Primary Phosphines and Phosphine Oxides with a Stereogenic Carbon Center Adjacent to the Phosphorus Atom: Synthesis and Anti-Markovnikov Radical Addition to Alkenes
Abstract
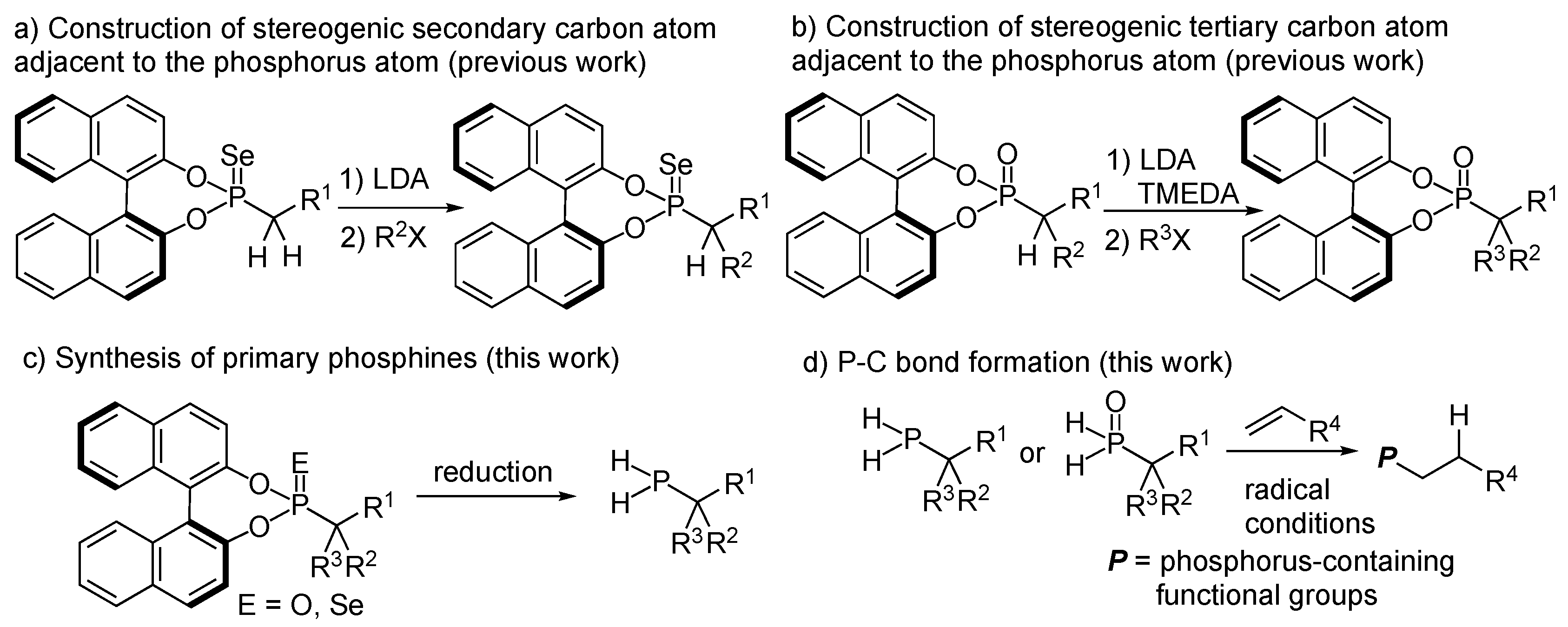
:1. Introduction
2. Materials and Methods
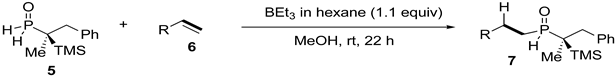
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Best Synthetic Methods: ORGANOPHOSPHORUS (V) CHEMISTRY; Timperley, C.M. (Ed.) Elsevier: London, UK, 2015. [Google Scholar]
- Kolodiazhnyi, O.I. Asymmetric Synthesis in Organophosphorus Chemistry; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Organophosphorus Chemistry; Iaroshenko, V. (Ed.) Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Fleming, J.T.; Higham, L.J. Primary phosphine chemistry. Coord. Chem. Rev. 2015, 297, 127–145. [Google Scholar] [CrossRef]
- Wang, X.; Xia, C.; Wu, L. Visible-Light-Promoted Photoredox Dehydrogenative Coupling of Phosphines and Thiophenols. Org. Lett. 2020, 22, 7373–7377. [Google Scholar] [CrossRef]
- Arockiam, P.B.; Lennert, U.; Graf, C.; Rothfelder, R.; Scott, D.J.; Fischer, T.G.; Zeitler, K.; Wolf, R. Versatile Visible-Light-Driven Synthesis of Asymmetrical Phosphines and Phosphonium Salts. Chem. Eur. J. 2020, 26, 16374–16382. [Google Scholar] [CrossRef]
- Bevern, D.; Goerls, H.; Krieck, S.; Westerhausen, M. Synthesis, Structure, and Stability of Lithium Arylphosphanidyl-diarylphosphine Oxide. Z. Anorg. Allg. Chem. 2020, 646, 948–958. [Google Scholar] [CrossRef] [Green Version]
- Gafurov, Z.N.; Sakhapov, I.F.; Kagilev, A.A.; Kantyukov, A.O.; Khayarov, K.R.; Sinyashin, O.G.; Yakhvarov, D.G. The formation of mesitylphosphine and dimesitylphosphine in the reaction of organonickel s-complex [NiBr(Mes)(bpy)] (Mes = 2, 4, 6-trimethylphenyl, bpy = 2, 2’-bipyridine) with phosphine PH3. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 726–729. [Google Scholar] [CrossRef]
- Oleg, G.; Yakhvarov, D.G.; Wilders, A.M.; Henle, J.; Haibach, M.C.; Swiatowiec, R.; Bien, J.; Henry, R.F.; Asare, S.O.; Wall, A.L.; et al. Pd-Catalyzed Cross-Coupling of Hindered, Electron-Deficient Anilines with Bulky (Hetero)aryl Halides Using Biaryl Phosphorinane Ligands. ACS Catal. 2020, 10, 15008–15018. [Google Scholar]
- Navratil, M.; Faria, E.N.; Panahy, G.; Cisarova, I.; Goicoechea, J.M.; Stepnicka, P. Novel ferrocenyl functionalized phosphinecarboxamides: Synthesis, characterization and coordination. Dalton Trans. 2020, 49, 8645–8651. [Google Scholar] [CrossRef]
