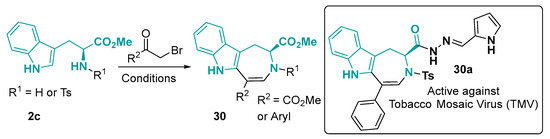
Figure 18.
Preparation of Azepino [4,5-b]indoles. Conditions: (1) K2CO3, DMF, rt, 1–2 h; (2) TfOH, DCE, rt, 2 h [124].
A similar core with indole fused to azepinone can be obtained in a catalyst-free Ugi-three-component reaction (Ugi-3CR) using 2-formyl-L-tryptophan as a bifunctional building block (Figure 19) [126].
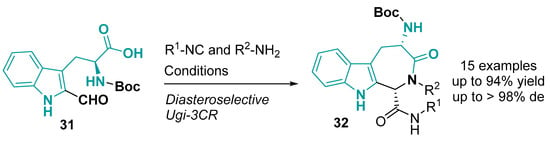
Figure 19.
Synthesis of 1-Carbamoyl-4-aminoindoloazepinone derivatives via the Ugi reaction. Conditions: MeOH, 70 °C, 48 h [126].
3.2. Tricyclic-Fused Heterocyclic Scaffolds by Heterocyclization at Indole C-4
The azepinone ring can also be fused to indole C-4 forming a tetrahydro-1H-azepino [5,4,3-cd]indol-1-one core 34. This core is present in natural products [127] and the FDA-approved drug Rucaparib (Figure 20), used to treat cancer [128]. Recently, rucaparib derivatives with the benzazepinone scaffold were described, using 2-aryl-tryptophan in a Pd-catalyzed remote C–H carbonylation, where molybdenum carbonyl was used as the source of the C=O (98% yield) (Figure 20) [129].
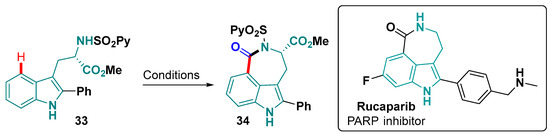
Figure 20.
Synthetic strategy for obtention of the 1,3,4,5-tetrahydrobenzo [cd]indole scaffold. Conditions: Pd(OAc)2, Mo(CO)5, AgOAc, BQ, 1,4-dioxane, Ar, 110 °C, 18 h [129].
3.3. Polycyclic-Fused Scaffolds Involving Indole Nitrogen and C-2
Like histidine, the tryptophan-derived fused-ring system involving heteroaromatic nitrogen cyclization is less common. Among them, the tetracyclic core diaza-cyclopenta-fluorene-dione is present in some natural products, such as the cytotoxic alkaloid Chaetominine [130] and Versiquinazoline H [131]. A synthetic route for these alkaloids has already been developed, where the tetracyclic system 36 is obtained using DMDO in acetone or THF (Figure 21) [132,133].
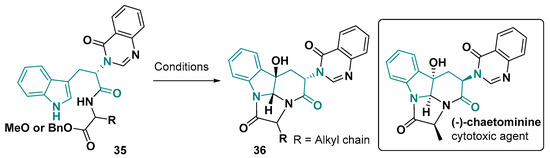
Figure 21.
Cyclization step for the diaza-cyclopenta-fluorene-dione core synthesis. Conditions: (1) DMDO, acetone or THF, −78 °C, 1 h; (2) Na2SO3, 0 °C, 1.5 h [132,133].
Other hexacyclic or pentacyclic skeletons can be prepared from tryptophan through an initial Pictet–Spengler-like reaction, followed by oxidative cyclization and aromatization (Figure 22) [134]. This framework is very similar to an aryl hydrocarbon receptor (AhR) select modulator, 11-Cl-BBQ [135], which suppresses lung cancer cell growth and is under patent protection for this application [136].
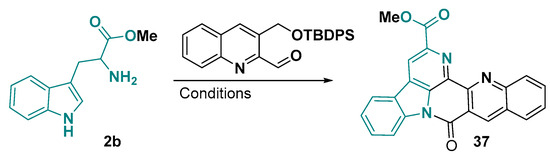
Figure 22.
Synthetic strategy to give the indolo-quinolino-naphthyridine core. Conditions: (1) p-xylene, reflux, 16 h; (2) n-Bu4NF, THF, r. t., 12 h; (3) DMP, Py, CH2Cl2, 2 days [134].
The hexahydropyrrolo [2,3-b]indole core 29 is common in pyrrolidinoindoline natural products, a growing family of bioactive alkaloids. Pyrrolidinoindolines consist mainly of a majority of diketopiperazines (DKPs) and a minority of diketomorpholines (DKMs) (Figure 23) [137]. Nocardioazines B is a DKP marine-derived natural product, where the key step for the 7-fused-mebered-ring scaffold is diketopiperazine formation by amide coupling [138,139]. Similar occurs with the total synthesis of Javanicunines A, where the diketomorpholine is built by amide coupling and ester cyclization to obtain the 4-fused-mebered-ring scaffold of this natural product [137]. For both of these natural products, the key intermediate 3a-bromo hexahydropyrrolo [2,3-b]indole core is synthetically obtained from tryptophan (Figure 17). Another DKP pyrrolidinoindoline natural product, Brevianamide E, explored another synthetic strategy by preparing one amide before the hexahydropyrrolo [2,3-b]indole core formation and, then, a last cyclization to form the diketopiperazine [140].
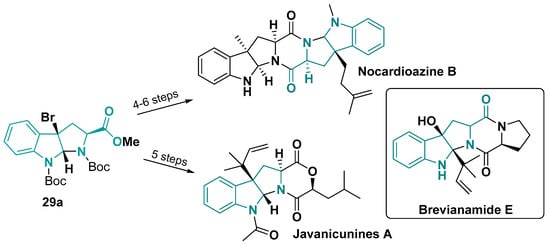
Figure 23.
Nocardioazine B and Javanicunines A synthetic pathway and Breviananide E structure [137,138,139].
Beyond the hexahydropyrrolo [2,3-b]indole core 29, the tetrahydro-1H-pyrido [3,4-b]indole 19 and pyrido [3,4-b]indole core (β-carboline, 22) are also used to prepared larger fused-ring systems. The tetrahydro-1H-pyrido [3,4-b]indole core has been used for the obtention of compounds fused with hydantoin and thiohydantoin rings (Figure 24) by reacting this core with isocyanate and isothiocyanate, respectively [54,141,142,143,144]. Monastroline (HR22C16) is an example of a bioactive compound with this skeleton, which is a cell-permeable non-tubulin-interacting mitosis inhibitor for cancer treatment [145,146].
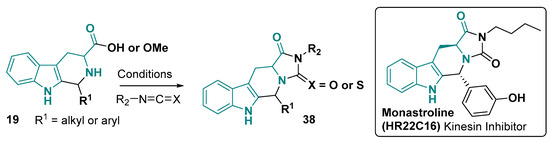
Figure 24.
Hydantoins and thiohydantoins derivatives from hexahydropyrrolo [2,3-b]indole core. Conditions: Acetone/DMSO, DCM, and MeCN, rt. 2–3 h [54,141,142,143,144,145,146].
The synthesis of tetrahydro-β-carboline fused to lactams is also reported and can be performed by different synthetic strategies. The condensation of tryptophan with dimethyl 2-oxopentanedioate as a key step to synthesize the natural product (+)-tabertinggine (Figure 25). This approach gave the (5S,11R)-39 as the major stereoisomer formed [147].
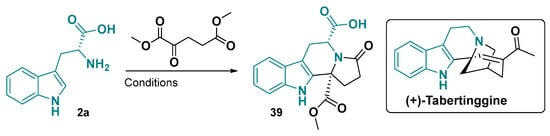
Figure 25.
Initial cyclization step for the synthesis of (+)-Tabertinggine. Conditions: (1) PTSA, H2O, toluene:THF (2:1), distill; (2) THF, reflux, 24 h [147].
