1. Introduction
A major byproduct of the petroleum refining process is petroleum coke or petcoke. It is the carbon-rich residue left over after all the low and medium boiling hydrocarbon fractions have been distilled from the crude oil. Being carbon-rich, petcoke is used as a raw material in gasifiers for the generation of energy and production of chemicals for industrial use. Gasifiers are usually designed to be capable of processing multiple feedstocks. As a result, very few gasifiers operate with 100% petcoke feedstock [
1]. Many of them employ various blends of fuels, as addition of a highly reactive fuel improves the chemical reactivity of petcoke [
2] as it is a fuel with low reactivity.
In addition to producing fuels and chemicals for industrial use, gasifiers produce ash that melts at high temperatures and especially under reducing conditions to form slag. The slag flows down on the gasifier walls and is removed through a slag tap. The walls of a gasifier are lined with refractory materials (usually alumina or chrome-lined alumina) to help protect the surface from the corrosive slags.
Slag viscosities greatly impact the rate and ease with which they can be tapped out of the gasifier. A viscosity in the range of 5–25 Pa-s is considered ideal for the operation of slagging gasifiers [
3,
4]. Higher viscosities result in solidification of the slag before tapping, thus clogging the tapping valves. Higher viscosities also cause the slag to be in contact with the refractory wall for longer periods and “sticking” to it. Because it is almost always in contact with molten slag, the refractory layer’s integrity is of utmost importance in gasifier operations. Maintenance and repair of the tapping valves and refractory linings are time consuming and lead to long shutdowns and excessive operational costs.
During viscosity measurement experiments in the current study, it was found that some compositions of slag caused very high dissolution of crucible material into the slag, while others did not. The viscosity experiments were conducted in a high-temperature viscometer (see
Section 3) using synthetic petcoke ash contained by high alumina crucible. It was initially assumed that the presence of significant amounts of only vanadium had a role to play in the dissolution. Bennett et al. [
5] stated that vanadium in petcoke ash can severely deteriorate alumina refractories. In their study, 90% (or higher) Cr
2O
3 spent refractories from gasifiers were analyzed using SEM (scanning electron microscopy), EDX (energy-dispersive X-ray spectroscopy) and XRD (X-ray diffraction) to understand failure mechanisms. Thermodynamic modeling was also performed to understand refractory dissolution in slag. Thermodynamic databases for vanadium compounds were limited at the high temperatures and reducing environments of entrained flow gasifiers. So, the focus of their work did not include vanadium compounds. The authors claimed that FeO from slag reacts with Cr
2O
3 lining to form FeCr
2O
4 and with Al
2O
3 to form FeAl
2O
4. Both these reactions lead to loss of refractory material from the wall to the slag at the interface and thus are reported to be the major reasons for refractory wear. However, in the viscosity experiments, two petcoke slag samples with a similar content of vanadium (about 20% by weight) but with different silica and alumina contents showed starkly different results. One composition caused extensive dissolution of the crucible, while the other did not. This observation indicated that vanadium content of a slag may not be the only cause for refractory depletion.
Interactions between high vanadium slags and the crucible materials have rarely been studied. One of the studies similar to the slag-crucible dissolution observed in this study was conducted by Ilyushechkin et al. [
6], who studied two slags with vanadium and nickel oxide contents of up to 20.7% and 2.7%, respectively. The slags were processed in four crucible materials—alumina, molybdenum, platinum, and nickel—and slags’ microstructures were studied by SEM-EDX. According to Bennett et al. [
5], petcoke slag can have an average V
2O
5 and NiO content of 57.0 wt. % and 8.4 wt. %. They reported that both molybdenum and alumina crucibles were suitable for working with slag containing up to 5% vanadium, while only alumina crucibles were suitable for working with slag compositions containing more than 5% vanadium. Even lower concentrations of vanadium were found to dissolve crucibles into the slag. Though the problem of dissolution was observed by the authors, no quantitative method to mitigate the problem was proposed. In a study by French et al. [
7], coals ash slags’ interactions with molybdenum crucibles from viscosity measurements were reported. The researchers determined that molybdenum reacts weakly with coal slags containing low iron levels (less than 10 wt. %), whereas it forms compounds in presence of higher levels of iron, while also generating particles of molybdenum within the molten slag. Vanadium and nickel are absent in coal ash slags [
5].
Jonayat et al. [
8] studied the interactions between Fe
2O
3, Cr
2O
3 and V
2O
3 by using density functional theory—oxides with similar crystal structure as Al
2O
3 (Corundum) under conditions similar to those found inside gasifiers and proposed a mechanism for these interactions. According to these authors, the surface of the major oxide acts as a base on which the minority oxide could be considered to be doped. The stability of such mixed oxides is determined by the surface energies of the oxides involved. Their observations suggest that surface energies in such oxide mixtures are determined by the affinity of these oxides towards oxygen. Under reducing conditions, the metal with higher tendency to reduce (lower bond energy) would segregate to the surface on the crystal, as it is more reducible.
In all the above studies, interaction of petcoke slag, containing typical high amount of vanadium and nickel, with alumina crucible/refractory were not investigated. Researchers need to determine viscosity of petcoke ash slag and alumina is a standard crucible material for high temperature slag viscosity experiments. Therefore, this study is an attempt in which vanadium and nickel amounts would be varied systematically in slag and their interactions with high alumina crucibles would be identified by performing high-temperature experiments and postmortem analysis. The systematic variation would be based on the average petcoke ash compositions reported by Bennett et al. [
5].
3. Experimental Setup and Sample Preparation
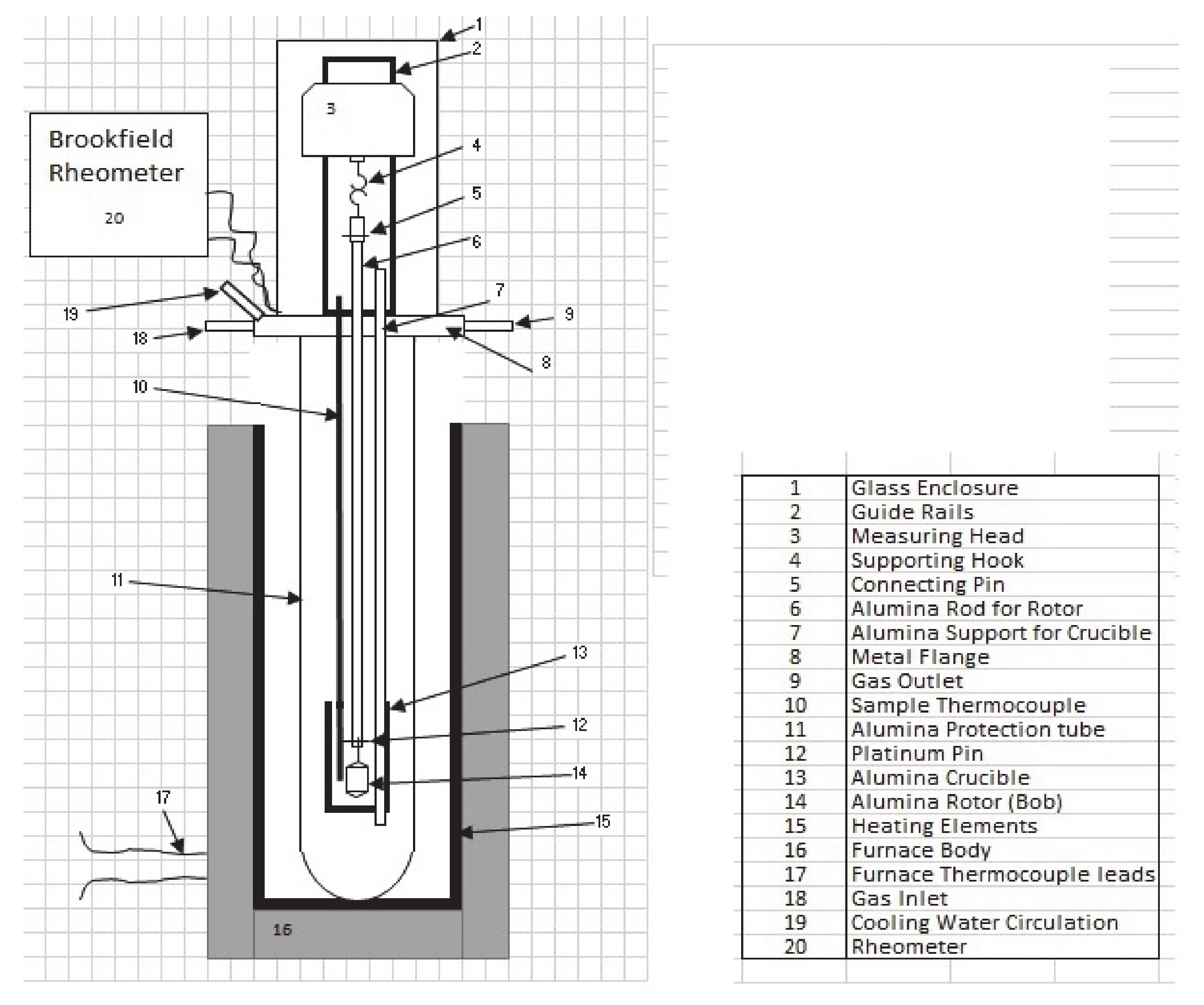
Viscosity measurements of synthetic petcoke slags were carried out with a high-temperature viscometer (Theta Industries, New Castle, DE, USA), capable of heating to 1700 °C using Molybdenum Silicide heating elements. The equipment consisted of a furnace, an alumina protection tube and a glass enclosure for atmosphere control during the experiments, an enclosure for the protection tube, a movable measuring head, and a rheometer. Three alumina rods fixed to the top of the metal flange supported the crucible during the experiment. Another alumina rod, connected to the rotor at one end with a platinum pin and the measuring head at the other, was used for measuring the shear stress, shear rate, and viscosity. Deflection of the spring in the measuring head indicated the viscosity of the fluid being tested. The metal flange has two sets of perforations, one for letting in the gases into and out of the protection tube and another one for cooling water that prevents the outside of the furnace from overheating.
Type B thermocouples, located on the body and inside the furnace, read the furnace and the approximate sample temperatures, respectively.
Figure 1 shows the schematic of the viscometer in detail.
The starting compositions of the synthetic slags were selected from the typical petcoke compositions reported by Conn et al. [
9], Bryers et al. [
10], Vassilev et al. [
11], and summarized by Bennett et al. [
5].
Table 1 lists the compositions used for this study. The oxides were varied systematically, so oxides in some of the tests do not add to 100% (see Results and Discussion).
FactSage (version 7.1), a thermodynamic equilibrium calculation software that uses Gibbs’ free energy minimization to predict the formation of compounds at equilibrium, was used for modeling the conditions and phases expected during the experiments. Initially, four slag compositions were tested under CO/CO2 (69.5/30.5), 100% H2, 100% O2 and 5% H2 (95% N2) atmospheres. The only variation in the four slag samples was that in their alumina and vanadium contents, while all other compositions were maintained in the same ratio as that in the slag sample that caused extensive dissolution of the crucible. While one composition was similar to the slag sample, the other had excess alumina in its composition. The third composition had no alumina and the fourth had no alumina as well as no vanadium in it. Equilibrium alumina content in the slag was determined for each composition and formed the basis for classification of slags as either deficit or rich in alumina, depending on whether they had lower or higher alumina content respectively than that predicted to be in the slag at equilibrium.