- Han, Z.; Rohner, D.; Samedov, K.; Gates, D.P. Isolable Phosphaalkenes Bearing 2, 4, 6-Trimethoxyphenyl and 2, 6-Bis(trifluoromethyl)phenyl as P Substituents. J. Org. Chem. 2020, 85, 14643–14652. [Google Scholar] [CrossRef]
- Rafols, L.; Torrente, S.; Aguila, D.; Soto-Cerrato, V.; Perez-Tomas, R.; Gamez, P.; Grabulosa, A. Expanding the Range of Pyrenylphosphines and Their Derived Ru(II)-Arene Complexes. Organometallics 2020, 39, 2959–2971. [Google Scholar] [CrossRef]
- Zimmerman, A.N.; Xu, R.S.; Reynolds, S.C.; Shipp, C.A.; Marshall, D.J.; Wang, G.; Blank, N.F.; Gibbons, S.K.; Hughes, R.P.; Glueck, D.S.; et al. Diastereoselective Synthesis of P-Stereogenic Secondary Phosphine Oxides (SPOs) Bearing a Stereogenic Substituent by Ring Opening of (+)-Limonene Oxide with Primary Phosphido Nucleophiles. J. Org. Chem. 2020, 85, 14516–14526. [Google Scholar] [CrossRef]
- Schoemaker, R.; Kossatz, P.; Schwedtmann, K.; Hennersdorf, F.; Weigand, J.J. Coordination Chemistry and Methylation of Mixed-Substituted Tetraphosphetanes (RP-PtBu)2 (R = Ph, Py). Chem. Eur. J. 2020, 26, 11734–11741. [Google Scholar] [CrossRef]
- Itazaki, M.; Matsutani, T.; Nochida, T.; Moriuchi, T.; Nakazawa, H. Convenient synthesis of phosphinecarboxamide and phosphinecarbothioamide by hydrophosphination of isocyanates and isothiocyanates. Chem. Commun. 2020, 56, 443–445. [Google Scholar] [CrossRef]
- Del, B.; Janet, E.; Alkorta, I.; Elguero, J. Complexes H2CO:PXH2 and HCO2H: PXH2 for X = NC, F, Cl, CN, OH, CCH, CH3, and H: Pnicogen Bonds and Hydrogen Bonds. ChemPhysChem 2020, 21, 741–748. [Google Scholar]
- Scott, D.J.; Cammarata, J.; Schimpf, M.; Wolf, R. Synthesis of monophosphines directly from white phosphorus. Nat. Chem. 2021, 13, 458–464. [Google Scholar] [CrossRef]
- Hadlington, T.J.; Kostenko, A.; Driess, M. Synthesis and Coordination Ability of a Donor-Stabilized Bis-Phosphinidene. Chem. Eur. J. 2021, 27, 2476–2482. [Google Scholar] [CrossRef]
- Moncea, O.; Poinsot, D.; Fokin, A.A.; Schreiner, P.R.; Hierso, J.-C. Palladium-Catalyzed C2-H Arylation of Unprotected (N-H)-Indoles on Water Using Primary Diamantyl Phosphine Oxides as a Class of Primary Phosphine Oxide Ligands. ChemCatChem 2018, 10, 2915–2922. [Google Scholar] [CrossRef]
