Influence of Inhibitory Compounds on Biofuel Production from Oxalate-Rich Rhubarb Leaf Hydrolysates Using Thermoanaerobacter thermohydrosulfuricus Strain AK91
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Media and Organisms
2.2. Collection of Rhubarb Biomass and Preparation of Hydrolysates
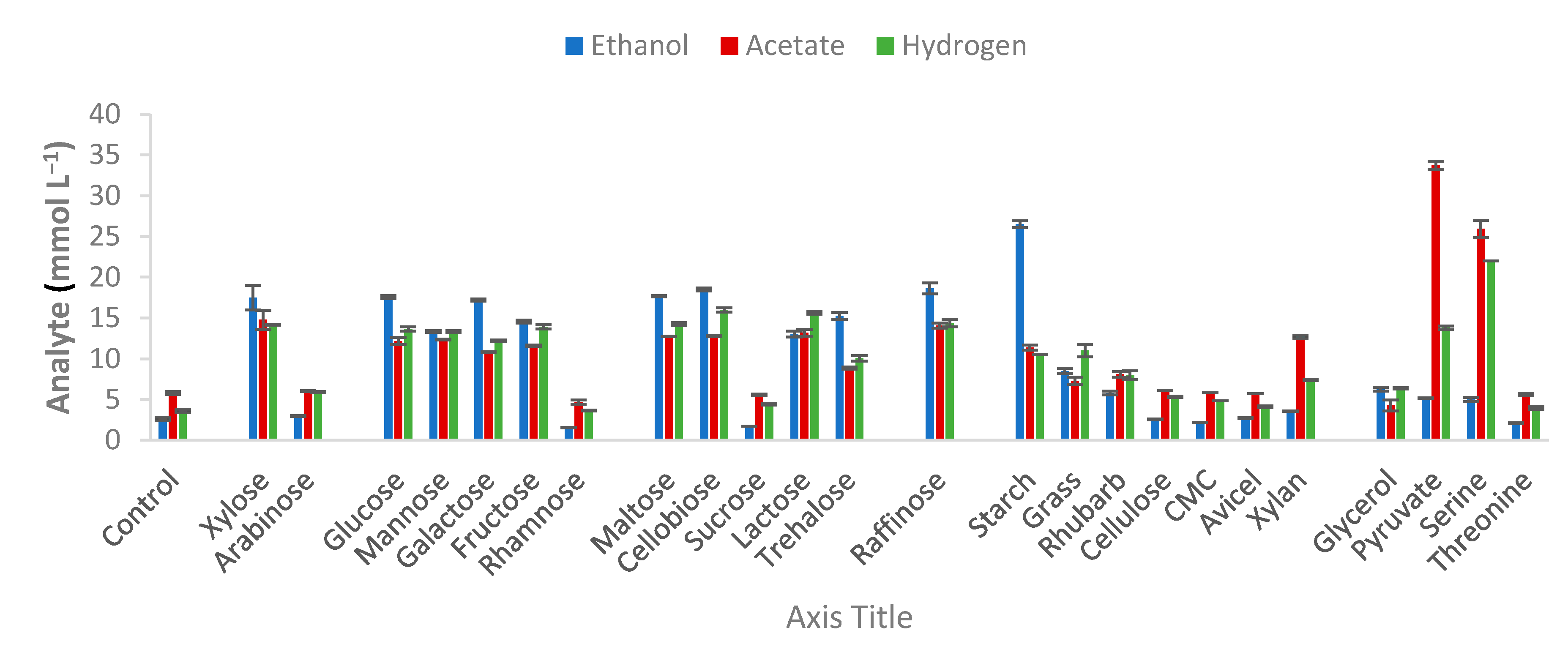
2.3. Characterization and Substrate Spectra
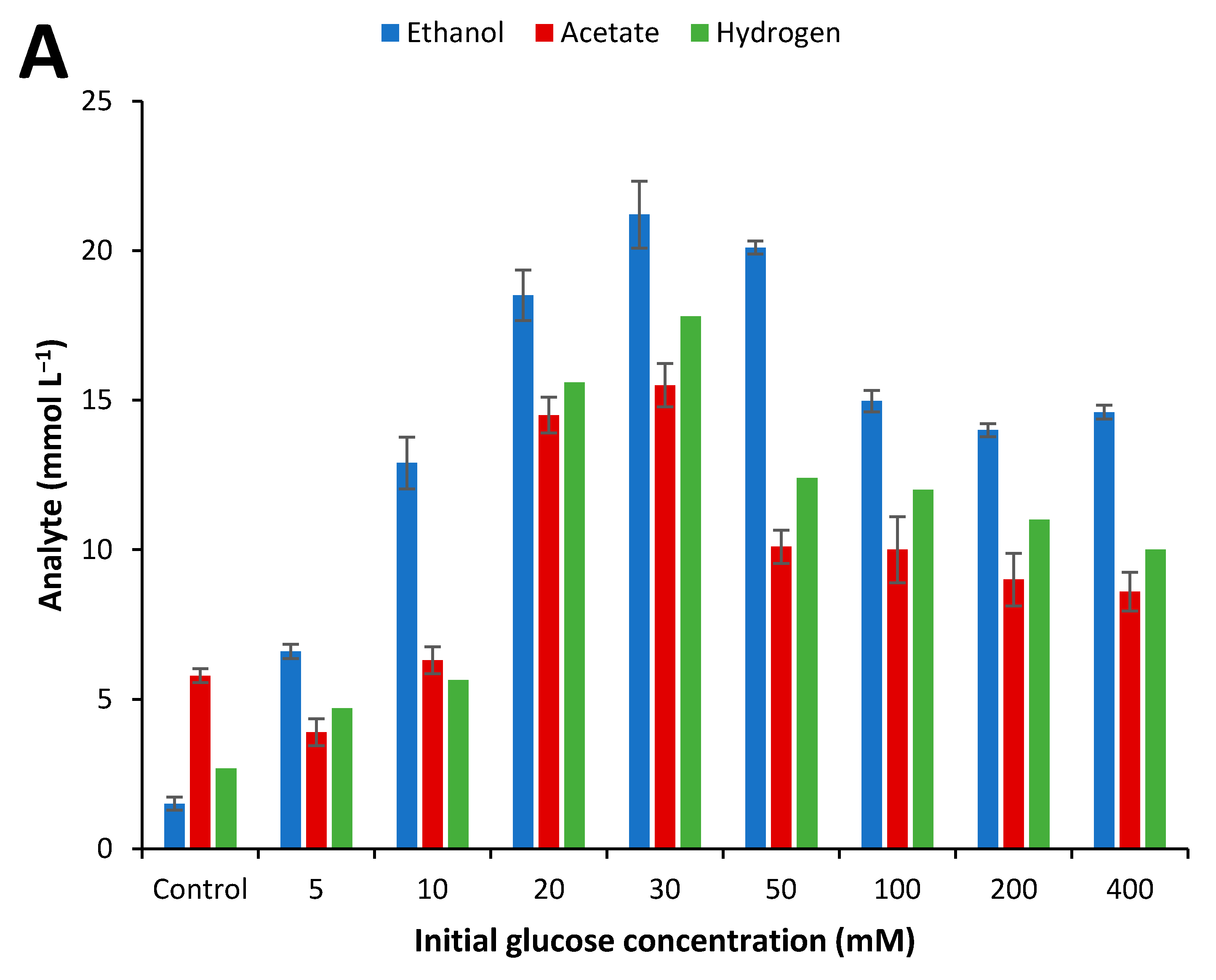
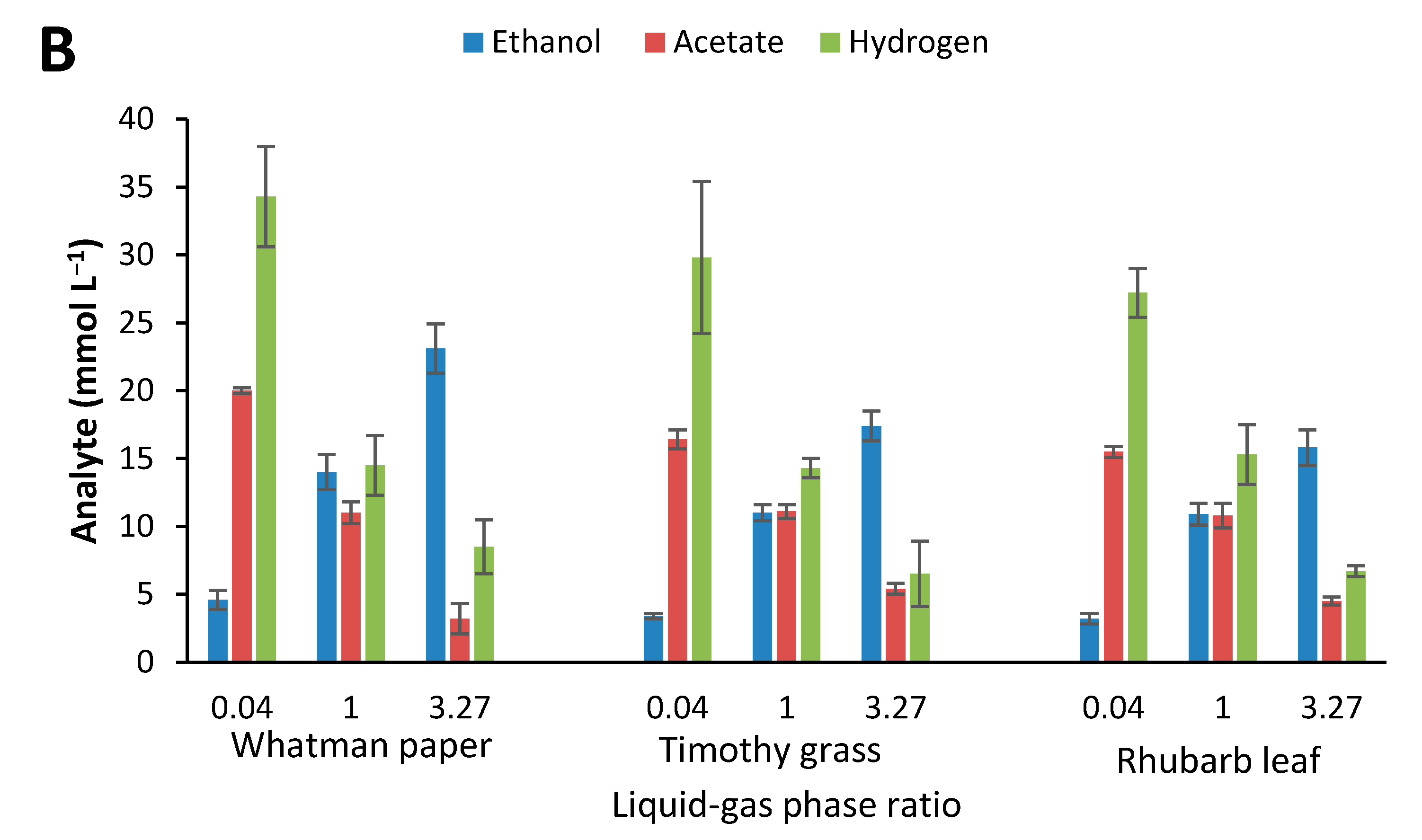
2.4. Influence of Initial Glucose Concentration and Liquid–Gas Phase Ratio