Five-membered heteroaromatic rings can also be built with a fourth ring fused to tetrahydro-β-carboline and β-carboline cores (Figure 26). The cyclization using dimethyl acetylenedicarboxylate (DMAD) in the presence of acetic anhydride gave the pyrrole-tetracyclic product 40 in 86%. This product after some steps gives an antitumor indolizino [6,7-b]indole 41 with multiple modes of action, including topoisomerase I and II inhibition [148]. Another antitumoral tetracyclic agent, 42a, can be prepared from β-carboline 22a forming an imidazole-fused-ring at 42 [149]. Depending on the desired position of the imidazole substituent, a different reactant is used. For the C-2-substituted pattern, an alfa-bromo-ketone in reflux of ethanol gives the desired product [148], while for the C-3-substituted pattern, an aldehyde together with triethylamine and octasulfur in a solution of DMSO/cyclohexane at high temperature affords the tetracyclic pattern [149].

Figure 26.
Five-membered heteroaromatic ring fused to tetrahydro-β-carboline and β-carboline. Conditions: (A) DMAD, Ac2O, 70 °C, 2 h; (B) MeOH or EtOH, 80–90 °C, 2–4 h; (C) DMSO/DMF, Et3N, S8, 120–150 °C, 2–4 h [148,149].
Ultimately, a significantly explored tetracyclic framework from tryptophan is the diketopiperazine fused to the tetrahydro-β-carboline core 43 (Figure 27). For the construction of this skeleton, two strategies can be employed—using other amino acids and amide coupling agents [150,151] and using chloroacetyl chloride and alkyl-amide [152,153,154,155].
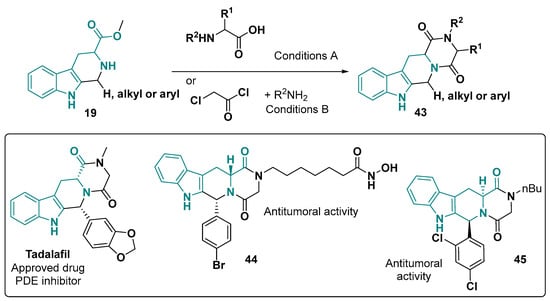
Figure 27.
Diketopiperazine-fused to tetrahydro-β-carboline core building and representative biological application examples. Conditions: (A) amide coupling followed by base addition (NH4OH or N-methylmorpholine), rt; (B) (1) Et3N or NaHCO3, DCM, rt, 2 h; (2) MeOH, 70 °C, 16 h [150,151,152,153,154,155,156].
Some biological applications for these diketopiperazines are the antitumor activity of 45 and 46 [154,155] and an FDA-approved drug, Tadalafil, that is used for erectile dysfunction [156].
4. Conclusions
The versatile use of histidine and tryptophan for building simple to complex fused heterocyclic framework can be verified in this review. Tryptophan is more extensively explored than histidine in this field. However, the similar chemical reactivity of these two heteroaromatic amino acids provides comparable skeleton and biological application, where the anti-infective and antitumor activity stands out (Supplementary Materials). The most common reaction involves the Pictet–Spengler reaction, and the influence of natural products in the development of synthetic strategies is notable. Despite the explored synthetic strategies, few studies investigate the biological activity of histidine and tryptophan derivatives, which indicates an opening for future directions in research on drug discovery. Lastly, it is possible to see the potential application of these two heteroaromatic amino acids in the drug development process since it calls for cheap and widely available starting material to prepare drug candidates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6020023/s1, Table with biological activity data for the compounds cited in the manuscript.
Author Contributions
F.F. performed the study design. I.A.S.d.B., J.B.P., A.R.J. and F.F. contributed to the acquisition and analysis of the data. Writing and critical review were performed by I.A.S.d.B., A.R.J. and F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through scholarships for de Borba, I.A.S. and Peripolli, J.B. Also, this work was financially supported by FAPERGS [Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul] (Grant number 19/2551-0001273-0).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADP | Adenosine diphosphate |
| AhR | Aryl hydrocarbon receptor |
| BQ | 1,4-benzoquinone |
| CDI | 1,1′-Carbonyldiimidazole |
| CNPq | Conselho Nacional de Desenvolvimento Científico e Tecnológico |
| DBN | 1,5-diazabicyclo [4.3.0]non-5-ene |
| DCE | 1,2-Dichloroethane |
| DCM | Dichloromethane |
| DEPBT | (3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one |
| DKM | Diketomorpholines |
| DKP | Diketopiperazines |
| DMAD | Dimethyl acetylenedicarboxylate |
| DMDO | Dimethyldioxirane |
| DMF | N,N-Dimethylformamide |
| DMP | Dess–Martin periodinane |
| DMSO | Dimethyl Sulfoxide |
| FAPERGS | Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul |
| FDA | Food and Drug Administration |
| GB | Gardenia blue |
| GCR | Glutathione recovery capacity |
| HDAC | Histone deacetylase |
| HIV | Human Immunodeficiency Viruses |
| HSV | Herpes simplex virus |
| IBD | Iodobenzene diacetate |
| IBX | 2-Iodoxybenzoic acid |
| JAK | Janus kinase |
| NBS | N-Bromosuccinimide |
| NCS | N-Chlorosuccinimide |
| PC | Prostate cancer |
| PDE5 | Phosphodiesterase type 5 |
| PG | Protecting group |
| PPSE | Trimethylsilyl polyphosphate |
| PTSA | p-toluene sulfonic acid |
| Py | Pyridine |
| RAF | Rapidly Accelerated Fibrosarcoma |
| SSAO | Semicarbazide-sensitive amine oxidase |
| TBDPS | tert-butyldiphenylsilyl |
| TCCA | Trichloroisocyanuric acid |
| TFA | Trifluoroacetic acid |
| TFAE | Trifluoroacetaldehyde ethyl hemiacetal |
| TfOH | Trifluoromethanesulfonic acid |
| THF | Tetrahydrofuran |
| TMV | Tobacco Mosaic Virus |
| Ugi-3CR | Ugi-three-component reaction |
References
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs: Miniperspective. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [CrossRef] [PubMed]
- Mendes Lampert, L.; Ruszczyk Machado, B.; Rocha Joaquim, A.; Fumagalli, F. Rings in “Lead-like Drugs”. Lett. Drug Des. Discov. 2024, 21, 3851–3857. [Google Scholar] [CrossRef]
- Stefanucci, A.; Pinnen, F.; Feliciani, F.; Cacciatore, I.; Lucente, G.; Mollica, A. Conformationally Constrained Histidines in the Design of Peptidomimetics: Strategies for the χ-Space Control. Int. J. Mol. Sci. 2011, 12, 2853–2890. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Murugan, R.N.; Jacob, B.; Hyun, J.-K.; Cheong, C.; Hwang, E.; Park, H.-N.; Seo, J.-H.; Srinivasrao, G.; Lee, K.S.; et al. Discovery of Novel Histidine-Derived Lipo-Amino Acids: Applied in the Synthesis of Ultra-Short Antimicrobial Peptidomimetics Having Potent Antimicrobial Activity, Salt Resistance and Protease Stability. Eur. J. Med. Chem. 2013, 68, 10–18. [Google Scholar] [CrossRef]
- Mahindra, A.; Bagra, N.; Wangoo, N.; Jain, R.; Khan, S.I.; Jacob, M.R.; Jain, R. Synthetically Modified L-Histidine-Rich Peptidomimetics Exhibit Potent Activity against Cryptococcus Neoformans. Bioorg. Med. Chem. Lett. 2014, 24, 3150–3154. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and Arginine-Rich Antimicrobial Peptides: Structures and Mechanisms of Action. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, H.; Shi, P.; Zhao, X.; Liu, H.; Li, X. Clickable Tryptophan Modification for Late-Stage Diversification of Native Peptides. Sci. Adv. 2024, 10, eadp9958. [Google Scholar] [CrossRef]
- Rai, N.; Tiwari, R.T.; Sahu, A.; Verma, E.; Rathore, S.; Patil, S.; Patil, A.G. Exploring Tryptophan-Based Short Peptides: Promising Candidate for Anticancer and Antimicrobial Therapies. Anticancer. Agents Med. Chem. 2025, 25, 124–133. [Google Scholar] [CrossRef]
- Han, Y.N.; Ryu, S.Y.; Han, B.H.; Woo, L.K. Spinacine fromPanax Ginseng. Arch. Pharm. Res. 1987, 10, 258–259. [Google Scholar] [CrossRef]
- Kong, D.; Cui, L.; Wang, X.; Wo, J.; Xiong, F. Fungus-Derived Opine Enhances Plant Photosynthesis. J. Adv. Res. 2024, S2090123224005472. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A.; Lamahewage, S.N.S. Ergothioneine, Ovothiol A, and Selenoneine—Histidine-Derived, Biologically Significant, Trace Global Alkaloids. Molecules 2022, 27, 2673. [Google Scholar] [CrossRef]
- Shengule, S.R.; Karuso, P. Concise Total Synthesis of the Marine Natural Product Ageladine A. Org. Lett. 2006, 8, 4083–4084. [Google Scholar] [CrossRef]
- Liu, J.; Ng, T.; Rui, Z.; Ad, O.; Zhang, W. Unusual Acetylation-Dependent Reaction Cascade in the Biosynthesis of the Pyrroloindole Drug Physostigmine. Angew. Chem. Int. Ed. 2014, 53, 136–139. [Google Scholar] [CrossRef]
- Alkaloids Derived from Tryptophan. In Alkaloids; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–102. ISBN 978-0-12-417302-6.