- Horky, F.; Cisarova, I.; Stepnicka, P. Synthesis, Reactivity, and Coordination of Semihomologous dppf Congeners Bearing Primary Phosphine and Primary Phosphine Oxide Groups. Organometallics 2021, 40, 427–441. [Google Scholar] [CrossRef]
- Horky, F.; Cisarova, I.; Stepnicka, P. A Stable Primary Phosphane Oxide and Its Heavier Congeners. Chem. Eur. J. 2021, 27, 1282–1285. [Google Scholar] [CrossRef]
- Lapshin, I.V.; Cherkasov, A.V.; Asachenko, A.F.; Trifonov, A.A. Ln(II) amido complexes coordinated by ring-expanded N-heterocyclic carbenes-promising catalysts for olefin hydrophosphination. Chem. Commun. 2020, 58, 12913–12916. [Google Scholar] [CrossRef]
- Dannenberg, S.G.; Waterman, R. A bench-stable copper photocatalyst for the rapid hydrophosphination of activated and unactivated alkenes. Chem. Commun. 2020, 56, 14219–14222. [Google Scholar] [CrossRef]
- Wang, C.; Huang, K.; Ye, J.; Duan, W.-L. Asymmetric Synthesis of P-Stereogenic Secondary Phosphine-Boranes by an Unsymmetric Bisphosphine Pincer-Nickel Complex. J. Am. Chem. Soc. 2021, 143, 5685–5690. [Google Scholar] [CrossRef]
- Rauhut, M.M.; Currier, H.A.; Semsel, A.M.; Wystrach, V.P. The free radical addition of phosphines to unsaturated compounds. J. Org. Chem. 1961, 26, 5138–5145. [Google Scholar] [CrossRef]
- Alvey, L.J.; Meier, R.; Soos, T.; Bernatis, P.; Gladysz, J.A. Syntheses and carbonyliridium complexes of unsymmetrically substituted fluorous trialkylphosphanes: Precision tuning of electronic properties, including insulation of the perfluoroalkyl groups. Eur. J. Org. Chem. 2000, 1975–1983. [Google Scholar] [CrossRef]
- Emnet, C.; Tuba, R.; Gladysz, J.A. Convenient Modular Syntheses of Fluorous Secondary Phosphines and Selected Derivatives. Adv. Synth. Catal. 2005, 347, 1819–1826. [Google Scholar] [CrossRef]
- Tuba, R.; Tesevic, V.; Dinh, L.V.; Hampel, F.; Gladysz, J.A. Synthesis, structure, and reactivity of fluorous phosphorus/carbon/phosphorus pincer ligands and metal Complexes. Dalton Trans. 2005, 13, 2275–2283. [Google Scholar] [CrossRef]
- Vlad, G.; Richter, F.U.; Horvath, I.T. Synthesis of fluorous trialkyl phosphines with the complete exclusion of PH3. Tetrahedron Lett. 2005, 46, 8605–8608. [Google Scholar] [CrossRef]
- Arbuzova, S.N.; Gusarova, N.K.; Trofimov, B.A. Nucleophilic and free-radical additions of phosphines and phosphine chalcogenides to alkenes and alkynes. Arkivoc 2006, V, 12–36. [Google Scholar] [CrossRef] [Green Version]
