Adamantane-Functionalized Phthalimide Scaffold: Pathways to Supramolecular Interactions and Drug Discovery
Abstract
:1. Introduction
2. Materials and Methods
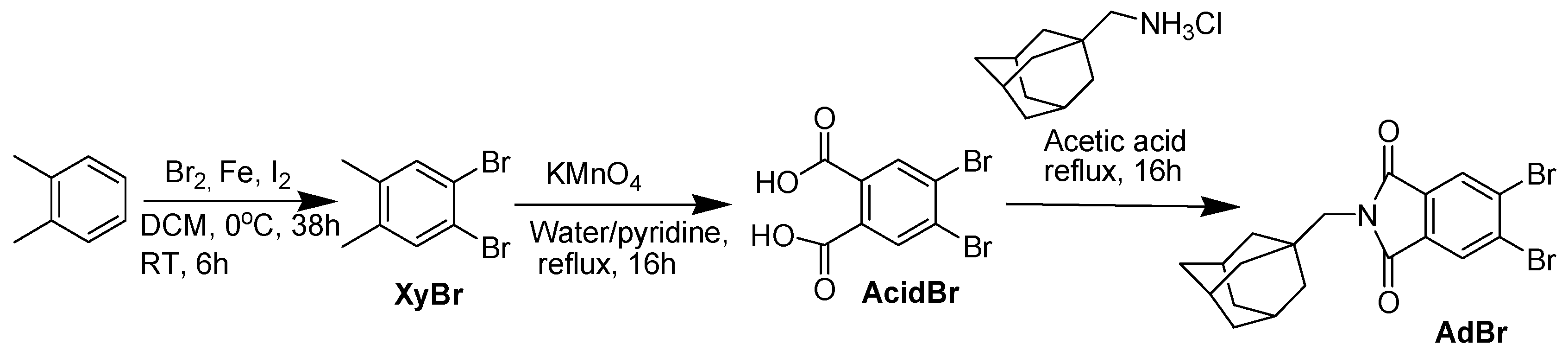
2.1. Preparation of 4,5-Dibromo Phthalic Acid (AcidBr)
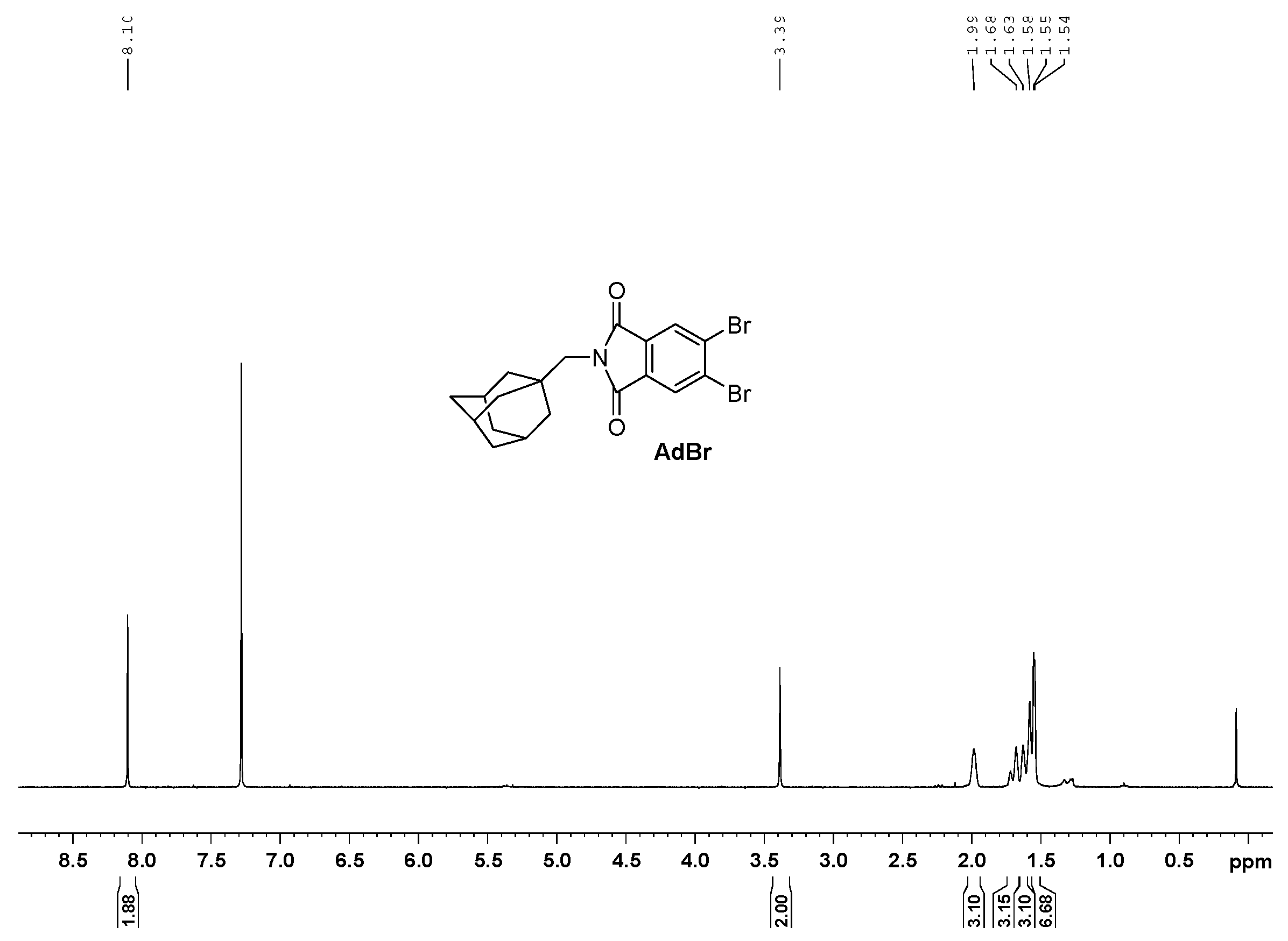
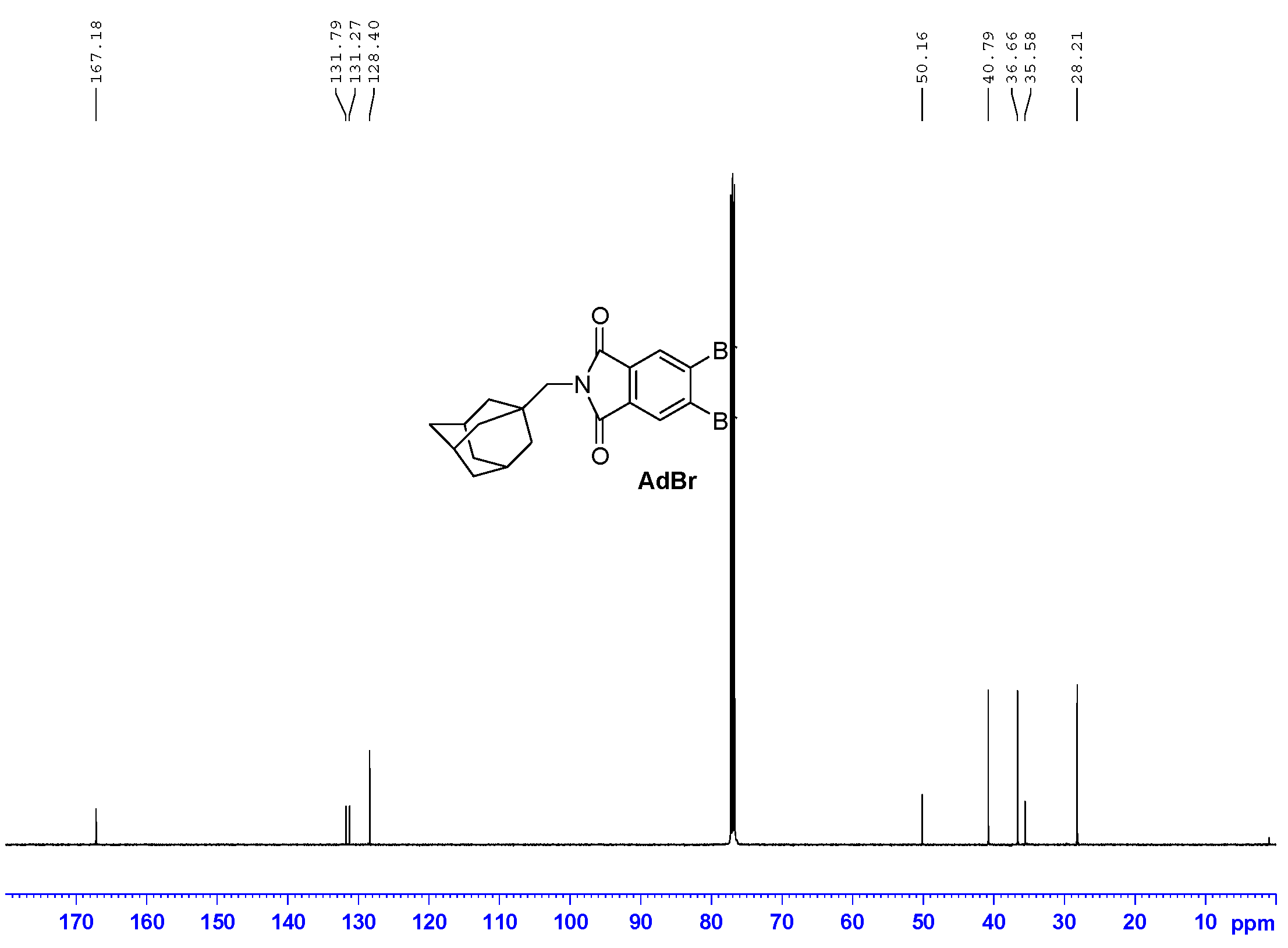
2.2. Preparation of 2-[(Adamantan-1-yl)Methyl]-5,6-Dibromo-1H-Isoindole-1,3(2H)-Dione (AdBr)
2.3. Crystal Structure Data Acquisition
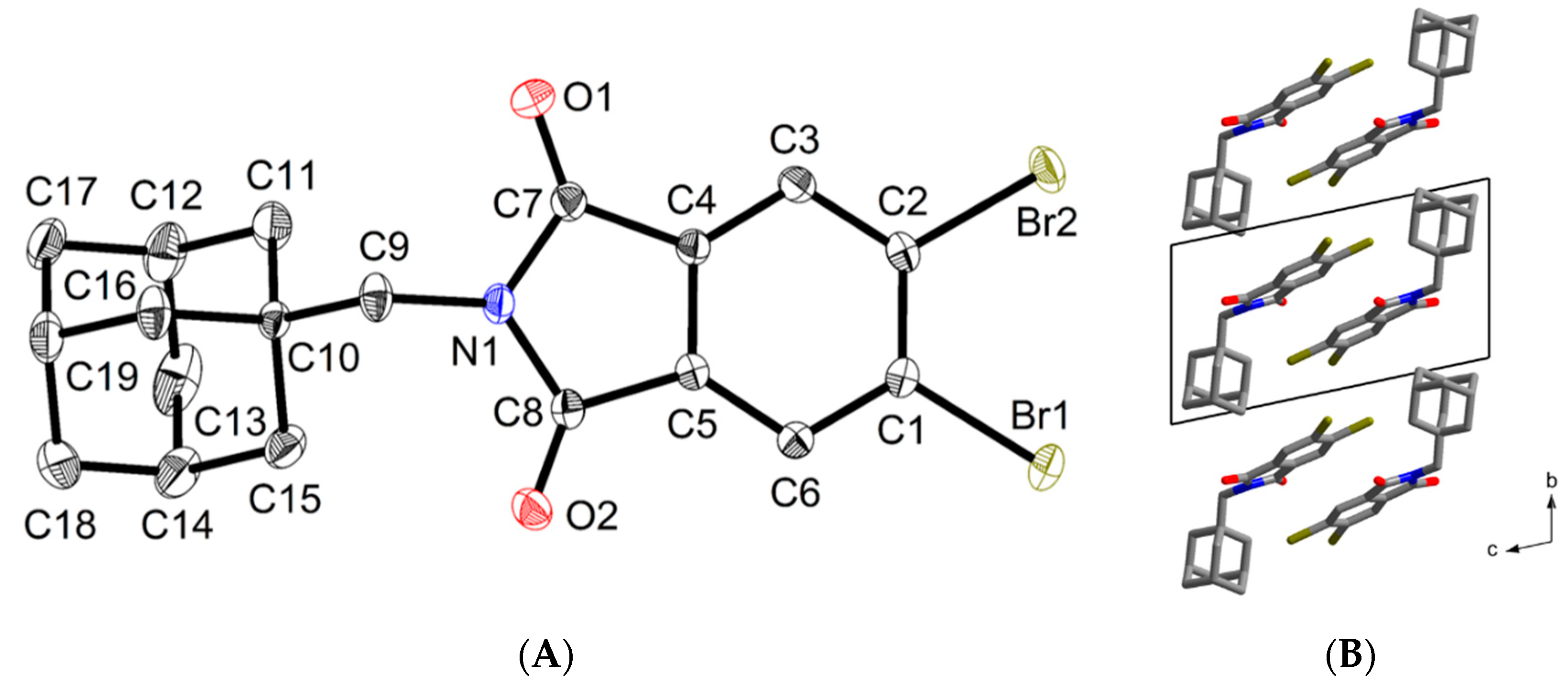
3. Results and Discussion
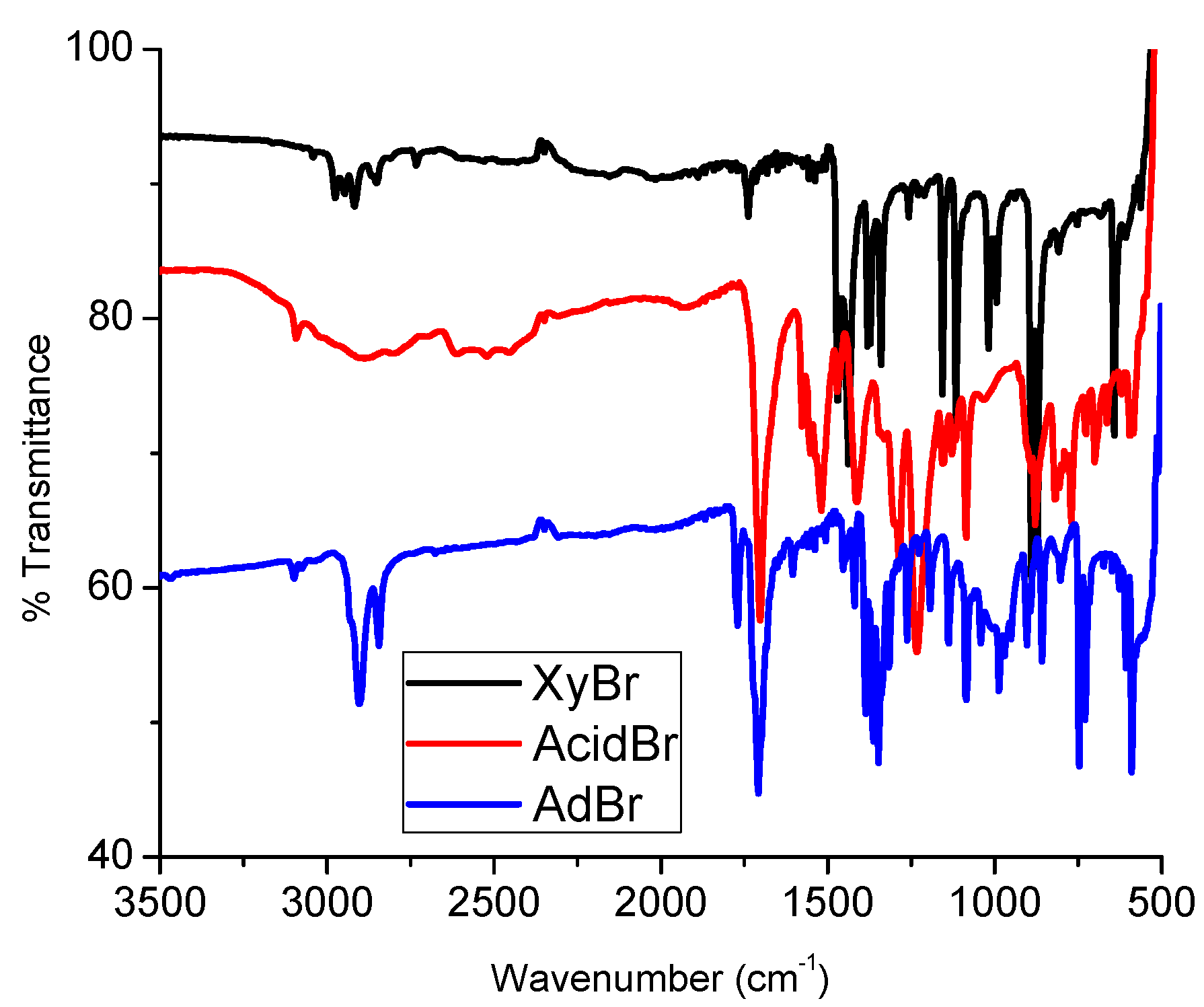
3.1. Synthesis and Spectroscopy
3.2. Crystallography
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nasrallah, H.; Hierso, J.-C. Porous Materials Based on 3-Dimensional Td -Directing Functionalized Adamantane Scaffolds and Applied as Recyclable Catalysts. Chem. Mater. 2019, 31, 619–642. [Google Scholar] [CrossRef]
- Von Schleyer, P.R. A Simple Preparation of Adamantane. J. Am. Chem. Soc. 1957, 79, 3292. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Donaldson, M.M.; Nicholas, R.D.; Cupas, C. Adamantane. Org. Synth. 1962, 42, 8. [Google Scholar]
- Davies, W.L.; Grunert, R.R.; Haff, R.F.; McGahen, J.W.; Neumayer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral Activity of 1-Adamantanamine (Amantadine). Science 1964, 144, 862. [Google Scholar] [CrossRef]