2.5. Effect of Inhibitory Compounds on Glucose Fermentation
2.6. Kinetic Study of Selected Inhibitory Compounds on Glucose Fermentation by Thermoanaerobacter Strain AK91
2.7. Fermentation of Biomass Hydrolysates
2.8. Analytical Methods
3. Results and Discussion
3.1. Biomass Composition
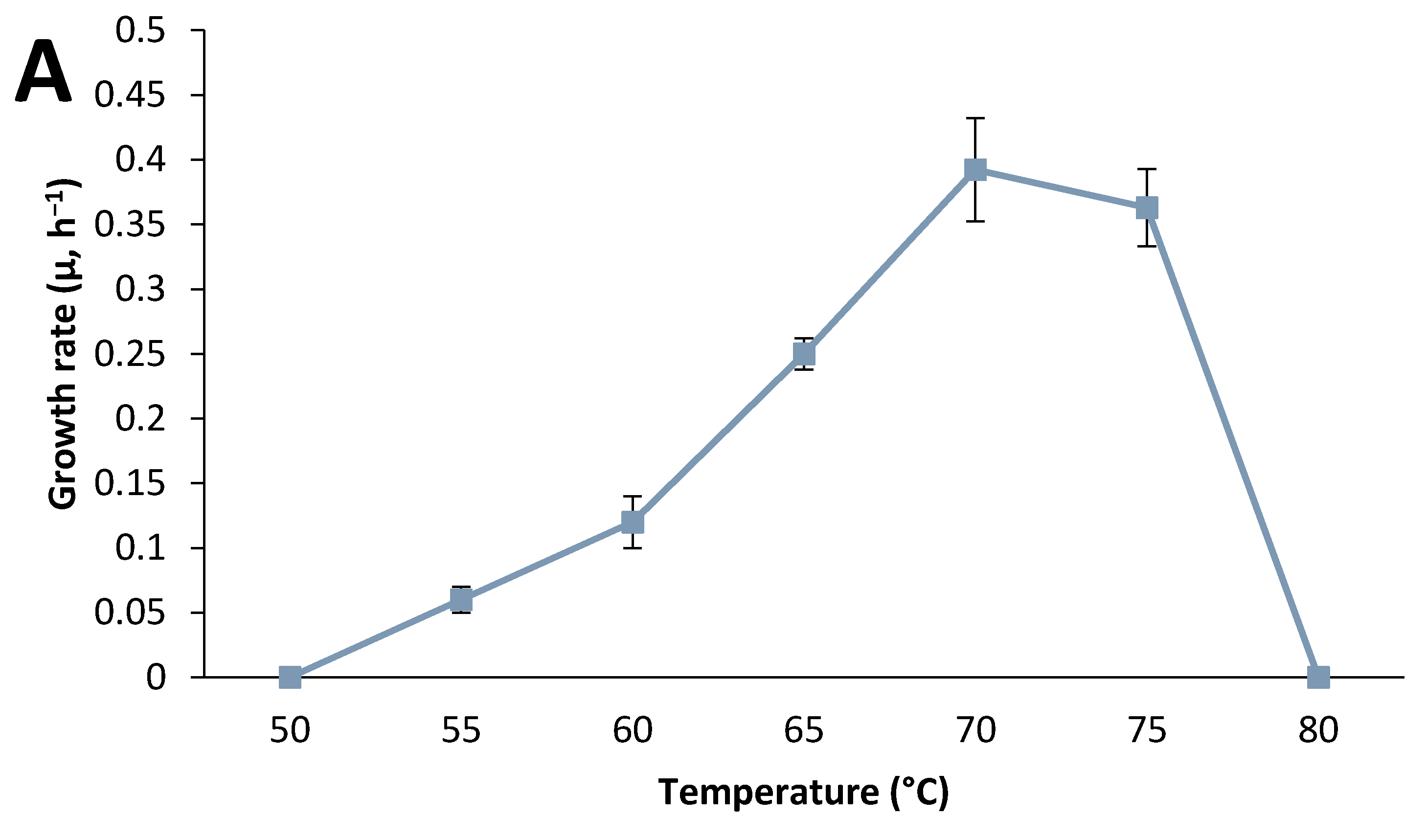
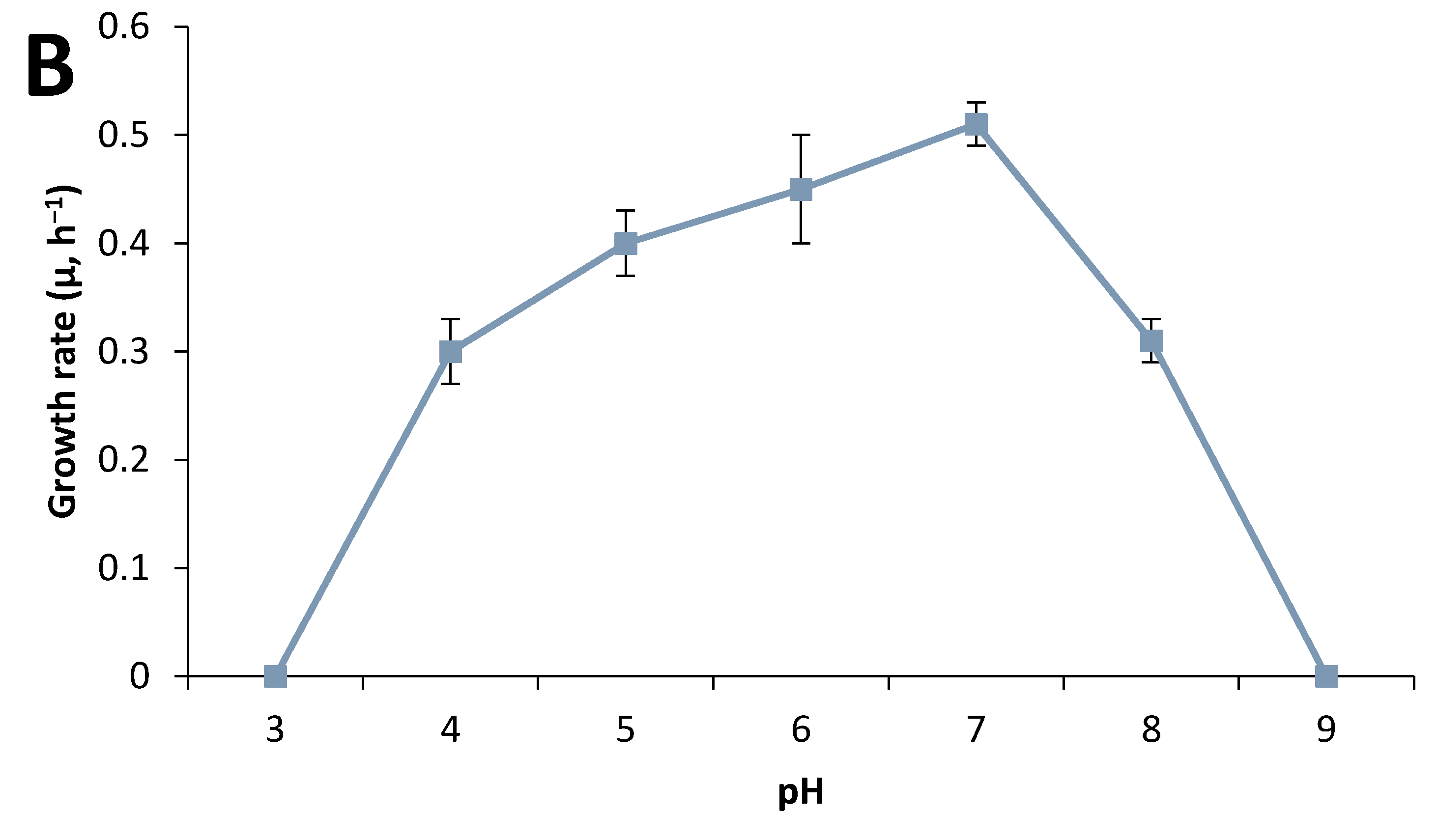
3.2. Strain Characterization
3.3. Effect of Culture Conditions on End-product Formation
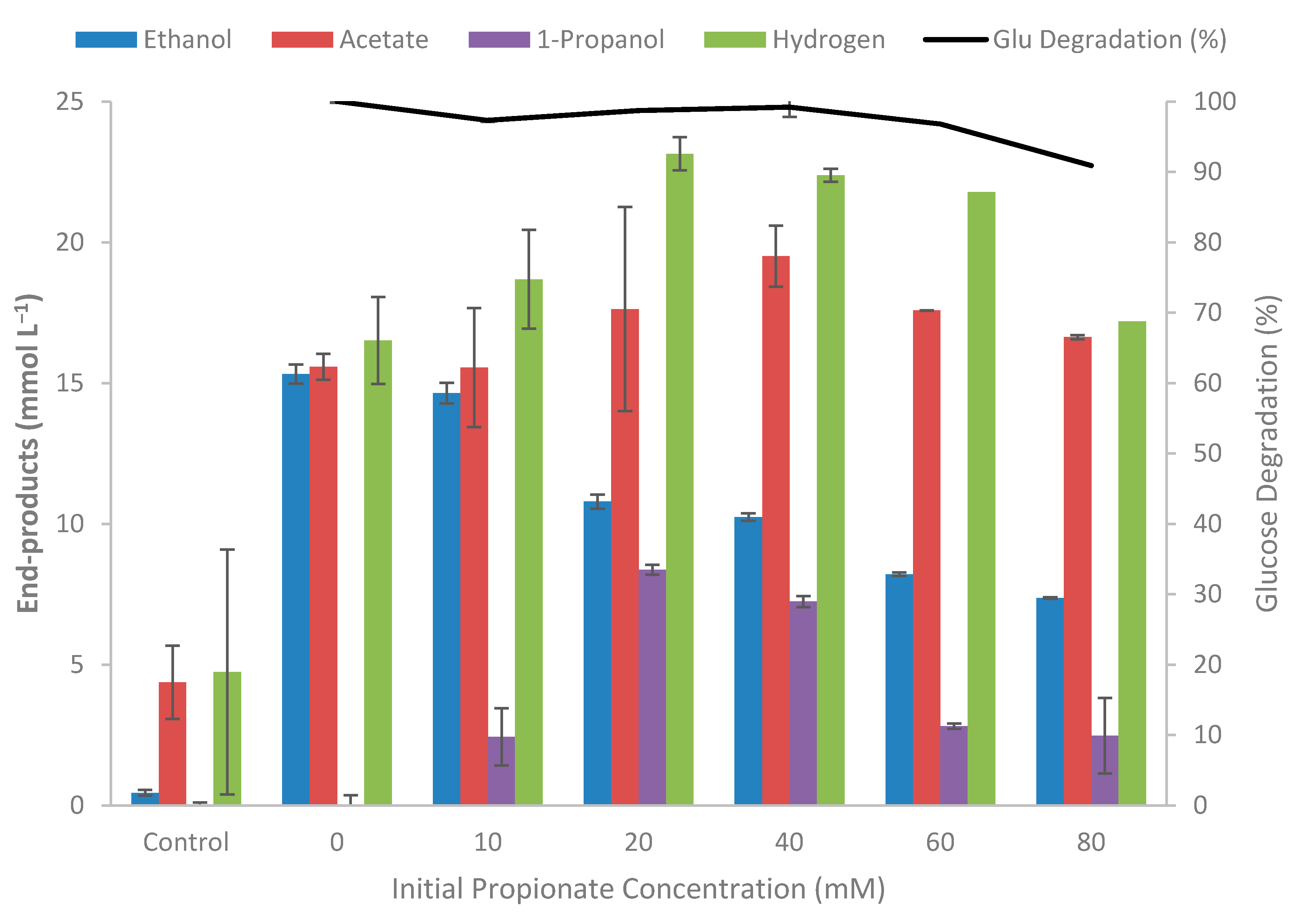
3.4. Effect of Inhibitory Compounds on Growth