- Coudray, L.; Montchamp, J.-L. Recent Developments in the Addition of Phosphinylidene-Containing Compounds to Unactivated Unsaturated Hydrocarbons: Phosphorus–Carbon Bond Formation by Hydrophosphinylation and Related Processes. Eur. J. Org. Chem. 2008, 2008, 3601–3613. [Google Scholar] [CrossRef] [Green Version]
- Parsons, A.F.; Wright, A. Synthesis of Bispyrrolidines by Radical Cyclization of Diallylamines Using phosphorus Hydrides. Synlett 2008, 2008, 2142–2146. [Google Scholar] [CrossRef]
- Leca, D.; Fensterbank, L.; Lacote, E.; Malacria, M. Recent advances in the use of phosphorus-centered radicals in organic chemistry. Chem. Soc. Rev. 2005, 34, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, M.; Paul, T. Reactivity of phosphorus centered radicals. In New Aspects in Phosphorus Chemistry V. Topics in Current Chemistry; Majoral, J.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 250, pp. 43–76. [Google Scholar]
- Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Recent advances in sulfur- and phosphorous-centered radical reactions for the formation of S-C and P-C Bonds. Tetrahedron 2015, 71, 7481–7529. [Google Scholar] [CrossRef]
- Quint, V.; Noel-Duchesneau, L.; Lagadic, E.; Morlet-Savary, F.; Lalevee, J.; Gaumont, A.-C.; Lakhdar, S. Metal-Free Generation of Phosphorus-Centered Radicals for the Synthesis of Phosphorus-Based Heterocycles. Synthesis 2017, 49, 3444–3452. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, G.; Zhao, Y. Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 589–596. [Google Scholar] [CrossRef]
- Ren, W.; Yang, Q.; Yang, S.-D. Applications of transition metal catalyzed P-radical for synthesis of organophosphorus compounds. Pure Appl. Chem. 2019, 91, 87–94. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yamaguchi, K.; Miwa, Y.; Kutsumizu, S.; Minoura, M.; Murai, T. N, N-Diarylthiazol-5-amines: Structure-Specific Mechanofluorochromism and White Light Emission in the Solid State. Bull. Chem. Soc. Jpn. 2020, 93, 927–935. [Google Scholar] [CrossRef]
- Pamungkas, K.K.P.; Maruyama, T.; Murai, T. Boron complexes of thiazole-bridged 1, 5-bidentate nitrogen ligands: Synthesis and acid-responsive photophysical properties. Org. Biomol. Chem. 2021, 19, 6804–6811. [Google Scholar] [CrossRef]