To experimentally confirm the findings of the simulations, the oxides in Tests 1–6 (see
Table 1) were heated under an oxidizing atmosphere in a Carbolite box-type furnace at 2 °C/min to 1500 °C, held at that temperature for 1 min, and cooled at 1.5 °C/min to ensure that all the elements were in their highest oxidation states. After cooling down, the mixture of oxides was separated from the crucible material by carefully breaking the crucible to avoid inclusion of crucible into the samples. The mixture was then ground to a powder, heated to 1500 °C at 2 °C/min, held at that temperature for 6 h to simulate the time taken for recording viscosities during the viscosity measurement experiments, and allowed to cool down at 1.5 °C/min.
SEM-EDS and XRD techniques were used for characterizing the samples and determining the impact of change in alumina content on crucible dissolution. A comparison with the FactSage simulation data generated by varying the alumina content in the slag would help in theoretical understanding of the effect of slag’s composition on the phenomenon of dissolution.
4. Results and Discussion
During viscosity measurement experiments with Test 1 (with 64.39% V content) and Test 4 (with 20.48% V content) samples, the crucible wall material was found to have dissolved into the slag, leaving perforations (holes) through which the slag escaped out. There was no apparent loss of wall thickness during viscosity measurement of the calibration test though its vanadium content was 20.69%, which was very close to the vanadium content in Test 4 (20.48%). This suggested that the loss of wall thickness could not be attributed to the high content of vanadium alone and was probably caused by other interactions between the slag and crucible.
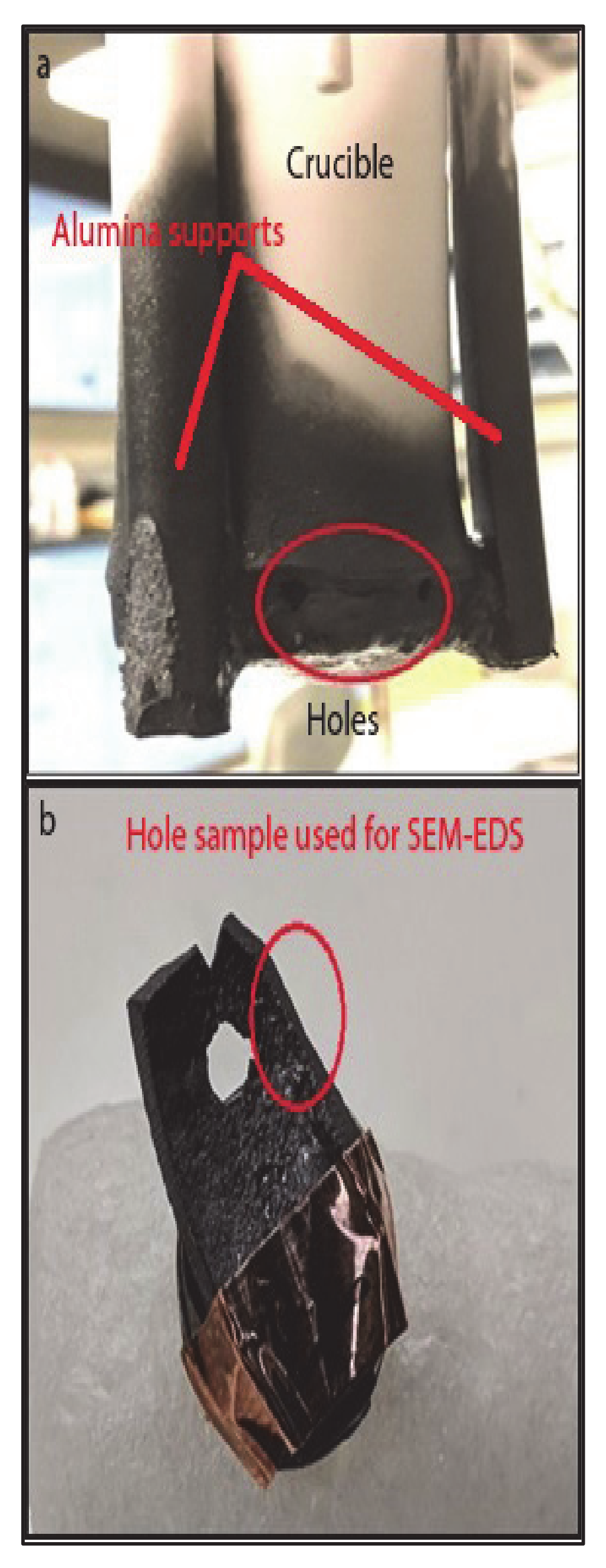
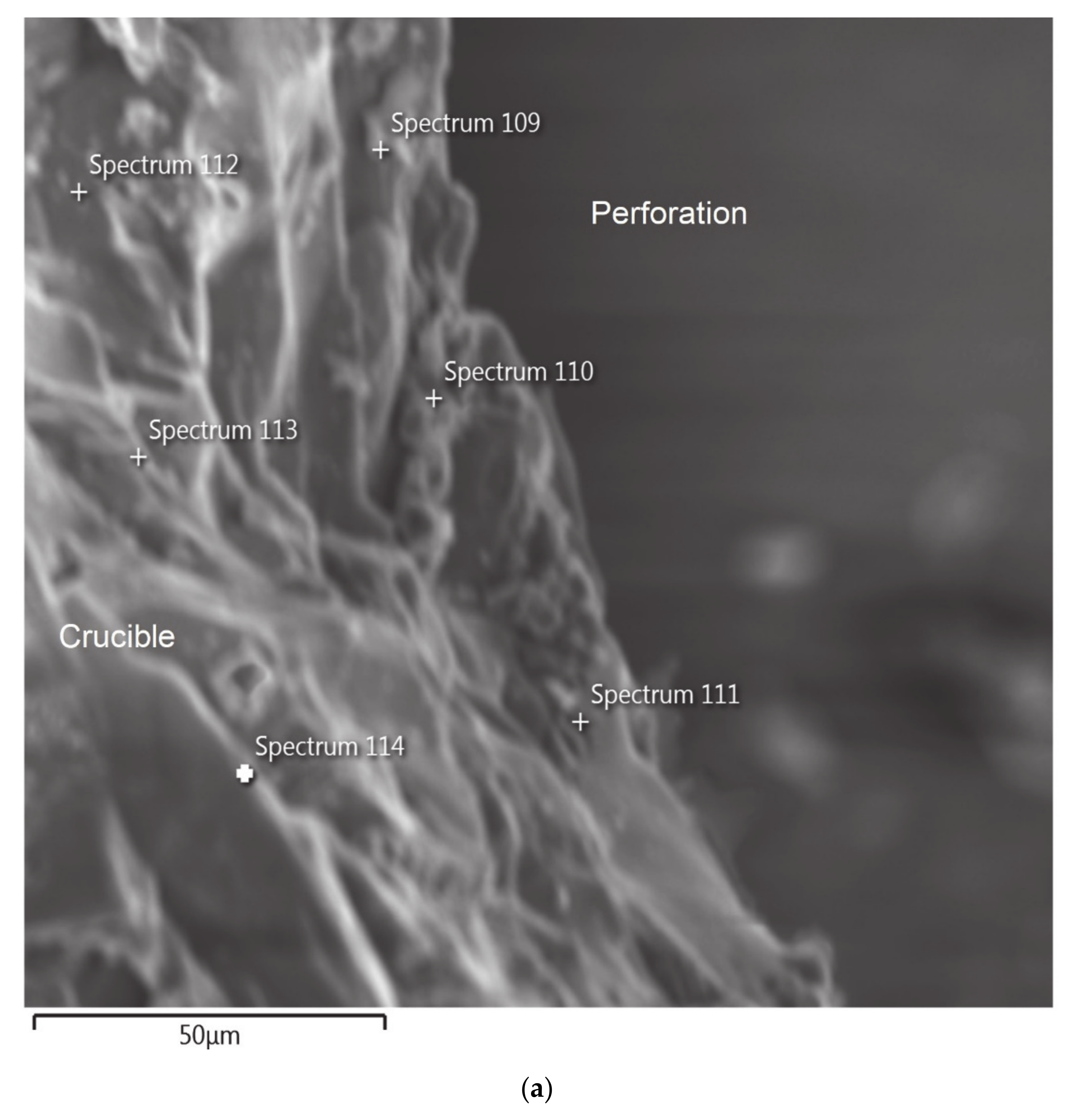
Liquid slag falling out of the crucible indicated that the perforations as shown in
Figure 2a,b were formed probably when the slag was fluid enough, at the lower end of the 1500–1425 °C temperature range, as viscosity measurement could not be continued below 1425 °C due to non-availability of slag inside the crucible.
Figure 2a shows photograph of the crucible after the completion of the viscosity measurement experiment (Test 1). The Figure shows circumferential warping and perforations near the bottom of the crucible.
Figure 2b shows one of the perforations as well as thinning of the crucible wall.
