Development of Water-Developable Negative Photoresist for i-Line Photolithography Using Cellulose Derivatives with Underlayer
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of HPC-Derived Polymers
2.2. Exposure Sensitivity Measurement
2.3. Selection of Underlayers
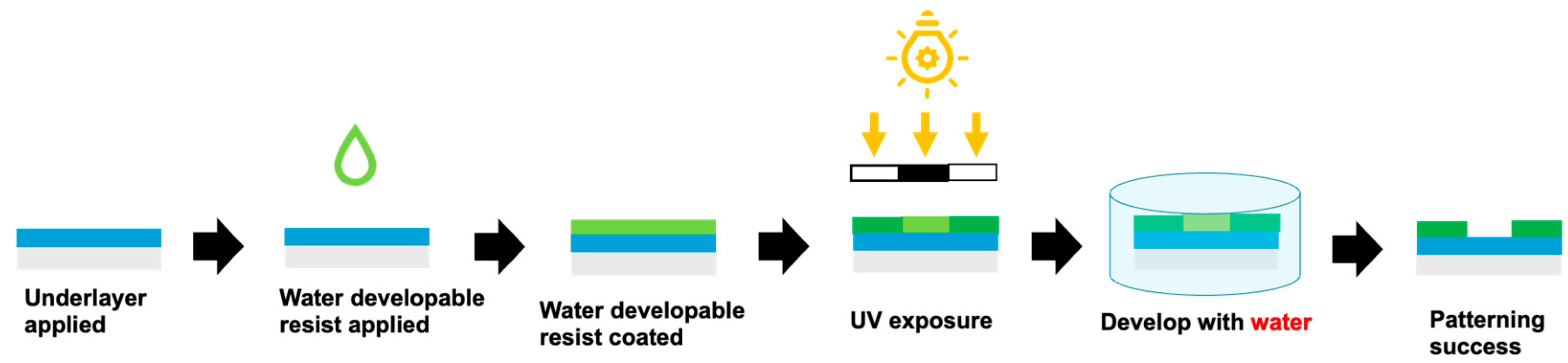
2.4. Lithography and Micropatterning
2.5. Etching Rate Measurement
3. Result and Discussion
3.1. Exposure Sensitivity Measurement
3.2. Selection of Underlayers
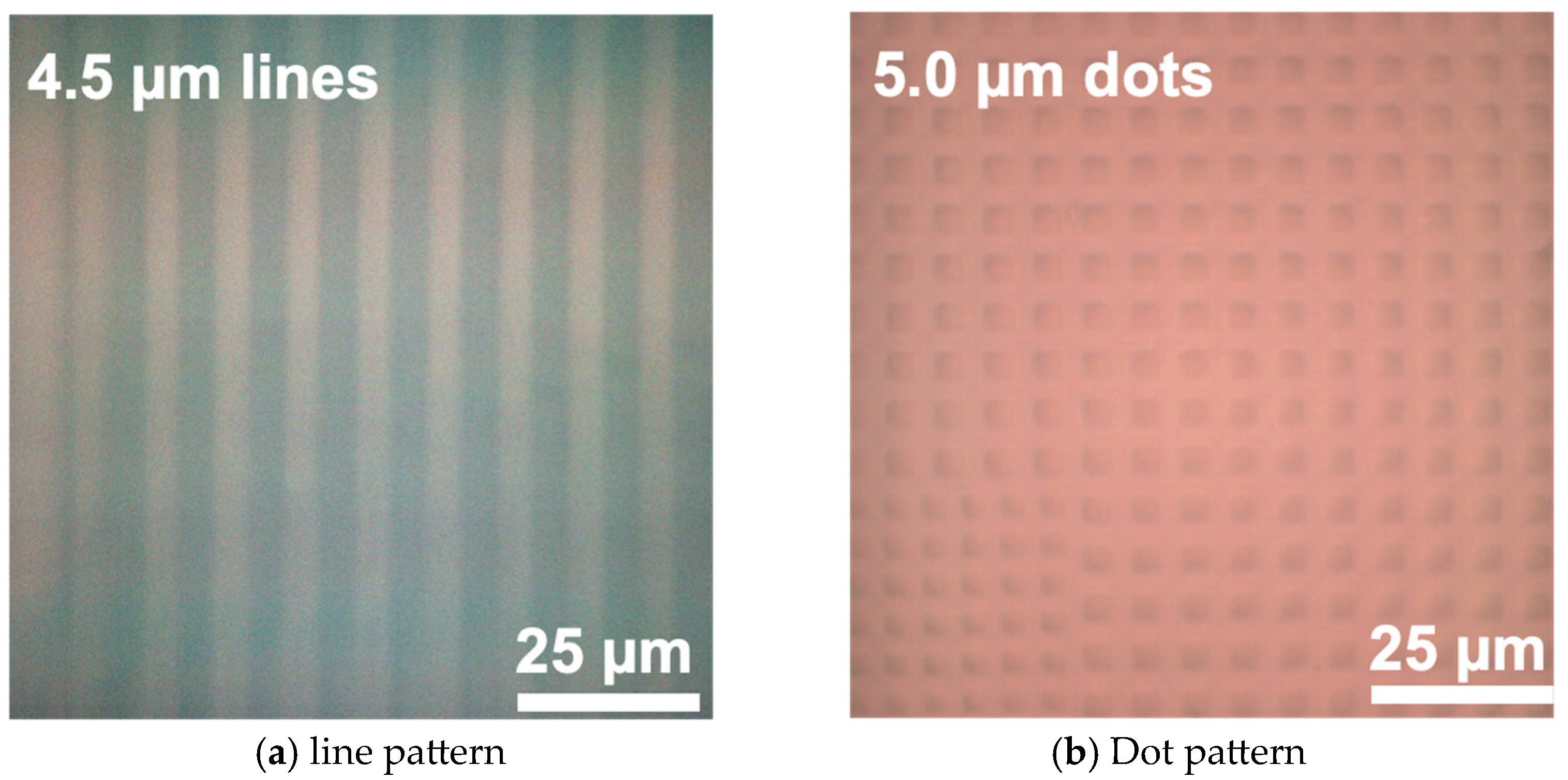
3.3. Micropatterning Evaluation
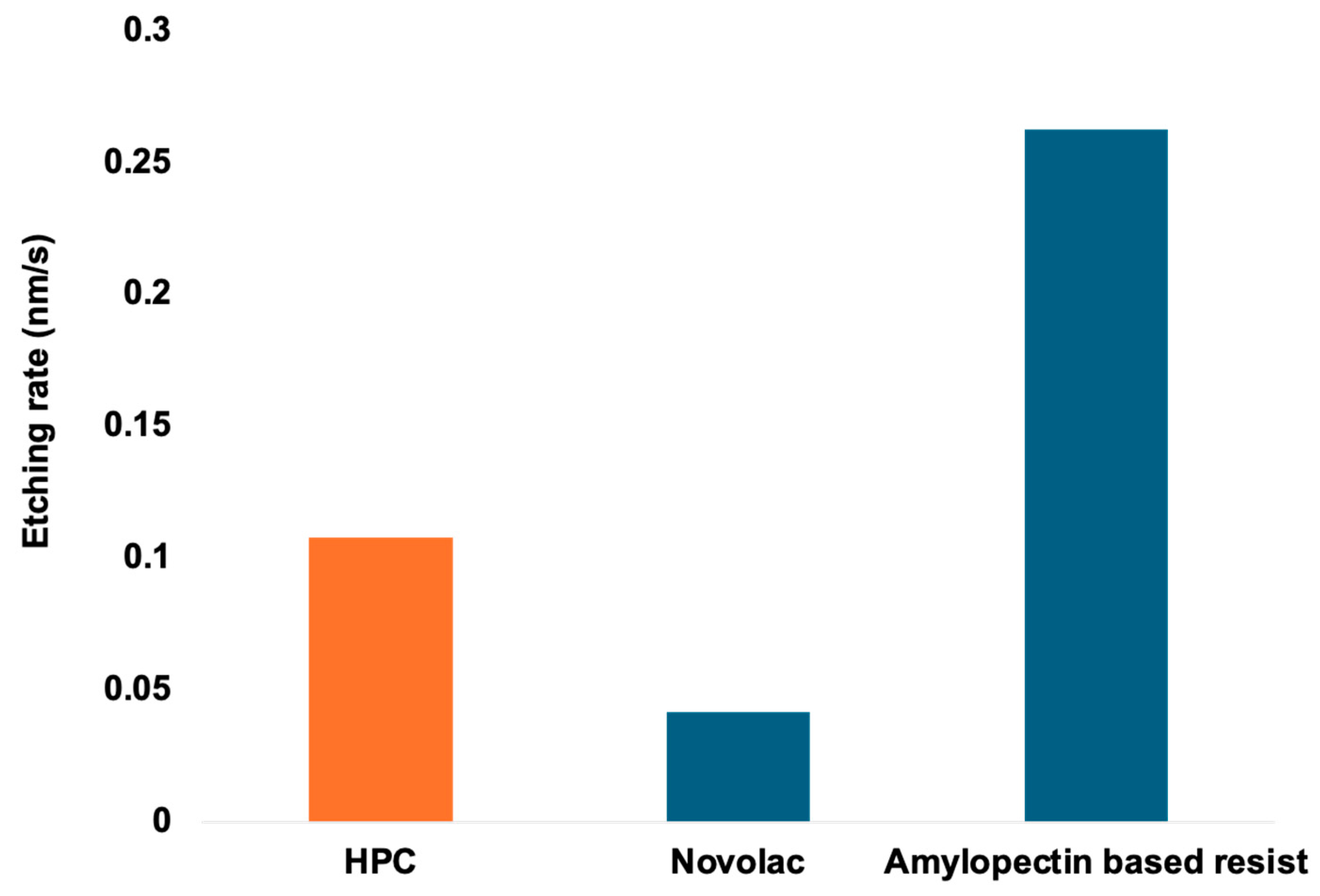
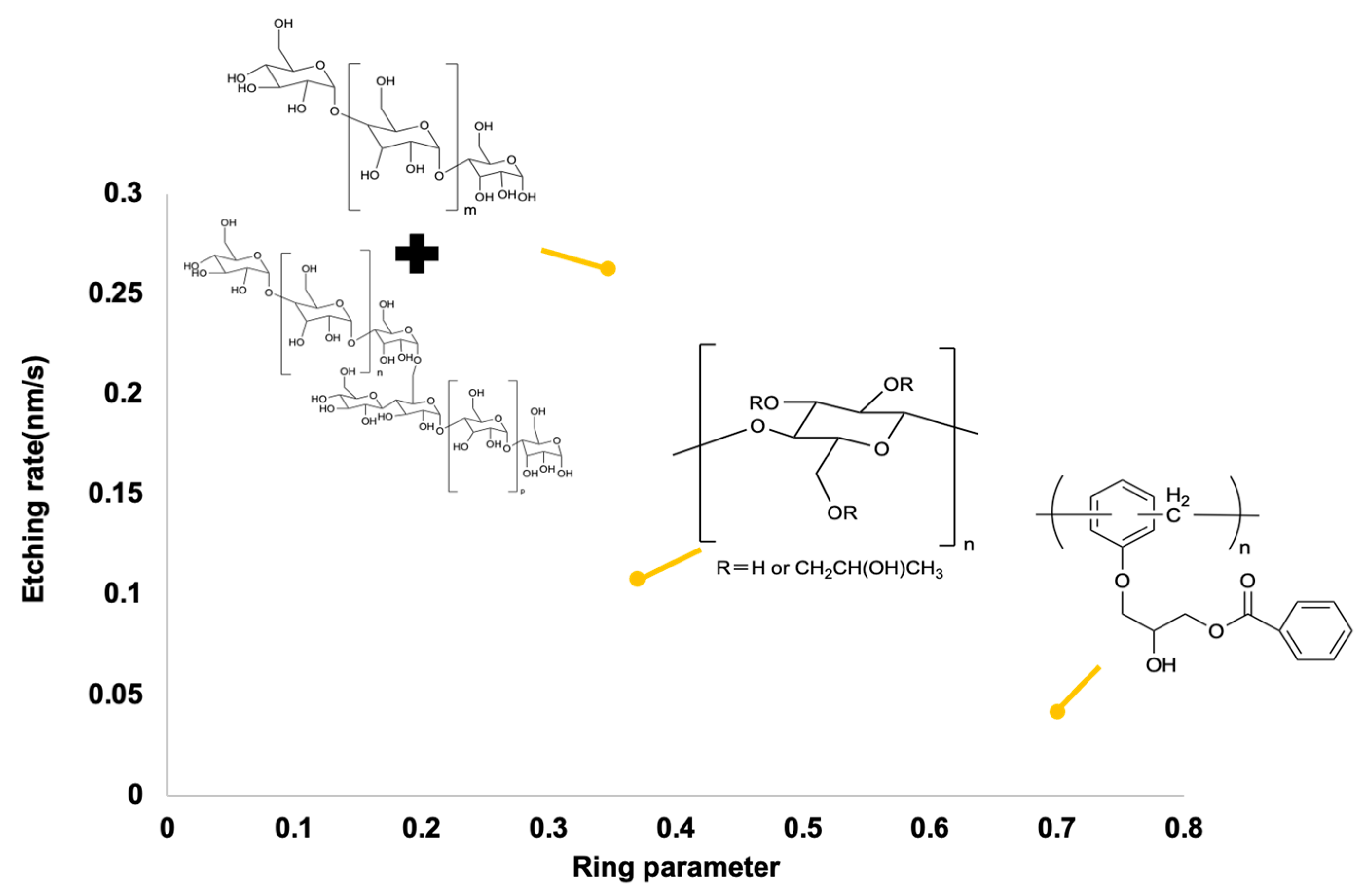
3.4. Etching Rate Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, C.; Xu, C.; Lv, L.; Li, H.; Huang, X.; Liu, W. Review of recent advances in inorganic photoresists. RSC Adv. 2020, 10, 8385–8395. [Google Scholar] [CrossRef]
- Yang, D.X.; Frommhold, A.; McClelland, A.; Roth, J.; Rosamond, M.; Linfield, E.H.; Robinson, A.P.G. Performance of a high resolution chemically amplified electron beam resist at various beam energies. Microelectron. Eng. 2016, 155, 97–101. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, J.A.; Park, H.H.; Yi, G.Y.; Chung, K.J.; Park, H.D.; Lee, I.S. Exposure to volatile organic compounds and possibility of exposure to by-product volatile organic compounds in photolithography processes in semiconductor manufacturing factories. Saf. Health Work. 2011, 2, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Havard, J.M.; Shim, S.Y.; Fréchet, J.M.; Lin, Q.; Medeiros, D.R.; Willson, C.G.; Byers, J.D. Design of photoresists with reduced environmental impact. 1. Water-soluble resists based on photo-cross-linking of poly (vinyl alcohol). Chem. Mater. 1999, 11, 719–725. [Google Scholar] [CrossRef]