- Maekawa, Y.; Kuwabara, K.; Sugiyama, A.; Iwata, K.; Maruyama, T.; Murai, T. Synthesis of P-Stereogenic Phosphinates via an Axis-to-Center Chirality Transfer by the Reaction of Phosphonates Having a Binaphthyloxy Group with Grignard Reagents. Chem. Lett. 2017, 46, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, K.; Maekawa, Y.; Minoura, M.; Murai, T. Hydrolysis of Phosphonothioates with a Binaphthyl Group: P-Stereogenic O-Binaphthyl Phosphonothioic Acids and Their Use as Optically Active Ligands and Stereogenic Discriminating Agents. Org. Lett. 2018, 20, 1375–1379. [Google Scholar] [CrossRef]
- Kuwabara, K.; Maekawa, Y.; Ebihara, M.; Maruyama, T.; Murai, T. Synthesis of P-stereogenic phosphonothioates via alcoholysis of phosphonothioates with a binaphthyl group. Heteroat. Chem. 2018, 29, e21448. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, K.; Maekawa, Y.; Minoura, M.; Maruyama, T.; Murai, T. Chemo- and Stereoselective Alcoholysis of Binaphthyl Phosphonothioates: Straightforward Access to both Stereoisomers of Biologically Relevant P-Stereogenic Phosphonothioates. J. Org. Chem. 2020, 85, 14446–14455. [Google Scholar] [CrossRef]
- Hirata, Y.; Kuwabara, K.; Takashima, M.; Murai, T. Hormetic Effects of Binaphthyl Phosphonothioates as Pro-oxidants and Antioxidant. Chem. Res. Toxicol. 2020, 33, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Maruyama, T.; Murai, T. Sequential Deprotonation-Alkylation of Binaphthyloxy-Substituted Phosphonochalcogenoates: Stereogenic Tri- and Tetrasubstituted Carbon Centers Adjacent to a Phosphorus Atom. Org. Lett. 2016, 18, 5264–5267. [Google Scholar] [CrossRef] [PubMed]
- Bonnaventure, I.; Charette, A.B. Probing the Importance of the Hemistable Site of Bis(phosphine) Monoxide Ligands in the Copper-Catalyzed Addition of Diethylzinc to N-Phosphinoylimines: Discovery of New Effective Stereogenic Ligands. J. Org. Chem. 2008, 73, 6330–6340. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, A.N.; Skvortsov, N.K. Synthesis of 5-[3-(diphenylphosphinoyl)propyl]-2-thiobarbituric acid. Russ. J. Gen. Chem. 2008, 78, 320–322. [Google Scholar] [CrossRef]
- Jessop, C.M.; Parsons, A.F.; Routledge, A.; Irvine, D.J. Radical addition reactions of phosphorus hydrides: Tuning the reactivity of phosphorus hydrides, the use of microwaves and Horner-Wadsworth-Emmons-type reactions. Eur. J. Org. Chem. 2006, 2006, 1547–1554. [Google Scholar] [CrossRef]
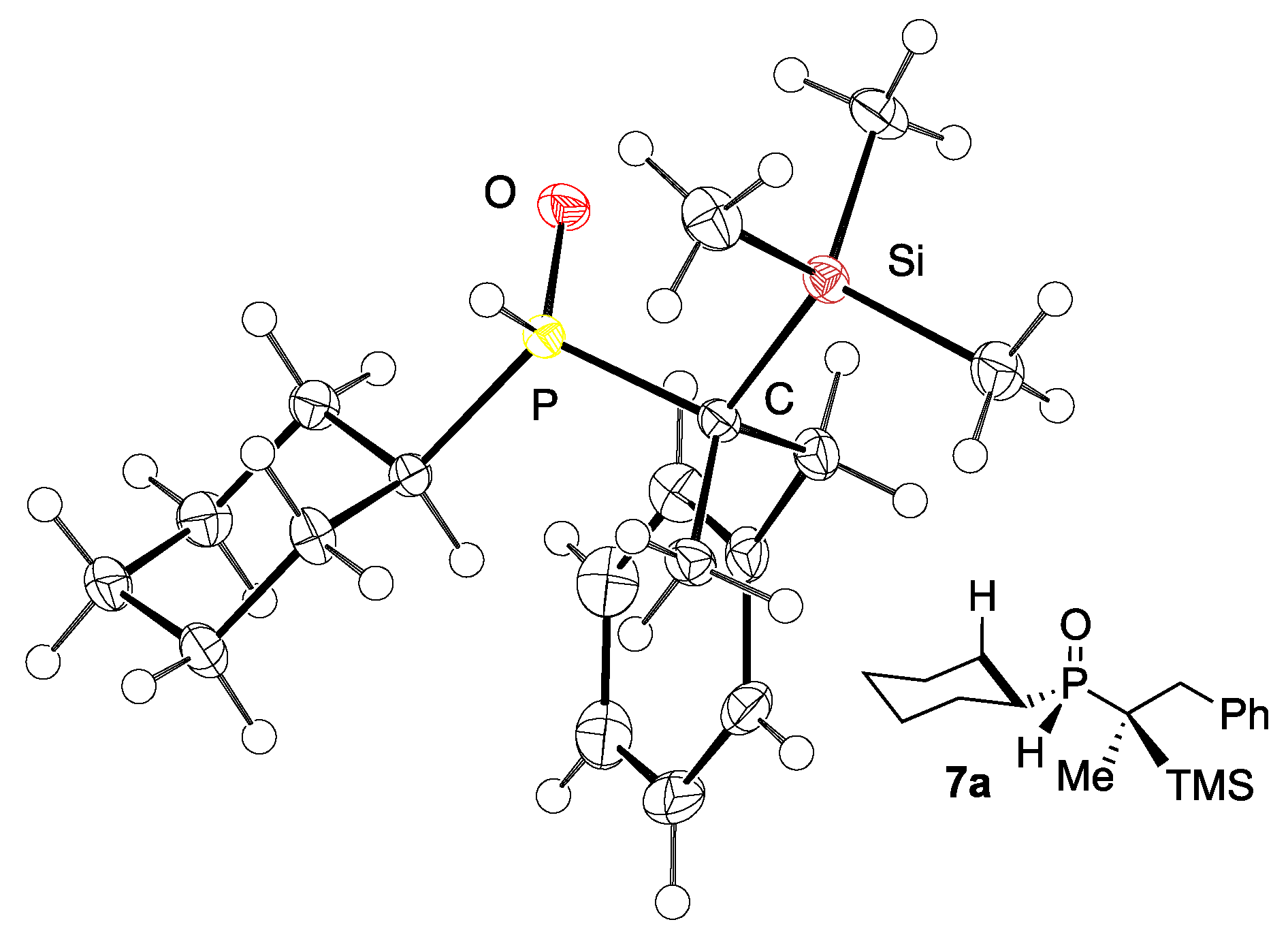