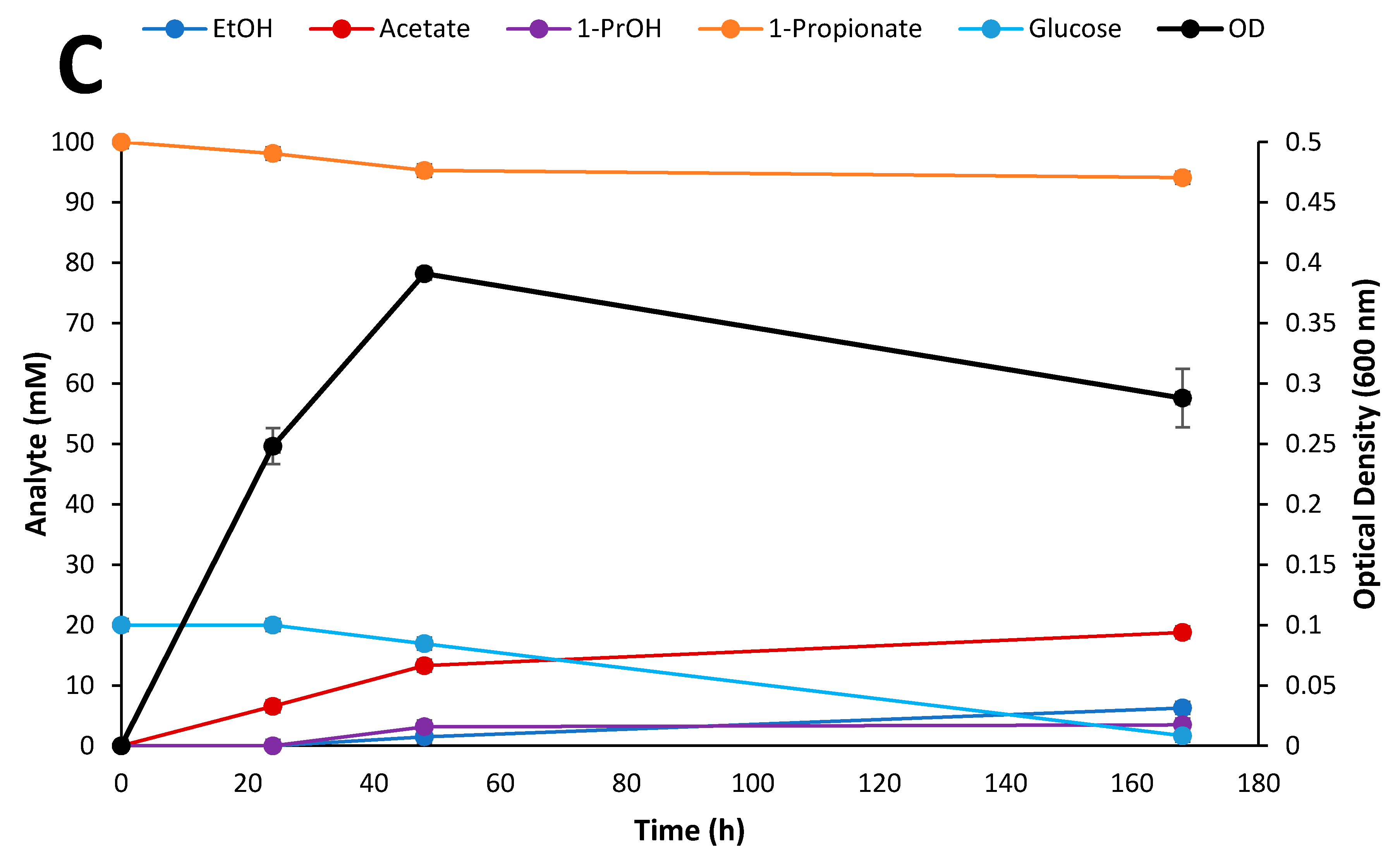
3.5. Kinetic Experiment on Glucose and Propionate
3.6. Fermentation of Biomass Hydrolysates
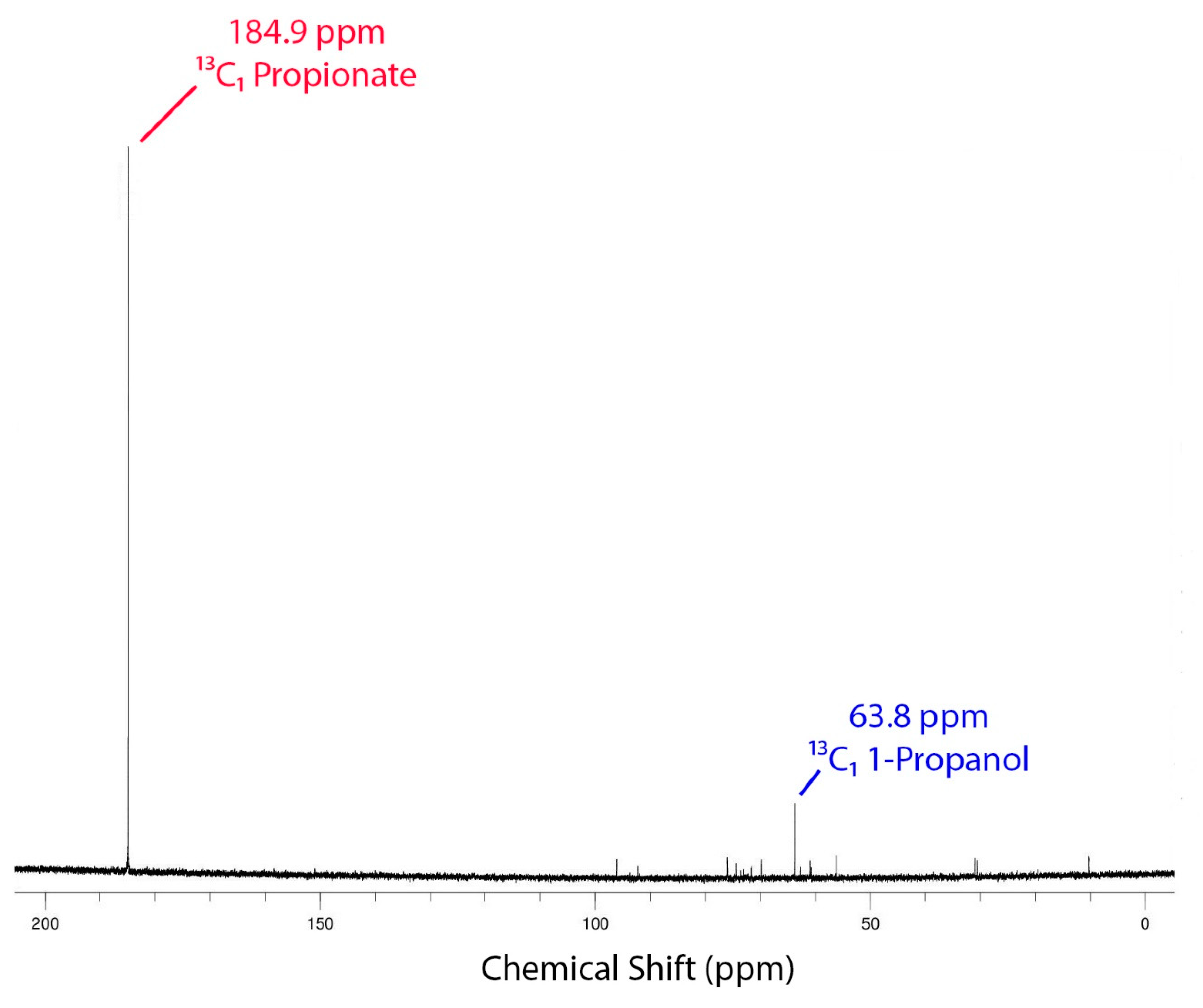
3.7. NMR Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salameh, T.; Tawalbeh, M.; Al-Shannag, M.; Saidan, M.; Melhem, K.B.; Alkasrawi, M. Energy saving in the process of bioethanol production from renewable paper mill sludge. Energy 2020, 196, 117085. [Google Scholar] [CrossRef]