- Chiang, S.Y.; Wei, C.C.; Chiang, T.H.; Chen, W.L. How can electronics industries become green manufacturers in Taiwan and Japan. Clean Technol. Environ. Policy 2011, 13, 37–47. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Electrodialysis process for the recycling and concentrating of tetramethylammonium hydroxide (TMAH) from photoresist developer wastewater. Ind. Eng. Chem. Res. 2013, 52, 18356–18361. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Doyle, A.M.; O’Dwyer, T.F.; Hodnett, B.K. Preparation and use of a mesoporous silicate material for the removal of tetramethyl ammonium hydroxide (TMAH) from aqueous solution. J. Chem. Technol. Biotechnol. 2001, 76, 1216–1222. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.; You, K.H.; Shin, H.C.; Kim, H.O. Tetramethylammonium hydroxide poisoning during a pallet cleaning demonstration. J. Occup. Health 2013, 55, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Inoue, K. Re-evaluation of the reduced heart weights in male rats in a 28-day oral repeated-dose toxicity study of tetramethylammonium hydroxide. Regul. Toxicol. Pharmacol. 2024, 153, 105712. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Huang, H.L.; Lin, Y.C.; Hsiao, P.Z.; Chang, C.W.; Liao, P.C. Determination of tetramethylammonium hydroxide in air using ion chromatography or ultra-performance liquid chromatography–high-resolution mass spectrometry. J. Environ. Exposure Assess. 2024, 3, 21. [Google Scholar] [CrossRef]
- Wu, C.L.; Su, S.B.; Chen, J.L.; Lin, H.J.; Guo, H.R. Mortality from dermal exposure to tetramethylammonium hydroxide. J. Occup. Health 2008, 50, 99–102. [Google Scholar] [CrossRef]