- Crystallographic Data for 7a: C18H31OPSi, Fw: 322.49, T = 100 K, orthorhombic, P212121, a = 6.4316(6), b = 13.8703(14), c = 20.916(2), V = 1865.9(3), Z = 4, Dcalcd = 1.148 g cm−3, 4209 Unique Reflections Out of 43417 with I > 2σ(I), Flack 0.05(6), GOF = 1.031, R1 = 00221, wR2 (All Data) = 0.062. Crystallographic Data Have Been Deposited with the Cambridge Crystallographic Data Centre: Deposition Number CCDC-2101001. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 25 October 2021).
- Deprele, S.; Montchamp, J.L. Triethylborane-Initiated Room Temperature Radical Addition of Hypophosphites to Olefins: Synthesis of Monosubstituted Phosphinic Acids and Esters. J. Org. Chem. 2001, 66, 6745–6755. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Douglass, M.R.; Stern, C.L.; Marks, T.J. Intramolecular Hydrophosphination/Cyclization of Phosphinoalkenes and Phosphinoalkynes Catalyzed by Organolanthanides: Scope, Selectivity, and Mechanism. J. Am. Chem. Soc. 2001, 123, 10221–10238. [Google Scholar] [CrossRef]
- Nielsen, M.; Jacobsen, B.C.; Jørgensen, K.A. Asymmetric Organocatalytic Electrophilic Phosphination. Angew. Chem. Int. Ed. 2011, 50, 3211–3214. [Google Scholar] [CrossRef]
- Zhao, D.; Mao, L.; Wang, L.; Yang, D.; Wang, R. Catalytic asymmetric construction of tetrasubstituted carbon stereocenters by conjugate addition of dialkyl phosphine oxides to β, β-disubstituted α, β-unsaturated carbonyl compounds. Chem. Commun. 2012, 48, 889–891. [Google Scholar] [CrossRef]
- Liu, S.; Tanabe, Y.; Kuriyama, S.; Sakata, K.; Nishibayashi, Y. Ruthenium-Catalyzed Enantioselective Propargylic Phosphinylation of Propargylic Alcohols with Phosphine Oxides. Angew. Chem. Int. Ed. 2021, 60, 11231–11236. [Google Scholar] [CrossRef]
- Gbubele, J.D.; Olszewski, T.K. Asymmetric synthesis of organophosphorus compounds using H–P reagents derived from stereogenic alcohols. Org. Biomol. Chem. 2021, 19, 2823–2846. [Google Scholar] [CrossRef] [PubMed]



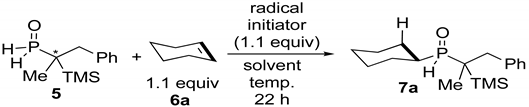 | |||||
|---|---|---|---|---|---|
| Entry | Radical Initiator | Solvent | Temp | Yield [%] [b] | dr [b] |
| 1 | AIBN | MeOH | rt | 0 [c] | |
| 2 | BEt3 | MeOH | rt | 10 | |
| 3 | BEt3 | MeOH | 0 °C | 62 | 91:9 |
| 4 | BEt3 | MeOH | 40 °C | 75 | 91:9 |
| 5 | BEt3 | CH2Cl2 | rt | 12 | |
| 6 | BEt3 | THF | rt | 0 [c] | |
 | |||||
|---|---|---|---|---|---|
| Entry | Alkene 6 | Product | Ratio of Product [%] a | Isolated Yield [%] b | dr c |
| 1 | 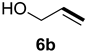 | 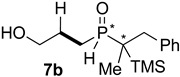 | 52 | 37 | 81:19 |