- Nowacka, A.; Śniegocka, M.; Śniegocki, M.; Ziółkowska, E.; Bożiłow, D.; Smuczyński, W. Multifaced Nature of Yohimbine—A Promising Therapeutic Potential or a Risk? Int. J. Mol. Sci. 2024, 25, 12856. [Google Scholar] [CrossRef]
- Wang, H.; Tian, R.; Chen, Y.; Li, W.; Wei, S.; Ji, Z.; Aioub, A.A.A. In Vivo and in Vitro Antifungal Activities of Five Alkaloid Compounds Isolated from Picrasma Quassioides (D. Don) Benn against Plant Pathogenic Fungi. Pestic. Biochem. Phys. 2022, 188, 105246. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-Ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Lim, L.R.; Tan, Y.Q.; Go, M.K.; Bell, D.J.; Freemont, P.S.; Yew, W.S. Reconstituting the Complete Biosynthesis of D-Lysergic Acid in Yeast. Nat. Commun. 2022, 13, 712. [Google Scholar] [CrossRef]
- Xue, H.; Wang, W. Effects of Carbetocin Combined with Ergometrine Maleate on Blood Loss and Coagulation Function of Puerperae with Postpartum Haemorrhage. Am. J. Transl. Res. 2023, 15, 556–562. [Google Scholar]
- Bharate, S.B.; Lindsley, C.W. Natural Products Driven Medicinal Chemistry. J. Med. Chem. 2024, 67, 20723–20730. [Google Scholar] [CrossRef]
- Fay, N.; Kouklovsky, C.; De La Torre, A. Natural Product Synthesis: The Endless Quest for Unreachable Perfection. ACS Org. Inorg. Au 2023, 3, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Dymińska, L. Spectroscopic Properties of Spinacine—An Active Component of Ginseng (Panax ginseng) and Spinach (Spinacia oleracea). Spectrosc. Lett. 2016, 49, 635–646. [Google Scholar] [CrossRef]
- De La Figuera, N.; Fiol, S.; Fernández, J.-C.; Forns, P.; Fernández-Forner, D.; Albericio, F. Role of the Acid Group in the Pictet-Spengler Reaction of α-Amino Acids. Synlett 2006, 2006, 1903–1907. [Google Scholar] [CrossRef]
- Hu, Q.; Kuki, A.; Nowlin, D.; Nowlin, M.; Plewe, M.; Plewe, B.; Wang, H.; Zhang, J. HIV-Integrase Inhibitors, Pharmaceutical Compositions, and Methods for Their Use. WO 2004/039803 A2, 13 May 2004. [Google Scholar]
- Chuaqui, C.; Cossrow, J.; Dowling, J.; Guan, B.; Hoemann, M.; Ishchenko, A.; Jones, J.H.; Kabigting, L.; Kumaravel, G.; Peng, H.; et al. Heteroaryl Compounds Useful as RAF Kinase Inhibitors. WO 2010/078408 A1, 8 July 2010. [Google Scholar]
- Mark, W.O.; Sawyer, J.S.; Lisa, M. Schultze Chemical Compounds. WO 02/38563 A2, 16 May 2002. [Google Scholar]
- Caldirola, P.; Besencon, O.; Olsson, R.; Öhman, J. New Use of 4, 5, 6, 7-Tetrahydroimidazo-[4,5-c]Pyridine Derivatives. WO 02/38153, 16 May 2002. [Google Scholar]
- Karuso, P.H.; Shengule, S.R. Synthesis of Ageladine A and Analogs Thereof. WO 2009/152584 A1, 23 December 2009. [Google Scholar]
- Guzman, F.; Cain, M.; Larscheid, P.; Hagen, T.; Cook, J.M.; Schweri, M.; Skolnick, P.; Paul, S.M. Biomimetic Approach to Potential Benzodiazepine Receptor Agonists and Antagonists. J. Med. Chem. 1984, 27, 564–570. [Google Scholar] [CrossRef]
- Fujii, S.; Maki, Y.; Kimoto, H.; Cohen, L.A. Facile Syntheses of 4-(Trifluoromethyl)-L-Spinacine and 4-(Trifluoromethyl)Spinaceamine. J. Fluor. Chem. 1987, 35, 581–589. [Google Scholar] [CrossRef]
- Neuberger, A. The Reaction between Histidine and Formaldehyde. Biochem. J. 1944, 38, 309–314. [Google Scholar] [CrossRef]
- Smith, D.; Gallagher, A.; Crowley, V.; Gergens, W.; Abel, P.; Hulce, M. An Efficient Synthesis of 4(5)-Benzyl-l-Histidines Employing Catalytic Transfer Hydrogenolysis at Elevated Temperatures. Synthesis 2013, 46, 515–521. [Google Scholar] [CrossRef]
- Daka, P.; Liu, A.; Karunaratne, C.; Csatary, E.; Williams, C.; Xiao, H.; Lin, J.; Xu, Z.; Page, R.C.; Wang, H. Design, Synthesis and Evaluation of XZH-5 Analogues as STAT3 Inhibitors. Bioorg. Med. Chem. 2015, 23, 1348–1355. [Google Scholar] [CrossRef]
- Guillen, F.; Brégeon, D.; Plaquevent, J.-C. (S)-Histidine: The Ideal Precursor for a Novel Family of Chiral Aminoacid and Peptidic Ionic Liquids. Tetrahedron Lett. 2006, 47, 1245–1248. [Google Scholar] [CrossRef]
- Voelter, W.; Wollmann, H. Process for the Preparation of N-Substituted Histidine Derivatives, N-Substituted Histidine Derivatives and Their Use. DE 3322117 A1, 20 December 1984. [Google Scholar]
- Smolyar, N.N.; Abramyants, M.G.; Yutilov, Y.M. Dehydrogenation of 4-Phenyl-Substituted Spinaceamine and Spinacine. Russ. J. Org. Chem. 2006, 42, 541–544. [Google Scholar] [CrossRef]
- Smolyar, N.N.; Abramyants, M.G.; Zavyazkina, T.I.; Matveeva, D.I.; Borodkin, Y.S.; Voloskii, I.A. Synthesis and Dehydrogenation of Spinaceamine and Spinacine 4-Hetaryl Derivatives. Russ. J. Org. Chem. 2009, 45, 1219–1223. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, K.C. Aromatization of Cyclohexenes and Cyclohexadienes with Selenium Dioxide-Trimethylsilyl Polyphosphate. Tetrahedron Lett. 1992, 33, 6363–6366. [Google Scholar] [CrossRef]
- Gi, U.S.; Baltes, W. Model Reactions on Roast Aroma Formation. 14. Formation of 2-Acetylpyrido[3,4-d]Imidazole by Heating of Glucose with Histidine. J. Agric. Food Chem. 1993, 41, 644–646. [Google Scholar] [CrossRef]
- Gi, U.-S.; Baltes, W. Model Reactions on Roast Aroma Formation. 15. Investigations on the Formation of Pyrido[3,4-d]Imidazoles during the Maillard Reactions. J. Agric. Food Chem. 1995, 43, 2226–2230. [Google Scholar] [CrossRef]
- Koo-ho, G.; Cucurbit; Jeong, H.; Ikjun, I. Novel Pyrrolo-Lactone and Pyrrole Compounds Exhibiting Glutathione Recovery Ability in Living Cells against Harmful Oxygen Groups and Their Preparation Method. KR1020160035931 A, 25 February 2019. [Google Scholar]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. J. Clin. Orthop. Trauma. 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Daniel, D.L.; Smith, C. Corbin Thompson Dimethyl Amino Azetidine Amides as JAK Inhibitors. US 2020/0071323 Al, 5 March 2020. [Google Scholar]
- Mahboobi, S.; Sellmer, A.; Pongratz, H.; Leon-Hardt, M.; Krämer, O.; Böhmer, F.-D.; Kelter, G. Novel HDAC6 Inhibitors and Their Uses. WO 2016/020369 A1, 11 February 2016. [Google Scholar]