- Kawashima, A.; Inoue, K.; Ushida, K.; Kai, K.; Yoshida-Yamashita, L.S.; Masumura, K. Derivation of human health hazard assessment values for tetramethylammonium hydroxide (TMAH) under the Japan Chemical Substances Control Law. Fundam. Toxicol. Sci. 2024, 11, 267–278. [Google Scholar] [CrossRef]
- Innocenzi, V.; Zueva, S.B.; Vegliò, F.; De Michelis, I. Pilot-scale experiences with aerobic treatment and chemical processes of industrial wastewaters from electronics and semiconductor industry. Energies 2021, 14, 5340. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Choi, Y.; Lee, S. Treatment of semiconductor wastewater containing tetramethylammonium hydroxide (TMAH) using nanofiltration, reverse osmosis, and membrane capacitive deionization. Membranes 2023, 13, 336. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Amato, A.; Ferella, F.; Prisciandaro, M.; Beolchini, F.; Vegliò, F.; Innocenzi, V. The application of the life cycle assessment and life cycle costing for the treatment of microelectronic industry effluents. Case Stud. Chem. Environ. Eng. 2024, 10, 100854. [Google Scholar] [CrossRef]
- Shibata, J.; Murayama, N.; Matsumoto, S. Recovery of tetramethyl ammonium hydroxide from waste solution by ion exchange resin. Resour. Process. 2006, 53, 199–203. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Yoshinaga, K.; Wu, J.H.; Chen, W.Y.; Terashima, M.; Goel, R.; Yasui, H. Kinetic analysis of biological degradation for tetramethylammonium hydroxide (TMAH) in the anaerobic activated sludge system at ambient temperature. Biochem. Eng. J. 2016, 114, 42–49. [Google Scholar] [CrossRef]
- Cao, C.B.; Zhou, C.; Sun, X.; Gao, J.P.; Wang, Z.Y. Photo-induced crosslinking of water-soluble polymers with a new photobase generator. Polymer 2010, 51, 4058–4062. [Google Scholar] [CrossRef]