- Maassab, H.F.; Cochran, K.W. Rubella Virus: Inhibition in vitro by Amantadine Hydrochloride. Science 1964, 145, 1443. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jihoon Jo, J.; Jo, D.; Kim, J.; Min, J.-J.; Yange, D.H.; Hyun, H. Supramolecular assembly based on host–guest interaction between beta-cyclodextrin and adamantane for specifically targeted cancer imaging. J. Ind. Eng. Chem. 2018, 57, 37–44. [Google Scholar] [CrossRef]
- Rodell, C.B.; Ahmed, M.S.; Garris, C.S.; Pittet, M.J.; Weissleder, R. Development of Adamantane-Conjugated TLR7/8 Agonists for Supramolecular Delivery and Cancer Immunotherapy. Theranostics 2019, 9, 8426–8436. [Google Scholar] [CrossRef]
- Welling, D.M.; Duszenko, N.; Van Willigen, D.M.; Smits, W.K.; Buckle, T.; Roestenberg, M.; Van Leeuwen, F.W.B. Cyclodextrin/Adamantane-Mediated Targeting of Inoculated Bacteria in Mice. Bioconjugate Chem. 2021, 32, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Orzeszko, B.; Maurin, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003. [Google Scholar] [CrossRef]
- Ondrusek, B.A.; Chung, H. Modified N-Heterocyclic Carbene Ligand for the Recovery of Olefin Metathesis Catalysts via Noncovalent Host–Guest Interactions. ACS Omega 2017, 2, 3951–3957. [Google Scholar] [CrossRef]