- Braña, M.F.; Guisado, C.; Pérez-Castells, J.; Pérez-Serrano, L. Synthesis of 4,7,8a,9-tetrahydro-3 H. -diimidazo-[1,5- a :4′,5′- d ]Pyridine Derivatives. J. Heterocycl. Chem. 2002, 39, 417–420. [Google Scholar] [CrossRef]
- Eschenbrenner-Lux, V.; Dückert, H.; Khedkar, V.; Bruss, H.; Waldmann, H.; Kumar, K. Cascade Syntheses Routes to the Centrocountins. Chem. Eur. J. 2013, 19, 2294–2304. [Google Scholar] [CrossRef]
- Kumar, K. Centrocountins—Synthesis and Chemical Biology of Nature Inspired Indoloquinolizines. In Small Molecule Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–265. ISBN 978-0-12-818349-6. [Google Scholar]
- Zhang, X.; Zhang, R.; Li, R.; Zhang, J.; Wang, Y.; Chai, X.; Wang, Y. Elucidating the Formation Mechanism of Gardenia Blue Pigment from Amino Acid and Genipin. Arab. J. Chem. 2025, 18, 106048. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, X.; Wang, Y.; Yu, H.; Wang, D.; Fang, S.; Yu, H.; Dong, X.; Li, R. A Cyclopentaquinolizine Imidazole Compound and Its Preparation Method and Applica-Tion. CN 114751906 A, 15 July 2022. [Google Scholar]
- Zhao, M.; Peng, S.; Gui, L.; Hao, Y. Hexacyclic Piperazine-Dione Compounds, Their Preparation, Biological Activity and Appli-Cation. CN 112010855 A, 1 December 2020. [Google Scholar]
- Afsah, E.M.; Hammouda, M.; Hamama, W.S. Pictet-Spengler Reactions of Tryptamine and Tryptophan with Cycloalkanones and ketonicMannich Bases. Monatsh. Chem. 1985, 116, 851–855. [Google Scholar] [CrossRef]
- Wu, G.; Wang, W.; Li, F.; Xu, C.; Zhou, Y.; Li, Z.; Liu, B.; Shao, L.; Chen, D.; Bai, S.; et al. Design, Synthesis and Biological Activity Evaluation of β-Carboline Derivatives Containing Nitrogen Heterocycles. Molecules 2024, 29, 5155. [Google Scholar] [CrossRef]
- Kuo, F.-M.; Tseng, M.-C.; Yen, Y.-H.; Chu, Y.-H. Microwave Accelerated Pictet–Spengler Reactions of Tryptophan with Ketones Directed toward the Preparation of 1,1-Disubstituted Indole Alkaloids. Tetrahedron 2004, 60, 12075–12084. [Google Scholar] [CrossRef]
- Rashid, N.; Alam, S.; Hasan, M.; Khan, N.; Khan, K.M.; Duddeck, H.; Pescitelli, G.; Kenéz, Á.; Antus, S.; Kurtán, T. Cis -Diastereoselectivity in Pictet–Spengler Reactions of L -Tryptophan and Electronic Circular Dichroism Studies. Chirality 2012, 24, 789–795. [Google Scholar] [CrossRef]
- Singh, J.; Shah, R.; Singh, D.; Jaggi, A.S.; Singh, N. Design, Synthesis, and Biological Evaluation of 2-substituted-2,3,4,9-tetrahydrospiro-β-carboline-3-carboxylic Acid Derivatives as First-in-class Mast Cell Stabilizers. Arch. Pharm. 2018, 351, 1800019. [Google Scholar] [CrossRef]
- Nazari Formagio, A.S.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Düsman Tonin, L.T.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and Antiviral Activity of β-Carboline Derivatives Bearing a Substituted Carbohydrazide at C-3 against Poliovirus and Herpes Simplex Virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef]
- Du, G.; Sun, C.; Liu, Y.; Dong, Q.; Gao, P.; Li, D.; Qu, L. In Vitro Anti-Parasitic Activity and Mechanism of β-Carboline Derivatives Isolated from the Extracellular Product of Salinivibrio Proteolyticus Strain YCSC6. Aquaculture 2021, 534, 736337. [Google Scholar] [CrossRef]
- Saini, K.; Singh, J.; Shah, R.; Kaur, J.; Singh, D.; Singh, N.; Jaggi, A.S.; Chopra, D.S.; Singh, R.S. Synthesis of 1-(4-Hydroxy-3-Methoxyphenyl)-2,3,4,9-Tetrahydro-1H-β-Carboline-3-Carboxylic Acid Derivatives as Mast Cell Stabilizers. Med. Chem. Res. 2020, 29, 1400–1412. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Y.; Zhu, R.; Qian, J.; Liu, J.; Gao, W.; Meng, C.; Miao, J.; Xiong, B.; Qiu, X.; et al. Hydroxamic Acid Derivatives of β-Carboline/Hydroxycinnamic Acid Hybrids Inducing Apoptosis and Autophagy through the PI3K/Akt/mTOR Pathways. J. Nat. Prod. 2019, 82, 1442–1450. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Liu, Y.; Huang, Y.; Li, Y.; Wang, Q. Design, Synthesis, Anti-TMV, Fungicidal, and Insecticidal Activity Evaluation of 1,2,3,4-Tetrahydro-β-Carboline-3-Carboxylic Acid Derivatives Based on Virus Inhibitors of Plant Sources. Bioorg. Med. Chem. Lett. 2014, 24, 5228–5233. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Chen, H.; Hou, X.; Guan, H.; Chen, Q.; Ma, Y.; Xu, A. Synthesis and in Vitro Cytotoxic Evaluation of 1,3-Bisubstituted and 1,3,9-Trisubstituted β-Carboline Derivatives. Eur. J. Med. Chem. 2005, 40, 249–257. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, J. Design, Synthesis, and Evaluation of a Novel Class of 2,3-Disubstituted-Tetrahydro-β-Carboline Derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3718–3722. [Google Scholar] [CrossRef]
- Xie, J.; Xu, W.; Song, H.; Liu, Y.; Zhang, J.; Wang, Q. Synthesis and Antiviral/Fungicidal/Insecticidal Activities Study of Novel Chiral Indole Diketopiperazine Derivatives Containing Acylhydrazone Moiety. J. Agric. Food Chem. 2020, 68, 5555–5571. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Bi, Y.; Gao, X.; Li, P.; Hou, S.; Zhang, Y.; Bammert, C.; Jockusch, S.; Legalley, T.D.; Michael Gibson, K.; et al. Indole-TEMPO Conjugates Alleviate Ischemia-Reperfusion Injury via Attenuation of Oxidative Stress and Preservation of Mitochondrial Function. Bioorg. Med. Chem. 2017, 25, 2545–2568. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Liu, Y.; Wang, L.; Wang, Q. Synthesis and Antiviral and Fungicidal Activity Evaluation of β-Carboline, Dihydro-β-Carboline, Tetrahydro-β-Carboline Alkaloids, and Their Derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef]
- Li, J.-L.; Liu, L.; Pei, Y.-N.; Zhu, H.-J. Copper(II)-Containing C2-Symmetric Bistetracarboline Amides in Enantioselective Henry Reactions. Tetrahedron 2014, 70, 9077–9083. [Google Scholar] [CrossRef]
- Singh, D.; Hazra, C.K.; Malakar, C.C.; Pandey, S.K.; Kaith, B.S.; Singh, V. Indium-Mediated Domino Allylation-Lactonisation Approach: Diastereoselective Synthesis of β-Carboline C-3 Tethered α-Methylene γ-Butyrolactones. ChemistrySelect 2018, 3, 4859–4864. [Google Scholar] [CrossRef]