- Sim, K.M.; Yoon, S.; Kim, S.K.; Ko, H.; Hassan, S.Z.; Chung, D.S. Surfactant-induced solubility control to realize water-processed high-precision patterning of polymeric semiconductors for full color organic image sensor. ACS Nano 2019, 14, 415–421. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, B.; Kumar, G. A simple approach for the synthesis of cellulose nanofiber reinforced chitosan/PVP bio nanocomposite film for packaging. J. Polym. Environ. 2019, 27, 2963–2973. [Google Scholar] [CrossRef]
- Sysova, O.; Durin, P.; Gablin, C.; Léonard, D.; Téolis, A.; Trombotto, S.; Soppera, O. Chitosan as a water-developable 193 nm photoresist for green photolithography. ACS Appl. Polym. Mater. 2022, 4, 4508–4519. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, J.; Li, C.; Zhang, L.; Zhang, X.; Yang, P. Water-Based Photo- and Electron-Beam Lithography Using Egg White as a Resist. Adv. Mater. Interfaces 2017, 4, 1601223. [Google Scholar] [CrossRef]
- Weißenborn, E.; Braunschweig, B. Hydroxypropyl cellulose as a green polymer for thermo-responsive aqueous foams. Soft Matter 2019, 15, 2876–2883. [Google Scholar] [CrossRef]
- Leminen, V.; Ovaska, S.S.C.; Tanninen, P.; Varis, J. Convertability and oil resistance of paperboard with hydroxypropyl-cellulose-based dispersion barrier coatings. J. Appl. Packag. Res. 2015, 7, 92–100. [Google Scholar]
- Le Dare, B.; Gicquel, T. Therapeutic applications of ethanol: A review. J. Pharm. Pharm. Sci. 2019, 22, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Lugani, Y.; Chandel, A.K.; Rai, R.; Kumar, S. Production of first- and second-generation ethanol for use in alcohol-based hand sanitizers and disinfectants in India. Biomass Convers. Biorefinery 2023, 13, 7423–7440. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.L.S.; Fighera, T.M.; Miasaki, F.; Mesa, C.O.; Paz Filho, G.J.D.; Graf, H.; Carvalho, G.A.D. Evaluation of percutaneous ethanol injections in benign thyroid nodules. Arq. Bras. Endocrinol. Metabol. 2014, 58, 912–917. [Google Scholar] [CrossRef][Green Version]