- Kim, C.; Ondrusek, B.A.; Chung, H. Removable Water-Soluble Olefin Metathesis Catalyst via Host–Guest Interaction. Org. Lett. 2018, 20, 736–739. [Google Scholar] [CrossRef]
- Gupta, S.; Sabbasani, V.R.; Su, S.; Wink, D.J.; Lee, D. Alkene-Chelated Ruthenium Alkylidenes: A Missing Link to New Catalysts. ACS Catal. 2021, 11, 1977–1987. [Google Scholar] [CrossRef]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Kraämer, K.W.; Liu, S.X.; Decurtins, S.; Domasevitch, K.V. Functionalized Adamantane Tectons Used in the Design of Mixed-Ligand Copper(II) 1,2,4-Triazolyl/Carboxylate Metal–Organic Frameworks. Inorg. Chem. 2013, 52, 863–872. [Google Scholar] [CrossRef]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent Dyes and Their Supramolecular Host/Guest Complexes with Macrocycles in Aqueous Solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef]
- Granadero, D.; Bordello, J.; Pérez-lvite, M.J.; Novo, M.; Al-Soufi, W. Host-Guest Complexation Studied by Fluorescence Correlation Spectroscopy: Adamantane–Cyclodextrin Inclusion. Int. J. Mol. Sci. 2010, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Kashani, S.K.; Jessiman, J.E.; Newman, S.G. Exploring Homogeneous Conditions for Mild Buchwald–Hartwig Amination in Batch and Flow. Org. Process Res. Dev. 2020, 24, 1948–1954. [Google Scholar] [CrossRef]