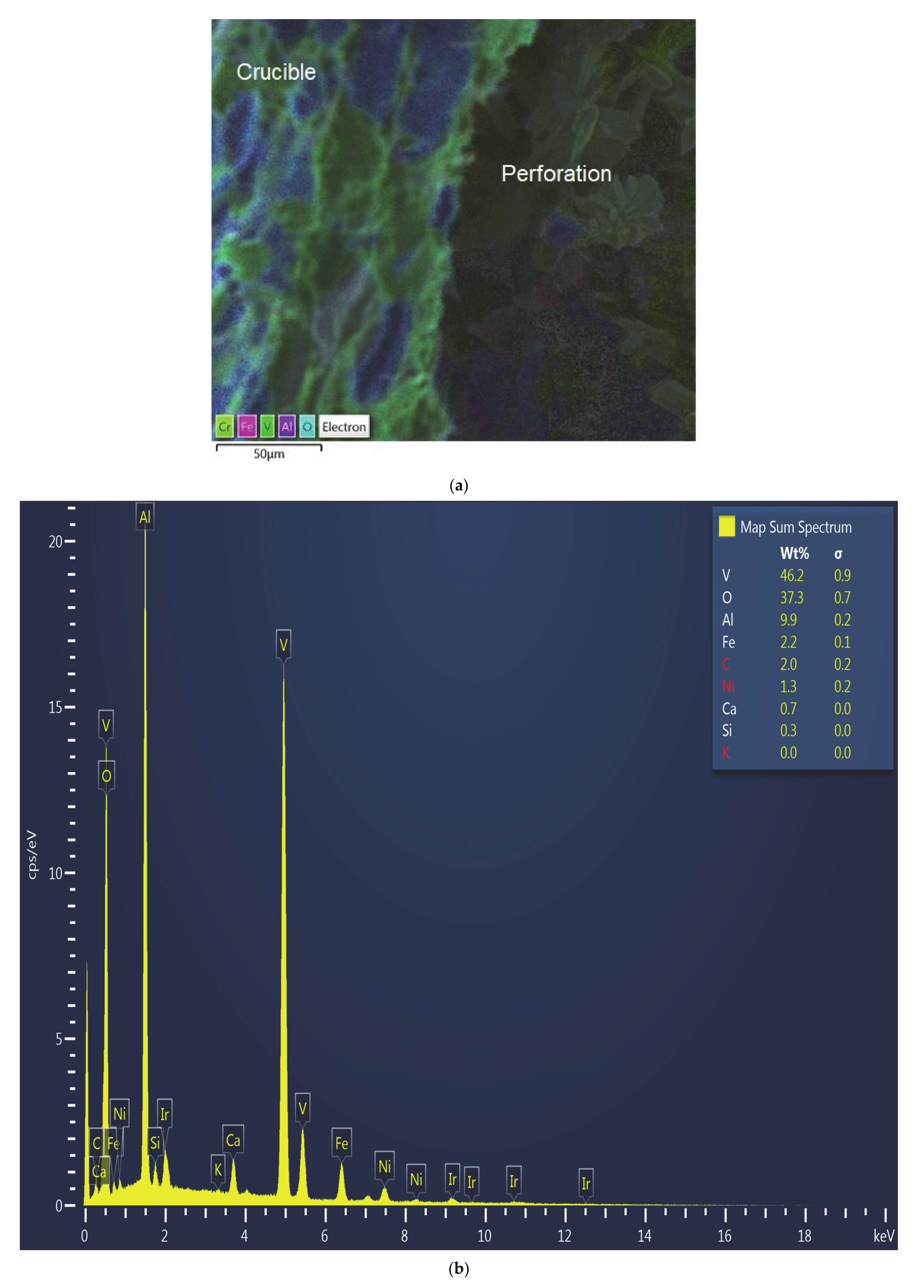
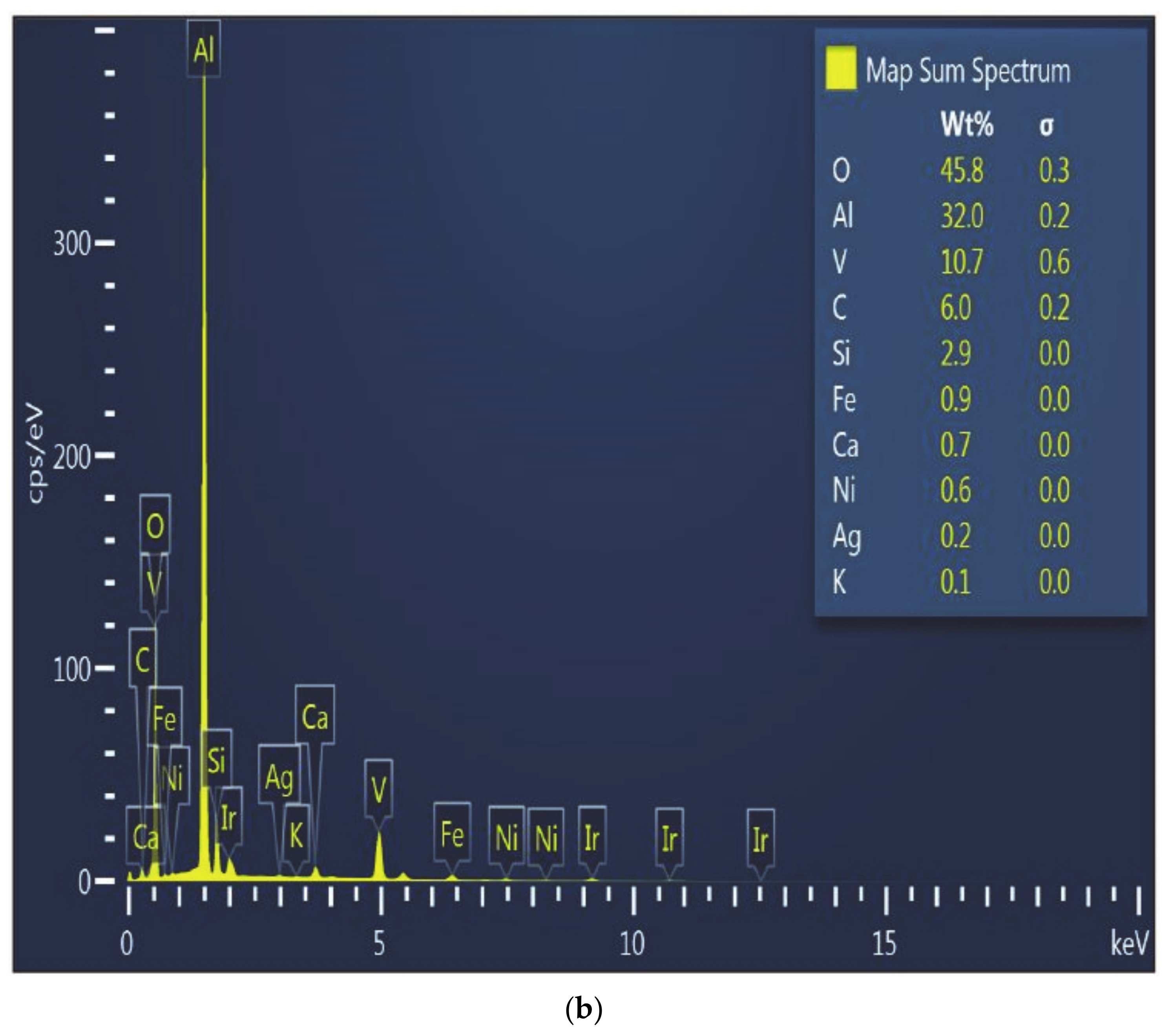
The SEM micrograph and the EDS pattern of the wall around the perforations in the crucible walls used in Test 1 (
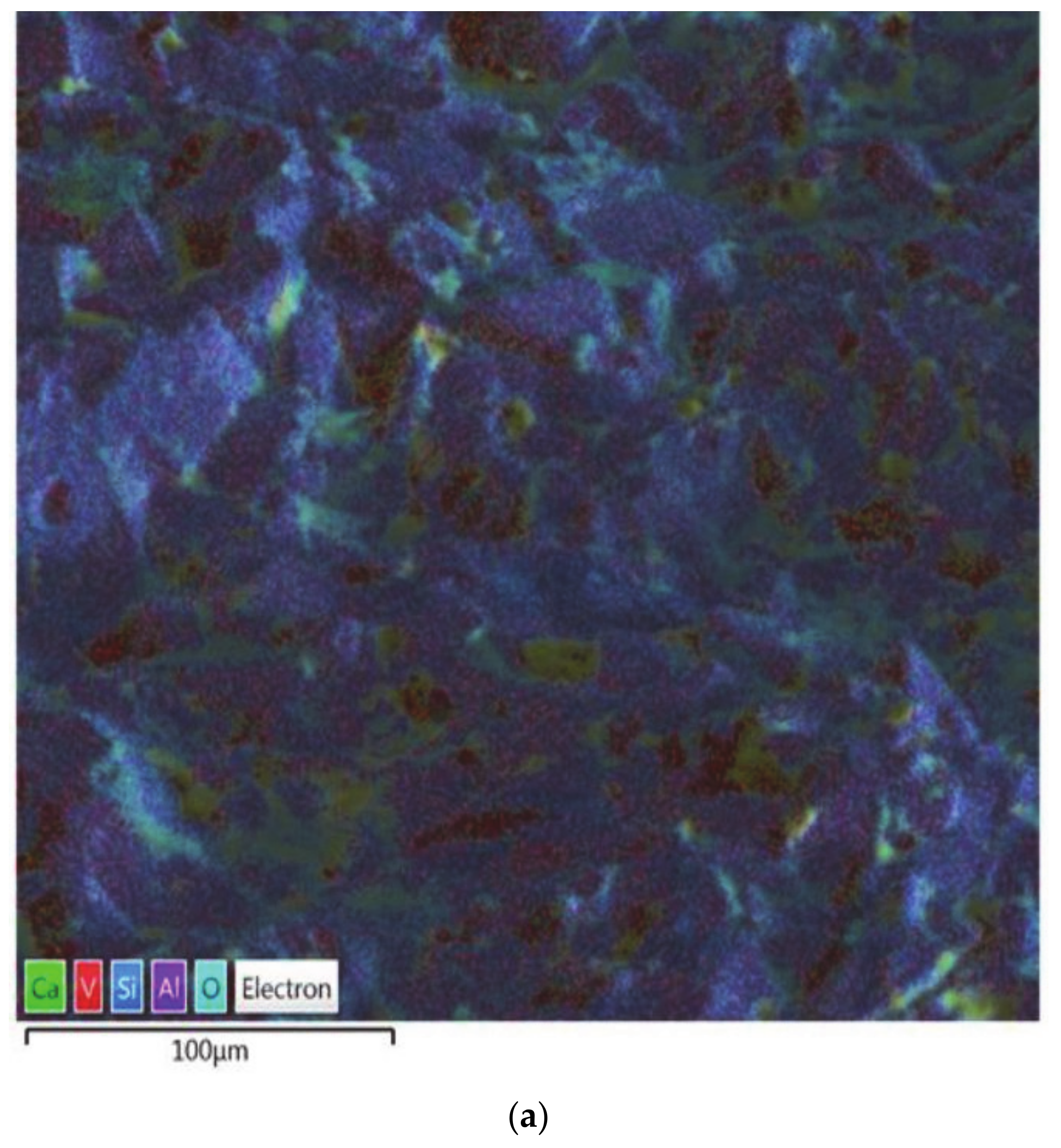
Figure 3) showed significant accumulation of vanadium (46.2%), while also showed a lower content of aluminum (9.9%), compared to 32% at the bottom of the crucible, which is affected to a lesser extent (
Figure 4). The bottom of the crucible was not found to have been affected during the experiment. As expected, alumina was the major constituent in the unaffected region of the crucible. Vanadium was identified to be 10.7% by weight at this location, which is a marked difference from the site that developed the perforation (~46%). All other oxide constituents were seen in roughly the same proportions as in the starting mixture.
Figure 4 also shows the presence of silicon and iron in minute quantities. This is due to the fact that silica has a low density (2.65 g/cm
3) whereas other oxides are denser (V
2O
3-4.87 g/cm
3, NiO-6.67 g/cm
3, Al
2O
3-3.95 g/cm
3 and CaO-3.34 g/cm
3). Since the holes were found at the bottom of the crucibles, it is believed that the heavier oxides escaped first, and silica was in contact with the crucible long enough to solidify at the interface before completely flowing out of the perforation (hole).
Figure 5 shows the SEM micrograph and EDS spectrum at a point close to the perforation found during Test 1. Accumulation of vanadium close to the perforation, accompanied by loss of aluminum can be clearly seen from the EDS pattern. In fact, the spectrum barely shows any other elements, apart from vanadium.