- Jeong, W.K.; Baek, J.H.; Rhim, H.; Kim, Y.S.; Kwak, M.S.; Jeong, H.J.; Lee, D. Radiofrequency ablation of benign thyroid nodules: Safety and imaging follow-up in 236 patients. Eur. Radiol. 2008, 18, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.H.; Hui, E.P.; Tang, P.; Chan, S.K.C.; Chu, C.C.M.; Hui, J.W.Y.; Yeo, W. Transarterial ethanol ablation for unresectable hepatocellular carcinoma: Analysis of clinical and tumor outcomes. J. Vasc. Interv. Radiol. 2016, 27, 639–649. [Google Scholar] [CrossRef][Green Version]
- Maier, A.; Ovesen, J.L.; Allen, C.L.; York, R.G.; Gadagbui, B.K.; Kirman, C.R.; Quinones-Rivera, A. Safety assessment for ethanol-based topical antiseptic use by health care workers: Evaluation of developmental toxicity potential. Regul. Toxicol. Pharmacol. 2015, 73, 248–264. [Google Scholar] [CrossRef]
- Miura, S.; Hachikubo, Y.; Yamagishi, R.; Ando, M.; Takei, S. Water-soluble biomass resist materials based on polyglucuronic acid for eco-friendly photolithography. Coatings 2023, 13, 2038. [Google Scholar] [CrossRef]
- Russom, C.L.; Drummond, R.A.; Huffman, A.D. Acute toxicity and behavioral effects of acrylates and methacrylates to juvenile fathead minnows. Bull. Environ. Contam. Toxicol. 1988, 41, 539–596. [Google Scholar] [CrossRef]
- Amano, T.; Kobayasi, M.; Takei, S. Effect of Sugar Chain Binding Mode on Water-soluble Micropatterning Performance and Physical Characteristics. J. Photopolym. Sci. Technol. 2021, 34, 187–193. [Google Scholar] [CrossRef]