- Fayssal, S.A.; Naret, T.; Huc, V.; Buendia, J.; Martini, C.; Schulz, E. Benzyloxycalix[8]arene supported Pd–NHC cinnamyl complexes for Buchwald–Hartwig C–N cross-couplings. Catal. Sci. Technol. 2021, 11, 5223–5231. [Google Scholar] [CrossRef]
- Seifinoferest, B.; Tanbakouchian, A.; Larijani, B.; Mahdavi, M. Ullmann-Goldberg and Buchwald-Hartwig C−N Cross Couplings: Synthetic Methods to Pharmaceutically Potential N-Heterocycles. Asian J. Org. Chem. 2021, 10, 1319. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Sun, X.; Bian, Y.; Ma, C.; Jiang, J. 2,3,9,10,16,17,24,25-Octakis(octyloxycarbonyl)phthalocyanines. Synthesis, Spectroscopic, and Electrochemical Characteristics. Inorg. Chem. 2007, 46, 7136–7141. [Google Scholar] [CrossRef]
- Lie Chen, L.; Chen, Y.; Yao, K.; Zhou, W.; Li, F.; Chen, L.; Hu, R.; Tang, B.Z. Synthesis and Helical Conformation of Novel Optically Active Liquid Crystalline Poly(p-phenylene)s Containing Cyanoterphenyl Mesogen as Pendant. Macromolecules 2009, 42, 5053–5061. [Google Scholar] [CrossRef]
- Medvedeva, I.V.; Stokes, M.E.; Eisinger, D.; Labrie, S.T.; Ai, J.; Trotter, M.W.B.; Schafer, P.; Yang, R. Large-scale Analyses of Disease Biomarkers and Apremilast Pharmacodynamic Effects. Sci. Rep. 2020, 10, 605–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, A.; Farajpour, B.; Knedel, T.-O.; Janiak, C. Synthesis of Substituted Phthalimides via Ultrasound-Promoted One-Pot Multicomponent Reaction. J. Org. Chem. 2021, 86, 574–580. [Google Scholar] [CrossRef]