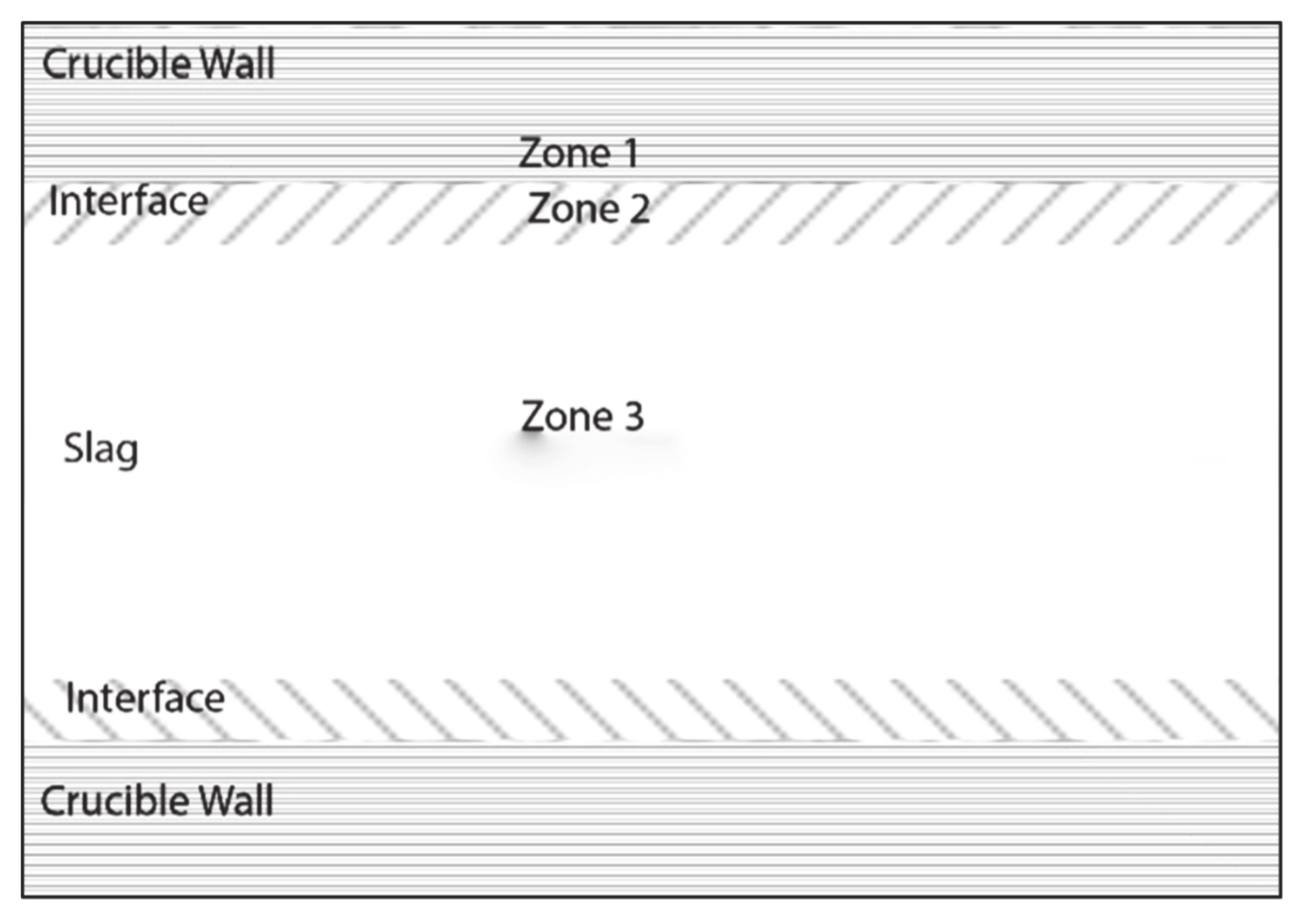
Table 2 shows the compositions obtained from the EDS spectra analysis for tests 1-6 carried out for this study. The different zones where measurements were carried out are shown graphically in
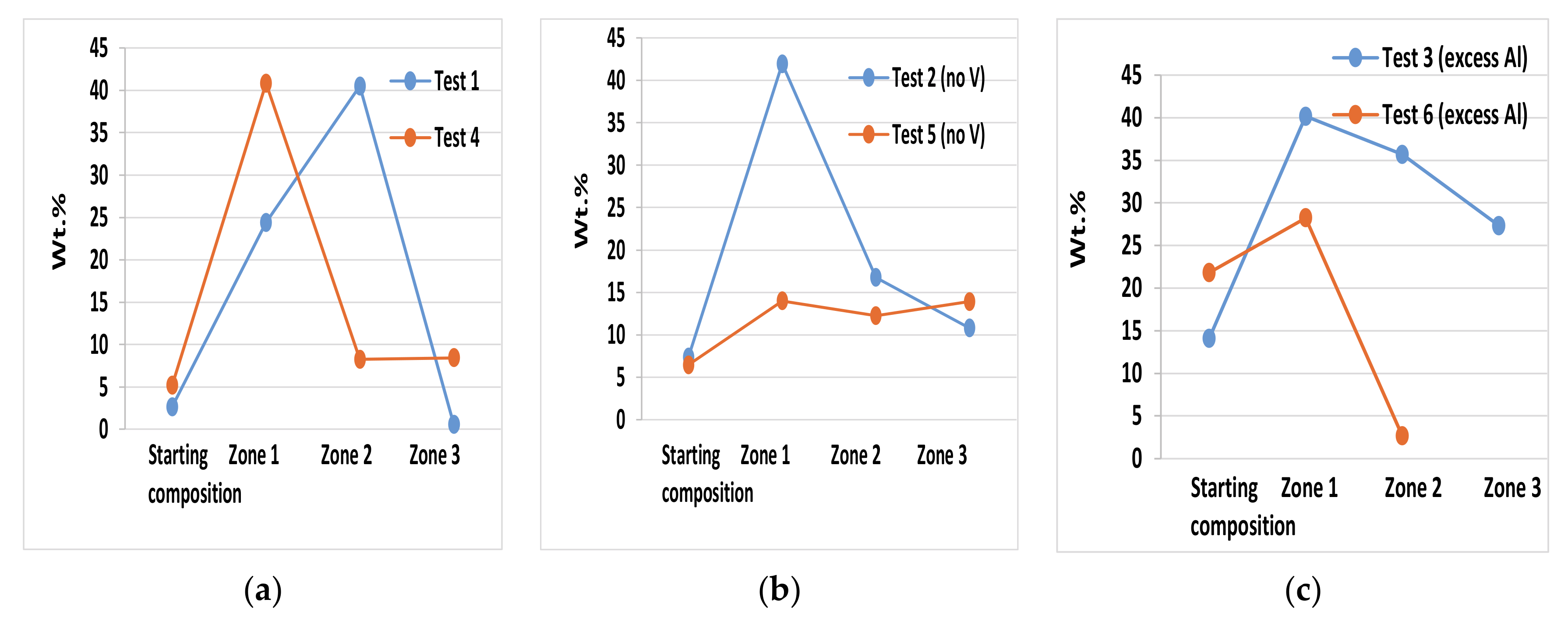
Figure 6. Al concentrations in the different zones and initial slag composition are shown in
Figure 7.
Zone 1 is the area close to the interface of crucible and slag, on the crucible side. Zone 2 is the area close to the interface, on the slag side of the interface, while Zone 3 is close to the center of the crucible, where the boundary interactions have almost no effect on the composition. If the crucible is affected by the slag, the aluminum composition in Zone 1 would reduce, while the content of vanadium would increase. When the crucible is not attacked by the slag, the compositions in Zone 2 and Zone 3 should be similar to the initial compositions of the respective test samples.
Figure 7a–c show that in tests with no vanadium in the starting samples (Test 2 and Test 5), the Al compositions in Zones 2 and 3 increased with respect to the initial slag composition. So, in absence of vanadium, dissolution of alumina into slag is observed. In Test 1, the Al concentration in Zone 3 is much less than the initial slag composition, which implies that some of the alumina could have migrated towards interface (Zone 2). However, in case of test 4, the aluminum concentration is higher in zone 3 than the initial composition. In tests 3 and 6, wt. % of vanadium in zone 1 (6.19% and 0%, respectively) are much lower than the initial slag compositions (49.74% and 7.50%, respectively). Vanadium might not have attacked the crucible wall due to the excess alumina. Measurements could not be done for Zone 3 in case of test 6. In
Table 2, corresponding elemental composition of the initial synthetic mixture has been reported in the column for initial slag composition. Some of the weight percentages do not add to 100% as the SEM-EDS data had carbon and other elements that were not present in the initial slag composition and the crucible material. So, those elements were discarded in the analysis. The only change between Test 1 and Test 3 was the relative composition of alumina in the starting slag (4.96% vs. 26.61%). Similarly, the only difference between Test 4 and Test 6 was the alumina content (9.78% vs. 41.17%). Addition of alumina in both the cases led to reduction of vanadium content in zone 1.
In
Table 2, tests 1 and 4 (which led to formation of holes in the crucible) show significant presence of vanadium and nickel and other elements in Zone 1 (crucible). Zone 1 should ideally have only alumina. These results also show significant amount of aluminum (greater wt. % than initial slag composition) in Zone 2. This aluminum can come from the crucible material due to elements present in the corrosive slag.