- Jadala, C.; Sathish, M.; Reddy, T.S.; Reddy, V.G.; Tokala, R.; Bhargava, S.K.; Shankaraiah, N.; Nagesh, N.; Kamal, A. Synthesis and in Vitro Cytotoxicity Evaluation of β-Carboline-Combretastatin Carboxamides as Apoptosis Inducing Agents: DNA Intercalation and Topoisomerase-II Inhibition. Bioorg. Med. Chem. 2019, 27, 3285–3298. [Google Scholar] [CrossRef]
- Marçal, L.; Garden, S. Synthesis of Spiro-Pyrrolidinyloxindoles by Oxidative Rearrangement of N-Acyltetrahydro-β-Carbolines Using an Oxone/Aqueous Acetone Mixture. J. Braz. Chem. Soc. 2018, 30, 19–32. [Google Scholar] [CrossRef]
- Alberch, L.; Bailey, P.D.; Clingan, P.D.; Mills, T.J.; Price, R.A.; Pritchard, R.G. The Cis- Specific Pictet−Spengler Reaction. Eur. J. Org. Chem. 2004, 2004, 1887–1890. [Google Scholar] [CrossRef]
- Lin, G.; Wang, Y.; Zhou, Q.; Tang, W.; Wang, J.; Lu, T. A Facile Synthesis of 3-Substituted 9H-Pyrido[3,4-b]Indol-1(2H)-One Derivatives from 3-Substituted β-Carbolines. Molecules 2010, 15, 5680–5691. [Google Scholar] [CrossRef]
- Lin, Y.; Xia, X.; Yao, R.; Ni, L.; Hu, J.; Guo, W.; Zhu, B. Synthesis and in Vitro Biological Evaluation of Hybrids from Tetrahydro-β-Carboline and Hydroxylcinnamic Acid as Antitumor Carcinoma Agents. Chem. Pharm. Bull. 2014, 62, 343–349. [Google Scholar] [CrossRef]
- Chen, L.; Xie, J.; Song, H.; Liu, Y.; Gu, Y.; Wang, L.; Wang, Q. Design, Synthesis, and Biological Activities of Spirooxindoles Containing Acylhydrazone Fragment Derivatives Based on the Biosynthesis of Alkaloids Derived from Tryptophan. J. Agric. Food Chem. 2016, 64, 6508–6516. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Majumder, S.; Clayton, T.; Petrou, S.; VanLinn, M.L.; Namjoshi, O.A.; Ma, C.; Cromer, B.A.; Roth, B.L.; Platt, D.M.; et al. Design, Synthesis, and Subtype Selectivity of 3,6-Disubstituted β-Carbolines at Bz/GABA(A)Ergic Receptors. SAR and Studies Directed toward Agents for Treatment of Alcohol Abuse. Bioorg. Med. Chem. 2010, 18, 7548–7564. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sarkar, T.; Datta, S.; Maiti, A.; Chakrabarti, M.; Mondal, T.; Mondal, C.; Banerjee, A.; Roy, S.; Mukherjee, S.; et al. Structure-based Discovery of (S)-2-amino-6-(4-fluorobenzyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]Pyrido[3,4-b]Indole-1,3(2H)-dione as Low Nanomolar, Orally Bioavailable Autotaxin Inhibitor. Chem. Biol. Drug. Des. 2022, 99, 496–503. [Google Scholar] [CrossRef]
- Li, S.; Yang, B.; Zhang, Q.; Zhang, J.; Wang, J.; Wu, W. Synthesis and Bioactivity of β-Carboline Derivatives. Nat. Prod. Commun. 2010, 5, 1934578X1000501016. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Teng, S.F.; Kesuma, D.; Deng, Y.; Duan, J.; Wang, J.H.; Qi, R.Z.; Sim, M.M. β-Carbolines as Specific Inhibitors of Cyclin-Dependent Kinases. Bioorg. Med. Chem. Lett. 2002, 12, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; Wang, X.; Luo, L.; Guo, J.; Peng, Y.; Xu, Q.; Miao, J.; Zhang, Y.; Ling, Y. Development of Novel β-Carboline-Based Hydroxamate Derivatives as HDAC Inhibitors with DNA Damage and Apoptosis Inducing Abilities. Med. Chem. Commun. 2017, 8, 1213–1219. [Google Scholar] [CrossRef]
- Ling, Y.; Feng, J.; Luo, L.; Guo, J.; Peng, Y.; Wang, T.; Ge, X.; Xu, Q.; Wang, X.; Dai, H.; et al. Design and Synthesis of C3-Substituted β-Carboline-Based Histone Deacetylase Inhibitors with Potent Antitumor Activities. ChemMedChem 2017, 12, 646–651. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Xiao, T.; Song, Z.; Csuk, R.; Li, S. Design and Discovery of Novel Chiral Antifungal Amides with 2-(2-Oxazolinyl)Aniline as a Promising Pharmacophore. J. Agric. Food Chem. 2018, 66, 8957–8965. [Google Scholar] [CrossRef]
- Ling, Y.; Guo, J.; Yang, Q.; Zhu, P.; Miao, J.; Gao, W.; Peng, Y.; Yang, J.; Xu, K.; Xiong, B.; et al. Development of Novel β-Carboline-Based Hydroxamate Derivatives as HDAC Inhibitors with Antiproliferative and Antimetastatic Activities in Human Cancer Cells. Eur. J. Med. Chem. 2018, 144, 398–409. [Google Scholar] [CrossRef]
- Xu, Q.-B.; Chen, X.-F.; Feng, J.; Miao, J.-F.; Liu, J.; Liu, F.-T.; Niu, B.-X.; Cai, J.-Y.; Huang, C.; Zhang, Y.; et al. Design, Synthesis and Biological Evaluation of Hybrids of β-Carboline and Salicylic Acid as Potential Anticancer and Apoptosis Inducing Agents. Sci. Rep. 2016, 6, 36238. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Zhu, S.; Luo, J.; Zhang, Y.; Weng, Q. Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.-M.; Lu, Y.; Jin, J.-L.; Guo, H.; Lin, G.-W.; Wang, Y.; Lu, T. Synthesis, Characterization, DNA Binding Ability and Cytotoxicity of the Novel Platinum(II), Copper(II), Cobalt(II) and Nickel(II) Complexes with 3-(1 H -Benzo[ d ]Imidazol-2-Yl)- β -Carboline. Inorganica Chim. Acta 2014, 421, 91–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, Y.; Jiang, Z.; Shu, B.; Sethuraman, V.; Zhong, G. Design, Synthesis, Fungicidal Property and QSAR Studies of Novel β -carbolines Containing Urea, Benzoylthiourea and Benzoylurea for the Control of Rice Sheath Blight. Pest. Manag. Sci. 2018, 74, 1736–1746. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, V.; Devi, N.; Malakar, C.C.; Shankar, R.; Singh, V. Metal–Free Decarboxylative Amination: An Alternative Approach Towards Regioselective Synthesis of β-Carboline N-fused Imidazoles. Adv. Synth. Catal. 2017, 359, 1213–1226. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, C.; Luo, L.; Cao, J.; Feng, J.; Xue, Y.; Zhu, Q.; Ju, C.; Li, F.; Zhang, Y.; et al. Novel β-Carboline/Hydroxamic Acid Hybrids Targeting Both Histone Deacetylase and DNA Display High Anticancer Activity via Regulation of the P53 Signaling Pathway. J. Med. Chem. 2015, 58, 9214–9227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Q.; Lin, G.; Yang, T.; Wang, Z.; Lu, Y.; Tang, Y.; Liu, L.; Lu, T. Synthesis and Structure of the β-Carboline Derivatives and Their Binding Intensity with Cyclin-Dependent Kinase 2. Chem. Pharm. Bull. 2012, 60, 435–441. [Google Scholar] [CrossRef]