- Alkasrawi, M.; Rudolf, A.; Lidén, G.; Zacchi, G. Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzym. Microb. Technol. 2006, 38, 279–286. [Google Scholar] [CrossRef]
- Alkasrawi, M.; Abu Jrai, A.; Al-Muhtaseb, A.H. Simultaneous saccharification and fermentation process for ethanol production from steam-pretreated softwood: Recirculation of condensate streams. Chem. Eng. J. 2013, 225, 574–579. [Google Scholar] [CrossRef]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic ethanologenesis: Future prospects for second-generation bioethanol production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Onuki, S.; Koziel, J.A.; Jenks, W.S.; Cai, L.; Grewell, D.; van Leeuwen, J.H. Taking ethanol quality beyond fuel grade: A review. J. Inst. Brew. 2016, 122, 588–598. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef]
- Robertson, G.P.; Hamilton, S.K.; Barham, B.L.; Dale, B.E.; Izaurralde, R.C.; Jackson, R.D.; Landis, D.A.; Swinton, S.M.; Thelen, K.D.; Tiedje, J.M. Cellulosic biofuel contributions to a sustainable energy future: Choices and outcomes. Science 2017, 356, eaal2324. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Modig, T. Kinetics and inhibition effects of furfural and hydroxymethyl furfural on enzymes in yeast. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Pucher, G.W.; Wakeman, A.J.; Vickery, H.B. The Organic Acids of Rhubarb (Rheum hybridium). III: The Behavior of the Organic Acids During Culture of Excised Leaves. J. Biol. Chem. 1938, 126, 43–54. [Google Scholar] [CrossRef]
- USDA. USDA Database for Oxalic Acid Content of Selected Vegetables; U.S. Department of Agriculture: Washington, DC, USA, 1984; ISBN 978-1-61583-987-2.