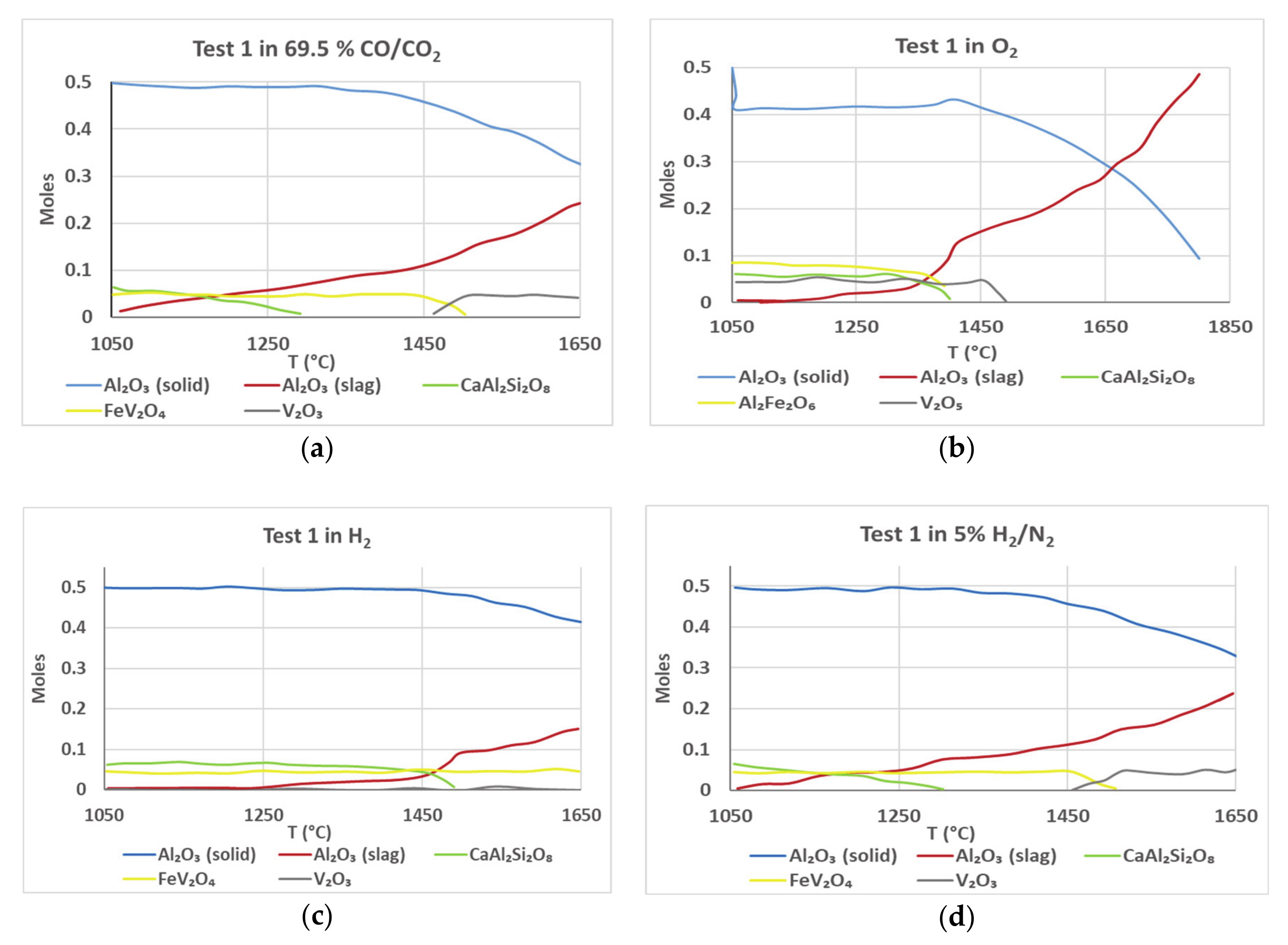
Figure 8 shows the FactSage simulation plots for Test 1 sample for various gaseous atmospheres. The plots show the composition in moles (on
y-axis) as a function of temperature (
x-axis). To simulate the conditions that are encountered during viscosity measurement, two different phases of alumina—gamma and corundum—were included in the input to FactSage. This was done solely based on their different melting points and stability at 1500 °C. Gamma alumina is used to represent the powdered alumina in the slag and corundum is used to represent the alumina from the crucible. Due to this setup, a comparison of the levels and rates of changes between these two components would be indicative of the dissolution of powdered alumina and the crucible into the molten slag at different temperatures. The blue plots indicate the content of alumina in the crucible/refractory as a function of temperature, while the red plots show the content of alumina predicted to be present in the slag at equilibrium. As the crucible/refractory dissolves, the equilibrium moles of alumina in the crucible wall would drop, while the number of moles of alumina in the slag rise.
The simulation shows that dissolution of crucible is the maximum under oxidizing conditions (pure O
2) and minimum in a pure H
2 atmosphere. At 1500 °C—the highest temperature achieved during the viscosity measurement—FactSage predicts the presence of about 0.17 moles of Al
2O
3 in the liquid slag (
Figure 8b). However, the initial slag composition consisted of only 0.04 moles of Al
2O
3. Thus, there is a deficit of about 0.13 moles of Al
2O
3 in the liquid slag. Similarly, analysis under CO/CO
2 and pure H
2 atmospheres shows a deficit of 0.1 moles and 0.05 moles, respectively. This deficit could be one of the reasons for the dissolution of alumina into slag, as the system tries to reach thermodynamic equilibrium.
The plots of V2O3 and FeV2O4 concentration are symmetric, suggesting that the drop in concentration of one is directly related to the rise in the concentration of the other. Vanadium is predicted to exist as FeV2O4 at temperatures below 1450 °C in all conditions. At higher temperatures, depending on the atmosphere, it dissociates into V2O3, simultaneously causing a sharp drop in the alumina content of the crucible. This leads credence to the observation that the thermal stability of FeV2O4 is related to the stability of the alumina crucible, and that V2O3 is the component that reacts with alumina.
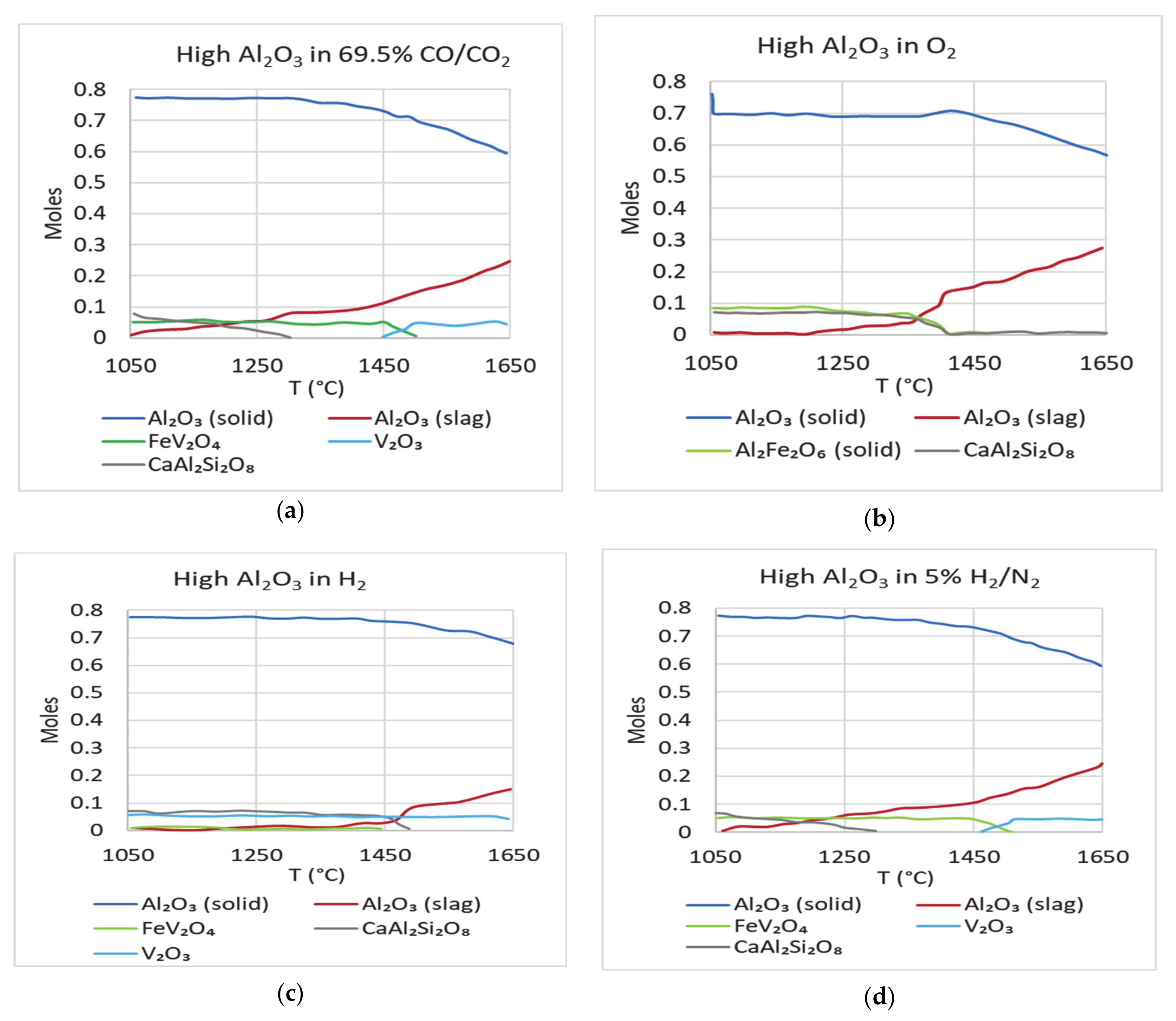
Figure 9 shows the plots obtained for Test 1 sample with additional alumina added (0.3 moles against 0.04 moles), while keeping all other components at the same levels. The amount of additional alumina was decided based on the deficit observed in the previous simulations (
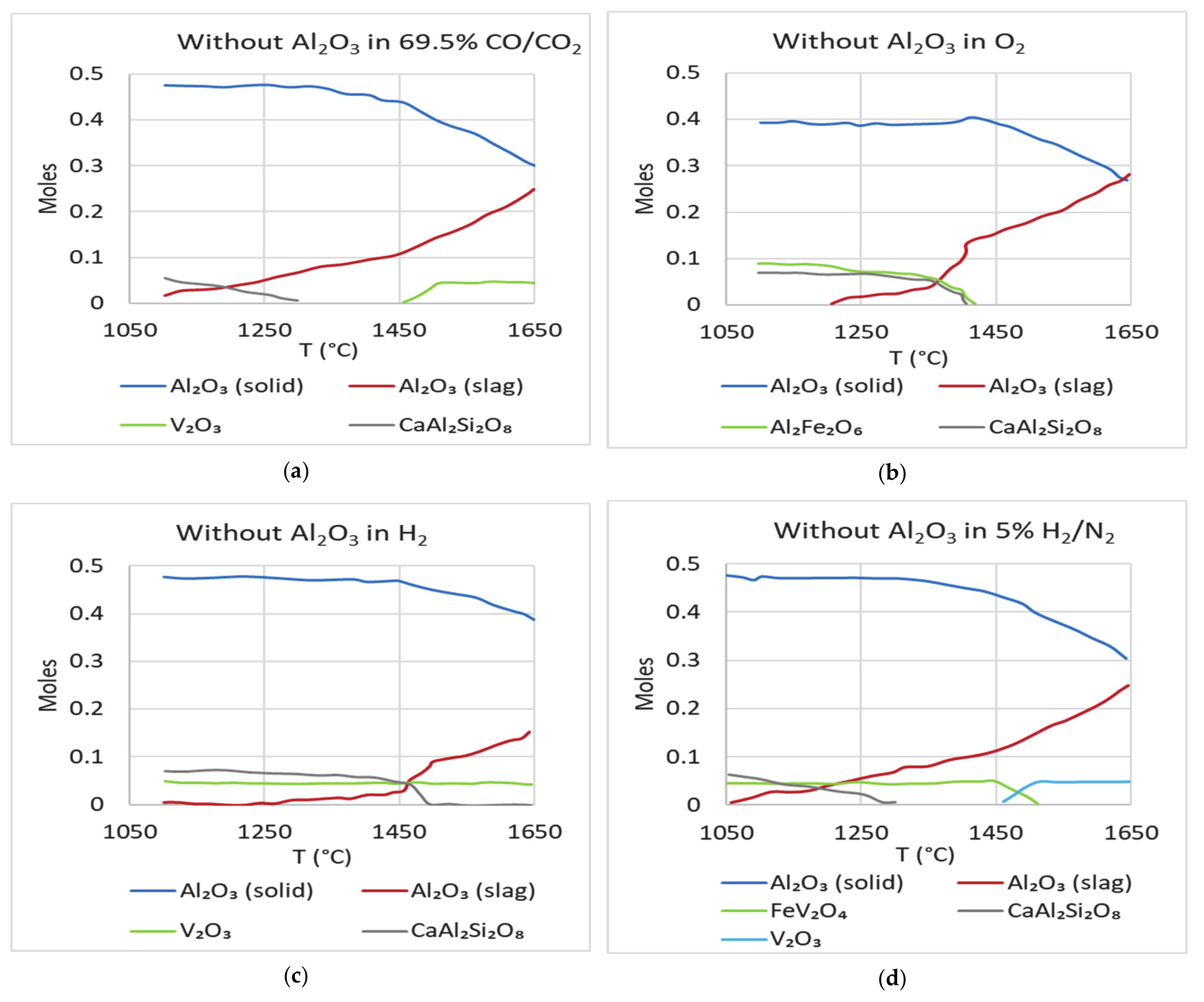
Figure 8). The plots show that addition of alumina did not change the equilibrium content of alumina in the slag. It did, however, lead to lesser dissolution of alumina from the crucible/refractory. The loss of alumina, which was approximately 40% earlier, dropped to about 25%. To study how the absence of alumina in the starting mixture affects the equilibrium prediction, a simulation for Test 1 mixture without alumina was carried out. The equilibrium plots (
Figure 10a–d) are the same as in the previous case, indicating that alumina in this case would definitely be coming from the refractory, as the system tries to reach equilibrium.