- Basarić, N.; Horvat, M.; Franković, O.; Mlinarić-Majerski, K.; Jörg Neudörfl, J.; Griesbeck, A.G. Photoinduced hydrogen atom abstraction in N-(adamantyl)phthalimides: Structure–reactivity study in the solid state. Tetrahedron 2009, 65, 1438–1443. [Google Scholar] [CrossRef]
- Wilkinson, S.M.; Barron, M.L.; O’Brien-Brown, J.; Janssen, B.; Stokes, L.; Werry, E.L.; Chishty, M.; Skarratt, K.K.; Ong, J.A.; Hibbs, D.E.; et al. Pharmacological Evaluation of Novel Bioisosteres of an Adamantanyl Benzamide P2X7 Receptor Antagonist. ACS. Chem. Neurosci. 2017, 8, 2374–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danopoulos, A.A.; Cole, P.; Downing, S.P.; Pugh, D. Copper and palladium complexes with N-heterocyclic carbene ligands functionalised with carboxylate groups. J. Organomet. Chem. 2008, 693, 3369. [Google Scholar] [CrossRef]
- Sabbasani, V.R.; Lee, H.; Xia, Y.; Lee, D. Complementary Iron(II)-Catalyzed Oxidative Transformations of Allenes with Different Oxidants. Angew. Chem. Int. Ed. 2016, 55, 1151. [Google Scholar] [CrossRef]
- Mack, H.; Della Vedova, C.O.; Willner, H. Structures and conformations of carbonyl and carbonyl azides. An experimental and investigation. J. Mol. Struct. 1993, 291, 197–209. [Google Scholar] [CrossRef]