- Ahring, B.K.; Jensen, K.; Nielsen, P.; Bjerre, A.B.; Schmidt, A.S. Pretreament of wheat straw and conversion of xylose and xylan to ethanol by thermophilic anaerobic bacteria. Bioresour. Technol. 1997, 58, 107–113. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Almarsdottir, A.R.; Sigurbjornsdottir, M.A.; Orlygsson, J. Effect of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK 17. Biotechnol. Bioeng. 2012, 109, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Brynjarsdottir, H.; Wawiernia, B.; Orlygsson, J. Ethanol production from sugars and complex biomass by Thermoanaerobacter AK5: The effect of electron-scavenging systems on end-product formation. Energy Fuels 2012, 26, 4568–4574. [Google Scholar] [CrossRef]
- Brynjarsdottir, H.; Scully, S.M.; Orlygsson, J. Production of biohydrogen from sugars and lignocellulosic biomass using Thermoanaerobacter GHL15. Int. J. Hydrog. Energy 2013, 38. [Google Scholar] [CrossRef]
- Vipotnik, Z.; Jessen, J.E.; Scully, S.M.; Orlygsson, J. Effect of culture conditions on hydrogen production by Thermoanaerobacter strain AK68. Int. J. Hydrog. Energy 2016, 41. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Orlygsson, J.; Baldursson, S.R.B. Phylogenetic and physiological studies of four hydrogen-producing thermoanareobes. Icel. Agric. Sci. 2007, 20, 93–105. [Google Scholar]
- Scully, S.M.; Brown, A.; Ross, A.B.; Orlygsson, J. Biotransformation of organic acids to their corresponding alcohols by Thermoanaerobacter pseudoethanolicus. Anaerobe 2019, 57, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Iloranta, P.; Myllymaki, P.; Orlygsson, J. Branched-chain alcohol formation by thermophilic bacteria within the genera of Thermoanaerobacter and Caldanaerobacter. Extremophiles 2015, 19, 809–818. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Jain, M.K.; Lee, C.; Zeikus, J.G. Taxonomic Distinction of Saccharolytic Thermophilic Anaerobes: Description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; Reclassification of Thermoanaerobium. Int. J. Syst. Bacteriol. 1993, 43, 41–51. [Google Scholar] [CrossRef]
- Wagner, I.D.; Wiegel, J. Diversity of thermophilic anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 1–43. [Google Scholar] [CrossRef]
- Wagner, I.D.; Zhao, W.; Zhang, C.L.; Romanek, C.S.; Rohde, M.; Wiegel, J. Thermoanaerobacter uzonensis sp. nov., an anaerobic thermophilic bacterium isolated from a hot spring within the Uzon Caldera, Kamchatka, Far East Russia. Int. J. Syst. Evol. Microbiol. 2008, 58, 2565–2573. [Google Scholar] [CrossRef]
- Tomás, A.F.; Karakashev, D.; Angelidaki, I. Thermoanaerobacter pentosaceus sp. nov., an anaerobic, extreme thermophilic, high ethanol-yielding bacterium isolated from household waste. Int. J. Syst. Evol. Microbiol. 2013, 63, 2396–2404. [Google Scholar] [CrossRef]
- Parte, A.C. LPSN—List of prokaryotic names with standing in nomenclature. Nucleic Acids Res. 2014, 42, D613–D616. [Google Scholar] [CrossRef] [PubMed]
- Hitschler, L.; Kuntz, M.; Langschied, F.; Basen, M. Thermoanaerobacter species differ in their potential to reduce organic acids to their corresponding alcohols. Appl. Microbiol. Biotechnol. 2018, 102, 8465–8476. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Orlygsson, J. Biotransformation of carboxylic acids to alcohols: Characterization of Thermoanaerobacter strain AK152 and 1-propanol production via propionate reduction. Microorganisms 2020, 8, 945. [Google Scholar] [CrossRef]
- Jessen, J.E.J.; Orlygsson, J. Production of ethanol from sugars and lignocellulosic biomass by Thermoanaerobacter J1 isolated from a hot spring in Iceland. J. Biomed. Biotechnol. 2012, 1869–1882. [Google Scholar] [CrossRef]