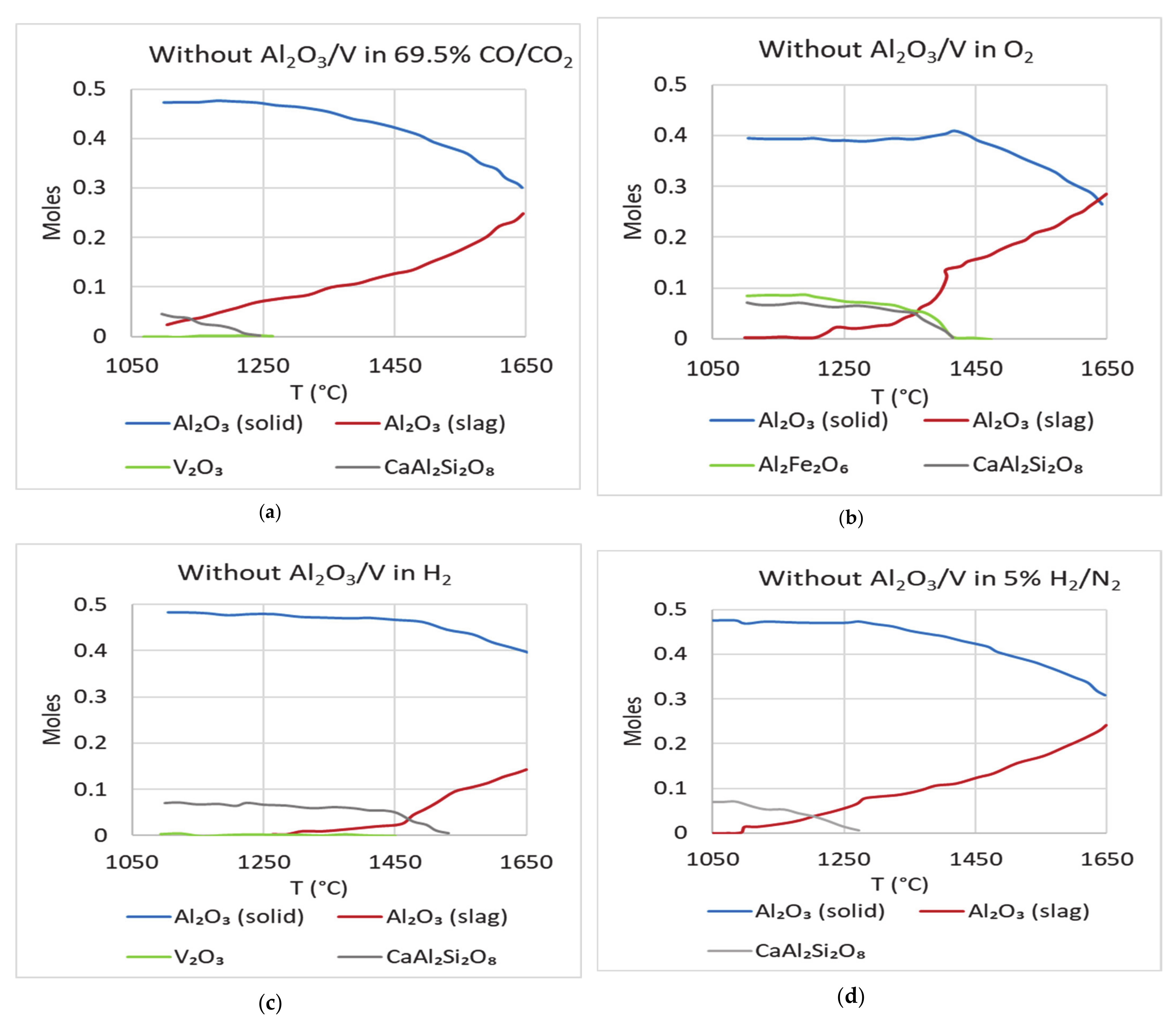
Figure 11 shows the simulations of the synthetic petcoke slag with both alumina and vanadium absent. The dissolution of alumina into the slag is predicted to be like other cases and is not affected in the absence of both these components. The dissolution of alumina from the crucible starts when the slag starts melting and continues until the system reaches equilibrium. This shows that when there is no alumina in the slag melted in alumina crucibles, it picks up alumina from the crucibles to reach thermodynamic equilibrium. The quantity of alumina picked up is dependent on the prevailing atmosphere and the slag temperature.
From the 16 simulations carried out (four compositions in four atmospheres each shown in
Figure 8,
Figure 9,
Figure 10 and
Figure 11), it is clear that FactSage predicts lower dissolution of Al
2O
3 into the slag when processed under 100% H
2 atmosphere than when processed under an atmosphere of CO/CO
2, which in turn shows less dissolution when compared to slags processed in O
2 atmosphere. Though H
2 provides the best atmosphere for preventing refractory dissolution, it also carries significant risk of explosion owing to the flammability of H
2.
The plots also show that under a given atmosphere and at a given temperature, the quantity of Al2O3 present in slag form at equilibrium is a constant. It is also clear that the prediction of formation of various compounds does not change when the CO/CO2 atmosphere is replaced by 5% H2 atmosphere. Dissolution is found to be of the same order in both the cases. Thus, 5% H2/N2 gas mix may be used as a substitute for CO/CO2 mix for carrying out experimental validation of FactSage results.
FactSage simulations show that the dissociation of anorthite and dissolution of alumina from the crucible start simultaneously. However, most of the alumina picked up by the slag initially comes from the dissociation of anorthite. Dissolution of alumina from crucible increases after complete dissociation of anorthite. This might indicate the slag’s preference towards the different phases of alumina, especially alumina that is freely available (in powder form as part of slag). This preference strongly supports the assertion that slag starts dissolving alumina from the crucible when there is not enough of it in the initial mix.
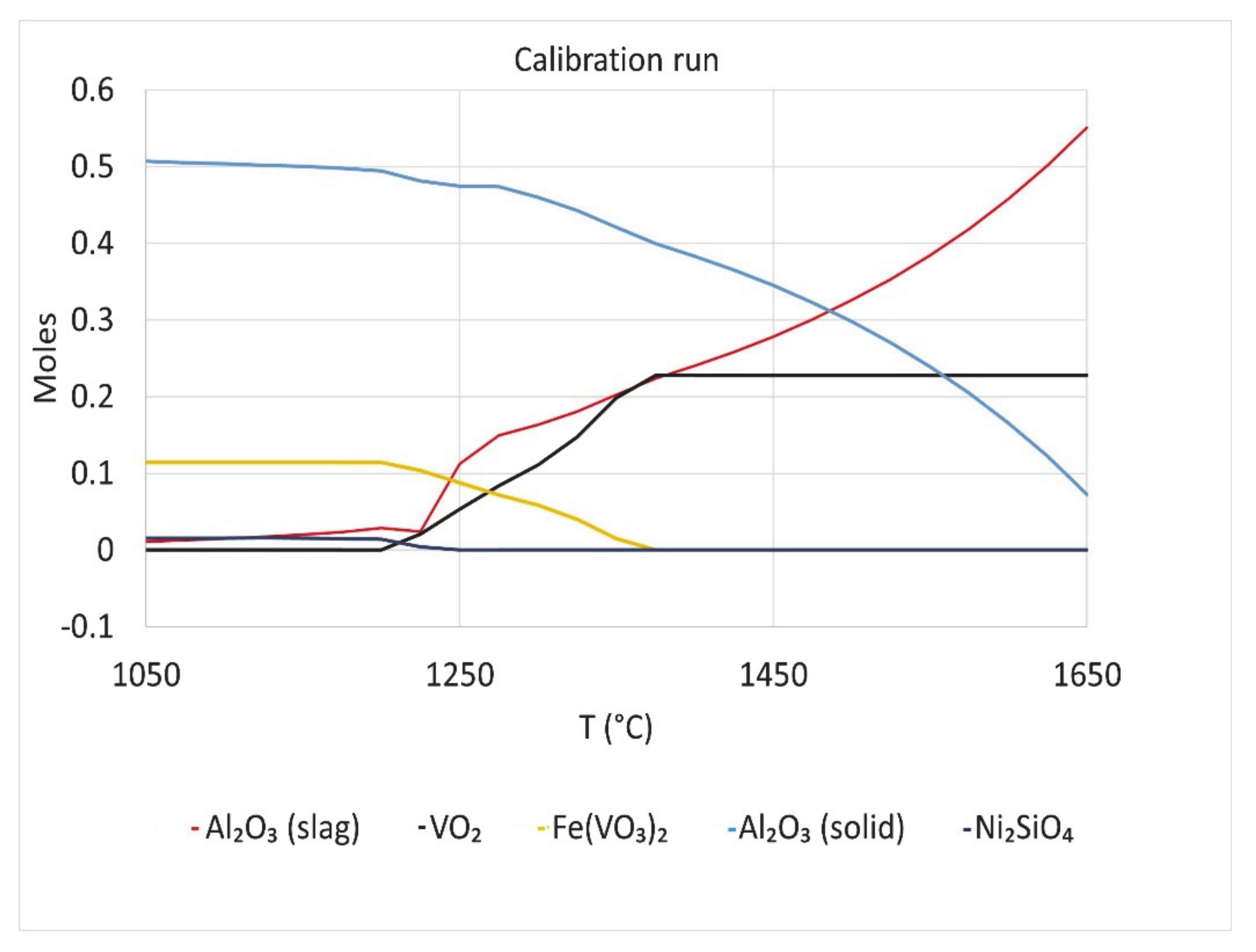
FactSage calculations were done with the composition used in the Calibration run. The results are shown in
Figure 12. The additional compounds in the P2 sample of the work of Duchesne [
12] were included in the simulations done in N
2 atmosphere. The two curves of moles of Al
2O
3 indicate dissolution of Al
2O
3 from solid to liquid slag phase as temperature increases. This was seen in the bulk composition of P2 slag before and after viscosity test done in alumina crucible in the work of Duchesne. Al
2O
3 content increased by about 6% in the slag after the viscosity test [
12]. Ni
2SiO
4 is present in the thermodynamic calculation results. Ni
2SiO
4 was found by Li et al. in petcoke ash slag obtained under oxidizing conditions [
13,
14].