- Lin, G.; Wang, Y.; Zhou, Q.; Wang, J.; Yang, T.; Wang, Z.; Lu, T. Synthesis of a Novel Series of 1,6-Disubstituted-3-(Cyclohexylmethoxy)-β-Carboline Derivatives via Minisci Reaction. Synth. Commun. 2012, 42, 1895–1910. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, M.; Wang, Y.; Zhu, H.; Wang, Y.; Zhao, S.; Wu, J.; Peng, S. Design, Synthesis, and in Vivo Evaluations of Benzyl Nω-Nitro-Nα-(9H-Pyrido[3,4-b]Indole-3-Carbonyl)-l-Argininate as an Apoptosis Inducer Capable of Decreasing the Serum Concentration of P-Selectin. Med. Chem. Commun. 2016, 7, 1730–1737. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Gohil, V.M.; Kushwah, V.; Neelagiri, S.; Jain, S.; Singh, S.; Bhutani, K.K. Design, Synthesis and Biological Evaluation of 1,3,6-Trisubstituted β-Carboline Derivatives for Cytotoxic and Anti-Leishmanial Potential. Bioorg. Med. Chem. Lett. 2016, 26, 789–794. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Jadala, C.; Nekkanti, S.; Senwar, K.R.; Nagesh, N.; Shrivastava, S.; Naidu, V.G.M.; Sathish, M.; Kamal, A. Design and Synthesis of C3-Tethered 1,2,3-Triazolo-β-Carboline Derivatives: Anticancer Activity, DNA-Binding Ability, Viscosity and Molecular Modeling Studies. Bioorg. Chem. 2016, 64, 42–50. [Google Scholar] [CrossRef]
- Lan, J.-S.; Xie, S.-S.; Li, S.-Y.; Pan, L.-F.; Wang, X.-B.; Kong, L.-Y. Design, Synthesis and Evaluation of Novel Tacrine-(β-Carboline) Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease. Bioorg. Med. Chem. 2014, 22, 6089–6104. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, W.; Fan, W.; Ma, Q.; Sun, R.; Shao, G.; Cao, R. Synthesis and Preliminary Evaluation of Novel Alkyl Diamine Linked Bivalent β-Carbolines as Angiogenesis Inhibitors. Med. Chem. Commun. 2016, 7, 2177–2183. [Google Scholar] [CrossRef]
- Kamal, A.; Srinivasulu, V.; Nayak, V.L.; Sathish, M.; Shankaraiah, N.; Bagul, C.; Reddy, N.V.S.; Rangaraj, N.; Nagesh, N. Design and Synthesis of C3-Pyrazole/Chalcone-Linked Beta-Carboline Hybrids: Antitopoisomerase I, DNA-Interactive, and Apoptosis-Inducing Anticancer Agents. ChemMedChem 2014, 9, 2084–2098. [Google Scholar] [CrossRef]
- Sun, R.; Liu, R.; Zhou, C.; Ren, Z.; Guo, L.; Ma, Q.; Fan, W.; Qiu, L.; Yu, H.; Shao, G.; et al. Synthesis and Biological Evaluation of Piperazine Group-Linked Bivalent β-Carbolines as Potential Antitumor Agents. Med. Chem. Commun. 2015, 6, 2170–2174. [Google Scholar] [CrossRef]
- Kamal, A.; Narasimha Rao, M.P.; Swapna, P.; Srinivasulu, V.; Bagul, C.; Shaik, A.B.; Mullagiri, K.; Kovvuri, J.; Reddy, V.S.; Vidyasagar, K.; et al. Synthesis of β-Carboline–Benzimidazole Conjugates Using Lanthanum Nitrate as a Catalyst and Their Biological Evaluation. Org. Biomol. Chem. 2014, 12, 2370–2387. [Google Scholar] [CrossRef]
- Savariz, F.C.; Foglio, M.A.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Duarte, M.C.T.; Da Rosa, M.F.; Meyer, E.; Sarragiotto, M.H. Synthesis and Evaluation of New β-Carboline-3-(4-Benzylidene)-4H-Oxazol-5-One Derivatives as Antitumor Agents. Molecules 2012, 17, 6100–6113. [Google Scholar] [CrossRef]
- Abdelsalam, M.A.; AboulWafa, O.M.; M Badawey, E.-S.A.; El-Shoukrofy, M.S.; El-Miligy, M.M.; Gouda, N.; Elaasser, M.M. Design, Synthesis, Anticancer Screening, Docking Studies and In Silico ADME Prediction of Some β-Carboline Derivatives. Future Med. Chem. 2018, 10, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, L.; Ma, Q.; Chen, W.; Fan, W.; Zhang, J. Design, Synthesis, and Biological Evaluation of Novel N-Acylhydrazone Bond Linked Heterobivalent β-Carbolines as Potential Anticancer Agents. Molecules 2019, 24, 2950. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lin, Y.-C.; Chen, J.-P.; Chan, H.-C.; Hsu, M.-H.; Lin, H.-Y.; Kuo, S.-C.; Huang, L.-J. Synthesis and Biological Evaluation of Novel 3,9-Substituted β-Carboline Derivatives as Anticancer Agents. Bioorg. Med. Chem. Lett. 2015, 25, 3873–3877. [Google Scholar] [CrossRef]
- Guo, L.; Ma, Q.; Chen, W.; Fan, W.; Zhang, J.; Dai, B. Synthesis and Biological Evaluation of Novel N9 -Heterobivalent β-Carbolines as Angiogenesis Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 375–387. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Zhang, B.; Chen, X.; Zhou, Y.; Zhang, J.; Han, X.; Dai, B. Synthesis and Fungicidal Evaluation of Novel β -Carboline-Benzimidazole and β-Carboline-Benzothiazole Hybrids. Chin. J. Org. Chem. 2018, 38, 3356. [Google Scholar] [CrossRef]
- Abramyants, M.G.; Lomov, D.A.; Zavyazkina, T.I. Dehydrogenation of 1-Aryl(Hetaryl)-1,2,3,4-Tetrahydro-9H-β-Carboline-3-Carboxylic Acids and Their Esters with Dimethyl Sulfoxide. Russ. J. Org. Chem. 2016, 52, 1610–1615. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, Z.; Dong, J.; Shi, X.-X.; Lu, X. Novel Asymmetric Total Syntheses of (R)-(−)-Pyridindolol, (R)-(−)-Pyridindolol K1, and (R)-(−)-Pyridindolol K2 via a Mild One-Pot Aromatization of N-Tosyl-Tetrahydro-β-Carboline with (S)-2,3-O-Isopropylidene-l-Glyceraldehyde as the Source of Chirality. Tetrahedron Asymmetry 2013, 24, 633–637. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Wang, L.; Chen, X.; Sun, Y.; Lin, L.; Tang, Y.; Li, F.; Chen, D. Organic Base-Promoted Efficient Dehydrogenative/Decarboxylative Aromatization of Tetrahydro-β-Carbolines into β-Carbolines under Air. Tetrahedron Lett. 2019, 60, 800–804. [Google Scholar] [CrossRef]
- Venkataramana Reddy, P.O.; Mishra, S.; Tantak, M.P.; Nikhil, K.; Sadana, R.; Shah, K.; Kumar, D. Design, Synthesis and in Vitro Cytotoxicity Studies of Novel β-Carbolinium Bromides. Bioorg. Med. Chem. Lett. 2017, 27, 1379–1384. [Google Scholar] [CrossRef]
- Kamal, A.; Sathish, M.; Prasanthi, A.V.G.; Chetna, J.; Tangella, Y.; Srinivasulu, V.; Shankaraiah, N.; Alarifi, A. An Efficient One-Pot Decarboxylative Aromatization of Tetrahydro-β-Carbolines by Using N-Chlorosuccinimide: Total Synthesis of Norharmane, Harmane and Eudistomins. RSC Adv. 2015, 5, 90121–90126. [Google Scholar] [CrossRef]