| 2 | 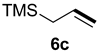 | 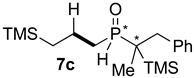 | 87 | 22 | 78:22 |
| 3 | 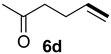 | 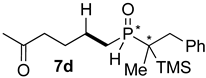 | 56 | 20 | 81:19 |
| 4 | 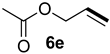 | 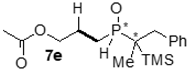 | 16 | 17 | 79:21 |
| 5 | 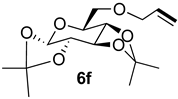 | 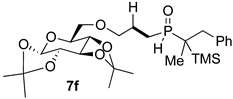 | 100 | 27 | 85:15 |
| 6 | 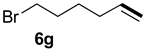 | 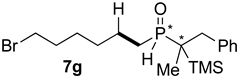 | 68 | 35 | 83:17 |
| 7 | 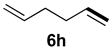 | 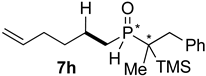 | 35 | 23 | 81:19 |
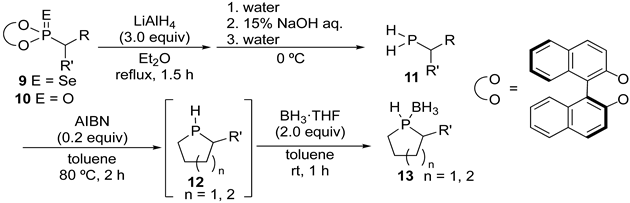 | ||||
|---|---|---|---|---|
| Entry | Substrate 9 or 10 (dr) | Product | Yield [%] a | dr b |
| 1 | 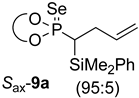 | 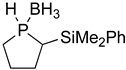 | 87 | 88:12 |
| 2 |  | 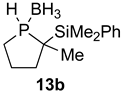 | 65 | 87:13 |
| 3 | 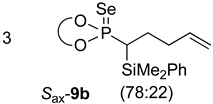 | 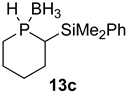 | 78 | 87:13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murai, T.; Wada, R.; Iwata, K.; Maekawa, Y.; Kuwabara, K.; Minoura, M. Primary Phosphines and Phosphine Oxides with a Stereogenic Carbon Center Adjacent to the Phosphorus Atom: Synthesis and Anti-Markovnikov Radical Addition to Alkenes. Organics 2021, 2, 395-403. https://doi.org/10.3390/org2040023
Murai T, Wada R, Iwata K, Maekawa Y, Kuwabara K, Minoura M. Primary Phosphines and Phosphine Oxides with a Stereogenic Carbon Center Adjacent to the Phosphorus Atom: Synthesis and Anti-Markovnikov Radical Addition to Alkenes. Organics. 2021; 2(4):395-403. https://doi.org/10.3390/org2040023
Chicago/Turabian StyleMurai, Toshiaki, Ryota Wada, Kouji Iwata, Yuuki Maekawa, Kazuma Kuwabara, and Mao Minoura. 2021. "Primary Phosphines and Phosphine Oxides with a Stereogenic Carbon Center Adjacent to the Phosphorus Atom: Synthesis and Anti-Markovnikov Radical Addition to Alkenes" Organics 2, no. 4: 395-403. https://doi.org/10.3390/org2040023
APA StyleMurai, T., Wada, R., Iwata, K., Maekawa, Y., Kuwabara, K., & Minoura, M. (2021). Primary Phosphines and Phosphine Oxides with a Stereogenic Carbon Center Adjacent to the Phosphorus Atom: Synthesis and Anti-Markovnikov Radical Addition to Alkenes. Organics, 2(4), 395-403. https://doi.org/10.3390/org2040023