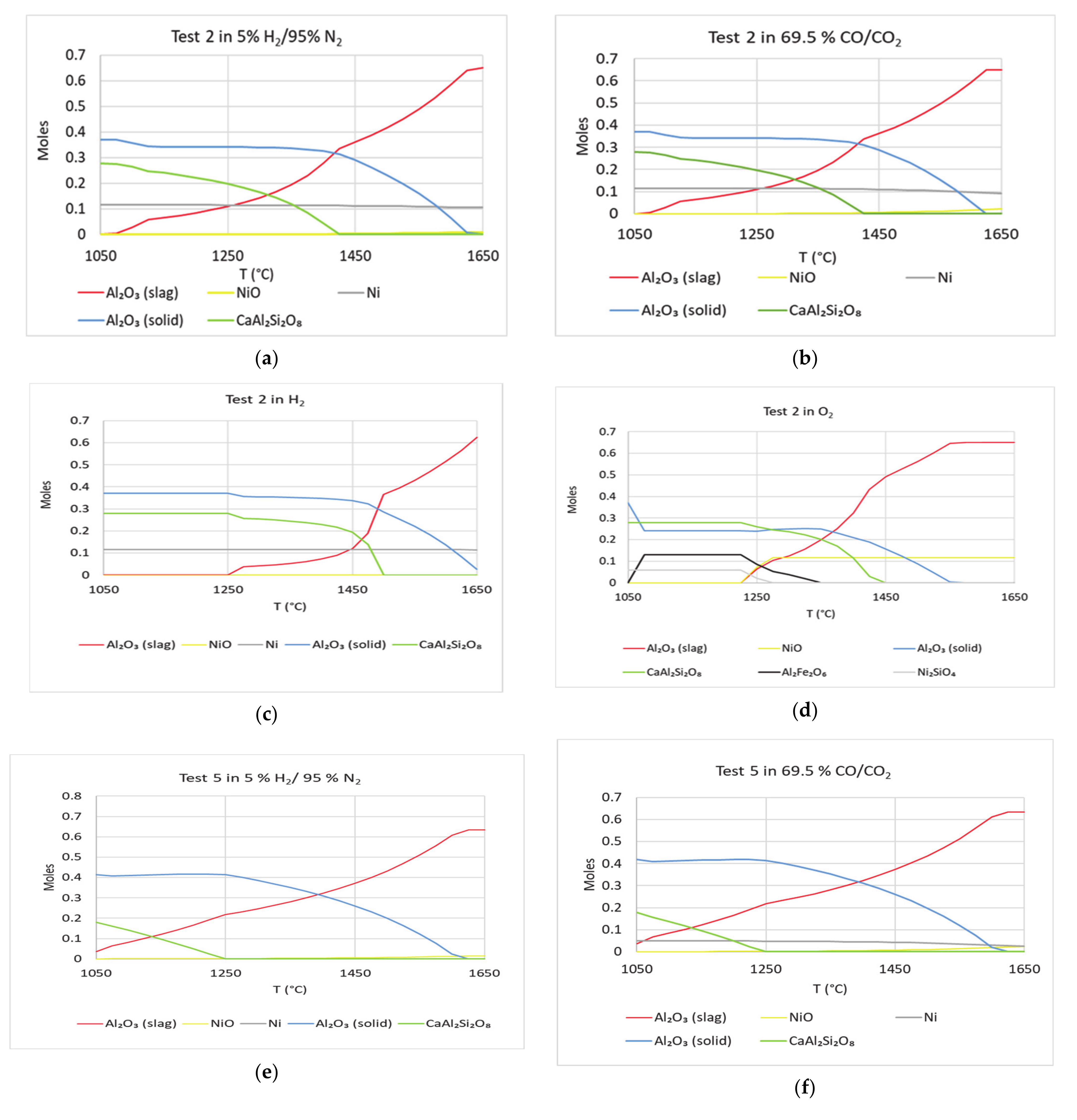
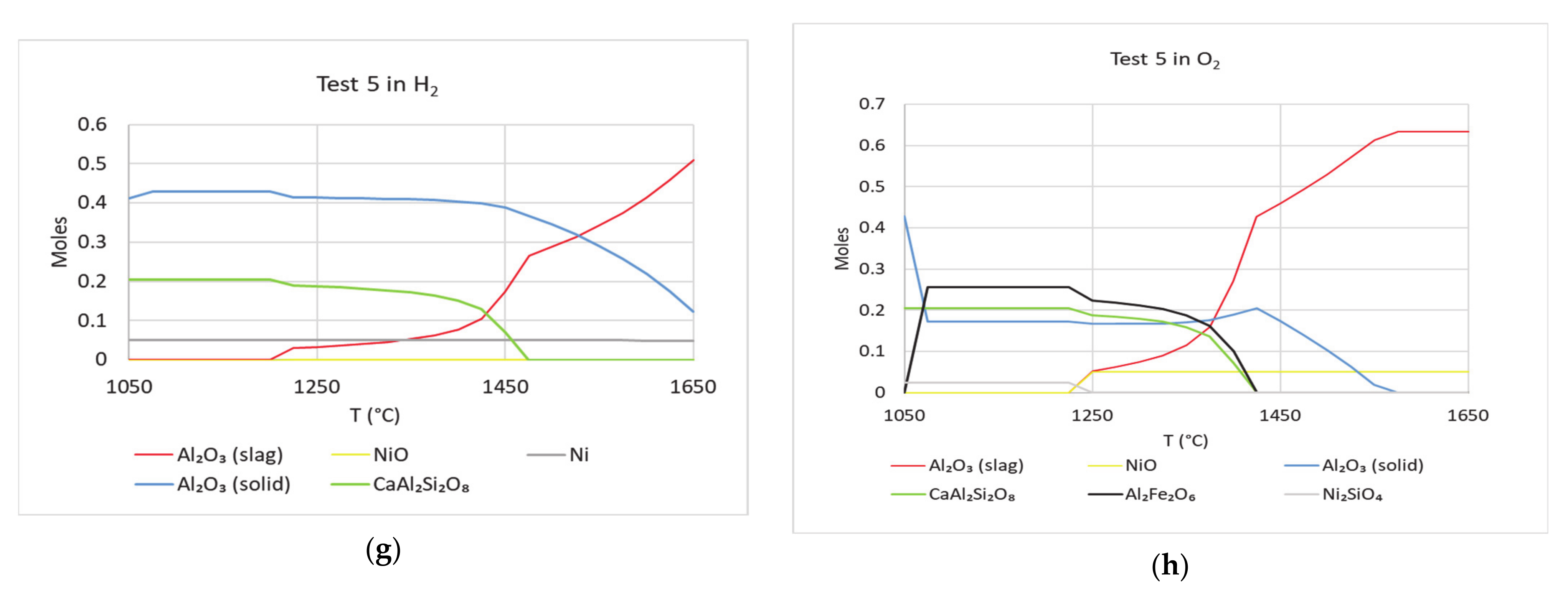
In
Figure 13, FactSage calculations with compositions from test 2 and 5 are shown under four different atmospheres. These calculations were performed to study the effect of Ni in dissolving Al
2O
3 crucible. Under oxidizing conditions, in tests 2 and 5, Ni
2SiO
4 is found in the FactSage calculations. This parallels the stated findings of Li et al. [
13,
14]. In comparison with
Figure 8, the plots in
Figure 13 show that drop in Al
2O
3 (solid), occurs at lower temperatures. This is valid for all the cases shown in
Figure 13. Thus, dissolution of Al
2O
3 crucible can be dependent on the presence of Ni compounds. This parallels the results of
Table 2—Al concentrations in Zone 3 of tests 2 and 5 are lower than their corresponding concentrations in the initial slag compositions. In addition, Ni was found in Zones 1 and 2 in significant amounts in tests 2 and 5. Under oxidizing conditions, similar to
Figure 8, rate of drop in Al
2O
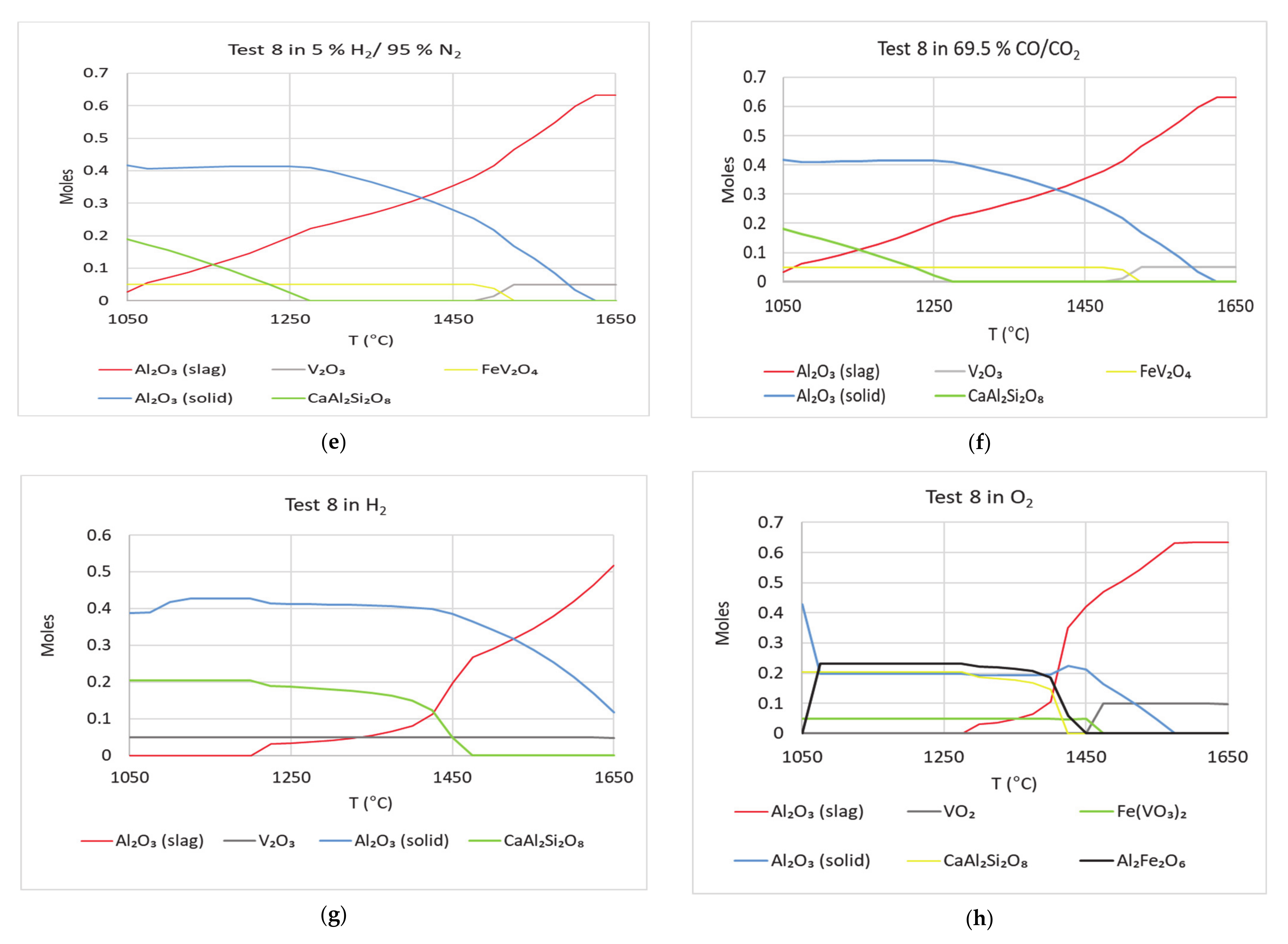
3 (solid) is highest. Compositions in tests 7 and 8 were derived from the compositions in test 2 and 5, respectively. Except NiO, the rest of the oxides were kept the same—amounts of NiO and V
2O
5 were interchanged. Tests 7 and 8 were not part of the experiment test matrix. These were added to determine the effect of vanadium in alumina dissolutions from the crucible using simulations and compare with literature. FactSage simulations were run for tests 7 and 8. Results of the calculations are shown in
Figure 14. The curves of Al
2O
3 (solid) are similar in
Figure 13 and
Figure 14. When NiO is replaced by V
2O
5, the rate of dissolution is about the same. In oxidizing atmosphere, the rate of drop in Al
2O
3 (solid) is highest. It is known that vanadium can corrode alumina refractories [
5]. Under oxidizing conditions Fe(VO
3)
2 is seen in Tests 7 and 8. This phase was also found in FactSage calculations by Li et al. [
14]. Other oxides used in tests 2 and 5 can be responsible for dissolving alumina from crucible. If nickel did not play any role in dissolving alumina, then the effect would have been more with the addition of vanadium. Thus, nickel can play a significant role in Al
2O
3 refractory degradation. The phases V
2O
3 and FeV
2O
4 appeared in simulations of tests 7 and 8. These phases have been found in petcoke ash experiments by other researchers [
14,
15,
16,
17,
18].