- Mohamad Arshad, A.S.; Meesala, R.; Hanapi, N.A.; Mordi, M.N. A Convenient Synthesis of β-Carbolines by Iron-Catalyzed Aerobic Decarboxylative/Dehydrogenative Aromatization of Tetrahydro-β-Carbolines under Air. Tetrahedron 2021, 83, 131960. [Google Scholar] [CrossRef]
- Drung, B.; Scholz, C.; Barbosa, V.A.; Nazari, A.; Sarragiotto, M.H.; Schmidt, B. Computational & Experimental Evaluation of the Structure/Activity Relationship of β-Carbolines as DYRK1A Inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 4854–4860. [Google Scholar] [CrossRef]
- Guo, L.; Chen, W.; Cao, R.; Fan, W.; Ma, Q.; Zhang, J.; Dai, B. Synthesis and Structure-Activity Relationships of Asymmetric Dimeric β-Carboline Derivatives as Potential Antitumor Agents. Eur. J. Med. Chem. 2018, 147, 253–265. [Google Scholar] [CrossRef]
- Gohil, V.M.; Brahmbhatt, K.G.; Loiseau, P.M.; Bhutani, K.K. Synthesis and Anti-Leishmanial Activity of 1-Aryl-β-Carboline Derivatives against Leishmania Donovani. Bioorganic Med. Chem. Lett. 2012, 22, 3905–3907. [Google Scholar] [CrossRef]
- Xin, B.; Tang, W.; Wang, Y.; Lin, G.; Liu, H.; Jiao, Y.; Zhu, Y.; Yuan, H.; Chen, Y.; Lu, T. Design, Synthesis and Biological Evaluation of β-Carboline Derivatives as Novel Inhibitors Targeting B-Raf Kinase. Bioorg. Med. Chem. Lett. 2012, 22, 4783–4786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Xiang, J.-C.; Cheng, Y.; Ma, J.-T.; Wu, Y.-D.; Wu, A.-X. Direct Biomimetic Synthesis of β-Carboline Alkaloids from Two Amino Acids. J. Org. Chem. 2018, 83, 12247–12254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, X.; Xu, B.; Bijian, K.; Wan, S.; Li, G.; Alaoui-Jamali, M.; Jiang, T. Total Synthesis and Bioactivity of the Marine Alkaloid Pityriacitrin and Some of Its Derivatives. Eur. J. Med. Chem. 2011, 46, 6089–6097. [Google Scholar] [CrossRef]
- Choudhary, A.N.; Kumar, A.; Joshi, A.; Kohli, M.S. Synthesis of Tryptoline-3-Carboxylic Acid Derivatives A Novel Antidiabetic Agent. J. Young Pharm. 2011, 3, 132–137. [Google Scholar] [CrossRef]
- Triggle, D.J.; Mitchell, J.M.; Filler, R. The Pharmacology of Physostigmine. CNS Drug Rev. 1998, 4, 87–136. [Google Scholar] [CrossRef]
- Kamenecka, T.M.; Danishefsky, S.J. Discovery through Total Synthesis: A Retrospective on the Himastatin Problem. Chem. Eur. J. 2001, 7, 41–63. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, P.; De Lera, A.R. Puzzling Out the Structure of Novofumigatamide: Total Synthesis of Constitutional Isomers. Part II. J. Org. Chem. 2022, 87, 12528–12546. [Google Scholar] [CrossRef]
- Luo, L.; Zhai, X.; Wang, Y.; Peng, Y.; Gong, H. Divergent Total Syntheses of C3 a−C7′ Linked Diketopiperazine Alkaloids (+)-Asperazine and (+)-Pestalazine A Enabled by a Ni-Catalyzed Reductive Coupling of Tertiary Alkyl Chloride. Chem. Eur. J. 2019, 25, 989–992. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kato, S.; Kataoka, S.; Kodato, S.; Watanabe, H.; Okajima, H.; Hino, T.; Witkop, B. Dye-Sensitized Photooxygenation of Tyrptophan: 3a-Hydroperoxypyrroloindole as a Labile Precursor of Formylkynurenine. Chem. Pharm. Bull. 1981, 29, 1013–1026. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, C.; Jiang, S.; Zhang, Y.; Li, Q.; Bai, W.; Wang, X. Directed Evolution of a Tryptophan 2,3-Dioxygenase for the Diastereoselective Monooxygenation of Tryptophans. Angew. Chem. Int. Ed. 2020, 59, 3043–3047. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, L.; Liu, J.; Liu, Y.; Song, H.; Wang, Q. Design, Synthesis, and Bioactivity Study of Novel Tryptophan Derivatives Containing Azepine and Acylhydrazone Moieties. Molecules 2022, 27, 6700. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Li, X.; Xu, L.; Ma, J.; Sun, L.; Zhang, B.; Lin, B.; Cheng, M.; Liu, Y. Diels-Alder Cycloaddition of Azepino[4,5-b]Indoles Towards Hydrocarbazole Derivatives and Related Heterocycles. Adv. Synth. Catal. 2022, 364, 873–889. [Google Scholar] [CrossRef]
- Jida, M.; Betti, C.; Urbanczyk-Lipkowska, Z.; Tourwé, D.; Ballet, S. Highly Diastereoselective Synthesis of 1-Carbamoyl-4-Aminoindoloazepinone Derivatives via the Ugi Reaction. Org. Lett. 2013, 15, 5866–5869. [Google Scholar] [CrossRef]
- Ryan, K.L.; Akhmedov, N.G.; Panaccione, D.G. Identification and Structural Elucidation of Ergotryptamine, a New Ergot Alkaloid Produced by Genetically Modified Aspergillus Nidulans and Natural Isolates of Epichloë Species. J. Agric. Food Chem. 2015, 63, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cheon, C.-H. Total Synthesis of Rucaparib. J. Org. Chem. 2022, 87, 4813–4817. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mingo, M.; Rodríguez, N.; Gómez Arrayás, R.; Carretero, J.C. Access to Benzazepinones by Pd-Catalyzed Remote C–H Carbonylation of γ-Arylpropylamine Derivatives. Org. Lett. 2019, 21, 4345–4349. [Google Scholar] [CrossRef]
- Jiao, R.H.; Xu, S.; Liu, J.Y.; Ge, H.M.; Ding, H.; Xu, C.; Zhu, H.L.; Tan, R.X. Chaetominine, a Cytotoxic Alkaloid Produced by Endophytic Chaetomium Sp. IFB-E015. Org. Lett. 2006, 8, 5709–5712. [Google Scholar] [CrossRef]
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A–K, Fumiquinazoline-Type Alkaloids from the Gorgonian-Derived Fungus Aspergillus Versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef]
- Wu, J.-F.; Huang, P.-Q. Concise, Enantioselective Total Syntheses of Both the Proposed and Revised Structures of (−)-Versiquinazoline H. Chin. Chem. Lett. 2020, 31, 61–63. [Google Scholar] [CrossRef]
- Xu, C.-P.; Luo, S.-P.; Wang, A.-E.; Huang, P.-Q. Complexity Generation by Chemical Synthesis: A Five-Step Synthesis of (−)-Chaetominine from l-Tryptophan and Its Biosynthetic Implications. Org. Biomol. Chem. 2014, 12, 2859. [Google Scholar] [CrossRef]