- Sugane, K.; Mishima, T.; Shibata, M. Biobased epoxy nanocomposites composed of sorbitol polyglycidyl ether, biobased carboxylic acids and microfibrillated cellulose. J. Polym. Res. 2021, 28, 257. [Google Scholar] [CrossRef]
- Marques, C.; Tarek, R.; Sara, M.; Brar, S.K. Sorbitol production from biomass and its global market. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 217–227. [Google Scholar]
- Bieleski, R.L. Sugar alcohols. In Plant Carbohydrates I: Intracellular Carbohydrates; Springer: Berlin/Heidelberg, Germany, 1982; pp. 158–192. [Google Scholar]
- Hachikubo, Y.; Miura, S.; Yamagishi, R.; Ando, M.; Kobayashi, M.; Ota, T.; Takei, S. Amylopectin-based eco-friendly photoresist material in water-developable lithography processes for surface micropatterns on polymer substrates. J. Photopolym. Sci. Technol. 2023, 36, 197–204. [Google Scholar] [CrossRef]
- Amano, T.; Hirata, D.; Hasegawa, Y.; Takei, S. Evaluation of nano-patterning performance of water-soluble material for photoresist using sugar chain. J. Photopolym. Sci. Technol. 2020, 33, 445–450. [Google Scholar] [CrossRef]
- Deruiter, J. Ethers and Thioethers; William, B., Pratt, P.T., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Yilgör, E.; Burgaz, E.; Yurtsever, E.; Yilgör, I. Comparison of hydrogen bonding in polydimethylsiloxane- and polyether-based urethane and urea copolymers. Polymer 2000, 41, 849–857. [Google Scholar] [CrossRef]
- Ung, H.U.; Moehlig, A.R.; Khodagholian, S.; Berden, G.; Oomens, J.; Morton, T.H. Proton-bridge motions in amine conjugate acid ions having intramolecular hydrogen bonds to hydroxyl and amine groups. J. Phys. Chem. A 2013, 117, 1360–1369. [Google Scholar] [CrossRef]