The mechanism for vanadium-alumina interactions were discussed by Jonayat et al. [
8]. Al-O bond energy is much lower than V-O bond energy. Thus, under reducing conditions, V
2O
3 tends to settle in the subsurface of Al
2O
3′s crystal. In rich slags, this Al
2O
3 comes from the oxide mixture, while in deficit slags, it comes from the crucible. This corroborates the observation in this study that addition of alumina to the slag reduced the dissolution of crucible alumina into the slag. Under oxidizing conditions, this phenomenon would be reversed, causing vanadium to segregate to the surface and thus increasing the chances of interaction of those surface atoms with the crucible. This mechanism also explains the higher dissolution of alumina into the slag under oxidizing conditions.
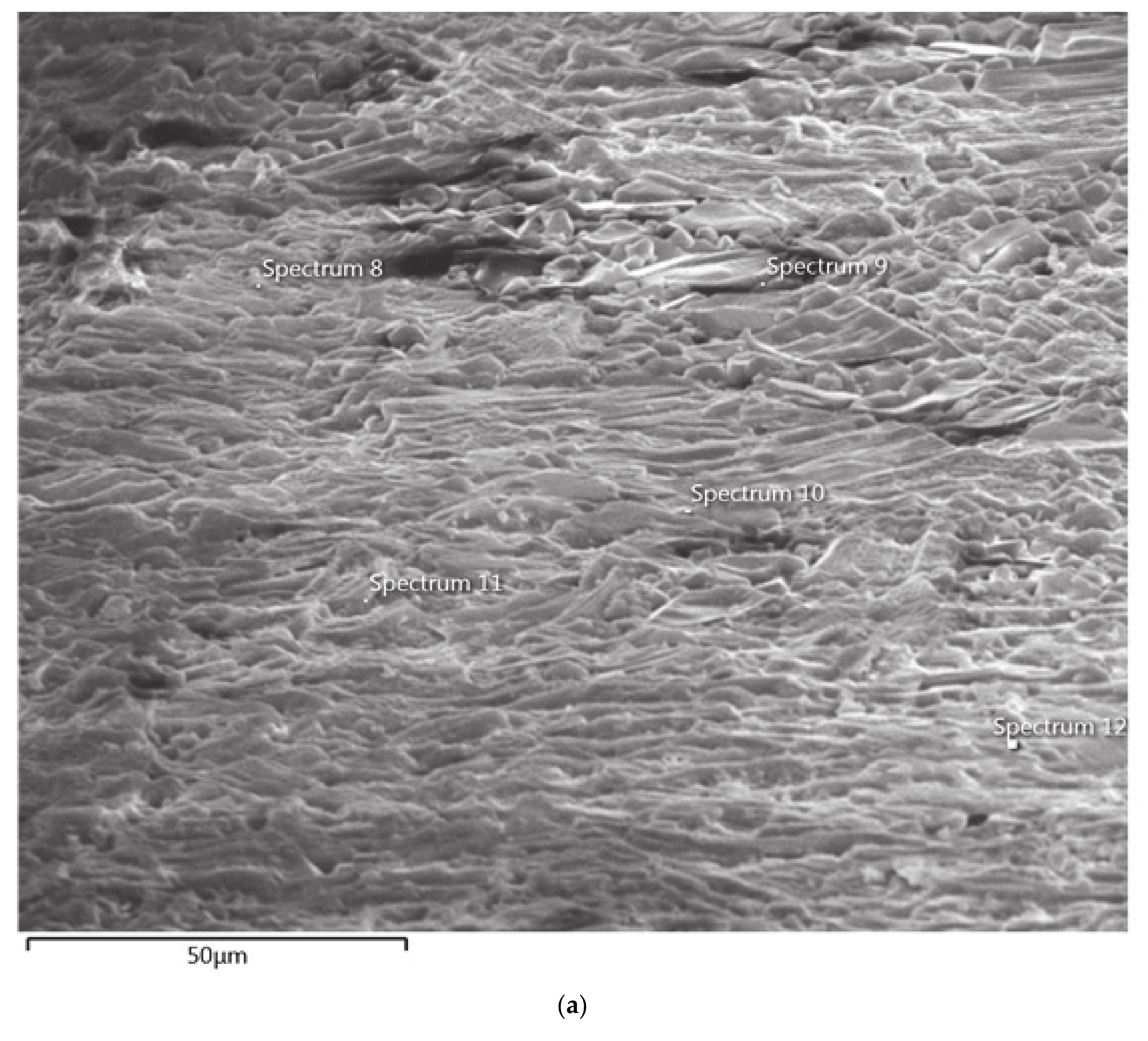
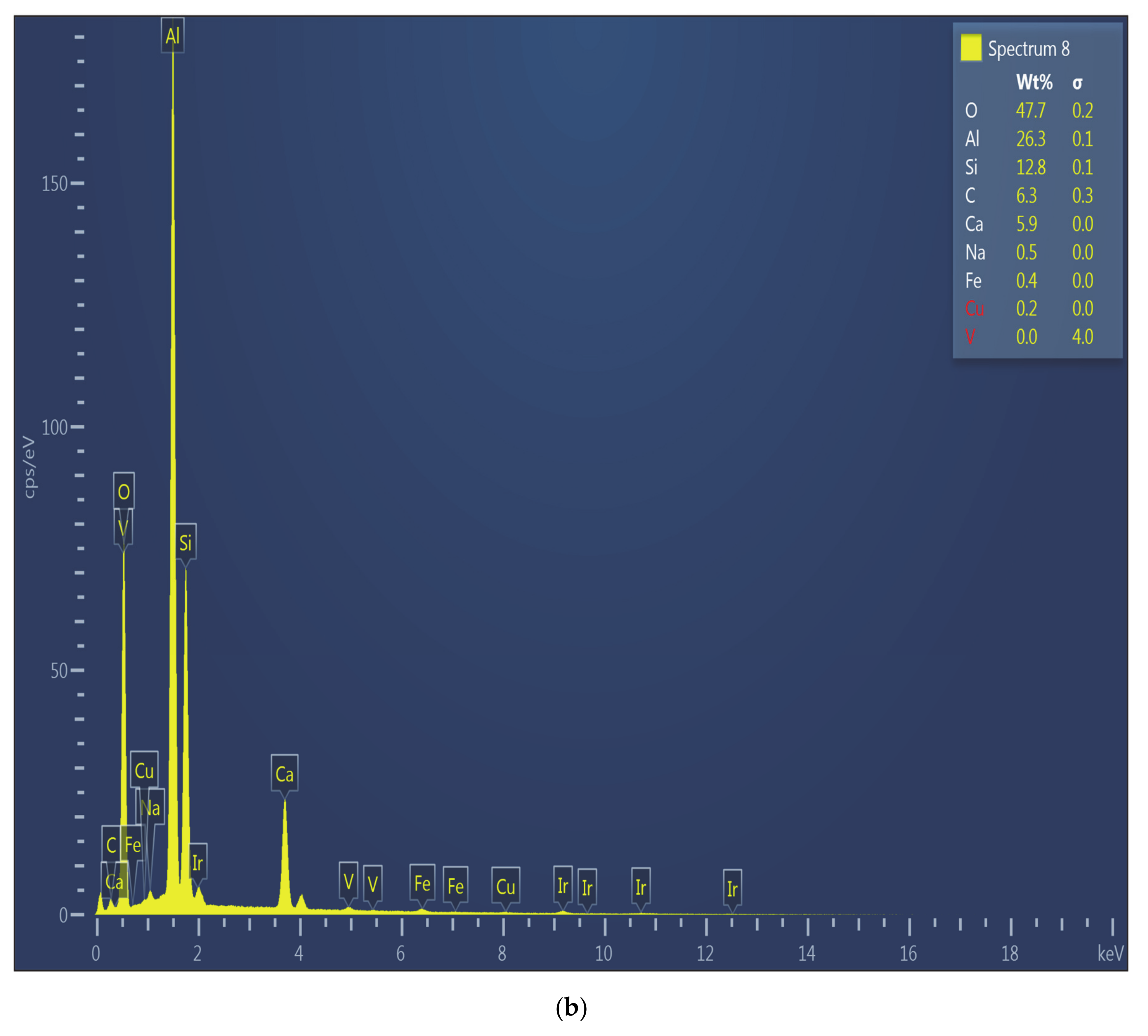
Figure 15 shows the SEM micrograph and EDS spectrum of Zone 1 in Test 6. The wall of the crucible was not involved in any significant interactions with the slag, evidenced by the absence of any interface/multi-component regions (SEM micrograph). Zone 1 composition of aluminum (EDS spectrum) by weight (26.3%) is almost the same as that of the slag composition.
A comparison of XRD patterns of Test 4 and Test 6 (
Figure 16 shows that the plots are quite similar but for the lack of a peak showing vanadium in Test 4 (red plot), while there is evidence of presence of vanadium compound in a phase similar to magnesiocoulsonite (MgV
2O
4) in Test 6. Since FeV
2O
4 and MgV
2O
4 crystals have similar structure and behave in similar ways under high temperatures and reducing atmosphere [
19] and we had no Mg in the starting composition, it may be concluded that this product actually is FeV
2O
4 with Mg being replaced by Fe in the crystal structure. The lack of vanadium peaks in Test 4 could also be explained by the fact that most of the slag from Test 4 sample leaked out from the perforations in the crucible wall, thus depleting vanadium from the test sample.
From the discussion above, we can conclude that 69.5% CO/30.5% CO2 and 5% H2 atmospheres have a similar effect on the oxide mixture. No evidence of V2O3, and detection of anorthite on the XRD plot agrees with the prediction made by FactSage.