- Sigurbjornsdottir, M.A.; Orlygsson, J. Combined hydrogen and ethanol production from sugars and lignocellulosic biomass by Thermoanaerobacterium AK54, isolated from hot spring. Appl. Energy 2012, 97, 785–791. [Google Scholar] [CrossRef]
- Georgieva, T.I.; Mikkelsen, M.J.; Ahring, B.K. Ethanol production from wet-exploded wheat straw hydrolysate by thermophilic anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous immobilized reactor. Appl. Biochem. Biotechnol. 2008, 145, 99–110. [Google Scholar] [CrossRef]
- Georgieva, T.I.; Ahring, B.K. Evaluation of continuous ethanol fermentation of dilute-acid corn stover hydrolysate using thermophilic anaerobic bacterium Thermoanaerobacter BG1L1. Appl. Microbiol. Biotechnol. 2007, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.; Thomsen, A.; Ahring, B. Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2001, 57, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.S.; Swamy, M.V.; Seenayya, G. Increased ethanol production by metabolic modulation of cellulose fermentation in Clostridium thermocellum. Biotechnol. Lett. 1997, 19, 819–823. [Google Scholar] [CrossRef]
- Soboh, B.; Linder, D.; Hedderich, R. A multisubunit membrane-bound [NiFe] hydrogenase and an NADH-dependent Fe-only hydrogenase in the fermenting bacterium Thermoanaerobacter tengcongensis. Microbiology 2004, 2451–2463. [Google Scholar] [CrossRef]



| Proximate Analysis (% on a Dry Weight Basis) | ||||
|---|---|---|---|---|
| Biomass | Fat | Protein | Ash | Carbohydrates 1 |
| Rhubarb leaf | 3.29 ± 0.90 | 10.07 ± 2.32 | 10.78 ± 0.19 | 75.87 |
| Timothy grass | 3.73 ± 0.28 | 15.72 ± 0.16 | 5.96 ± 0.04 | 74.59 |
| Whatman paper | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 100.00 |
| Minimum Inhibitory Concentration (mM) | |
|---|---|
| Acetate | >80 mM |
| Propionate | >80 mM |
| n-Butyrate | >80 mM |
| Lactate | >50 mM |
| Ethanol | >100 mM |
| Malate | >40 mM |
| Oxalate | >80 mM |
| Levulinic acid | >20 mM |
| p-Coumaric acid | <10 mM |
| 2-furfuraldehyde | <20 mM |
| 5-HMF | <30 mM |
| Organisms | Substrate | Conc. (g L−1) | Pre-treatment | Ethanol Yields (mM g−1) | References |
|---|---|---|---|---|---|
| Thermoanaerobacter strain AK 91 | Timothy grass | 2.5 | Ac/E | 7.0 | This study |
| Thermoanaerobacter strain AK 91 | Rhubarb leaf | 2.5 | Ac/E | 6.3 | This study |
| Clostridium thermocellum | Paddy straw | 8.0 | None | 6.10–8.00 | [34] |
| Thermoanaerobacter mathranii | Wheat straw | 6.7 | WO/E | 2.61 | [33] |
| Thermoanaerobacter BG1L1 | Corn stover | 25.0–150.0 | WO/E | 8.50–9.20 | [32] |
| Thermoanaerobacter BG1L1 | Wheat straw | 30.0–120.0 | WO/E | 8.50–9.20 | [31] |
| Thermoanaerobacter strain J1 | Hemp | 4.5 | Ac/E | 4.3 | [29] |
| Thermoanaerobacterium strain AK17 | Grass | 2.5 | Ac/Alk/E | 5.5 | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlygsson, J.; Scully, S.M. Influence of Inhibitory Compounds on Biofuel Production from Oxalate-Rich Rhubarb Leaf Hydrolysates Using Thermoanaerobacter thermohydrosulfuricus Strain AK91. Fuels 2021, 2, 71-86. https://doi.org/10.3390/fuels2010005
Orlygsson J, Scully SM. Influence of Inhibitory Compounds on Biofuel Production from Oxalate-Rich Rhubarb Leaf Hydrolysates Using Thermoanaerobacter thermohydrosulfuricus Strain AK91. Fuels. 2021; 2(1):71-86. https://doi.org/10.3390/fuels2010005
Chicago/Turabian StyleOrlygsson, Johann, and Sean Michael Scully. 2021. "Influence of Inhibitory Compounds on Biofuel Production from Oxalate-Rich Rhubarb Leaf Hydrolysates Using Thermoanaerobacter thermohydrosulfuricus Strain AK91" Fuels 2, no. 1: 71-86. https://doi.org/10.3390/fuels2010005
APA StyleOrlygsson, J., & Scully, S. M. (2021). Influence of Inhibitory Compounds on Biofuel Production from Oxalate-Rich Rhubarb Leaf Hydrolysates Using Thermoanaerobacter thermohydrosulfuricus Strain AK91. Fuels, 2(1), 71-86. https://doi.org/10.3390/fuels2010005