- Nourry, A.; Legoupy, S.; Huet, F. Synthesis of an Analogue of Lavendamycin and of Conformationally Restricted Derivatives by Cyclization via a Hemiaminal Intermediate. Tetrahedron Lett. 2007, 48, 6014–6018. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Stevens, B.L.; Elson, D.J.; Finlay, D.; Gamble, J.T.; Kopparapu, P.R.; Tanguay, R.L.; Buermeyer, A.B.; Kerkvliet, N.I.; Kolluri, S.K. 11-Cl-BBQ, a Select Modulator of AhR-Regulated Transcription, Suppresses Lung Cancer Cell Growth via Activation of P53 and p27Kip1. FEBS J. 2023, 290, 2064–2084. [Google Scholar] [CrossRef]
- Nancy, I. Kerkvliet; Sebas-Tian Bernales; Jit Chakravarty; Brahmam Pujala; Pasha Khan; Varun Kumar; Abhinandan Danodia; Gon-Zalo Ureta Aryl Hydrocarbon Receptor Activators. WO 2021/02206 A1, 4 February 2021. [Google Scholar]
- Wang, M.-Z.; Si, T.-X.; Ku, C.-F.; Zhang, H.-J.; Li, Z.-M.; Chan, A.S.C. Synthesis of Javanicunines A and B, 9-Deoxy-PF1233s A and B, and Absolute Configuration Establishment of Javanicunine B. J. Org. Chem. 2019, 84, 831–839. [Google Scholar] [CrossRef]
- Khopade, T.M.; Ajayan, K.; Vincent, D.M.; Lane, A.L.; Viswanathan, R. Biomimetic Total Synthesis of (+)-Nocardioazine B and Analogs. J. Org. Chem. 2022, 87, 11519–11533. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, X.; Cai, L.; Xu, Z.; Ye, T. Total Synthesis and Absolute Configuration of Nocardioazine B. Chem. Commun. 2012, 48, 4344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; May, J.P.; Huang, J.; Perrin, D.M. Stereoselective Synthesis of Brevianamide E. Org. Lett. 2012, 14, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Li, X.; Hikawa, H.; Suzuki, T.; Tsutsumi, K.; Sato, M.; Takikawa, O.; Suzuki, H.; Yokoyama, Y. Synthesis and Biological Evaluation of Novel Tryptoline Derivatives as Indoleamine 2,3-Dioxygenase (IDO) Inhibitors. Bioorg. Med. Chem. 2013, 21, 1159–1165. [Google Scholar] [CrossRef]
- Sunder-Plassmann, N.; Sarli, V.; Gartner, M.; Utz, M.; Seiler, J.; Huemmer, S.; Mayer, T.U.; Surrey, T.; Giannis, A. Synthesis and Biological Evaluation of New Tetrahydro-β-Carbolines as Inhibitors of the Mitotic Kinesin Eg5. Bioorg. Med. Chem. 2005, 13, 6094–6111. [Google Scholar] [CrossRef]
- Walton, J.G.A.; Patterson, S.; Liu, G.; Haraldsen, J.D.; Hollick, J.J.; Slawin, A.M.Z.; Ward, G.E.; Westwood, N.J. Synthesis and Biological Evaluation of Functionalised Tetrahydro-β-Carboline Analogues as Inhibitors of Toxoplasma Gondii Invasion. Org. Biomol. Chem. 2009, 7, 3049. [Google Scholar] [CrossRef]
- Abadi, A.H.; Lehmann, J.; Piazza, G.A.; Abdel-Halim, M.; Ali, M.S.M. Synthesis, Molecular Modeling, and Biological Evaluation of Novel Tetrahydro- β -Carboline Hydantoin and Tetrahydro-β-Carboline Thiohydantoin Derivatives as Phosphodiesterase 5 Inhibitors. Int. J. Med. Chem. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Hotha, S.; Yarrow, J.C.; Yang, J.G.; Garrett, S.; Renduchintala, K.V.; Mayer, T.U.; Kapoor, T.M. HR22C16: A Potent Small-Molecule Probe for the Dynamics of Cell Division. Angew. Chem. Int. Ed. 2003, 42, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.I.; Peters, U.; Thomas, S.L.; Garrett, S.; Zelnak, A.; Kapoor, T.M.; Giannakakou, P. Mitotic Kinesin Inhibitors Induce Mitotic Arrest and Cell Death in Taxol-Resistant and -Sensitive Cancer Cells. J. Biol. Chem. 2005, 280, 11569–11577. [Google Scholar] [CrossRef]
- Dan, L.; Liu, Z.; Huo, L.; Yang, H.; Yi, J.; Xie, X.; She, X. Total Synthesis of (+)-Tabertinggine. Tetrahedron 2022, 115, 132781. [Google Scholar] [CrossRef]
- Chaniyara, R.; Tala, S.; Chen, C.-W.; Zang, X.; Kakadiya, R.; Lin, L.-F.; Chen, C.-H.; Chien, S.-I.; Chou, T.-C.; Tsai, T.-H.; et al. Novel Antitumor Indolizino[6,7-b]Indoles with Multiple Modes of Action: DNA Cross-Linking and Topoisomerase I and II Inhibition. J. Med. Chem. 2013, 56, 1544–1563. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, J.; Zhang, J.; Chen, S.; Wei, J.; Xiong, L.; Qiu, D. A β-Carboline [1’, 2’: 1, 2] Imidazole Derivative and Its Preparation Method and Application, and Pharmaceutical Composition. CN118164983A, 11 June 2024. [Google Scholar]
- Liu, J.; Wu, G.; Cui, G.; Wang, W.-X.; Zhao, M.; Wang, C.; Zhang, Z.; Peng, S. A New Class of Anti-Thrombosis Hexahydropyrazino-[1′,2′:1,6]Pyrido-[3,4-b]-Indole-1,4-Dions: Design, Synthesis, logK Determination, and QSAR Analysis. Bioorg. Med. Chem. 2007, 15, 5672–5693. [Google Scholar] [CrossRef]
- Bai, H.; Cui, P.; Zang, C.; Li, S. Enantioselective Total Synthesis, Divergent Optimization and Preliminary Biological Evaluation of (Indole-N-Alkyl)-Diketopiperazines. Bioorg. Med. Chem. Lett. 2019, 29, 126718. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, Y.; Sun, B.; Cheng, C.; Qiao, Y.; Jiang, Y.; Zhao, S.; Xie, Z.; Tan, J.; Lou, H. Discovery of Furyl/Thienyl β-Carboline Derivatives as Potent and Selective PDE5 Inhibitors with Excellent Vasorelaxant Effect. Eur. J. Med. Chem. 2018, 158, 767–780. [Google Scholar] [CrossRef] [PubMed]
- El-Gamil, D.S.; Ahmed, N.S.; Gary, B.D.; Piazza, G.A.; Engel, M.; Hartmann, R.W.; Abadi, A.H. Design of Novel β-Carboline Derivatives with Pendant 5-Bromothienyl and Their Evaluation as Phosphodiesterase-5 Inhibitors. Arch. Pharm. 2013, 346, 23–33. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Girgis, N.M.R.; Wilcken, R.; Bauer, M.R.; Tinsley, H.N.; Gary, B.D.; Piazza, G.A.; Boeckler, F.M.; Abadi, A.H. Synthesis and Molecular Modeling of Novel Tetrahydro-β-Carboline Derivatives with Phosphodiesterase 5 Inhibitory and Anticancer Properties. J. Med. Chem. 2011, 54, 495–509. [Google Scholar] [CrossRef]
- ElHady, A.K.; Shih, S.-P.; Chen, Y.-C.; Liu, Y.-C.; Ahmed, N.S.; Keeton, A.B.; Piazza, G.A.; Engel, M.; Abadi, A.H.; Abdel-Halim, M. Extending the Use of Tadalafil Scaffold: Development of Novel Selective Phosphodiesterase 5 Inhibitors and Histone Deacetylase Inhibitors. Bioorg. Chem. 2020, 98, 103742. [Google Scholar] [CrossRef]
- Coward, R.M.; Carson, C.C. Tadalafil in the Treatment of Erectile Dysfunction. Ther. Clin. Risk Manag. 2008, 4, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).