- Amano, T.; Kobayasi, M.; Takei, S. Micropatterning Performance and Physical Characteristics of Water-soluble High Molecular Weight Polysaccharide Photoresist Materials. J. Photopolym. Sci. Technol. 2021, 34, 181–186. [Google Scholar] [CrossRef]
- Dammel, R.R. New developments in high-performance resist materials. J. Photopolym. Sci. Technol. 1998, 11, 687–703. [Google Scholar] [CrossRef]
- Hachikubo, Y.; Miura, S.; Yamagishi, R.; Ando, M.; Takei, S. Photoresist for Water-developable Photolithography Process Using Plant-derived Hemicellulose. J. Photopolym. Sci. Technol. 2024, 37, 363–370. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, H.; Hachikubo, Y.; Ando, M.; Oshima, M.; Morita, M.; Takei, S. Development of Water-Developable Negative Photoresist for i-Line Photolithography Using Cellulose Derivatives with Underlayer. Electron. Mater. 2025, 6, 13. https://doi.org/10.3390/electronicmat6040013
Hayashi H, Hachikubo Y, Ando M, Oshima M, Morita M, Takei S. Development of Water-Developable Negative Photoresist for i-Line Photolithography Using Cellulose Derivatives with Underlayer. Electronic Materials. 2025; 6(4):13. https://doi.org/10.3390/electronicmat6040013
Chicago/Turabian StyleHayashi, Hiryu, Yuna Hachikubo, Mano Ando, Misaki Oshima, Mayu Morita, and Satoshi Takei. 2025. "Development of Water-Developable Negative Photoresist for i-Line Photolithography Using Cellulose Derivatives with Underlayer" Electronic Materials 6, no. 4: 13. https://doi.org/10.3390/electronicmat6040013
APA StyleHayashi, H., Hachikubo, Y., Ando, M., Oshima, M., Morita, M., & Takei, S. (2025). Development of Water-Developable Negative Photoresist for i-Line Photolithography Using Cellulose Derivatives with Underlayer. Electronic Materials, 6(4), 13. https://doi.org/10.3390/electronicmat6040013