- Al-Far, R.; Ali, B.F.; Al-Sou’oud, K. Br···HN, N···HC, Br···HC, and Br···Br intermolecular interactions as supramolecular synthons: Synthesis and crystal structure of bis(2-amino-5-methylpyridinium) tetrabromomercurate(II). J. Chem. Crystallogr. 2006, 36, 523–529. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Il’in, M.V.; Suslonov, V.V.; Novikov, A.S. Symmetrical Noncovalent Interactions Br···Br Observed in Crystal Structure of Exotic Primary Peroxide. Symmetry 2020, 12, 637. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keita, H. Adamantane-Functionalized Phthalimide Scaffold: Pathways to Supramolecular Interactions and Drug Discovery. Organics 2021, 2, 388-394. https://doi.org/10.3390/org2040022
Keita H. Adamantane-Functionalized Phthalimide Scaffold: Pathways to Supramolecular Interactions and Drug Discovery. Organics. 2021; 2(4):388-394. https://doi.org/10.3390/org2040022
Chicago/Turabian StyleKeita, Hamidou. 2021. "Adamantane-Functionalized Phthalimide Scaffold: Pathways to Supramolecular Interactions and Drug Discovery" Organics 2, no. 4: 388-394. https://doi.org/10.3390/org2040022
APA StyleKeita, H. (2021). Adamantane-Functionalized Phthalimide Scaffold: Pathways to Supramolecular Interactions and Drug Discovery. Organics, 2(4), 388-394. https://doi.org/10.3390/org2040022





